Introduction and Case Study
Mrs. Peters is a 65-year old married woman with a known history of right breast cancer, diagnosed and treated 10 years ago. In the past several weeks, she has a new nonproductive cough and is a bit fatigued and short of breath with physical activity. Her husband finally convinces her to see her health care provider who obtains her history and completes a physical exam. On listening to her lungs, an absence of breath sounds is found in the lower half of the right lung. When percussed, there is dullness in the areas of absent breath sounds. Heart sounds are normal. There is no pedal edema. An in office pulse oximetry is 91% on room air. A chest x-ray including lateral, anterioposterior and decubitus films demonstrate a right-sided pleural effusion. Mrs. Peters is admitted for a work up of a presumptive diagnosis of metastatic breast cancer with malignant pleural effusion (MPE). This article will discuss the clinical presentation, diagnosis, treatment and nursing management of the patient with a MPE.
What is MPE?
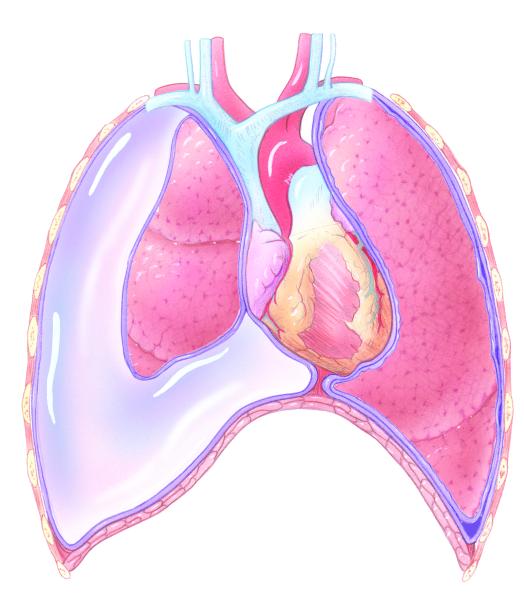
A pleural effusion is a collection of fluid between the parietal and visceral pleural layers of the lung. Pleural effusions are often associated with advanced malignancies such as carcinoma of the lung or breast. However, many other malignancies are known to produce MPE, such as mesothelioma, renal, ovarian, and sarcomas (Porcel & Vives, 2003). Relatively common, over 150,000 new cases of MPE are diagnosed each year (Neragi-Miandoab, 2006).
The pleural space lies between the 2 layers of the pleura. The visceral layer covers the lungs and the parietal layer lines the chest wall cavity. Under normal circumstances, a maximum of 50 mLs of pleural fluid is present in the pleural space. The fluid allows the lung layers to move easily during respiration. The capillaries contained in the parietal pleura manufacture pleural fluid and the visceral pleura reabsorb the fluid (Taubert, 2001). Anything that disturbs this balance can result in the development of a pleural effusion, such as a reduction in or obstruction of lymphatic drainage, increased or decreased oncotic pressure in the capillaries, increased capillary permeability, or increased negative pressure in the lungs from atelectasis (Putman, 2002; Taubert). A common etiology of pleural effusion in patients with a malignancy is lymphatic obstruction (Shuey & Payne, 2005). Effusions may be unilateral or bilateral.
Several types of pleural effusions occur including transudative and exudative, but most MPE are exudative (Taubert) characterized by high protein and high LDH levels, and a high white blood cell count (Goldman, 2004). The fluid is yellowish, cloudy or blood-tinged (Goldman). Fluid obtained during a diagnostic procedure is always sent for these diagnostic studies. In addition, other diagnostic studies useful in pleural fluid assessment are Gram stain, culture and sensitivity, cytology, glucose, amylase, and albumin (Light, 1999). However, the fluid LDH and the fluid to serum ratio of total protein are the most accurate tests available to distinguish a transudative effusion from an exudative effusion with the pleural fluid LDH; more than two-thirds of the upper limit of normal of the serum LDH is diagnostic for an exudative effusion (Tassi, Cardillo, Marchetti, Carleo, Martelli, 2006). Transudative effusions are seen in patients with lymphoma but most often are associated with nonmalignant conditions (Taubert). Characterized by low levels of protein and a watery fluid, transudative effusions are associated with cirrhosis or congestive heart failure.
