Summary
Research findings on the hypothalamic-pituitary-adrenal (HPA) axis and pediatric depression reflect a variety of methodological approaches that tap different facets of HPA-axis functions. Partly owing to the methodological heterogeneity of studies, descriptive reviews of this area have produced inconsistent conclusions. Therefore, we conducted formal meta-analyses of pertinent studies in order to advance our understanding of HPA-axis dysregulation in pediatric depression. We examined: a) 17 published studies of HPA-axis response to the dexamethasone suppression test in depressed youth (DST; N=926) and b) 17 studies of basal HPA-axis functioning (N=1,332). We also examined descriptively studies that used corticotropin releasing hormone (CRH) infusion, and those that used psychological probes of the HPA-axis. The global standardized mean effect size difference in HPA-axis response to the DST between depressed and non-depressed youth was .57, z = 4.18 p< .01. The global standardized mean difference effect size in basal HPA-axis functioning was .20, z = 4.53, p < .01. Age, sex, timing of sampling, dexamethasone dosage, or type of control group was not a significant source of variability for the DST or basal studies. In addition, when compared to non-depressed peers, depressed youth have a normative response to CRH infusion but an overactive response to psychological stressors. In conclusion, the HPA-axis system tends to be dysregulated in depressed youth, as evidenced by atypical responses to the DST, higher baseline cortisol values, and an overactive response to psychological stressors. This pattern of dysregulation suggests anomalies within the axis's negative feedback system and CRH production, but intact pituitary and adrenal sensitivity.
Keywords: HPA-Axis, Depression, Children, Adolescents, Dexamethasone Suppression Test
In the quest for biological markers of depression, researchers have explored the link between hypothalamic-pituitary-adrenal (HPA) axis functioning and adult depression for at least forty years, extending the inquiry to pediatric depression in more recent decades. Much of this work has involved the examination of cortisol response to biological and psychological probes or the assessment of basal cortisol levels. However, recent descriptive reviews of the pediatric literature have yielded inconsistent findings, with some reviewers concluding that the association between dysregulated HPA-axis and child depression is inconclusive at best (Birmaher et al., 1996; Birmaher & Heydl, 2001), while others suggesting that HPA-dysregulation in pediatric depression is consistent with that found in adults (Kaufman et al., 2001).
While earlier studies typically compared responses to the dexamethasone suppression test (DST) of depressed and non-depressed pediatric samples, there has been an increase in research using other methodologies to examine the HPA-axis, such as CRH infusion tests, psychological stress exposure, and measurements of basal levels at key times during the day. These different methodologies tap different components of the HPA-axis. Therefore, a careful analysis of group differences in cortisol functioning, as assessed by various methods, is needed to better understand of the specific components of the HPA-axis that may be associated with major depression in pediatric populations.
For example, upon administration of dexamethasone, the HPA-axis suppresses the production of corticotropin-releasing hormone (CRH) by the hypothalamus and adrenocorticotropic releasing hormone (ACTH) by the pituitary, eventually leading to a decrease of cortisol secretion (normative response). The non-suppression of cortisol after DST challenge indicates a dysregulation within the HPA-axis negative feedback mechanism (Burke et al., 2005). Such dysregulation appears to reflect tonic HPA-axis functioning and is usually considered an indication of hypercortisolaemia. In contrast, exogenous infusion of CRH results in the rapid release of ACTH by the pituitary gland followed by an increase in cortisol production by the adrenal gland. Relative suppression (limited increase) of ACTH and cortisol release after CRH infusion suggests pituitary under-sensitivity to CRH. Likewise, non-stress induced cortisol levels (basal) obtained at key hours of the day may reflect distinct components of the HPA-axis. For example, while high levels of total cortisol production during a 24-hour cycle indicate possible tonic hypercortisolaemia, samples obtained upon awakening (during the cortisol awakening response) are not necessarily reflective of tonic HPA-axis functioning and instead reflect stress reactivity (Edwards et al., 2001; Schmidt-Reinwald et al., 1999). Finally, while DST and CRH tests, and to a lesser extent basal cortisol levels, can mirror key areas of the HPA-axis system, responses to psychological probes may reflect differences within various HPA-axis components, including dysregulation of the negative feedback system, oversensitivity to CRH or ACTH, and/or differences in cognitive factors (e.g., attention, depressive rumination) that could lead to acute activation of the HPA-axis.
In addition to differences in the methods used to assess HPA-axis functioning, studies have also differed along a number of dimensions that may have contributed to the inconsistencies of past findings, such as age (e.g., Dahl et al., 1992), time of data collection (e.g., Forbes et al., 2006; Mannie et al., 2007), or whether the comparison group consisted of youths with psychiatric diagnoses other than depression (e.g., Casat et al., 1994) or normal controls (e.g., Feder et al., 2004; Forbes et al., 2006). While previous descriptive reviews have examined these factors as possible moderators of the MDD-cortisol relationship, there is no quantitative review of the effects of these variables on the various indices of HPA-axis functioning in depression.
We conducted a meta-analytic review of the HPA-axis literature on pediatric depression with the overall goal of addressing several of the above noted issues. Specifically, we focused on comparing the different research methodologies used. To this end, we grouped studies according to whether they relied on a) biological probes such as the dexamethasone suppression test or corticotropin releasing hormone infusion, b) basal cortisol levels throughout the day, and c) psychological stress probes. In addition to assessing the main effects of each experimental approach, we examined the putative effects of age, sex, and age and sex distribution imbalances across MDD and control samples, timing of sampling during the day, and type of control group.
Methods
Study Selection and Search Strategy
We restricted our search to peer-reviewed journals and sought published articles, which reported on HPA-axis functioning of depressed youth. We used PubMed, PsycINFO, and Google Scholar™ to search for studies from the earliest available date up to February 15th, 2009. We searched abstracts and titles using iterations of the following search terms: depression, depressed, WITH child, children, childhood, adolescents, adolescence, WITH cortisol, HPA, CRH, and hormones. We also searched the reference lists of recent reviews of this area, as well as the reference lists of primary sources. Finally, we contacted key researchers in the field to ensure we did not miss any eligible studies.
After duplicate findings were eliminated, our search strategy yielded 157 unique articles. The abstracts and methods of these articles were reviewed and those that met full inclusion criteria were chosen for the meta-analysis. Articles were included if all of the following conditions were met: a) the design included a depressed group consisting of children or adolescents with diagnosed major depressive disorder or dysthymia, b) the depressed group did not include bipolar cases, c) the design included a non-depressed comparison group, d) the methodology entailed either a biological challenge relevant to the HPA axis, experimental or naturally occurring psychological stress, or characterization of diurnal variation of plasma, salivary, or urinary cortisol levels, and e) the outcome was reported as mean cortisol differences between MDD and non-depressed groups, or differences in rates of non-suppressors in the case of DST studies.
We then examined the selected studies for potential sample overlap. When two articles had sample overlap but presented non-redundant data (e.g., obtained at different times or during different protocols) the two articles were kept in the analysis and clustered as described in the statistical section below. If the two articles presented sample overlap and reported redundant data, the study with the largest sample size was selected.
Based on our inclusion criteria and procedures, we identified 17 DST articles, 17 articles presenting basal cortisol levels, 4 articles using corticotropin releasing hormone (CRH) infusion, and 3 articles using psychological stressors. Therefore, we were able to conduct separate meta-analysis only on studies using the DST and those reporting basal cortisol levels. The limited number of CRH infusion and psychological stressor studies precluded a formal meta-analysis and thus for these methodologies we present only a descriptive comparison of effect sizes.
Effect Size Calculation
Effect sizes were calculated for two types of outcome data, namely: 1) mean cortisol differences between MDD and control groups and 2) difference between MDD and control group in rate (χ2) of suppression vs. non-suppression to the DST. Hedge's G (Hedges, 1982) weighted effect size was used as metric for all mean comparison. Hedge's G adjusts for differences in sample sizes and yields a more conservative metric than Cohen's D (Grissom & Kim, 2005). Studies presenting χ2 statistics were also transformed to a Hedge's G scale and adjusted with Hedge's sample size weight adjustment. Studies that reported comparisons between an MDD group and more than one control sample were treated as independent samples nested within studies, yielding 18 DST and 27 basal samples nested within 17 and 17 studies, respectively.
Meta Analytic Approach
We used a hierarchical multilevel mixed-model approach (Kalaian & Raudenbush, 1996) similar to that employed by Dickerson and Kemeny (Dickerson & Kemeny, 2004). This approach allows the use of multiple effect sizes per study, as it controls for the non-independence of observations when a single study provides multiple comparisons varying across potential moderator variables (e.g., time of the day). Therefore, this model accounts for the embedded nature of the data in which some effect sizes are embedded within studies. We included multiple effect sizes per study as long as they represented comparisons that differed in at least one potential moderator. However, when the effect sizes were simple repeated observations of the same condition (e.g., morning basal samples obtained on 3 consecutive days), the data were pooled and presented as a single effect size. For all computations we used SAS PROC MIXED syntax designed for multi-level models (Singer, 1998) with special application for meta-analysis (van Houwelingen et al., 2002; Normand, 1999).
