Abstract
We measured the developmental time course for temporal contrast sensitivity in macaque monkeys. The animals, aged 5 wks to 4 yrs, detected an unpatterned field of light sinusoidally modulated over time at frequencies ranging from 1 to 40 Hz. Young infants showed reduced sensitivity for all frequencies, and a reduced range of detectable frequencies. Sensitivity to high and low frequencies developed at different rates, but the shape of the temporal contrast sensitivity function did not change significantly with age. Temporal contrast sensitivity matures earlier than spatial contrast sensitivity. The development of high, but not low, frequency sensitivity may be limited by maturation of the magnocellular pathway.
Keywords: Temporal vision, Visual development, Macaque monkey, Temporal contrast sensitivity
Introduction
Vision is immature at birth in primates and develops over some months or years thereafter. While much is known about the development of spatial vision (see Daw, 1995; Teller, 1997), comparatively little is known of the developmental time course for temporal vision.
A broad descriptor of temporal visual sensitivity is provided by the temporal contrast sensitivity function (tCSF), which relates the observer’s contrast sensitivity to the temporal frequency of the stimulus (DeLange, 1958; 1952). The adult human tCSF is well characterized as band-pass in shape, with a peak at intermediate temporal frequencies. There is a gradual fall off at low temporal frequencies and a steep fall off at higher temporal frequencies to the high frequency cut-off, known as the critical flicker frequency (CFF). Many parameters have been shown to influence the shape of the tCSF (see DeLange, 1958; Kelly, 1971; Kelly, 1972; Merigan, 1980; Snowden, Hess, & Waugh, 1995).
Few studies have investigated temporal contrast sensitivity development in human infants, and the existing data are conflicting. Initially, the CFF was measured in attempt to characterize the development of temporal vision (Horsten & Winkelman, 1964; Regal, 1981; see also, Banks, 1983). Regal (1981) made direct, behavioral measurements of high frequency flicker sensitivity in 1, 2, and 3 month-olds and found that CFF approaches adult levels as early as age 3 months. Subsequent studies have estimated the CFF by extrapolation rather than by direct measurement. In these studies, CFF was estimated by extrapolation from measured sensitivity to lower frequency flicker or from modulation of drifting or counter-phase low spatial frequency gratings. Dobkins & Teller (1996) tested 3 month old infants using moving gratings of 0.25 cycles/degree (c/deg) at a range of temporal frequencies; they obtained an extrapolated CFF close to Regal’s findings. However, a follow-up study of 3 and 4 month olds showed temporal resolution (in this case measured by the point at which the curve falls to one-half maximum) of roughly 10Hz lower than adults tested under similar conditions (Dobkins, Anderson, & Lia, 1999). Several other studies used uniform field flicker at a range of temporal frequencies to compute extrapolated CFFs for infants as old as 4 months and found the values to be considerably immature compared to adults (Rasengane et al., 1997; Hartmann & Banks, 1992). While it is difficult to make direct comparisons across studies due to the different parameters and methods employed, Regal’s (1981) direct measurement of CFF stands alone in showing very early development of temporal vision.
Several studies measured contrast sensitivity for temporal modulation over a range of frequencies below the CFF to study tCSF development. In spite of wide differences in stimuli, they have uniformly found substantial immaturities in overall sensitivity for low (1–2 Hz) and intermediate (5–10 Hz) temporal frequencies in infants as old as 8 months (Dobkins et al, 1999; Hartmann & Banks, 1992; Rasengane et al., 1997; Swanson & Birch, 1990; Teller, Lindsey, Mar, Succop, & Mahal, 1992). Furthermore, data from some of these studies suggest that sensitivity to high temporal frequencies matures at a different rate than sensitivity to low temporal frequencies implying that the tCSF changes shape during development (Hartmann & Banks, 1992; Rasengane et al., 1997; Teller et al., 1992). Interestingly, the notion that the tCSF changes shape was not borne out; Dobkins et al. (1999) found that the shape of the tCSF in 3–4 month old infants was similar to adults, as was peak temporal frequency. However, the idea that sensitivity to high temporal frequencies matures at a different rate than low temporal frequencies was bolstered by a study of tCSF in children, ages 4–7 years (Ellemberg, Lewis, Liu, & Maurer, 1999). They found that sensitivity to low temporal frequencies does not reach maturity until age 7, while sensitivity to higher temporal frequencies (including CFF) was mature in all age groups tested. Taken together, the human infant temporal contrast sensitivity studies suggest a differential development of high and low temporal frequencies and a considerable increase in temporal contrast sensitivity over the course of development. However, it is difficult to draw clear conclusions since there is wide variation in methodology across studies and there are no data from children between the ages of 8 months and 4 years.
We undertook to clarify the developmental profile for temporal vision by measuring full tCSFs over the complete course of maturation in an animal model. The macaque monkey provides an excellent model for the human visual system and monkeys produce quantitative, reliable data at virtually any age throughout development. Data describing the tCSF of the adult monkey have been long established (Merigan, 1980; Merigan, Pasternak, & Zehl, 1981; Harwerth, Smith, Boltz, Crawford, & von Noorden, 1983). These studies demonstrate that, in general, the adult monkey tCSF is similar in shape to the human tCSF, but humans are slightly more sensitive at intermediate and low temporal frequencies while monkeys typically have higher CFFs. No prior studies of temporal visual development have been published for infant monkeys.
In the study described here, tCSFs were obtained from fourteen monkeys at different stages of development to track the maturation of temporal contrast sensitivity for unpatterned sinusoidal flicker. We considered three important questions concerning temporal contrast sensitivity development. First, does the range of temporal resolution expand over development? Second, at what age does temporal contrast sensitivity reach maturity? And third, does development of sensitivity to high and low temporal frequencies proceed at different rates, i.e., does the curve change shape? We also compared the rate of development for temporal and spatial contrast sensitivity. We found temporal contrast sensitivity to be fully mature by approximately 20 weeks, following small increases in temporal frequency range and substantial increases in overall sensitivity. Further, we found that sensitivity to high temporal frequencies reaches maturity more rapidly than sensitivity to lower temporal frequencies. However, it appears that the tCSF does not change shape significantly during maturation. We also found that temporal vision develops more quickly than spatial vision. Lastly, comparison of our behavioral data with studies examining the development of temporal responsiveness in single neurons suggests that at least some of temporal development is limited at or before the level of the LGN.
Materials and Methods
Subjects
Subjects in this study were fourteen visually normal pigtailed monkeys (Macaca nemestrina) ranging in age from 5 weeks to 230 weeks. All animals were born at the Washington National Primate Research Center and hand-reared at the New York University Visual Neuroscience Laboratory. The monkeys were provided with a normal visual environment, which was enriched with a variety of appropriate visual and tactile stimuli. Four monkeys were tested longitudinally from infancy, 2 were tested at multiple ages from 6 months postnatal, and 8 were tested at only one age (range: 16 weeks to 227 weeks). All testing was conducted in accordance with the NIH Guide for Care and Use of Laboratory Animals and approved New York University IACUC protocols.