Signs and symptoms/patient assessment
Patients such as Mrs. Peters complain of feeling short of breath with activity or even at rest [dependent on the size of the effusion], fatigue, a dry cough, and chest pain or pleuritic pain or chest heaviness. These symptoms often cause the patient to seek health care from the physician or CRNP. Baseline vital signs and weight are done. A nursing history should include the onset and exacerbating factors for the shortness of breath (SOB) or dyspnea on exertion, its effect on the patient’s performance status and quality of life. Ask about the cough and whether or not it is productive and if chest pain is present. Have the patient explain the severity of the chest pain, any accompanying symptoms, and if the pain is worse with a deep breath which would be a sign of pleuritic pain. Observe the trachea and look for deviation from the midline. Auscultation of the lungs reveals absent breath sounds in the area of the effusion and percussion over the area of involvement is dull. Using percussion, a line can often be drawn between the fluid level and aerated lung showing the size of the effusion. Pulse oximetry may be normal or low dependent on the size of the effusion and the amount of normal lung tissue present. If the pleural effusion is associated with cardiac disease, other signs of excess fluid should be present such as lower extremity edema or tachycardia. If associated with liver disease, there should be other signs of disease such as ascites or varicies (telangectasia).
Symptomatic Management of MPE
Supplemental oxygen therapy will often improve the patient’s symptoms by improving blood oxygen levels. The respiratory and heart rates will decrease and the pulse oximetry readings increase. Medications such as oral morphine may also provide comfort and pain relief. Fatigue is common in patients with MPE. The patient needs to have care planned so that as little effort on the part of the patient is required until the MPE is drained and lung function improves. The nurse assists the patient with basic care needs such as bathing and toileting to conserve energy. The patient should consume a high calorie diet to meet their respiratory caloric needs by consuming small amounts of calorie concentrated foods that are easy to swallow. Anxiety needs to be managed as it will increase the respiratory rate and lead to more anxiety (Taubert, 2001) and morphine is often useful for this purpose. MPE in patients with solid tumors is often associated with end-of-life and patients benefit from consultation with a social worker and referral to hospice. The nurse needs to take the time to listen to the patient’s needs and anticipate physical care needs to reduce the burden on the patient. Education of the patient and family will help reduce anxiety and allow them to be active participants in their care.
Diagnostic Studies
A chest x-ray with anterior-posterior, lateral and decubitus films, will demonstrate a pleural effusion. Other diagnostic studies include ultrasound and chest CT scan which will assist the physician with obtaining a sample of pleural fluid for diagnosis. A thoracentesis is done to obtain not just fluid for diagnosis but to be therapeutic and remove the fluid filling the pleural space and crowding the lung preventing expansion and is the test and procedure of choice in the evaluation of pleural effusion (DeCamp, Mentzer, Swanson, Sugarbaker, 1997). Under local anesthesia, and ultrasound if the effusion is small, a needle in placed in the pleural space to remove a fluid sample for analysis. Additional fluid is removed to improve the patient’s breathing and reduce the feeling of shortness of breath and if possible, all the fluid is removed however, excess fluid removal may cause hypotension from fluid shifting from the intravascular space and pulmonary edema (Taubert, 2001). A pleural biopsy may also be done. After a thoracentesis, a chest x-ray is done to make sure that a pneumothorax or hemothorax did not occur during the procedure. The fluid from a first time thoracentesis may not be diagnostic and a second one may be needed to obtain a diagnosis. Patient education after a thoracentesis includes the need to report the acute onset of shortness of breath which may be associated with a pneumothorax or hemoptysis from bleeding and changes in heart rate. The nurse monitors the patient for hypoxia, cyanosis, tachycardia, respiratory changes, hemoptysis, and changes in breath sounds indicating the reaccumulation of the pleural effusion and checks the vital signs. Repeated thoracenteses can cause fluid loculations, infection or the implantation of tumor along the needle tracts (Genc, Petrou, Ladas, Goldstraw, 2000). Thoracentesis and fluid removal is helpful in the short term relief of shortness of breath for 1-3 days after which reaccumulation is possible with the majority recurring within 30 days (Anderson, Philpott, Ferguson, 1974). So if MPE is recurrent, additional therapeutic measures are necessary.