First, we calculated the overall effect size using maximum likelihood in a random effects model. Our model employed a two level structure. At the first level (within-study level), each observed effect size within a study (dij) is a function of the true effect size for the study (β0j) and within-study measurement error (Rij):
At the second level (between-study level), each true study effect size is a function of the overall effect size across all studies (γ00) and random residual error:
This structure was used to calculate 1) the overall effect size (γ00), 2) whether the overall effect size was significantly different from zero, and 3) the heterogeneity of effect sizes across studies. The heterogeneity, as observed in the variance of the random residual error (U0j), indicates whether there is variance that was not accounted for by the random effects model. However, we proceeded to examine possible factors that explain differences between studies even if heterogeneity was not observed, as estimates of heterogeneity have some limitations (Ioannidis, 2008), and significant moderating factors may be present even when the variability between studies is not large (see Hall & Rosenthal, 1995).
Then, we expanded the previous model to examine the effects of two types of within- and between-study factors. First, we entered time of the day for each cortisol assessment as a within-study level 1 factor. Second, between-study level 2 factors included type of control group (non-psychiatric vs. psychiatric), mean age of subjects (years), and sex (% male). For the DST studies only we also included DST dosage (.5mg vs. 1mg) as a between-study factor.
Publication Bias
Assessment and control for publication bias was conducted via a modified version of the Trim and Fill method as proposed by Duval and Tweedie (2000). First, a funnel plot was created by plotting the effect sizes against standard error (specifically 1/SE). The possible number of missing studies was estimated via a modified rank-base multiple iteration method leading to the L0 statistic (Duval & Tweedie, 2000). The L0 statistic was originally defined on a “naive” random effect size which assumed that all measurements were independent (i.e. sampled in clusters of size 1). To ensure that our analysis was not unduly weighted by studies with replicate measures as expected, given our multilevel hierarchical modeling, we estimated the mean effect size and pooled standard error within each study sample, and used these as the primary points in our trim-and-fill analysis. We computed a “Clustered L0”, by comparing the mean effect sizes to a “grand mean” intercept from a random effects model that assumed that measurements were sampled in clusters of varying size. When one or more missing studies were identified (Cluster L0 > 0.50), the missing data were estimated based on the symmetric data (in our case, cluster mean effect sizes and pooled variances) to fill the most extreme lower left cluster from our funnel plot. Using this estimated data, a new overall fixed effects model was fitted and reported.
Results
Dexamethasone Suppression Test: Meta-Analysis
Main effects
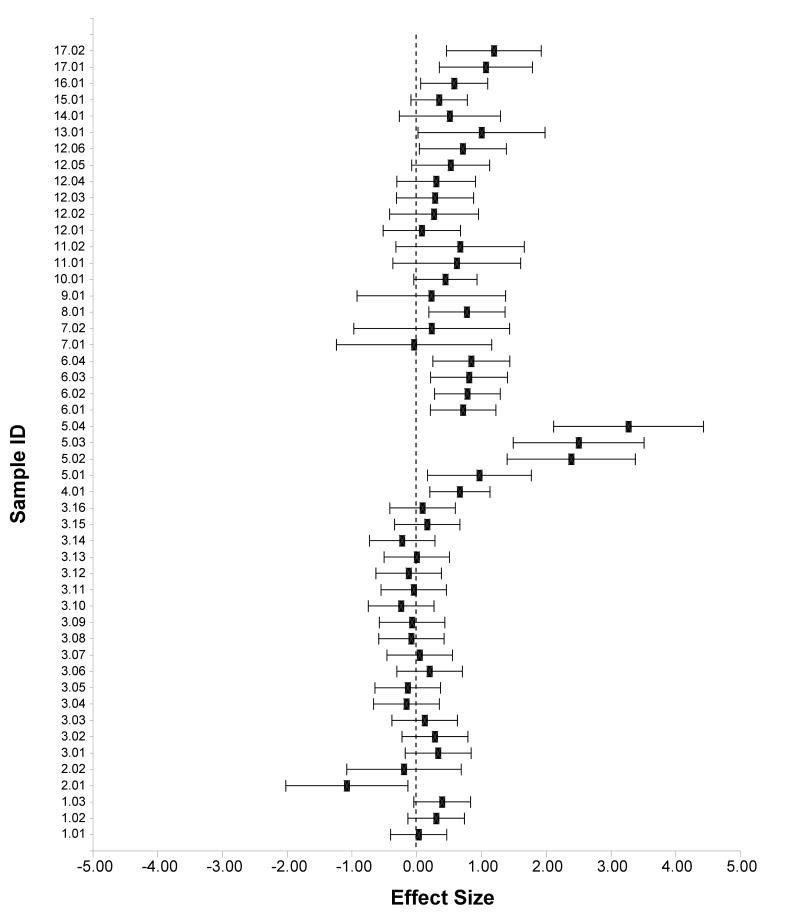
We identified 17 studies comparing MDD and non-MDD controls on post-DST cortisol levels (total 18 samples with N = 926; see Table 1). The studies contributed a total of 49 effect sizes. Using a mixed, random effects model, the pooled effect size (k = 49) for group differences in DST response was .57 (z = 4.18, p < .01; 95% CI .28 – .86) indicating greater cortisol production (or less suppression) after the DST among depressed children and adolescents than among non-MDD controls. The estimate of heterogeneity was significant (.26), z = 2.33, p < .01, suggesting large between-study variability. Figure 1 presents the effect sizes and 95% confidence intervals for the studies included in this analysis.
Table 1.
Study characteristics and mean effect size differences in cortisol response to the DST among depressed youth and comparison groups.
| ID | Author | Year | % Inpatient | Control Type | Sex MDD (%males) | Sex-Cont (%males) | Age-MDD | Age-Cont | Time | Dosage | N-MDD | N-Cont | G |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.01 | Birmaher et al. | 1992 | 0 | Norm | 50 | 66 | 14.9 | 15.3 | 23 | 1 | 44 | 38 | 0.03 |
| 1.02 | Birmaher et al. | 1992 | 0 | Norm | 50 | 66 | 14.9 | 15.3 | 16 | 1 | 44 | 38 | 0.30 |
| 1.03 | Birmaher et al. | 1992 | 0 | Norm | 50 | 66 | 14.9 | 15.3 | 8 | 1 | 44 | 38 | 0.39 |
| 2.01 | Casat et al. | 1994 | 100 | Psych | 55 | 78 | 10.1 | 10.8 | 16 | 0.5 | 11 | 9 | -1.08 |
| 2.02 | Casat et al. | 1994 | 100 | Psych | 55 | 78 | 10.1 | 10.8 | 8 | 0.5 | 11 | 9 | -0.20 |
| 3.01 | Dahl et al. | 1992 | 48 | Norm | 41 | 41 | 15.3 | 14.8 | 8 | 1 | 27 | 34 | 0.33 |
| 3.02 | Dahl et al. | 1992 | 48 | Norm | 41 | 41 | 15.3 | 14.8 | 9 | 1 | 27 | 34 | 0.28 |
| 3.03 | Dahl et al. | 1992 | 48 | Norm | 41 | 41 | 15.3 | 14.8 | 10 | 1 | 27 | 34 | 0.12 |
| 3.04 | Dahl et al. | 1992 | 48 | Norm | 41 | 41 | 15.3 | 14.8 | 11 | 1 | 27 | 34 | -0.16 |
| 3.05 | Dahl et al. | 1992 | 48 | Norm | 41 | 41 | 15.3 | 14.8 | 12 | 1 | 27 | 34 | -0.14 |