Stimulus
Stimuli were large Gaussian-windowed patches of spatially unpatterned light displayed on a computer screen in a dark room. We chose a large stimulus size and a Gaussian profile to minimize edge effects (Kelly, 1972). The stimuli were generated on a 21 inch Eizo FlexScan FX-E8 color display monitor (frame rate = 160 Hz) driven by a Dell Optiflex GX1 computer via a VSG2/3 graphics card (Cambridge Research Systems). The standard viewing distance for all monkeys was 50 cm, at which distance the usable monitor area subtended 39 deg (w) × 27 deg (h). Stimuli subtended 17 deg of visual angle and appeared to the left or right of the screen center at an eccentricity of 11 deg. To obtain temporal contrast sensitivity measurements, the luminance of the stimulus was modulated sinusoidally over time about its mean (56 cd/m2). The surround had constant mean luminance which was equal to the time-average luminance of the stimulus (cf. DeLange, 1958). We tested the following temporal frequencies at all ages: 1, 2, 4, 8, 16, 25, and 40 Hz. The stimulus ramped on and stayed on for 500 msec for adult monkeys, after which time the animals were free to respond. Infants were given up to 1000 msec to respond (see below).
Behavioral Task
All monkeys were tested binocularly in a dark room using our standard psychophysical procedures in a two-alternative forced choice operant task (see Kiorpes & Kiper, 1996; Kiorpes & Movshon, 1998). The monkeys were either free to wander in a large testing cage (infants and juveniles) or were seated in a primate chair (adults). Trials were initiated by the monkey placing its face in a mask that centered the monkey’s eyes before the computer screen and controlled the viewing distance. Stimuli were randomly presented on either the left or right side of the computer monitor. The monkey’s task was to indicate the side of the monitor on which the stimulus appeared. Most monkeys older than 24 wks were trained to grasp one of two available pull-bars to indicate their choice; younger monkeys were trained to make an eye movement to the flickering stimulus (see below). Correct responses were rewarded with a 0.25 mL squirt of an age-appropriate liquid: diluted apple juice for older monkeys, infant formula for young ones. Incorrect responses resulted in a brief time-out signaled by a tone (1 kHz).
The youngest monkeys were tested with a reinforced preferential looking technique based on an observer’s judgment of the infants’ eye movements (for details, see Kiorpes & Kiper, 1996). The monkeys were trained to direct their gaze to a crosshair located in the center of the screen at the beginning of each trial; the crosshair stayed on the screen throughout the trial. Maintenance of fixation on the crosshair was confirmed by the human observer who was viewing the animal’s eyes via a video camera and monitor. Once the monkey’s gaze was directed to the crosshair, the stimulus was presented. Infants were trained to make an eye movement toward the side of the display that contained the temporally varying stimulus. Stimuli for the infants could be displayed for up to 1000 msec. Observers judged the infant’s first rightward or leftward glance after stimulus presentation as the response to the stimulus. Measured response latencies were in the range of 800–1000 msec.
Fixation control was an important factor for several reasons. First, temporal resolution for unpatterned modulation varies with eccentricity (Hartmann, Lachenmayr, & Brettel, 1979; Tyler, 1985). Also, the shape of the tCSF varies with retinal locus (Wilson, 1980; Merigan et al., 1981; Snowden & Hess, 1992). Second, eye movement pattern or viewing strategy can affect the shape of the tCSF (Merigan, 1980; Merigan et al., 1981). Variation in any of these factors with age could result in an apparent, but artifactual, developmental trend. Therefore, to maintain consistent viewing behavior and eccentricity across trials and ages we primarily used the reinforced looking procedure. However, older monkeys, who indicated responses with bar-pulls, were allowed to freely view the display and hence presentation locus was not explicitly controlled. We attempted to reduce the influence of eye movements by limiting stimulus duration to 500 msec and presenting a crosshair at the center of the screen at all times. Lack of fixation control for older monkeys is unlikely to have substantially affected our results since one presumes that the monkeys used the locus of highest sensitivity for temporal modulation (the peripheral retina) to solve the task and would strive to perform reliably from trial to trial (thus maximizing reward); this would be easily accomplished by using the crosshair to maintain consistent eye position. If fact, there were no important differences found between monkeys tested with and without fixation control (e.g., see Figure 3), therefore we do not believe our adult data were affected by the free-viewing conditions.
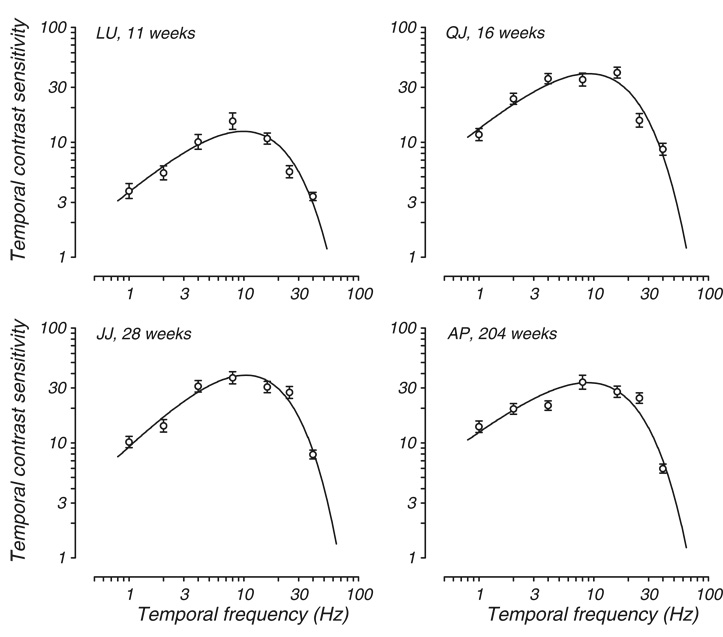
Fig. 3.
Representative cross-sectional data. tCSF is plotted for four macaques tested for the first time at the ages indicated. Axes and symbols are the same as in Fig. 1.
Data Analysis
Data were collected using the method of constant stimuli, where stimuli of a fixed number of contrasts were presented in random order during a given test run at a single temporal frequency. One test run for a given temporal frequency consisted of stimuli of four or five different levels of contrast, each level of contrast presented 25 times, for a total of 100 – 125 trials per run. Each threshold estimate was based on a minimum of 375 such trials. Data collection was counterbalanced across temporal frequency to control for practice effects. Following data collection, thresholds for each temporal frequency were calculated as the contrast level that produced responses at the 75% correct level by submitting log-transformed data to Probit analysis.
For individual functions, the reciprocal of threshold contrast (contrast sensitivity) was plotted as a function of temporal frequency. We fit the data using a double exponential function that has been shown to describe macaque spatial contrast sensitivity data well (e.g., Kozma and Kiorpes, 2003); it also seems to robustly capture temporal contrast sensitivity:
Here ω is temporal frequency. The three free parameters affect the steepness of the low and high frequency portions of the curve, and vertical scale along the sensitivity axis. The high frequency limbs of the functions were extrapolated to a sensitivity of 1, which we took as our estimate of the CFF. CFF was not directly measured due to limitations of the computer monitor.