Mrs. Peters underwent a thoracentesis which drained 500 ml of fluid of which a sample sent to the lab revealed that the fluid was exudative. The cytology of the specimen was positive for breast cancer. While awaiting the results of the diagnostic studies, her effusion reaccumulated. Since her prior cancer therapy consisted of surgery, radiation therapy and adjuvant chemotherapy, the health care providers recommended single agent chemotherapy to palliate her MPE after receiving additional local therapy for the effusion.
Management of Recurrent MPE
When managing a MPE the clinician should take into consideration the overall wellbeing of the patient. The general treatment for MPE is palliative in nature. Those patients who have developed MPE associated with a primary breast tumor as Mrs. Peters has may have a survival of 1-2 more years, whereas patients with a lung cancer primary have a much shorter time span such as 4-6 months. The prognosis may influence the treatment that the patient can tolerate (Burrows, Mathews, Colt, 2000).
If the effusion is small, systemic treatment may not be required, however if the primary tumor can be treated, clinicians may decide to treat it as mentioned above in our case study with chemotherapy after treating the effusion first.
The objective in treatment is assisting the patient to breathe without difficulty by administering supplemental oxygen if required, and removal of the fluid that has built up between the parietal and visceral pleural layers of the lung which can help with both symptom management and diagnosis of the cause of the fluid build up (Tyson, 2004).
Mrs. Peters has already had fluid removed by thoracentesis for diagnosis which was positive for breast cancer and now the methods of more permanent fluid removal need to be explored.
Thoracentesis
This is a procedure in which a catheter is placed under sterile conditions into the pleural space for drainage of the fluid. The procedure can either be performed at the bedside, using local anesthesia and anatomical landmarks to guide catheter insertion, or radiologically, using fluoroscopy to direct catheter location. No matter which method is used there are some common potential complications to assess for. Having the patient awake and able to report symptoms that they are experiencing is valuable. Complaints of shortness of breath, coughing or pain could be an indication of a problem.
Fluid should be removed slowly so as to prevent some complications and care should be given not to remove too much fluid at one time, generally no more than 1000ml despite the fact that some research shows removal of more fluid may be tolerated (Feller-Kopman, 2007). Removal of too much fluid too quickly along with pain or coughing could result in reexpansion pulmonary edema, hypotension and circulatory collapse from the rapid reexpansion of the lung. Another difficulty from removing too much fluid quickly is that it may allow for fluid to reaccumulate rapidly again.
Other complications from thoracentesis, especially if they are being performed repeatedly, are infection, pneumothorax, bleeding and fluid loculations from scar tissue formation from repeated catheter insertions. Post procedure chest x-ray always needs to be completed to determine if the procedure and the manipulation caused a pneumothorax. Thoracentesis is also considered only a temporary measure for symptomatic relief, and with the diagnosis of cancer cell shedding, will cause fluid reaccumulation over and over.
Tube Thoracoscopy
Placement of a chest tube for drainage and management of a MPE is another method which could be utilized. Chest tubes could come in two basic forms the first being poly vinyl chloride (PVC) type catheters which are large and rigid, usually used after surgery because of the thick, bloody drainage. These tubes can be placed at the bedside by blunt dissection in the area of the fifth intercostal space using anatomical landmarks to guide placement (Jain, Deoskar, Barthwal & Rajan, 2006). However, since the PVC tubes are usually large (26 to 36 French sized), a great deal of pain is usually involved and the patients seem to do better with moderate sedation or if the tube is placed under anesthesia. These are tubes that are commonly used as chest tubes after surgery in the thoracic cavity. Patients will rarely go home with a larger chest drainage system as there is not always the support needed at home for this type of system.
The second type of tube for tube thoracoscopy is a pigtail catheter which can be placed at the bedside as well, but is generally placed in Interventional Radiology under fluoroscopy. This catheter is much smaller in size (8-12 French sized), and is made of silicone, so it is much more flexible and comfortable for the patient. Once the proper placement is determined in the pleural space and the catheter is placed accurately, the tip of the catheter is curled to lock it into place and to prevent a penetrating injury (Gammie, et al., 1999; Tsai, et al. 2006)- thus the name “pigtail”.