| 3.06 | Dahl et al. | 1992 | 48 | Norm | 41 | 41 | 15.3 | 14.8 | 13 | 1 | 27 | 34 | 0.20 |
| 3.07 | Dahl et al. | 1992 | 48 | Norm | 41 | 41 | 15.3 | 14.8 | 14 | 1 | 27 | 34 | 0.05 |
| 3.08 | Dahl et al. | 1992 | 48 | Norm | 41 | 41 | 15.3 | 14.8 | 15 | 1 | 27 | 34 | -0.08 |
| 3.09 | Dahl et al. | 1992 | 48 | Norm | 41 | 41 | 15.3 | 14.8 | 16 | 1 | 27 | 34 | -0.07 |
| 3.10 | Dahl et al. | 1992 | 48 | Norm | 41 | 41 | 15.3 | 14.8 | 17 | 1 | 27 | 34 | -0.24 |
| 3.11 | Dahl et al. | 1992 | 48 | Norm | 41 | 41 | 15.3 | 14.8 | 18 | 1 | 27 | 34 | -0.05 |
| 3.12 | Dahl et al. | 1992 | 48 | Norm | 41 | 41 | 15.3 | 14.8 | 19 | 1 | 27 | 34 | -0.12 |
| 3.13 | Dahl et al. | 1992 | 48 | Norm | 41 | 41 | 15.3 | 14.8 | 20 | 1 | 27 | 34 | 0.00 |
| 3.14 | Dahl et al. | 1992 | 48 | Norm | 41 | 41 | 15.3 | 14.8 | 21 | 1 | 27 | 34 | -0.22 |
| 3.15 | Dahl et al. | 1992 | 48 | Norm | 41 | 41 | 15.3 | 14.8 | 22 | 1 | 27 | 34 | 0.16 |
| 3.16 | Dahl et al. | 1992 | 48 | Norm | 41 | 41 | 15.3 | 14.8 | 23 | 1 | 27 | 34 | 0.09 |
| 4.01 | Doherty et al. | 1986 | 100 | Psych | 57 | 57 | 11.5 | 11.5 | 8,4.11 | 1 | 26 | 64 | 0.67 |
| 5.01 | Extein et al. | 1982 | 100 | Psych | 40 | 50 | 15 | 16 | 24 | 1 | 15 | 12 | 0.97 |
| 5.02 | Extein et al. | 1982 | 100 | Psych | 40 | 50 | 15 | 16 | 12 | 1 | 15 | 12 | 2.39 |
| 5.03 | Extein et al. | 1982 | 100 | Psych | 40 | 50 | 15 | 16 | 8 | 1 | 15 | 12 | 2.50 |
| 5.04 | Extein et al. | 1982 | 100 | Psych | 40 | 50 | 15 | 16 | 16 | 1 | 15 | 12 | 3.27 |
| 6.01 | Fristad et al. | 1988 | 100 | Norm | 70 | 29 | 9.7 | 9.3 | 16 | 0.5 | 63 | 21 | 0.72 |
| 6.02 | Fristad et al. | 1988 | 100 | Norm | 70 | 29 | 9.7 | 9.3 | 8 | 0.5 | 63 | 21 | 0.78 |
| 6.03 | Fristad et al. | 1988 | 100 | Psych | 70 | 71 | 9.7 | 9.2 | 8 | 0.5 | 63 | 14 | 0.81 |
| 6.04 | Fristad et al. | 1988 | 100 | Psych | 70 | 71 | 9.7 | 9.2 | 16 | 0.5 | 63 | 14 | 0.84 |
| 7.01 | Ha et al. | 1984 | 100 | Psych | 75 | 75 | 15.38 | 14.75 | 23 | 1 | 8 | 4 | -0.04 |
| 7.02 | Ha et al. | 1984 | 100 | Psych | 75 | 75 | 15.38 | 14.75 | 16 | 1 | 8 | 4 | 0.23 |
| 8.01 | Hsu et al. | 1983 | 100 | Psych | 51 | 51 | 15 | 15 | 16 | 1 | 14 | 66 | 0.77 |
| 9.01 | Livingston et al. | 1984 | 100 | Psych | 50 | 73 | 9 | 10.18 | 16 | 0.5 | 4 | 11 | 0.23 |
| 10.01 | Naylor et al. | 1990 | 100 | Psych | 68 | 73 | 11.12 | 9.68 | 4 | 1 | 25 | 48 | 0.44 |
| 11.01 | Petty et al. | 1984 | 100 | Psych | 77 | 77 | 9.35 | 10.58 | 16 | 0.5 | 13 | 6 | 0.62 |
| 11.02 | Petty et al. | 1984 | 100 | Psych | 77 | 77 | 9.35 | 10.58 | 23 | 0.5 | 13 | 6 | 0.67 |
| 12.01 | Pfeffer et al. | 1989 | 100 | Psych | 71 | 71 | 10.5 | 10.5 | 8 | 1 | 18 | 27 | 0.08 |
| 12.02 | Pfeffer et al. | 1989 | 100 | Psych | 71 | 71 | 10.5 | 10.5 | 23 | 1 | 12 | 26 | 0.27 |
| 12.03 | Pfeffer et al. | 1989 | 100 | Psych | 71 | 71 | 10.5 | 10.5 | 16 | 1 | 19 | 26 | 0.28 |
| 12.04 | Pfeffer et al. | 1989 | 100 | Psych | 71 | 71 | 10.5 | 10.5 | 16 | 0.5 | 16 | 31 | 0.30 |
| 12.05 | Pfeffer et al. | 1989 | 100 | Psych | 71 | 71 | 10.5 | 10.5 | 8 | 0.5 | 17 | 31 | 0.52 |
| 12.06 | Pfeffer et al. | 1989 | 100 | Psych | 71 | 71 | 10.5 | 10.5 | 23 | 0.5 | 14 | 25 | 0.71 |
| 13.01 | Poznanski et al. | 1982 | 0 | Psych | 55 | 55 | 10.2 | 9 | 16 | 0.5 | 9 | 9 | 1.00 |
| 14.01 | Robbins et al. | 1983 | 100 | Psych | 50 | 50 | 15.5 | 15.5 | 0.5 | 23 | 9 | 0.51 | |
| 15.01 | Steingard et al. | 1990 | 0 | Psych | 94 | 94 | 11.2 | 11.2 | 16 | 0.1 | 56 | 32 | 0.35 |
| 16.01 | Targun & Capodano | 1983 | 100 | Psych | 51 | 51 | 15.05 | 15.05 | 1 | 17 | 103 | 0.58 | |
| 17.01 | Young et al. | 2006 | 0 | Norm | 46 | 47 | 10 | 10.3 | 8 | 1 | 11 | 32 | 1.07 |
| 17.02 | Young et al. | 2006 | 0 | Norm | 46 | 47 | 10 | 10.3 | 16 | 0.5 | 11 | 32 | 1.19 |
Figure 1.
Forrest plot of standardized effect size and 95% confident intervals for comparative studies examining response to DST between depressed and non-depressed children and adolescents.
Effect Size Predictors
We used mixed-modeling meta-regression techniques to examine possible predictors of effect sizes. Table 2 presents the regression coefficients of all predictors tested. None of the factors examined significantly predicted effect sizes in DST response.
Table 2.
Summary of regression estimates for predictors of mean effect size differences in cortisol response to the DST among depressed youth and comparison groups.
| Estimate | SEM | Z | |
|---|---|---|---|
| Intercept | 0.5731 | 0.1372 | 4.18** |
| Level 1 Variable | |||
| Time (Vs. Afternoon) | |||
| Daily Average | 0.0403 | 0.3725 | 0.11 |
| Morning | 0.0859 | 0.1076 | 0.80 |
| Evening | -0.0726 | 0.1186 | -0.61 |
| Level 2 Variables | |||
| Non Psych Control | -0.1026 | 0.3094 | -0.33 |
| MDD Group % Inpatient | 0.0005 | 0.0032 | 0.17 |
| Mean Age (Years) | 0.0181 | 0.0559 | 0.32 |
| Mean % Male | -0.0135 | 0.0097 | -1.39 |
| Dosage DST (0.5 mg) | 0.1058 | 0.1835 | 0.58 |
Publication Bias
No missing studies in the direction of the null hypothesis were detected via the trim and fill method (Duval & Tweedie, 2000; Cluster Lo = -0.40 after 1 iteration). Figure 2 presents the funnel plot of cluster effect sizes by the inverse mean study sample standard error.
Figure 2.