It has previously been shown that the shape of the spatial contrast sensitivity function (sCSF) is relatively invariant over the course of development under free-viewing conditions (Movshon & Kiorpes, 1988) and at different eccentricities (Kiorpes & Kiper, 1996). To learn whether the same invariance of shape during development held for temporal contrast sensitivity data, we performed a multiple data set fitting analysis in which we simultaneously fit the unpatterned tCSF data from two age groups, infants younger than 11 weeks and animals tested with reinforced looking at the oldest ages: 16 –32 weeks. We used only reinforced looking data for this analysis to ensure that fixation strategy was not a factor in evaluating curve shape. This analysis generates a shape template based on the full data set. Those shape parameters are then evaluated against the separate data for the younger and older groups and the difference in chi square error tested for significance.
To estimate the age at maturation of a given parameter of the tCSF, or sensitivity to a particular temporal frequency, we fit a Naka-Rushton function to the data:
where a is the asymptotic value, b is age at half-asymptotic performance, and c is the exponent. For the purpose of comparison, we used the age at half-asymptotic performance as our measure of relative maturation.
Results
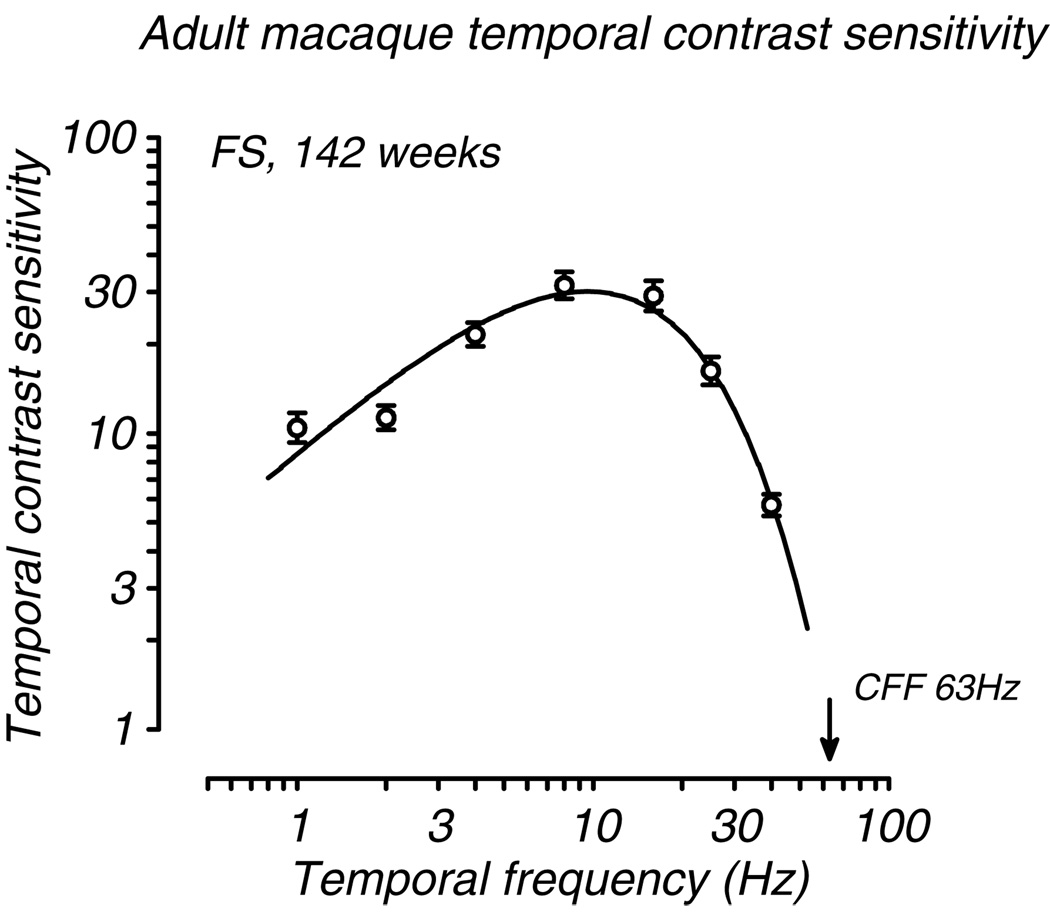
We found substantial development of sensitivity to temporal modulation during the first six postnatal months. All monkeys tested regardless of age could resolve flicker from 2 – 25 Hz and their tCSFs reached maximum sensitivity between 8 and 13 Hz. However, sensitivity was quite low at all temporal frequencies in the youngest animals and CFF was not yet fully adult. Data from a typical adult are shown in Fig. 1. This function shows typical bandpass tuning with a peak near 10Hz and a CFF of 63Hz. These parameters are consistent with those reported by Merigan (1980) for normal adult macaques.
Fig. 1.
A typical adult macaque temporal contrast sensitivity function (tCSF). Contrast sensitivity (+/− 1 SE) is plotted for 7 temporal frequencies. CFF (indicated by the filled arrow) is estimated by extrapolation of the fitted tCSF to 1.
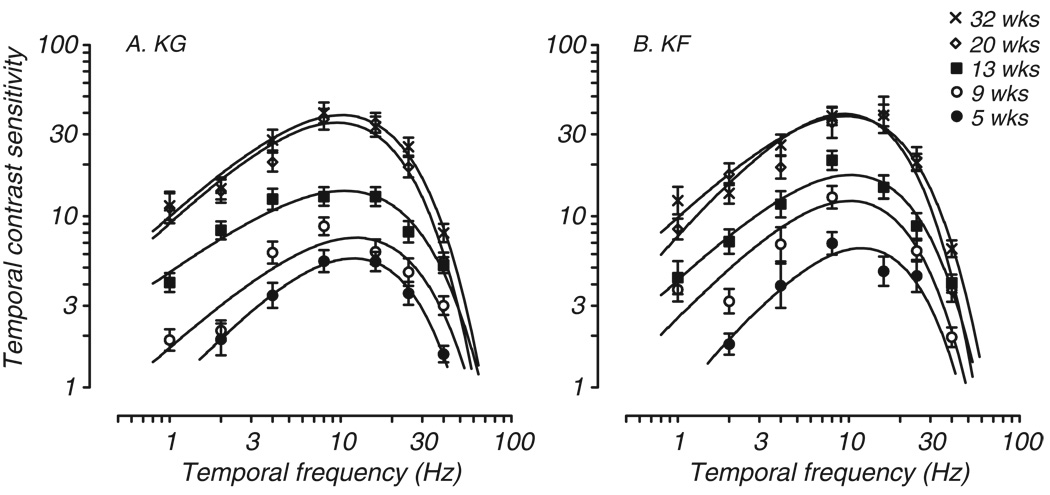
Four monkeys were tested longitudinally during the early postnatal months. Developmental data from two of those monkeys are shown in Fig. 2. Both animals demonstrated a reduced range of resolvable temporal frequencies at the youngest age tested (filled circles). At 5 weeks, neither infant had a measurable threshold for flicker of 1 Hz, although KG responded at above chance levels to 1Hz flicker presented at 100% contrast. KF was unable to resolve either 1 Hz or 40Hz flicker at 5 weeks under any conditions and thus demonstrated a reduced range of flicker sensitivity compared to adults. Extrapolated CFFs for these monkeys were 46Hz for KG and 44 Hz for KF, which is considerably lower than the CFF extrapolated for adult monkeys (average adult CFF = 69 Hz; range 55 – 89Hz). Thus, in comparison to adults and older infants, these 5 week old infants showed reduced temporal resolution as well as remarkably reduced sensitivity at all temporal frequencies below the CFF.