In either case, once the preferred tube is placed it can be connected to a chest drainage system or a drainage bag with a one way valve at the discretion of the clinician. In this way fluid can be drained and measured accordingly. Complications associated with this procedure are again infection, pneumothorax, bleeding, and reexpansion pulmonary edema if a large amount of fluid is removed too quickly. A post procedure chest x-ray is needed after this procedure to rule out a pneumothorax. If patients are very near to the end of their lives management of the MPE with a pigtail catheter to a gravity bag with a one way valve is a reasonable option to control the patient’s fluid build up and SOB for their remaining days.
There has been a recent push for more portable chest drains that allow patients to manage chest drainage problems at home. In having a more mobile or portable approach patients can go home sooner and care for their tubes as outpatients, costs will be deceased and the patients may have an improved quality of life (Carroll, 2005). Several of these tubes are geared toward patients with a pneumothorax and hold very little volume of drainage, so they would not be good options for a patient with a MPE. One of these mobile types of drains is made by Atrium and is called the Express Mini 500®. This drain can actually be connected to suction if required and holds up to 500 ml of fluid which can be emptied if needed with a Luer-Lok syringe. This drainage system could be utilized for patients if the drainage in not too large in volume, they want to be more mobile, and have good dexterity for emptying the container, and the patient’s clinician has no further plans for treatment of the MPE (Carroll, 2005).
Tube Thoracostomy with Administration of a Sclerosing Agent
Generally, placement of a chest tube alone is insufficient to completely treat the effusion. If a patient’s life expectancy is longer than just a few weeks, then attempting to relieve the effusion more permanently is considered necessary. This can be can be accomplished by placement of any chest tube of a desired size and instillation of a substance to causes a chemical sclerosing. The basic principle to sclerosing is that the chest tube itself will work to completely drain the pleural space, and the placement of a sclerosing agent into the tube causes a chemical pleuritis with scar tissue formation or adhesions which causes the visceral and parietal pleura to “stick together” and allows no space for reaccumulation of fluid (Taubert, 2004). The process works best if the pleural space is empty in order in order not to decrease the potency of the sclerosing agent.
A number of substances have been used as a sclerosing agent namely antibiotics, talc, and some chemotherapeutic agents. Tetracycline used to be the agent used most commonly, but is only available in oral form since the mid 1990’s so is no longer used and was effective about 80% of the time. Bleomycin Sulfate is one of the more common chemotherapeutic agents used and is effective about 60-80% of the time. These substances would be administered directly into the pleural space via the chest tube and the tube clamped to keep the agent in place. The patient would be asked to change positions to allow the agent to come in contact with all surfaces if possible, then the chest tube is unclamped. Monitoring chest x-rays and the volume of drainage will account for the effectiveness of the particular sclerosing agent for that patient.
Side effects with this method of elimination of the MPE are similar to general tube placement, and in addition fever and pain from the irritation of the agent to the pleural surface. Infection is always of concern just with the initial tube placement, tube manipulation (opening and closing the system) and because of the addition of a foreign substance. Once it is determined that the pleurodesis is successful, the chest tube is removed, and chest x-ray determines that there is not a pneumothorax, and the patient is sent home allowing for the chest tube site to heal in a few days.
Video Assisted Thorascopic Surgery with Talc Sclerosing
Video Assisted Thorascopic Surgery (VATS) is a procedure using a thoracoscope through a small incision in the chest, allowing viewing of the pleural space and a way to administrate the talc directly by insufflation (aerosolized) directly onto the pleural surface (Heffner, 2008). With this method it ensures that all surfaces are covered and therefore better results with the irritation and adhesion formation, close to 100% (Taubert, 2004). This procedure is considered surgical and performed under general anesthesia. Side effects to monitor for are those with chest tube placement and fever and pain. The patient will probably have to stay in the hospital a few days recovering from the surgical procedure. Talc may be mixed into a slurry or suspension and administered directly through the chest tube. Similar to sclerosing with tetracycline or bleomycin the patient would have to turn to attempt to get all surfaces covered and there is a great deal of pain involved in the irritation component. Patients would have to be monitored just as any other post operative patient would for bleeding and infection.
Other Surgical Methods for Treating MPE
Two surgical procedures which could be considered by the physician when a patient has a MPE are a pleurectomy or placement of a pleuroperitoneal shunt. The shunt is placed surgically and consists of a tube or passage allowing the fluid from the effusion to move from the pleural space to the peritoneal space where it is hoped the fluid will be slowly absorbed. Many times this procedure is done if the patient failed chemical pleurodesis. It requires manual pumping of the shunt and could have complications from blockage of the shunt (Taubert, 2004; Heffner, 2008).