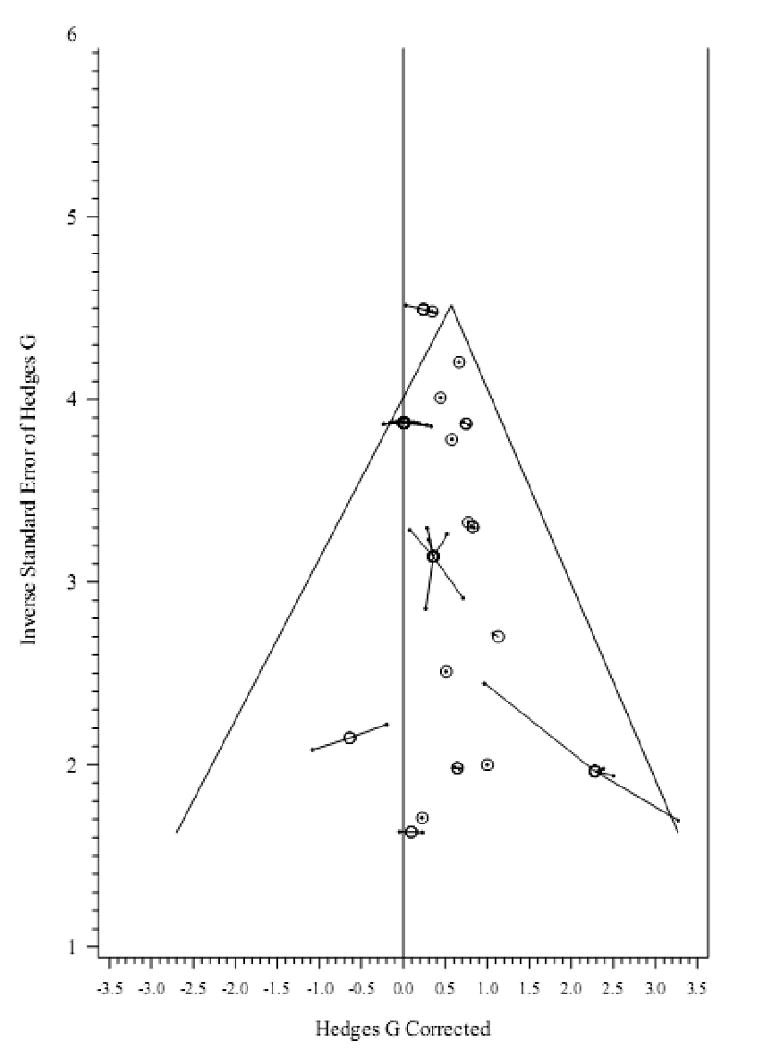
Inverse standard error vs. effect size in DST cortisol studies. Connected dots represent comparisons in the same sample/cluster. Circles represent average effect size and pooled precision of each cluster.
Basal HPA Axis Functioning Studies: Meta Analysis
Main Effects
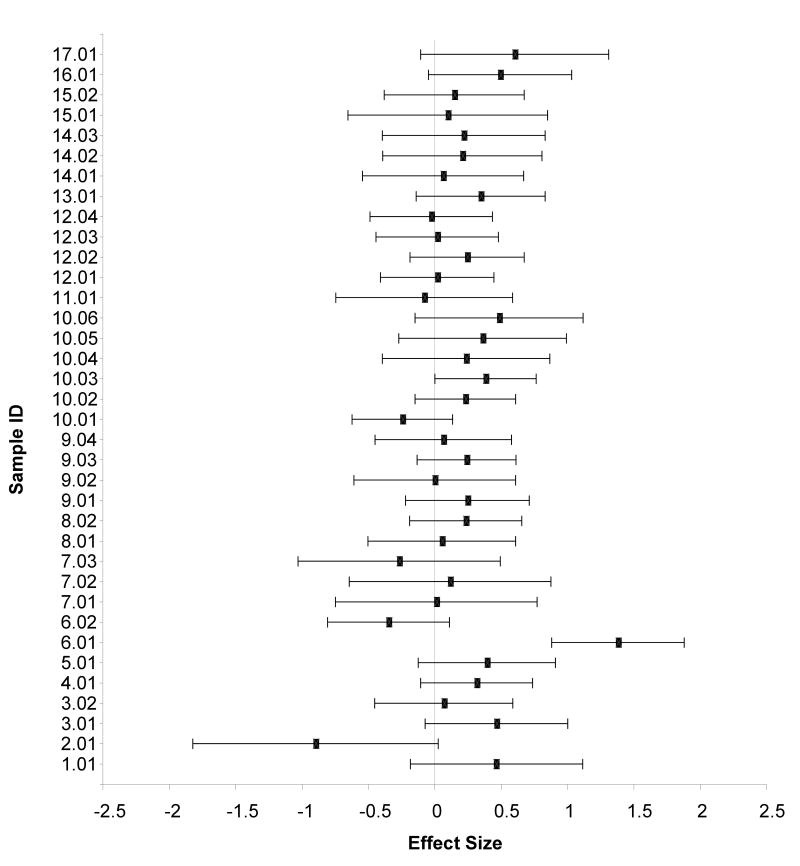
We identified 17 studies comparing MDD and non-MDD controls on basal (not stress induced) cortisol levels at different times during the day (27 total samples with N = 1,332; see Table 3). The studies contributed a total of 28 effect sizes. Using a random effects model, the pooled effect size (k = 36) for group differences in basal cortisol levels was .20 (z = 4.53, p < .01; 95% CI .11 – .29) indicating a tendency for depressed children and adolescents to have higher basal cortisol levels than non-MDD controls. The estimate of heterogeneity was not significant (.0000), z = .00, p > .20, suggesting limited between-study variability. Figure 3 presents the effect sizes and 95% confidence intervals for the studies included in this analysis.
Table 3.
Study characteristics and mean effect size differences in basal cortisol among depressed youth and comparison groups.
| ID | Author | Year | % Inpatient | Control Type | Sex MDD (%males) | Sex-Cont (%males) | Age -MDD | Age-Cont | Time* (hour) | N-MDD | N-Cont | G |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.01 | Birmaher et al. | 1996 | 39 | Norm | 71 | 50 | 10.4 | 10.1 | 17 | 28 | 14 | 0.47 |
| 2.01 | Casat et al. | 1994 | 100 | Psych | 55 | 78 | 10.1 | 10.8 | 8 | 11 | 9 | -0.90 |
| 3.01 | Coplan et al. | 2002 | 0 | Norm | 53 | 50 | 13.43 | 13.8 | UK | 22 | 36 | 0.47 |
| 3.02 | Coplan et al. | 2002 | 0 | Psych | 53 | 53 | 13.43 | 13.4 | UK | 22 | 40 | 0.07 |
| 4.01 | Dahl et al. | 1989 | 8 | Norm | 46 | 65 | 14.8 | 15.7 | M24h | 48 | 40 | 0.32 |
| 5.01 | Dahl et al. | 1991 | 48 | Norm | 37 | 41 | 15.2 | 14.9 | M24h | 32 | 27 | 0.39 |
| 6.01 | Doherty et al. | 1986 | 100 | Psych | 57 | 57 | 11.5 | 11.5 | 8 | 26 | 64 | 1.38 |
| 6.02 | Doherty et al. | 1986 | 100 | Psych | 57 | 57 | 11.5 | 11.5 | 23 | 26 | 64 | -0.35 |
| 7.01 | Extein et al. | 1982 | 100 | Psych | 40 | 50 | 15 | 16 | 8 | 15 | 12 | 0.01 |
| 7.02 | Extein et al. | 1982 | 100 | Psych | 40 | 50 | 15 | 16 | 16 | 15 | 12 | 0.12 |
| 7.03 | Extein et al. | 1982 | 100 | Psych | 40 | 50 | 15 | 16 | 24 | 15 | 12 | -0.27 |
| 8.01 | Feder et al. | 2004 | 15 | Norm | 59 | 59 | 9.3 | 9.2 | M24h | 72 | 15 | 0.05 |
| 8.02 | Feder et al. | 2004 | 15 | Psych | 59 | 59 | 9.3 | 8.7 | M24h | 72 | 31 | 0.23 |
| 9.01 | Forbes et al. | 2006 | 0 | Norm | 37 | 56 | 13.98 | 13.4 | 17 | 40 | 32 | 0.25 |
| 9.02 | Forbes et al. | 2006 | 0 | Psych | 37 | 64 | 13.98 | 13.4 | 17 | 40 | 14 | 0.00 |
| 9.03 | Forbes et al. | 2006 | 0 | Norm | 68 | 61 | 10.49 | 10.4 | 17 | 76 | 44 | 0.24 |
| 9.04 | Forbes et al. | 2006 | 0 | Psych | 68 | 67 | 10.49 | 10.5 | 17 | 76 | 18 | 0.06 |
| 10.01 | Goodyer et al. | 1996 | 0 | Norm | 42 | 43 | 12.98 | 13.08 | 8 | 82 | 40 | -0.24 |
| 10.02 | Goodyer et al. | 1996 | 0 | Norm | 42 | 43 | 12.98 | 13.08 | 12 | 82 | 40 | 0.23 |
| 10.03 | Goodyer et al. | 1996 | 0 | Norm | 42 | 43 | 12.98 | 13.08 | 20 | 82 | 40 | 0.38 |
| 10.04 | Goodyer et al. | 1996 | 0 | Psych | 42 | 27 | 12.98 | 12.09 | 8 | 82 | 11 | .24 |
| 10.05 | Goodyer et al. | 1996 | 0 | Psych | 42 | 27 | 12.98 | 12.09 | 12 | 82 | 11 | .36 |
| 10.06 | Goodyer et al. | 1996 | 0 | Psych | 42 | 27 | 12.98 | 12.09 | 20 | 82 | 11 | .49 |
| 11.01 | Kaufman et al. | 1997 | 50 | Norm | 46 | 54 | 9.75 | 9.5 | 17 | 26 | 13 | -0.08 |
| 12.01 | Luby et al. | 2003 | 0 | Norm | 45 | 41 | 4.76 | 4.7 | 11 | 40 | 44 | 0.02 |
| 12.02 | Luby et al. | 2003 | 0 | Norm | 45 | 41 | 4.76 | 4.7 | 20 | 40 | 44 | 0.24 |
| 12.03 | Luby et al. | 2003 | 0 | Psych | 45 | 54 | 4.76 | 4.5 | 11 | 40 | 33 | 0.02 |
| 12.04 | Luby et al. | 2003 | 0 | Psych | 45 | 54 | 4.76 | 4.5 | 20 | 40 | 33 | -0.03 |
| 13.01 | Naylor et al. | 1990 | 100 | Psych | 68 | 73 | 11.12 | 9.7 | 11 | 25 | 48 | 0.35 |
| 14.01 | Pfeffer et al. | 1989 | 100 | Psych | 71 | 71 | 10.5 | 10.5 | 8 | 17 | 27 | 0.06 |
| 14.02 | Pfeffer et al. | 1989 | 100 | Psych | 71 | 71 | 10.5 | 10.5 | 16 | 17 | 29 | 0.21 |
| 14.03 | Pfeffer et al. | 1989 | 100 | Psych | 71 | 71 | 10.5 | 10.5 | 23 | 17 | 26 | 0.22 |
| 15.02 | Puig-Antich et al. | 1989 | 0 | Norm | 64 | 38 | 9.5 | 9.1 | M24h | 45 | 8 | 0.10 |
| 15.01 | Puig-Antich et al. | 1989 | 0 | Psych | 64 | 60 | 9.5 | 8.7 | M24h | 45 | 20 | 0.15 |
| 16.01 | Rao et al. | 2008 | 0 | Norm | 48 | 43 | 15 | 15.1 | 20 | 30 | 25 | 0.49 |
| 17.01 | Rao & Poland. | 2008 | 0 | Norm | 44 | 38 | 15.8 | 15.6 | M12p | 16 | 16 | 0.60 |
Figure 3.