Fig. 2.
Development of tCSF in two infant macaques tested longitudinally at the ages indicated. The curves predominately shift vertically with age. Axes and symbols are the same as in Fig. 1.
Temporal contrast sensitivity at subsequent stages of development is also shown in Fig. 2. By 9 weeks both infants had measurable thresholds at 1 and 40 Hz, but their sensitivity was still quite low (open circles). As these infants aged, their temporal contrast sensitivity at all frequencies tested increased, reaching levels comparable to adult monkeys by approximately 20 weeks. Testing of these two monkeys beyond 20 weeks revealed little further change in sensitivity at any temporal frequency (compare open diamonds and Xes in Fig. 2).
Since the data shown in Fig. 2 are longitudinal, it is possible that the developmental profile we found and the rapid rate of improvement in sensitivity is due to practice, or repeated exposure to the task. To examine that possibility, we plot in Fig. 3 data from the first test age for animals that began testing at older ages or were tested only once. Comparison of these four data sets with each other and with the longitudinal data in Fig. 2 confirms that the developmental profile demonstrated by KG and KF is in fact representative. Data from an animal tested for the first time at 11 weeks (Fig. 3A, LU) are comparable to those of KG at 13 weeks. The other panels all show similar tCSFs for a wide range of ages.
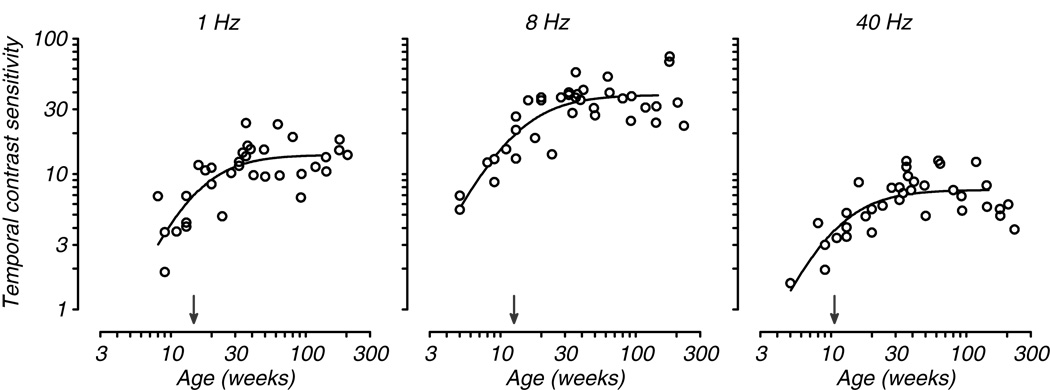
Examination of the improvement in tCSF with age suggests the possibility that the curve develops uniformly, simply shifting vertically to higher sensitivity. If that were true, we would expect uniform development at all temporal frequencies. That would further suggest that the curve does not change shape during development and thus could perhaps be dependent on a single underlying mechanism. To explore this possibility, we compared the rate of development of sensitivity to low, mid, and high temporal frequencies for all animals tested. Fig. 4 plots contrast sensitivity as a function of age for three temporal frequencies: 1, 8, and 40 Hz. These plots illustrate a progressive increase in sensitivity with age at each temporal frequency, with 8Hz showing somewhat greater overall change compared to low and high frequency. To quantify developmental rate we fit a Naka-Rushton equation, a non-linear saturating function that is often used to fit contrast response data, to each data set (see Methods). We took the semi-saturation point to be our index of maturation (arrows pointing to abscissa in Fig. 4). We found a progression of maturation with frequency such that the highest frequency reached asymptote earlier than the mid-frequency, which in turn matured earlier than the low frequency. This result suggests that in fact development is not uniform across temporal frequency and that the curve may change shape.
Fig. 4.
Temporal contrast sensitivity as a function of age is plotted for three temporal frequencies. The data at each frequency are taken from the full population of animals tested. The smooth curves are Naka-Rushton functions fit to the data; the filled arrows indicate the semi-saturation point for each data set (see Methods).
To explore the question of whether the tCSF curve changes shape with development we performed a multiple-data-set fitting analysis (see Methods; Movshon and Kiorpes, 1988). For this analysis, only data sets from the youngest infants (under 11 weeks) and those from the oldest animals tested with reinforced looking (ages 16 – 32 weeks) were included. We simultaneously fit the combined data to generate a template curve whose shape parameters were constant, but whose scale parameters varied for individual data sets; we repeated the fitting separately for the younger and older groups. The χ2 error for the combined fit, 110.5, was not significantly different than the sum of the error for the two group fits, 109.05 (χ2 difference 1.45, df 3, p > .05) suggesting that the template curve fit all of the data equally well. To specifically test whether the shape of the tCSF was different for the two age ranges, we used the shape parameters from the older group to fit the infant data. The resulting χ2 error, 57.77, was not different from that for the original infant group fit, 57.33. This analysis clearly indicates that the tCSF does not change shape during development, but instead can be modeled as a single mechanism shifting vertically with age.
Spatial vs. Temporal Vision
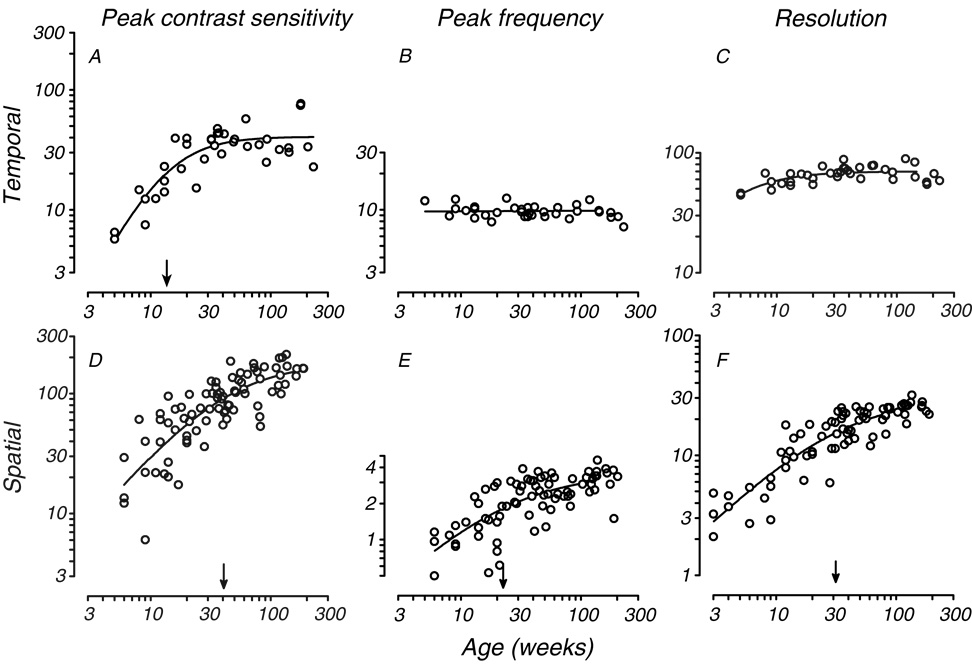
Our data show that temporal contrast sensitivity matures by about 20 weeks postnatal in monkeys. To compare the rate of temporal vision development with that of spatial vision, we selected three features of the CSFs: peak sensitivity, peak frequency and resolution (high frequency cutoff). For spatial vision, we used data from prior studies (Kiorpes & Bassin, 2003; Kiorpes & Movshon, 2004) as well as data collected from infants in the current study. The data are shown in Figure 5, where temporal contrast sensitivity parameters are in the top row and spatial contrast sensitivity parameters are on the bottom. Fig. 5A shows peak sensitivity (derived from the fits) as a function of age for temporal vision. As for individual frequencies (Fig. 4), we fit a Naka-Rushton function to the data. Peak temporal contrast sensitivity matures over the first 20 weeks, with the half-asymptotic value 13.6 weeks. Peak temporal frequency did not appear to shift with age (Fig. 5B), ranging from 7.2 to 12.5 Hz (mean 9.8 Hz) independently of age. Fig. 5C shows extrapolated CFF, our measure of temporal resolution, as a function of age. CFF also changes little over the age range tested with immature values evident only at ages below 10 weeks. Although we plot the function along with the data, the half-asymptote was well below the range of the data (3.4 weeks) indicating very early maturation.