The other surgical procedure considered only in patients who can withstand a long surgical procedure and with a good life expectancy is a pleurectomy. A pleurectomy involves removal of the parietal pleura and manual irritation of the visceral pleura causing formation of adhesions and scar tissue and therefore no more fluid build up in the space (Taubert, 2004; Heffner, 2008). This is a major surgical procedure done under general anesthesia as an open chest case. A partial pleurectomy could be done via a thoracoscope. Patients having this procedure would need to be monitored and cared for as any thoracotomy patient would with high risk for pneumonia and deep vein thrombosis.
Tunnelled Indwelling Pleural Catheter with Intermittent Drainage
The final method for managing a MPE is use of an indwelling pleural catheter. These silicone catheters are generally placed in Interventional Radiology using fluoroscopy and tunnelled directly into the pleural space. The catheters of which there are several on the market have a one way valve that prevents air from entering the chest cavity and fluid from coming out without accessing the valve (Taubert, 2004). Once the catheter is placed and chest x-ray has confirmed that there is no pneumothorax, patients can go home and manage their effusion as an outpatient by draining the catheter using the appropriate supplies 2-3 times a week or as ordered by the physician. The nurse monitors the patient for all the usual side effects of placement of a chest tube, and teaches the patient and their family how to drain, manage problems and care for the patient with this type of catheter. Patients feel a sense of control with this catheter, allowing them to be at home and not connected to a drainage system all of the time for the remaining time that they have.
Overall nursing care for any of these methods of managing a MPE is to monitor patients for signs of bleeding, increased SOB and infection. Care for any of the post surgical patients by monitoring for respiratory problems, infection and administering pain medications. Patients having their pleural effusion drained will often have some pain with the procedure and the nurse needs to adequately medicate the patient to control that pain.
Mrs. Peters had an indwelling pleural catheter placed and her husband was taught how to empty the drain three times a week. This lasted for about 10 weeks before the effusion was only producing 75ml at a time. The catheter was pulled. Mrs. Peters went on hospice about six months later and died peacefully at home.
Quality of Life and End-of-Life Issues
MPE has a significant impact on the patient’s quality of life as it is often common in the last 4-6 months of life (Burrows, Mathews, Colt, 2000; von Gruenigen, Frasure, Reidy, Gil, 2003). A patient’s physical condition with MPE helps predict how well they will do and their survival after MPE treatment. In patients who underwent a thorascopic pleurodesis for the management of MPE, the patients who had the best performance status (functional status) were the ones that had better post-operative survival (Burrows, et al). In this study, the patients who were able to perform self care lived 395 days and the patients who required hospitalization only survived 34 days (Burrows). Management of dyspnea at this point is paramount and nurses play a major role in symptom management. Besides morphine, oxygen therapy is useful along with a fan which helps reduce the feeling of breathlessness. Positioning the patient for maximal lung expansion by sitting them up in bed and teaching them how to use the diaphragm for maximal lung expansion is helpful. Pursed lip breathing helps reduce the feeling of breathlessness and controls the respiratory rate. When activity is required, the patient needs to be taught how to pace himself to not become short of breath. Referral to hospice and social work is appropriate to maximize the quality of the patient’s remaining life span.
Figure 1.