Forrest plot of standardized effect size and 95% confident intervals for comparative studies examining basal cortisol levels between depressed and non-depressed children and adolescents.
Effect Size Predictors
We used mixed-modeling meta-regression techniques to examine possible predictors of effect sizes. Table 4 presents the regression coefficients for these predictors. None of the factors examined predicted average effect size differences between the MDD and control groups.
Table 4.
Summary of regression estimates for predictors of mean effect size differences in basal cortisol among depressed youth and comparison groups.
| Estimate | SEM | Z | |
|---|---|---|---|
| Intercept | 0.1990 | 0.0439 | 4.53** |
| Level 1 Variable | |||
| Time (Vs. Afternoon) | |||
| Daily Average | 0.0986 | 0.1971 | 0.50 |
| Morning | 0.0502 | 0.1945 | 0.26 |
| Evening | -0.0353 | 0.1998 | -0.18 |
| Level 2 Variables | |||
| Non Psych Control | 0.0499 | 0.0879 | 0.57 |
| MDD Group % Inpatient | 0.0004 | 0.0011 | 0.35 |
| Mean Age (Years) | 0.0190 | 0.0134 | 1.41 |
| Mean % Male | 0.0003 | 0.0042 | 0.08 |
p < .01
Publication Bias
No missing studies in the direction of the null hypothesis were detected via the trim and fill method (Duval & Tweedie, 2000; Cluster L0 = -1.0 after 1 iteration). Figure 4 presents the funnel plot of cluster effect sizes by the inverse mean study sample standard error.
Figure 4.
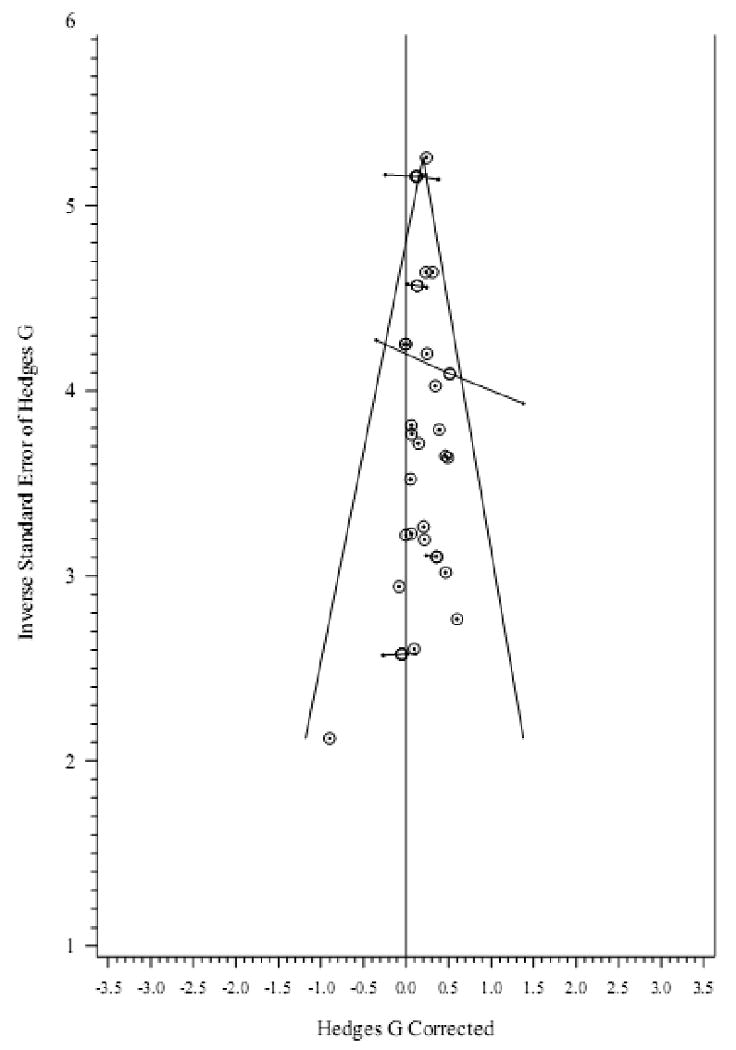
Inverse standard error vs. effect size in basal cortisol studies. Connected dots represent comparisons in the same sample/cluster. Circles represent average effect size and pooled precision of each cluster.
Additional Descriptive Analysis of HPA-Axis Probes
CRH Infusion Studies
Four studies compared cortisol levels post CRH infusion, but none found a significant difference in cortisol or ACTH secretion between MDD and non-MDD groups (Birmaher et al., 1996; Dorn et al., 1996; Kaufman et al., 1997; Ronsaville et al., 2006). Effect sizes could be calculated from only one study (Kaufman et al., 1997), which provided data on differences in total cortisol production, peak cortisol level, and pre-to-post test change after administration of CRH in MDD and non-MDD controls. The effect sizes were -.13, -.14, and -.11, respectively.
Psychological (or naturally occurring) Challenge Studies
Surprisingly, we found only three relevant studies of HPA-axis reactivity to psychological stressors in children or adolescents with depression. Effect sizes could be calculated only for Luby et al. (2003) and Rao et al. (2008). For Luby et al. (2003), the mean effect size differences between the MDD group and psychiatric and healthy controls at 30 minutes post-stress were .30 and .21, suggesting higher post stress cortisol in the depressed group. However, Luby et al. (2003) reported blunted reactivity (percentage change) in the depressed group, likely due to already elevated baseline values. Luby et al. (2004) also compared cortisol responses to a laboratory stressor in young children and found reduced percentage change in the MDD group when compared to healthy controls. Furthermore, Rao et al (2008) compared the cortisol response of depressed adolescents and a non-psychiatric control group after a social stress task and found mean effect size differences of .66 in peak cortisol levels, and of .91 to .96 during recovery.
Discussion
Results of our meta-analyses support an association between HPA-axis dysregulation and pediatric depression. Specifically, as compared to control peers, depressed youth tend to have a dysregulated response to the dexamethasone suppression test and moderately higher cortisol levels throughout the day. However, we found no evidence of a dysregulated response to corticotropin releasing hormone in depressed pediatric samples. Furthermore, data on HPA-axis response to psychological stressors in this clinical population were noticeably scant. The three available studies suggest that, compared to non-depressed peers, depressed preschoolers show blunted reactivity (percentage change) but higher peak cortisol levels, while depressed adolescents show higher reactivity and delayed recovery.