Fig. 5.
Comparison of the development of temporal and spatial contrast sensitivity. Peak contrast sensitivity, best frequency, high frequency resolution are plotted in the top row for temporal vision and in the bottom row for spatial vision. Temporal CSF data are plotted as a function of age for the full population of animals tested in the present study. Data are derived from the tCSF fits. Spatial CSF data are plotted as a function of age for animals tested as part of this study as well as other studies (see text). Data are derived from sCSF fits. Smooth curves and arrows are as in Fig. 4. No semi-saturation point is indicated for either temporal frequency data set since they fall outside of the range of the data and therefore lack reliability.
The maturation of spatial vision, as measured by peak sensitivity, peak spatial frequency, and spatial resolution, is shown in Fig. 5D–F. These indicators of spatial vision maturation reveal a substantially slower time course, with half-asymptotes beyond 20 weeks in all cases. Also, peak spatial frequency increases with age unlike peak temporal frequency revealing a change in scale as well as sensitivity with age. Clearly, spatial vision develops more slowly, with a different profile, than temporal vision.
Discussion
We used a combination of longitudinal and cross-sectional data to chart the development of temporal vision in macaque monkeys. We found that the greatest change in the tCSF during development was a dramatic increase in overall sensitivity with age, while CFF changed rather little. From these data, we are able to draw several conclusions about the pattern of development of temporal vision. First, the data indicate that the range of temporal resolution is reduced at both the high and low frequency ends of the tCSF in infants as young as 5 weeks old. Second, the longitudinal and cross-sectional data collectively suggest that temporal contrast sensitivity is mature by about 20 weeks, earlier than spatial vision. And finally, sensitivity to high and low temporal frequencies develops at slightly different rates but, according to a multiple-data-set fitting analysis, the shape of the function does not change significantly with age.
This study is the first comprehensive examination of temporal vision development in primates. We measured full tCSFs for all animals at all test ages, and covered the full range of maturation. As discussed in the Introduction, there is disagreement in the human literature on the relative development of high and low temporal frequencies and the age of maturation of CFF. Our results are in general agreement with Regal (1981) showing very early maturation of CFF compared with lower temporal frequencies. Our data are also in agreement with multiple studies in human infants suggesting somewhat later maturation of low and intermediate temporal frequency sensitivity (Swanson & Birch, 1990; Hartmann & Banks, 1992; Teller et al., 1992; Rasengane et al., 1997; Ellemberg et al., 1999), although most of these studies used spatially patterned stimuli. A few of the human development studies used unpatterned stimuli or very low spatial frequency stimuli (Teller et al., 1992; Hartmann & Banks, 1992; Rasengane et al., 1997). These studies suggest different rates of development for different temporal frequencies, with curves appearing low-pass at the youngest ages and later becoming bandpass. However, Dobkins et al., (1999) showed a consistent curve shape for infants and adults. Interestingly, we show that although there is non-uniform maturation of sensitivity to different ranges of temporal frequency, there is no reliable, significant change in tCSF shape or peak temporal frequency; the monkey tCSFs were bandpass at all ages. It is possible to reconcile these seemingly contradictory results by accepting the possibility that the lowest temporal frequencies are mediated by a separate mechanism than that for intermediate and higher temporal frequencies (see below; Merigan and Eskin, 1986; Dobkins et al., 1999). The relatively small difference in developmental rate across a restricted range of the full data set, coupled with some individual variation, would not significantly impact tCSF shape with our curve fitting procedure. Regardless, our results indicate that temporal maturation is best described by a single mechanism that shifts vertically, to higher sensitivity with age.
It would be of interest to determine the relative rate of development of temporal vision for monkeys and humans. Teller and colleagues established this relationship for spatial vision as being 4:1, with monkey grating acuity developing 4 times faster than human acuity, or 1 week of monkey age being functionally equivalent to one month of human age (Boothe, Dobson, & Teller, 1985). Unfortunately, there are no human temporal contrast sensitivity data between the ages of 4 months and 4 years using unpatterned stimuli. The existing data suggest immature sensitivity for all measured temporal frequencies tested and immature extrapolated CFF at 4 months (Teller et al., 1992; Hartmann & Banks, 1992; Rasengane et al., 1997; but see Regal, 1981). Ellemberg et al. (1999) found mature extrapolated CFF at their youngest test age, 4 years, but slightly reduced sensitivity to low temporal frequencies up to 7 years. Our longitudinal data show maturation of the tCSF by 20 to 30 weeks in macaque monkey, with substantially earlier development of the CFF. Applying the 4:1 translation to this case, then, human temporal contrast sensitivity should mature by age 2–3 years (20–30 months). This comparison suggests substantially slower development in humans than in monkeys. On the other hand if we compare the development of CFF, using Regal’s direct measurements (Regal, 1981), we find monkey and human infants maturing at approximately the same age – 2 to 3 months. The exercise suggests dramatically different relative developmental time courses for temporal vision in humans and monkeys depending on the metric. Procedural differences or differences in stimulus configuration between the human studies and ours might explain this result. For example, it is not always possible to control fixation during trials with human infants. Stimulus size, eccentricity, and viewing conditions all affect relative temporal sensitivity, and may impact the mechanism used for detection. Additionally, while monkey infants may grow tired or fussy, as do human infants, because the monkeys participate for a milk reward, we are able to obtain many more trials for animal subjects than is possible for humans. Whatever the case, resolution will await additional studies in children that fill in the age range between infancy and later childhood.
We were especially concerned with avoiding limitations on performance due to stimulus properties. Therefore, we used relatively large, unpatterned, Gaussian-vignetted stimuli at moderate luminance levels with controlled fixation over the critical age range. Although our adult subjects were tested with uncontrolled fixation, their tCSF profiles look very much like those collected under controlled stimulus presentation (see, for example, Fig. 3). There was a fair degree of individual variation in sensitivity among the adults, particularly at the low temporal frequency range, suggesting that different animals may have adopted different strategies. Nonetheless, the developmental pattern is clear and the important features of the curve are consistent across animals and testing paradigms.