MPE References
- Anderson CB, Philpott GW, Gerguson TB. The treatment of malignant pleural effusion. Cancer. 1974;33(4):916–922. doi: 10.1002/1097-0142(197404)33:4<916::aid-cncr2820330405>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Brubacher S, Gobel B. Homles. Use of the Pleurx Pleaural Catheter for the management of malignant pleural effusions. CJON. 2003;7(1):35–38. doi: 10.1188/03.CJON.35-38. [DOI] [PubMed] [Google Scholar]
- Burrows CM, Mathews WC, Colt HG. Predicting survival in patients with recurrent symptomatic malignant pleural effusions: an assessment of the prognostic values of physiologic, morphologic, and quality of life measures of extent of disease. Chest. 2000;117(1):73–78. doi: 10.1378/chest.117.1.73. [DOI] [PubMed] [Google Scholar]
- Carroll P. Keeping up with mobile chest drains. RNweb. 2005;68(10):26–31. [PubMed] [Google Scholar]
- DeCamp MM, Jr., Mentzer SJ, Swanson SJ, Sugarbaker DJ. Malignant effusive disease of the pleura and peri cardium. Chest. 1997;112(4):291S–295S. doi: 10.1378/chest.112.4_supplement.291s. [DOI] [PubMed] [Google Scholar]
- Feller-Koman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. The Annals of Thoracic Surgery. 2007;84:1656–1662. doi: 10.1016/j.athoracsur.2007.06.038. [DOI] [PubMed] [Google Scholar]
- Gammie JS, Banks MC, Fuhrman CR, Pham SM, Griffith BP, Keenan RJ, Luketich JD. The pigtail catheter for pleural drainage: A less invasive alternative to tube thoracostomy. JSLS. 1999;3:57–61. 7. [PMC free article] [PubMed] [Google Scholar]
- Genc O, Petrou M, Ladas G, Goldstraw P. The long-term morbidity of pleuroperitoneal shunts in the management of recurrent malignant effusions. European Journal of Cardio-thoracic Surgery. 2000;18(2):143–146. doi: 10.1016/s1010-7940(00)00422-x. [DOI] [PubMed] [Google Scholar]
- Goldman DA. Effusions. In: Yarbro CH, Frogge JH, Goodman M, editors. Cancer symptom management. 3rd ed Jones & Bartlett Publishers; Sudbury, MA: 2004. pp. 420–4436. [Google Scholar]
- Heffner JE. Management of malignant pleural effusions. Up to Date. Feb. 11, 2008. pp. 1–7. 2000. Last Updated. [DOI] [PubMed]
- Jain S, Deoskar RB, Barthwal MS, Rajan KE. Study of pigtail catheters for tube thoracostomy. MJAFI. 2006;62(1):40–41. doi: 10.1016/S0377-1237(06)80153-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light RW. Useful tests on the pleural fluid in the management of patients with pleural effusions. Current Opinion in Pulmonary Medicine. 1999;5:245–249. doi: 10.1097/00063198-199907000-00012. [DOI] [PubMed] [Google Scholar]
- Neragi-Miandoab S. Malignant pleural effusion, current and evolving approaches for its diagnosis and management. Lung Cancer. 2006;54:1–9. doi: 10.1016/j.lungcan.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Porcel JM, Vives M. Etiology and pleural fluid characteristics of large and massive effusions. Chest. 2003;124:978–983. doi: 10.1378/chest.124.3.978. [DOI] [PubMed] [Google Scholar]
- Putnam JB., Jr. Malignant pleural effusions. Surgical Clinics of North America. 2002;82:867–883. doi: 10.1016/s0039-6109(02)00036-1. [DOI] [PubMed] [Google Scholar]
- Shuey K, Payne Y. Malignant pleural effusion. Clinical Journal of Oncology Nursing. 2005;9:529–532. doi: 10.1188/05.CJON.529-532. [DOI] [PubMed] [Google Scholar]
- Tabuert J. Management of malignant pleural effusion. Nursing Clinics of North America. 2001;36:665–683. [PubMed] [Google Scholar]
- Tyson LB. Oncologic Urgencies and Emergencies. In: Houlihan NG, editor. Lung Cancer: Site specific cancer series. Oncology Nursing Society; Pittsburgh: 2004. pp. 45–55. [Google Scholar]
- Tassi GF, Cardillo G, Marchetti GP, Carleo F, Martelli M. Diagnostic and therapeutical management of malignant pleural effusion. Annals of Oncology. 2006;17(suppl 2):ii11–ii12. doi: 10.1093/annonc/mdj911. [DOI] [PubMed] [Google Scholar]
- Tsai WK, Chen W, Lee J,C, Cheng WE, Chen CH, Hsu WH, Shih CM. Pigtail catheters vs large-bore chest tubes for management of secondary spontaneous pneumothoraces in adults. AJEM. 2006;24:795–800. doi: 10.1016/j.ajem.2006.04.006. [DOI] [PubMed] [Google Scholar]
- von Gruenigen VE, Frasure HE, Reidy AM, Gil KM. Clinical disease course during the last year in ovarian cancer. Gynecologic Oncology. 2003;90(3):619–624. doi: 10.1016/s0090-8258(03)00418-9. [DOI] [PubMed] [Google Scholar]