Depressed youth's dysregulated HPA-axis response to the DST suggests possible alterations in the mechanism in charge of regulating tonic cortisol levels. Hypercortisolemia has been consistently found in depressed adults (Christensen & Kessing, 2001; Gillespie & Nemeroff, 2005), and has been examined as a potential predictor of various outcomes (e.g., treatment response; Ribeiro et al., 1993) as well as one cause of the brain structural anomalies observed in depressed patients (Videbech & Ravnkilde, 2004). Our results indicate that such HPA-axis dysregulation can also be found in children and adolescents, a conclusion that is in line with, and extends previous reviews by Casat et al. (1989) and Kaufman et al. (2001). However, contrary to previous descriptive reviews, which proposed that age influences HPA axis response in depressed youngsters (Birmaher & Heydl, 2001), our analysis found no significant age differences. Likewise, we found no significant effects of time of sampling and type of control group (psychiatric vs. healthy peers).
Consistent with the DST results, our meta-analysis suggests that depressed youth have higher cortisol levels throughout the day than do non-depressed youth, also reflecting hypercortisolaemia. However, group differences in baseline cortisol did not appear to be a function of sex, age, type of control group, or time of the sample collection. The lack of timing effects are particularly remarkable, as this indicates that the dysregulation is not specific to any time point, as would be expected if the dysregulation was driven by anomalies within circadian systems. Group differences in baseline cortisol also appear to be markedly less robust than differences in DST responses, suggesting that dysregulated responses to the DST do not necessarily translate to tonic HPA-axis dysregulation. Other investigators have also observed this phenomenon. For example, in their meta-analysis Ribeiro et al. (1993) found that while responses to the DST reliably predicted specific outcomes, pre-DST baseline measures were not associated with such outcomes. Therefore, the DST appears to provide a more sensitive index of hypercortisolaemia in pediatric depression than baseline cortisol measures, extending the extant adult literature.
We also examined studies using probes to activate the HPA-axis in depressed youngsters. The 4 published studies that used infusion of corticotropin releasing hormone failed to find a difference in cortisol response between the depressed and control groups (Birmaher et al., 1996; Dorn et al., 1996; Kaufman et al., 1997; Ronsaville et al., 2006). Effect sizes were extremely weak and in the opposite direction of what was expected. This normative cortisol response to CRH infusion among depressed youngsters suggests intact hypothalamic sensitivity to CRH and/or intact adrenal sensitivity to ACTH.
Finally, the three studies of HPA-axis reactivity to psychological stressors (Luby et al., 2003, 2004; Rao et al., 2008) reported equivocal results. Depressed preschool children had blunted activation (reduced base to peak change) compared to non-depressed peers, but mean effect size differences in peak cortisol suggested higher post-stress levels in the depressed groups. In contrast, depressed adolescents showed higher post-stress and recovery cortisol levels when compared to non-depressed peers. These contradictory findings are possibly due to group differences in baseline cortisol levels prior to the stress tasks, with the depressed preschool groups showing higher cortisol levels across the entire protocol (whether because of elevated tonic levels or chronic stress response to the entire laboratory experience), which could constrain the stress response to specific tasks. Additional research on the effects of psychological stressors in pediatric depression using clinical samples of various ages and more methodological controls is warranted.
All in all, our results point towards possible anomalies within HPA-axis tonic system as reflected by dysregulated response to the DST and elevated basal cortisol levels. Evidence for a dysregulated response to psychological stressors is less clear however, as extant research is extremely limited. Yet, the apparent normative response to CRH challenges suggests that if there is a HPA-axis dysregulation to psychological stressors (as found in depressed adults; see Burke et al., 2005), such dysregulation is likely due to factors that affect the production of CRH by the hypothalamus, such as cognitive factors (e.g., rumination, attention) that could trigger hypothalamic activation, or endogenous differences in hypothalamic sensitivity to stress signals.
Although our findings are consistent with descriptive reviews of the adult literature (see Holsboer, 1995), the lack of relevant published meta-analyses of studies of adults precludes direct comparisons of the strength of the HPA-axis-depression association in these two age groups. While some meta-analyses of the DST in adult depression exist (see for example, Mann et al., 2006; Nelson & Davis, 1997; Ribeiro et al., 1993), none of them focused on the DST responses or hypercortisolaemia of depressed vs. non-depressed adults. The only relevant empirical review was conducted by Burke et al. (2005), who examined cortisol responses to psychological stressors in depressed vs. non-depressed adults. In their meta-analysis, the mean cortisol difference between the depressed and non-depressed groups was .27 in stress response and 1.39 during recovery. This is consistent with our findings on pediatric samples using psychological stressors that show a modest group difference in reactivity but much stronger difference during recovery.
The results of our meta-analysis should be considered in the context of various limitations. First, although we examined the effects of the type of comparison group (psychiatric vs. normative), we could not control for diagnostic differences within the psychiatric samples. Unfortunately, most of the psychiatric samples included participants with various diagnoses resulting in diagnostic heterogeneity. Second, although we only included comparisons between participants with documented MDD against controls, we could not control for comorbidity of other disorders within the MDD groups. It is imperative that future studies further examine the effects of comorbidity on the association between HPA-axis dysregulation and pediatric depression. Likewise, due to limited variance between the studies, we were unable to examine factors that had been proposed as possible moderators of the HPA-depression relation, such as depression severity, endogenous subtype, and atypicality (eg., Dahl et al., 1992; Nelson & Davis, 1997), and exposure to traumatic events (e.g., Kaufman et al., 1997; Rao et al., 2008). Finally, due to our strict selection criteria, a number of informative studies were omitted from the meta-analysis (see for example Goodyer et al., 1998, 2003, 2000a, 2000b, 2001, 1991; Halligan et al., 2007; Herbert et al., 1996; Rao et al., 1999, 1996, 1997). Yet, the general findings of the omitted studies are consistent with our results, suggesting HPA-axis dysregulation in pediatric depression.
Our results suggest at least two directions for future work. First, there is a great need for longitudinal research with pediatric populations at familial-risk for depression, who have not yet developed the disorder. Studying such groups will help establish whether HPA-axis dysregulation is a risk factor for eventual depression (potential cause) or a reflection of depressive states (potential symptom). For example, while Rao et al. (1997) reported significant reductions in basal cortisol after remission from an MDD episode, indicating that HPA-Axis dysregulation may be accentuated during depressive states, there is also evidence that HPA dysregulation is already present prior to the onset of depressive symptoms (Halligan et al., 2007) or MDD (Goodyer et al., 2000b). However, most longitudinal studies were conducted with children who were “at-risk” due to the presence of subclinical signs of depression, raising the possibility that the observed cortisol differences prior to MDD onset may have reflected the disease process already underway (see for example Goodyer et al., 2003, 2000a, 2000b). Future studies with asymptomatic children, at familial-risk for depression, may help to clarify the potential impact of HPA-dysregulation in the development of this condition.
Finally, more studies are needed of HPA-axis reactivity to psychological stress in pediatric depression. Such studies should examine the mechanisms by which psychological stressors elicit a stronger cortisol response and/or a more delay regulation phase in depressed individuals when compared to non-depressed peers. This is of great importance given that HPA-axis dysregulation is not unique to depression and is observed in many other disorders, such as post-traumatic stress disorder (de Kloet et al., 2006) and social anxiety (Beaton et al., 2006). Thus, mechanistic research with diverse clinical populations may help us understand how HPA-axis dysregulation may differentially affect the development or course of different psychiatric conditions. For example, is dysregulated cortisol response to stressors observed in depression the result of oversensitivity of the HPA-axis system to stress signals, or to neurocognitive factors prior to the activation of the system? The impact of rumination, attention and other aspects of information processing on HPA-axis functioning in this population should be a specific focus of study.
Acknowledgments
We would like to thank Dr. Jutta Joormann and Dr. Delia Vazquez for their helpful comments on previous versions of this manuscript, and Dr. Anne-Marie R. Iselin for her assistance with our meta-analytic procedures.