We found that the patterns of maturation of spatial and temporal vision follow different time courses. While temporal vision is largely mature by 20 weeks, the maturation of spatial vision continues for several additional months. Spatial vision development includes a dramatic increase in both spatial scale and sensitivity (Fig. 5; see also, Boothe et al., 1988; Kiorpes & Kiper, 1996) but temporal vision development involves little change in temporal scale, either peak temporal frequency or CFF. Together these differences between the patterns of maturation of spatial and temporal contrast sensitivity suggest that their development is subserved by different mechanisms.
It is natural to assume that these profiles reflect differential development of the magnocellular and parvocellular pathways, consistent with a two-channel model of early visual processing (Shapley & Perry, 1986; Merigan & Maunsell, 1993). Accordingly, the magnocellular channel governs the detection of low spatial, high temporal frequencies, while the parvocellular channel governs detection of high spatial, low temporal frequencies. However, there are clearly areas of overlap such that these pathways are not functionally exclusive (Merigan & Maunsell, 1990; 1993). Perhaps because of the extensive overlap in sensitivity of these pathways or perhaps because they develop at different rates, the development of spatial vision, using spatial resolution or contrast sensitivity as the metric, is not well captured by the development of either parvocellular or magnocellular neurons in the LGN exclusively (see Hawken, Morely & Blakemore, 1997; Movshon, Kiorpes, Hawken, & Cavanaugh, 2005; but see also, Blakemore & Vital-Durand, 1985).
Given prior neurophysiological evidence from retinal (Lee, Martin, & Valberg, 1989; Lee, Pokorny, Smith, Martin, & Valberg, 1990) and LGN (Merigan & Eskin, 1986; Merigan & Maunsell, 1990) recordings in adult macaques , it is reasonable to postulate that tCSF measurements using unpatterned stimuli, such as we used in this study, rely heavily on the maturation of the magnocellular pathway, with perhaps only the lowest temporal frequency depending on the parvocellular pathway. Indeed, sensitivity to 1 Hz modulation seemed to reach maturity slightly later than sensitivity to higher temporal frequencies. However, Anderson & Burr (1985) found no evidence of more than one temporal channel operating at very low spatial frequencies in human adults, strengthening our view that the pattern of development we identified is consistent with a single mechanism improving in sensitivity with age. Consistent with our results, Dobkins et al. (1999) argue convincingly for relatively early development of mechanisms having a magnocellular profile compared to chromatically sensitive mechanisms. Thus, the time course of temporal contrast sensitivity development for luminance stimuli could potentially be explained by that of neurons in the magnocellular pathway.
A few studies have charted the development of temporal responsiveness of neurons in the early visual pathways of macaque monkeys. There are no neurophysiological data from infant macaque retina, but several studies of LGN found immature temporal response properties for both magnocellular and parvocellular neurons. Optimizing sinusoidal gratings for preferred spatial frequency, and using high contrast levels, Hawken, Blakemore, & Morely (1997) and Movshon et al. (2005) found a one to two octave reduction in temporal resolution for both classes of neurons in newborn macaques compared to adults. Adult levels of high temporal frequency sensitivity were reached by 6 to 9 months. There were no important differences between the developmental profiles for parvocellular and magnocellular neurons aside from the higher resolution of magnocellular neurons as a group at all ages. Zheng, Zhang, Maruko, Watanaabe, Nakatsuka, Smith, & Chino (2007) recorded temporal response properties of neurons in V1 and V2 of young monkeys. They found immaturities remaining at 8 weeks (the oldest infants recorded) in V1 and V2, with temporal resolution lagging in V2 neurons compared to V1 neurons.
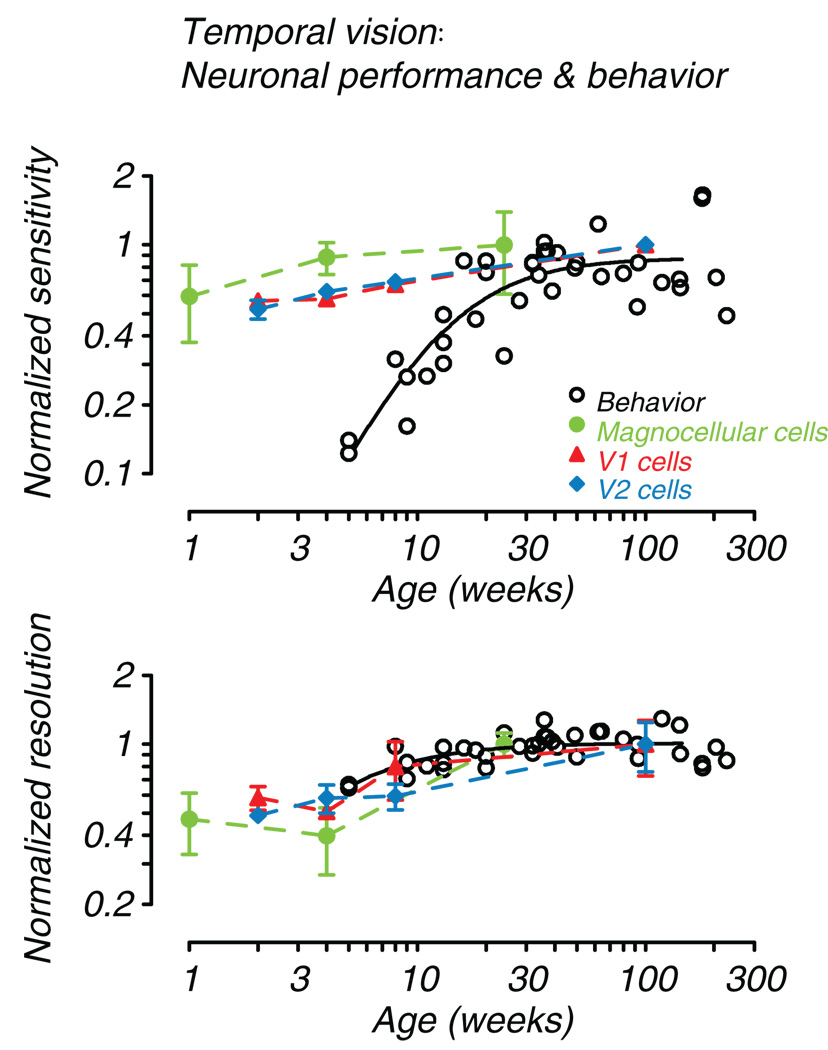
To directly compare the developmental profiles for neurons in the early visual pathway and behavior, we plotted normalized best sensitivity and resolution together in Figure 6. Behavioral data are taken from Fig. 5 (A and C); the physiological data are taken from Movshon et al., (2005) for LGN and Zheng et al., (2007) for cortical areas V1 and V2. Accepting that the neuronal data are collected using optimized, spatially patterned, temporally modulated stimuli rather than unpatterned flickering fields, Fig. 6 (bottom) shows that CFF can potentially be limited by temporal resolution of magnocellular cells at or before the LGN. Behavior and resolution of V1 and V2 cells largely follow the magnocellular pattern. However, Fig. 6 (top) reveals a very different relationship for temporal contrast sensitivity. Again development of V1 and V2 neurons seem to parallel the profile set by magnocellular neurons, but in no case do neuronal sensitivities set a limit on behavior. This result suggests that there is a downstream filter on overall temporal sensitivity that is developing more slowly than even V2 neurons. Kelly, Boynton, & Baron (1976) provided evidence for the existence of such post-receptoral filtering by comparing human psychophysical flicker sensitivity data with high frequency following of macaque photoreceptors. They postulated the existence of a “slow” filter process downstream from the photoreceptors that governs typical psychophysical flicker thresholds, but the physiological mechanism remains to be discovered. The comparison in Figure 6 suggests that for high frequency resolution, this process may act at or before the level of the LGN. Movshon et al. (2005) recorded S-potentials simultaneously with several LGN neuron recordings. They found that temporal resolution was poor at the input levels as well, so indeed this limit could be retinal. Substantial immaturities of the human infant ERG have been documented as well, bolstering the idea that maturation of the photoreceptors and early retinal circuitry may play a substantial role (Westall, Panton & Levi, 1999; Fulton, Hansen & Westall, 2003). For sensitivity in lower frequency ranges, the limiting process likely lies beyond the LGN.