Role of funding source: This study was supported by the NIMH Program Project Grant #MH56193, HHSA, Washington, DC, USA. The NIMH had no further role in study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
Footnotes
Conflict of Interest: All authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beaton EA, Schmidt LA, Ashbaugh AR, Santesso DL, Martin M. Low salivary cortisol levels among socially anxious young adults: Preliminary evidence from a selected and a non-selected sample. Pers Individ Dif. 2006;41:1217–1228. [Google Scholar]
- Birmaher B, Dahl R, Perel J, Williamson D, Nelson B, Stull S, et al. Corticotropin-releasing hormone challenge in prepubertal major depression. Biol Psychiatry. 1996;39:267–277. doi: 10.1016/0006-3223(95)00177-8. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Dahl R, Ryan N, Rabinovich H, Ambrosini P, Al-Shabbout M, et al. The dexamethasone suppression test in adolescent outpatients with major depressive disorder. Am J Psychiatry. 1992;149:1040–1045. doi: 10.1176/ajp.149.8.1040. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Heydl P. Biological studies in depressed children and adolescents. Int J Neuropsychopharmacol. 2001;4:149–157. doi: 10.1017/S1461145701002358. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Ryan N, Williamson D, Brent D, Kaufman J, Dahl R, et al. Childhood and adolescent depression: A review of the past 10 years. Part I. J Am Acad Child Adolesc Psychiatry. 1996;35:1427–1439. doi: 10.1097/00004583-199611000-00011. [DOI] [PubMed] [Google Scholar]
- Burke H, Davis M, Otte C, Mohr D. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Casat C, Arana G, Powell K. The DST in children and adolescents with major depressive disorder. Am J Psychiatry. 1989;146:503–507. doi: 10.1176/ajp.146.4.503. [DOI] [PubMed] [Google Scholar]
- Casat C, Pearson D, Ruiz-Nazario J, Rhoades H. Serial dexamethasone suppression tests (DST) in recently hospitalized children. Biol Psychiatry. 1994;36:203–205. doi: 10.1016/0006-3223(94)91227-0. [DOI] [PubMed] [Google Scholar]
- Christensen M, Kessing L. The hypothalamo-pituitary-adrenal axis in major affective disorder: A review. Nordic J Psych. 2001;55:359–363. doi: 10.1080/080394801317080873. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Moreau D, Chaput F, Martinez J, Hoven C, Mandell D, et al. Salivary cortisol concentrations before and after carbon-dioxide inhalations in children. Biol Psychiatry. 2002;51:326–333. doi: 10.1016/s0006-3223(01)01250-1. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Kaufman J, Ryan N, Perel J, Al-Shabbout M, Birmaher B, et al. The dexamethasone suppression test in children and adolescents: A review and a controlled study. Biol Psychiatry. 1992;32:109–126. doi: 10.1016/0006-3223(92)90015-r. [DOI] [PubMed] [Google Scholar]
- Dahl R, Puig-Antich J, Ryan N, Nelson B, Novacenko H, Twomey J, et al. Cortisol secretion in adolescents with major depressive disorder. Acta Psychiatr Scand. 1989;80:18–26. doi: 10.1111/j.1600-0447.1989.tb01295.x. [DOI] [PubMed] [Google Scholar]
- Dahl R, Ryan N, Puig-Antich J, Nguyen N, Al Shabbout M, Meyer V, et al. 24-hour cortisol measures in adolescents with major depression: A controlled study. Biol Psychiatry. 1991;30:25–36. doi: 10.1016/0006-3223(91)90067-v. [DOI] [PubMed] [Google Scholar]
- Dickerson S, Kemeny M. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Doherty MB, Madansky D, Kraft J, Carter-Ake LL, Rosenthal PA, Coughlin BF. Cortisol dynamics and test performance of the dexamethasone suppression test in 97 psychiatrically hospitalized children aged 3-16 years. J Am Acad Child Psychiatry. 1986;25:400–408. [Google Scholar]
- Dorn LD, Burgess ES, Susman EJ, Von Eye A, DeBellis MD, Gold PW, et al. Response to oCRH in depressed and nondepressed adolescents: Does gender make a difference? J Am Acad Child Adolesc Psychiatry. 1996;35:764–773. doi: 10.1097/00004583-199606000-00016. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R. Trim and Fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Edwards S, Clow A, Evans P, Hucklebridge F. Exploration of the awakening cortisol response in relation to diurnal cortisol secretory activity. Life Sci. 2001;68:2093–2103. doi: 10.1016/s0024-3205(01)00996-1. [DOI] [PubMed] [Google Scholar]
- Extein I, Rosenberg G, Pottash A, Gold M. The dexamethasone suppression test in depressed adolescents. Am J Psychiatry. 1982;139:1617–1619. doi: 10.1176/ajp.139.12.1617. [DOI] [PubMed] [Google Scholar]
- Feder A, Coplan J, Goetz R, Mathew S, Pine D, Dahl R, et al. Twenty-Four-Hour Cortisol Secretion Patterns in Prepubertal Children with Anxiety or Depressive Disorders. Biol Psychiatry. 2004;56:198–204. doi: 10.1016/j.biopsych.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Forbes E, Williamson D, Ryan N, Birmaher B, Axelson D, Dahl R. Peri-sleep-onset cortisol levels in children and adolescents with affective disorders. Biol Psychiatry. 2006;59:24–30. doi: 10.1016/j.biopsych.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fristad M, Weller E, Weller R, Teare M, Preskorn S. Self-report vs biological markers in assessment of childhood depression. J Affect Disord. 1988;15:339–345. doi: 10.1016/0165-0327(88)90030-4. [DOI] [PubMed] [Google Scholar]
- Gillespie C, Nemeroff C. Hypercortisolemia and depression. Psychosom Med. 2005;67 1:S26–S28. doi: 10.1097/01.psy.0000163456.22154.d2. [DOI] [PubMed] [Google Scholar]
- Goodyer I, Herbert J, Altham PM. Adrenal steroid secretion and major depression in 8- to 16-year-olds, III. Influence of cortisol/DHEA ratio at presentation on subsequent rates of disappointing life events and persistent major depression. Psychol Med. 1998;28:265–73. doi: 10.1017/s0033291797006314. [DOI] [PubMed] [Google Scholar]
- Goodyer I, Herbert J, Altham P, Pearson J, Secher S, Shiers H. Adrenal secretion during major depression in 8- to 16-year-olds, I. Altered diurnal rhythms in salivary cortisol and dehydroepiandrosterone (DHEA) at presentation. Psychol Med. 1996;26:245–256. doi: 10.1017/s0033291700034644. [DOI] [PubMed] [Google Scholar]
- Goodyer I, Herbert J, Moor S, Altham P. Cortisol hypersecretion in depressed school-aged children and adolescents. Psychiatry Res. 1991;37:237–244. doi: 10.1016/0165-1781(91)90060-3. [DOI] [PubMed] [Google Scholar]
- Goodyer I, Herbert J, Tamplin A. Psychoendocrine antecedents of persistent first-episode major depression in adolescents: A community-based longitudinal enquiry. Psychol Med. 2003;33:601–610. doi: 10.1017/s0033291702007286. [DOI] [PubMed] [Google Scholar]
- Goodyer I, Herbert J, Tamplin A, Altham P. First-episode major depression in adolescents: Affective, cognitive and endocrine characteristics of risk status and predictors of onset. Br J Psychiatry. 2000a;176:142–149. doi: 10.1192/bjp.176.2.142. [DOI] [PubMed] [Google Scholar]
- Goodyer I, Herbert J, Tamplin A, Altham P. Recent life events, cortisol, dehydroepiandrosterone and the onset of major depression in high-risk adolescents. Br J Psychiatry. 2000b;177:499–504. doi: 10.1192/bjp.177.6.499. [DOI] [PubMed] [Google Scholar]
- Goodyer I, Park R, Herbert J. Psychosocial and endocrine features of chronic first-episode major depression in 8-16 year olds. Biol Psychiatry. 2001;50:351–357. doi: 10.1016/s0006-3223(01)01120-9. [DOI] [PubMed] [Google Scholar]
- Grissom R, Kim J. Effect Sizes for Research: A Broad Practical Approach. Lawrence Erlbaum Associates; Mahwah, NJ: 2005. [Google Scholar]
- Ha H, Kaplan S, Foley C. The dexamethasone suppression test in adolescent psychiatric patients. Am J Psychiatry. 1984;141:421–423. doi: 10.1176/ajp.141.3.421. [DOI] [PubMed] [Google Scholar]
- Hall J, Rosenthal R. Interpreting and evaluating meta-analysis. Eval Health Prof. 1995;18:393–407. doi: 10.1177/016327879501800404. [DOI] [PubMed] [Google Scholar]
- Halligan SL, Herbert J, Goodyer I, Murray L. Disturbances in Morning Cortisol Secretion in Association with Maternal Postnatal Depression Predict Subsequent Depressive Symptomatology in Adolescents. Biological Psychiatry. 2007;62:40–46. doi: 10.1016/j.biopsych.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Hedges L. Estimation of effect size from a series of independent experiments. Psychol Bull. 1982;92:490–499. [Google Scholar]