Fig. 6.
Comparison of behavioral and neural development. Normalized sensitivity and resolution are plotted as a function of age. Open circles and smooth curves are from Fig. 5 (A and C). The behavioral data are normalized to average adult levels (age > 100 weeks). Also plotted are normalized (to the oldest available age) mean sensitivity and characteristic frequency for magnocellular LGN neurons (green, filled circles; Movshon et al., 2005), and mean sensitivity and temporal resolution for V1 (red, filled triangles) and V2 (blue, filled diamonds) cells (Zheng et al., 2007, Table 1). Error bars are +/− 1SE.
In summary, we found that as development progresses the shape of the tCSF remains essentially the same, the temporal scale increases slightly at both ends of the function, and infants demonstrate large increases in sensitivity at all temporal frequencies. The results characterize the development of temporal vision from infancy to adult levels and contribute to behavioral evidence that temporal vision follows a different maturation pattern than spatial vision. Development of the CFF is rapid and may be limited early in the magnocellular pathway. But overall sensitivity to temporal modulation seems to depend on a slower, downstream mechanism.
Acknowledgments
This work was supported in part by NIH grant EY05864 to L Kiorpes and grants from Fight for Sight and the New York University Dean’s Undergraduate Research Fund to KA Stavros. We also acknowledge the support of NIH/NCRR RR00166 to the Washington National Primate Research Center and Core Grant for Vision Research EY13079 to New York University. We thank Chao Tang, Elaine Hall, Tracy Price for their contributions to this work. We also thank Michael Gorman and Laura Albanese for assistance with animal care and testing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson SJ, Burr DC. Spatial and temporal selectivity of the human motion detection system. Vision Research. 1985;25:1147–1154. doi: 10.1016/0042-6989(85)90104-x. [DOI] [PubMed] [Google Scholar]
- Banks MS. The development of spatial and temporal contrast sensitivity. Current Eye Research. 1983;2:191–198. doi: 10.3109/02713688208997694. [DOI] [PubMed] [Google Scholar]
- Banton T, Bertenthal BI. Infants’ sensitivity to uniform motion. Vision Res. 1996;36:1633–1640. doi: 10.1016/0042-6989(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Blakemore C, Vital-Durand F. Organization and post-natal development of the monkey’s lateral geniculate nucleus. Journal of Physiology (London) 1986;308:451–491. doi: 10.1113/jphysiol.1986.sp016297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothe RG, Dobson MV, Teller DY. Postnatal development of vision in human and nonhuman primates. Annual Review of Neuroscience. 1985;8:495–545. doi: 10.1146/annurev.ne.08.030185.002431. [DOI] [PubMed] [Google Scholar]
- Boothe RG, Kiorpes L, Williams RA, Teller DY. Operant measurements of contrast sensitivity in infant macaque monkeys during normal development. Vision Res. 1988;28:387–396. doi: 10.1016/0042-6989(88)90181-2. [DOI] [PubMed] [Google Scholar]
- Daw NW. Visual Development. New York: Plenum Press; 1995. [Google Scholar]
- De Lange H. Research into the dynamic nature of the human fovea cortex system with intermittent and modulated light. Journal of the Optical Society of America. 1958;48:777–784. doi: 10.1364/josa.48.000777. [DOI] [PubMed] [Google Scholar]
- Dobkins KR, Anderson CM, Lia B. Infant temporal contrast sensitivity functions (tCSFs) mature earlier for luminance than for chromatic stimuli: evidence for precocious magnocellular development? Vision Res. 1999;39:3223–3239. doi: 10.1016/s0042-6989(99)00020-6. [DOI] [PubMed] [Google Scholar]
- Dobins KR, Teller DY. Infant contrast detectors are selective for direction of motion. Vision Res. 1996;36:281–294. doi: 10.1016/0042-6989(95)00094-g. [DOI] [PubMed] [Google Scholar]
- Ellemberg D, Lewis TL, Liu CH, Maurer D. Development of spatial and temporal vision during childhood. Vision Res. 1999;39:2325–2333. doi: 10.1016/s0042-6989(98)00280-6. [DOI] [PubMed] [Google Scholar]
- Fulton AB, Hansen RM, Westall CA. Development of ERG responses: The ISCEV rod, maximal and cone responses in normal subjects. Doc. Ophthalmol. 2003;107:235–241. doi: 10.1023/b:doop.0000005332.88367.b8. [DOI] [PubMed] [Google Scholar]
- Gunn A, Cory E, Atkinson J, Braddick O, Wattam-Bell J, Guzzeta A, Cioni G. Dorsal and ventral stream sensitivity in normal development and hemiplegia. Neuroreport. 2002;13:843–847. doi: 10.1097/00001756-200205070-00021. [DOI] [PubMed] [Google Scholar]
- Hamer RD, Norcia AM. The development of motion sensitivity during the first year of life. Vision Res. 1994;34:2387–2402. doi: 10.1016/0042-6989(94)90283-6. [DOI] [PubMed] [Google Scholar]
- Hartmann E, Lachenmayr B, Brettel H. The peripheral critical flicker frequency. Vision Research. 1979;19:1019–1023. doi: 10.1016/0042-6989(79)90227-x. [DOI] [PubMed] [Google Scholar]
- Hartmann EE, Banks MS. Temporal contrast sensitivity in human infants. Vision Res. 1992;32:1163–1168. doi: 10.1016/0042-6989(92)90018-e. [DOI] [PubMed] [Google Scholar]
- Harwerth RS, Smith EL, III, Boltz RL, Crawford MLJ, von Noorden GK. Behavioral studies on the effect of abnormal early visual experience in monkeys: temporal modulation sensitivity. Vision Res. 1983;23:1511–1517. doi: 10.1016/0042-6989(83)90163-3. [DOI] [PubMed] [Google Scholar]
- Hawken MJ, Blakemore C, Morely JW. Development of contrast sensitivity and temporal-frequency selectivity in primate lateral geniculate nucleus. Experimental Brain Research. 1997;114:86–98. doi: 10.1007/pl00005626. [DOI] [PubMed] [Google Scholar]
- Horsten GPM, Winkelman JE. Electro-retinographic critical fusion frequency of the retina in relation to the histological development in man and animals. Documenta Ophthalmologica. 1964;18:515–521. doi: 10.1007/BF00160603. [DOI] [PubMed] [Google Scholar]
- Kelly DH. Theory of flicker and transient responses, I. Uniform fields. Journal of the Optical Society of America. 1971;61:537–546. doi: 10.1364/josa.61.000537. [DOI] [PubMed] [Google Scholar]
- Kelly DH. Flicker. In: Jameson D, Hurvich LM, editors. Handbook of Sensory Physiology VII/4: Visual Psychophysics. Berlin: Springer-Verlag; 1972. pp. 273–302. [Google Scholar]
- Kelly DH, Boynton RM, Baron WS. Primate flicker sensitivity: Psychophysics and electrophysiology. Science. 1976;194:1077–1079. doi: 10.1126/science.824735. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Bassin SA. Development of contour integration in macaque monkeys. Visual Neuroscience. 2003;20:567–575. doi: 10.1017/s0952523803205101. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Kiper DC. Development of contrast sensitivity across the visual field in macaque monkeys (Macaca nemestrina) Vision Res. 1996;36:239–247. doi: 10.1016/0042-6989(95)00097-j. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Kiper DC, Movshon JA. Contrast sensitivity and vernier acuity in amblyopic monkeys. Vision Res. 1993;33:2301–2311. doi: 10.1016/0042-6989(93)90107-8. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Movshon JA. Behavioral Analysis of Visual Development. In: Coleman JR, editor. Development of Sensory Systems in Mammals. John Wiley & Sons, Inc; 1990. pp. 125–154. [Google Scholar]
- Kiorpes L, Movshon JA. Peripheral and central factors limiting the development of contrast sensitivity in macaque monkeys. Vision Research. 1998;38:61–70. doi: 10.1016/s0042-6989(97)00155-7. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Movshon JA. Neural limitation on visual development in primates. In: Chalupa LM, Werner JS, editors. The Visual Neurosciences. MIT Press; 2003. [Google Scholar]
- Kiorpes L, Movshon JA. Development of sensitivity to visual motion in macaque monkeys. Visual Neuroscience. 2004;21:851–859. doi: 10.1017/S0952523804216054. [DOI] [PubMed] [Google Scholar]
- Kozma P, Kiorpes L. Contour integration in amblyopic macaque monkeys. Visual Neuroscience. 2003;20:577–588. doi: 10.1017/s0952523803205113. [DOI] [PubMed] [Google Scholar]
- Lee BB, Martin PR, Valberg A. Sensitivity of macaque retinal ganglion cells to chromatic and luminance flicker. Journal of Physiology. 1989;414:223–243. doi: 10.1113/jphysiol.1989.sp017685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BB, Pokorny J, Smith VC, Martin PR, Valberg A. Luminance and chromatic sensitivity of ganglion cells and human observers. Journal of the Optical Society of America A. 1990;7:222–2236. doi: 10.1364/josaa.7.002223. [DOI] [PubMed] [Google Scholar]
- Merigan WH. Temporal modulation sensitivity of macaque monkeys. Vision Res. 1980;20:953–959. doi: 10.1016/0042-6989(80)90077-2. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Eskin TA. Spatio-temporal vision of macaques with severe loss of Pβ retinal ganglion cells. Vision Res. 1986;26:1751–1761. doi: 10.1016/0042-6989(86)90125-2. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Maunsell JHR. Macaque vision after magnocellular lateral geniculate lesions. Visual Neuroscience. 1990;5:347–352. doi: 10.1017/s0952523800000432. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Maunsell JHR. How parallel are the primate visual pathways. Annual Review of Neuroscience. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Pasternak T, Zehl D. Spatial and temporal vision of macaques after central retinal lesions. Invest. Ophthalmol. Vis. Sci. 1981;21:17–26. [PubMed] [Google Scholar]
- Movshon JA, Kiorpes L. Analysis of the development of spatial contrast sensitivity in monkey and human infants. J. Opt. Soc. Am. A. 1988;5:2166–2172. doi: 10.1364/josaa.5.002166. [DOI] [PubMed] [Google Scholar]
- Movshon JA, Kiorpes L, Hawken MJ, Cavanaugh JR. Functional maturation of the macaque’s lateral geniculate nucleus. J of Neuroscience. 2005;25:2712–2722. doi: 10.1523/JNEUROSCI.2356-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasengane TA, Allen D, Manny RE. Development of temporal contrast sensitivity in human infants. Vision Res. 1997;37:1747–1754. doi: 10.1016/s0042-6989(96)00300-8. [DOI] [PubMed] [Google Scholar]
- Regal DM. Development of critical flicker frequency in human infants. Vision Res. 1980;21:549–555. doi: 10.1016/0042-6989(81)90100-0. [DOI] [PubMed] [Google Scholar]
- Shapley R, Perry VH. Cat and monkey retinal ganglion cells and their visual functional roles. Trends in Neurosciences. 1986;9:229–235. [Google Scholar]
- Snowden RJ, Hess RF. Temporal frequency filters in the human peripheral visual field. Vision Research. 1992;32:61–72. doi: 10.1016/0042-6989(92)90113-w. [DOI] [PubMed] [Google Scholar]
- Snowden RJ, Hess RF, Waugh SJ. The processing of temporal modulation at different levels of retinal illuminance. Vision Research. 1995;35:775–789. doi: 10.1016/0042-6989(94)00158-i. [DOI] [PubMed] [Google Scholar]
- Swanson WH, Birch EE. Infant spatiotemporal vision: dependence of spatial contrast sensitivity on temporal frequency. Vision Res. 1990;30:1033–1048. doi: 10.1016/0042-6989(90)90113-y. [DOI] [PubMed] [Google Scholar]
- Teller DY. First glances: The vision of infants. Investigative Ophthalmology and Visual Science. 1997;38:2183–2203. [PubMed] [Google Scholar]
- Teller DY, Lindsey DT, Mar CM, Succop A, Mahal MR. Infant temporal contrast sensitivity at low temporal frequencies. Vision Res. 1992;32:1157–1162. doi: 10.1016/0042-6989(92)90017-d. [DOI] [PubMed] [Google Scholar]
- Tyler CW. Analysis of visual modulation sensitivity. II. Peripheral retina and the role of photoreceptor dimensions. Journal of the Optical Society of America A. 1985;2:393–398. doi: 10.1364/josaa.2.000393. [DOI] [PubMed] [Google Scholar]
- Westall CA, Panton CM, Levi AV. Time course for maturation of electroretinogram responses from infancy to adulthood. Doc. Ophthalmol. 1999;93:355–379. doi: 10.1023/a:1001856911730. [DOI] [PubMed] [Google Scholar]
- Wilson HR. Development of spatiotemporal mechanisms in infant vision. Vision Res. 1988;28:611–628. doi: 10.1016/0042-6989(88)90111-3. [DOI] [PubMed] [Google Scholar]
- Zheng J, Zhang B, Bi H, Maruko I, Watanabe I, Nakatsuka C, Smith EL, 3rd, Chino YM. Development of temporal response properties and contrast sensitivity of V1 and V2 neurons in macaque monkeys. Journal of Neurophysiology. 2007;97:3905–3916. doi: 10.1152/jn.01320.2006. [DOI] [PubMed] [Google Scholar]