- Herbert J, Goodyer I, Altham P, Pearson J, Secher S, Shiers H. Adrenal secretion and major depression in 8- to 16-year olds, II. Influences of co-morbidity at presentation. Psychol Med. 1996;26:257–264. doi: 10.1017/s0033291700034656. [DOI] [PubMed] [Google Scholar]
- Holsboer F. Neuroendocrinology of mood disorders. In: Bloom F, Kupfer D, editors. Psychopharmacology: the fourth generation of progress. Raven Press; New York: 1995. pp. 957–969. [Google Scholar]
- van Houwelingen H, Arends L, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med. 2002;21:589–624. doi: 10.1002/sim.1040. [DOI] [PubMed] [Google Scholar]
- Hsu L, Molcan K, Cashman M, Lee S, Lohr J, Hindmarsh D. The dexamethasone suppression test in adolescent depression. J Am Acad Child Psychiatry. 1983;22:470–473. doi: 10.1016/s0002-7138(09)61511-9. [DOI] [PubMed] [Google Scholar]
- Ioannidis J. Interpretation of tests of heterogeneity and bias in meta-analysis. J Eval Clin Pract. 2008;14:951–957. doi: 10.1111/j.1365-2753.2008.00986.x. [DOI] [PubMed] [Google Scholar]
- Kalaian H, Raudenbush S. A multivariate mixed linear model for meta-analysis. Psychol Methods. 1996;1:227–235. [Google Scholar]
- Kaufman J, Birmaher B, Perel J, Dahl R, Moreci P, Nelson B, et al. The corticotropin-releasing hormone challenge in depressed abused, depressed nonabused, and normal control children. Biol Psychiatry. 1997;42:669–679. doi: 10.1016/s0006-3223(96)00470-2. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Martin A, King RA, Charney D. Are child-, adolescent-, and adult-onset depression one and the same disorder? Biol Psychiatry. 2001;49:980–1001. doi: 10.1016/s0006-3223(01)01127-1. [DOI] [PubMed] [Google Scholar]
- de Kloet C, Vermetten E, Geuze E, Kavelaars A, Heijnen C, Westenberg H. Assessment of HPA axis function in posttraumatic stress disorder: Pharmacological and non-pharmacological challenge tests, a review. J Psychiatr Res. 2006;40:550–567. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Livingston R, Reis C, Ringdahl I. Abnormal dexamethasone suppression test results in depressed and nondepressed children. Am J Psychiatry. 1984;141:106–108. doi: 10.1176/ajp.141.1.106. [DOI] [PubMed] [Google Scholar]
- Luby JL, Heffelfinger A, Mrakotsky C, Brown K, Hessler M, Spitznagel E. Alterations in stress cortisol reactivity in depressed preschoolers relative to psychiatric and no-disorder comparison groups. Arch Gen Psychiatry. 2003;60:1248–1255. doi: 10.1001/archpsyc.60.12.1248. [DOI] [PubMed] [Google Scholar]
- Luby JL, Mrakotsky C, Heffelfinger A, Brown K, Spitznagel E. Characteristics of depressed preschoolers with and without anhedonia: Evidence for a melancholic depressive subtype in young children. Am J Psychiatry. 2004;161:1998–2004. doi: 10.1176/appi.ajp.161.11.1998. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Currier D, Stanley B, Oquendo MA, Amsel LV, Ellis SP. Can Biological Tests Assist Prediction of Suicide in Mood Disorders? Int J Neuropsychopharmacol. 2006;9:465–474. doi: 10.1017/S1461145705005687. [DOI] [PubMed] [Google Scholar]
- Mannie Z, Harmer C, Cowen P. Increased waking salivary cortisol levels in young people at familial risk of depression. Am J Psychiatry. 2007;164:617–621. doi: 10.1176/ajp.2007.164.4.617. [DOI] [PubMed] [Google Scholar]
- Naylor M, Greden J, Alessi N. Plasma dexamethasone levels in children given the dexamethasone suppression test. Biol Psychiatry. 1990;27:592–600. doi: 10.1016/0006-3223(90)90526-8. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Davis JM. DST studies in psychotic depression: a meta-analysis. American Journal of Psychiatry. 1997;154:1497–1503. doi: 10.1176/ajp.154.11.1497. [DOI] [PubMed] [Google Scholar]
- Normand S. Tutorial in Biostatistics Meta-analysis: Formulating, evaluating, combining, and reporting. Stat Med. 1999;18:321–359. doi: 10.1002/(sici)1097-0258(19990215)18:3<321::aid-sim28>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Petty L, Asarnow J, Carlson G, Lesser L. The dexamethasone suppression test in depressed, dysthymic, and nondepressed children. Am J Psychiatry. 1985;142:631–633. doi: 10.1176/ajp.142.5.631. [DOI] [PubMed] [Google Scholar]
- Pfeffer CR, Stokes P, Weiner A, Shindledecker R, Faughnan L, Mintz M, et al. Psychopathology and plasma cortisol responses to dexamethasone in prepubertal psychiatric inpatients. Biol Psychiatry. 1989;26:677–689. doi: 10.1016/0006-3223(89)90102-9. [DOI] [PubMed] [Google Scholar]
- Poznanski E, Carroll B, Banegas M, Cook S, Grossman J. The dexamethasone suppression test in prepubertal depressed children. Am J Psychiatry. 1982;139:321–324. doi: 10.1176/ajp.139.3.321. [DOI] [PubMed] [Google Scholar]
- Puig-Antich J, Dahl R, Ryan N, Novacenko H, Goetz D, Goetz R, et al. Cortisol secretion in prepubertal children with major depressive disorder. Episode and recovery Arch Gen Psychiatry. 1989;46:801–9. doi: 10.1001/archpsyc.1989.01810090043008. [DOI] [PubMed] [Google Scholar]
- Rao U, Ryan ND, Dahl RE, Birmaher B, Rao R, Williamson DE, et al. Factors associated with the development of substance use disorder in depressed adolescents. J Am Acad Child Adolesc Psychiatry. 1999;38:1109–17. doi: 10.1097/00004583-199909000-00014. [DOI] [PubMed] [Google Scholar]
- Rao U, Poland RE. Electroencephalographic sleep and hypothalamic-pituitary-adrenal changes from episode to recovery in depressed adolescents. J Child Adolesc Psychopharmacol. 2008;18:607–13. doi: 10.1089/cap.2008.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao U, Dahl RE, Ryan ND, Birmaher B, Williamson DE, Giles DE, et al. The relationship between longitudinal clinical course and sleep and cortisol changes in adolescent depression. Biol Psychiatry. 1996;40:474–484. doi: 10.1016/0006-3223(95)00481-5. [DOI] [PubMed] [Google Scholar]
- Rao U, Hammen C, Ortiz LR, Chen L, Poland RE. Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biological Psychiatry. 2008;64:521–526. doi: 10.1016/j.biopsych.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao U, McCracken JT, Lutchmansingh P, Edwards C, Poland RE. Electroencephalographic sleep and urinary free cortisol in adolescent depression: A preliminary report of changes from episode to recovery. Biological Psychiatry. 1997;41:369–373. doi: 10.1016/s0006-3223(96)00430-1. [DOI] [PubMed] [Google Scholar]
- Ribeiro S, Tandon R, Grunhaus L, Greden J. The DST as a predictor of outcome in depression: A meta-analysis. Am J Psychiatry. 1993;150:1618–1629. doi: 10.1176/ajp.150.11.1618. [DOI] [PubMed] [Google Scholar]
- Robbins D, Alessi N, Yanchyshyn G, Colfer M. The dexamethasone suppression test in psychiatrically hospitalized adolescents. J Am Acad Child Psychiatry. 1983;22:467–469. doi: 10.1016/s0002-7138(09)61510-7. [DOI] [PubMed] [Google Scholar]
- Ronsaville D, Municchi G, Laney C, Cizza G, Meyer S, Haim A, et al. Maternal and environmental factors influence the hypothalamic-pituitary-adrenal axis response to corticotropin-releasing hormone infusion in offspring of mothers with or without mood disorders. Dev Psychopathol. 2006;18:173–194. doi: 10.1017/S095457940606010X. [DOI] [PubMed] [Google Scholar]
- Schmidt-Reinwald A, Pruessner J, Hellhammer D, Federenko I, Rohleder N, Schurmeyer T, et al. The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sci. 1999;64:1653–1660. doi: 10.1016/s0024-3205(99)00103-4. [DOI] [PubMed] [Google Scholar]
- Singer J. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J Educ Beh Stat. 1998;24:323–355. [Google Scholar]
- Steingard R, Biederman J, Keenan K, Moore C. Comorbidity in the interpretation of dexamethasone suppression test results in children: A review and report. Biol Psychiatry. 1990;28:193–202. doi: 10.1016/0006-3223(90)90574-l. [DOI] [PubMed] [Google Scholar]
- Targum S, Capodanno A. The dexamethasone suppression test in adolescent psychiatric inpatients. Am J Psychiatry. 1983;140:589–591. doi: 10.1176/ajp.140.5.589. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: A meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Young EA, Vazquez D, Jiang H, Pfeffer CR. Saliva cortisol and response to dexamethasone in children of depressed parents. Biol Psychiatry. 2006;60:831–836. doi: 10.1016/j.biopsych.2006.03.077. [DOI] [PubMed] [Google Scholar]