INTRODUCTION
Abnormalities in serotonin neurotransmission have been implicated in depression (Mann, 1999). A recent positron emission tomography study observed lower serotonin transporter binding potential in the amygdala and midbrain of depressed subjects compared to controls (Parsey et al., 2006). The regulation of the serotonin system depends in part on the serotonin transporter. Serotonin transporter deficient mice are behaviorally inhibited, underexploratory, underactive, underaggressive, harm avoidant and anxious, oversensitive to stimulation, and prone to exaggerated ACTH and catecholamine responses (Murphy et al., 2008). These observations suggest that the serotonion transporter gene has a pleiotropic expression relevant to complex behavioral disorders including depression. The serotonin transporter is encoded by a single gene (SLC6A4) on chromosome 17q11.1–17q12. SLC6A4 has two prevalent polymorphisms. The first consists of a variable number of tandem repeats in intron 2 (Ogilvie et al., 1996). The second (5-HTTLPR) is a 44-base pair insertion (L allele) or deletion (S allele) in the promoter region (Lesch et al., 1996). Neither polymorphism influences the structure of the serotonin transporter protein but both affect gene expression. There is evidence that the S allele (14 repeat allele) of the 5HTTLPR polymorphism is inefficient in transcribing the serotonin transporter in neurons (Lesch et al., 1996). Human lymphoblasts with an S allele express roughly half as much serotonin transporter compared to homozygous LL lymphoblasts (Lesch et al., 1996). Functional variants of the L allele have been identified and designated as LA and LG (Nakamura et al., 2000). The LG allele expresses comparable levels of serotonin transporter to that of the S allele and lower than that of the L allele (Hu et al., 2005; Hu et al., 2006).
The S allele of 5-HTTLPR polymorphism may attenuate the response of major depression to selective serotonin reuptake inhibitors (SSRIs) (Alessandro and Kato, 2008) but may not influence response to nortriptyline (Pollock et al., 2000a). Negative studies also exist (Kraft et al., 2007). Remission is recognized as the optimal target of the acute treatment of depression. Depressed patients who achieve remission are three times less likely to relapse than depressed patients left with residual symptoms (Judd et al., 1998). A 12-week citalopram trial showed a negative association between the S allele and remission of major depression (Arias et al., 2003). Patients homozygous for the S allele were three times less likely to achieve remission than patients with other 5-HTTLPR genotypes.
Along with genetic factors, aging related changes compromising the integrity of frontolimbic circuitry may contribute to geriatric depression non-remission (Alexopoulos, 2005). Depressed elders have microstructural (Nobuhara et al., 2006) and macromolecular (Kumar et al., 2004; Gunning-Dixon et al., 2008) white matter abnormalities in frontolimbic pathways. White matter hyperintensities have been associated with chronicity of geriatric depression (Simpson et al., 1998), although some disagreement exists (Salloway et al., 2002). In two different samples, we observed that depressed elderly patients who remained symptomatic after treatment with escitalopram (Alexopoulos et al., 2008) or citalopram (Alexopoulos et al., 2002) had lower fractional anisotropy (FA) in several frontolimbic white matter areas compared to depressed elders who achieved remission. Low FA has been used as an index of microstructural white matter abnormalities because FA has been shown to decline during the progression of degenerative disorders and in demyelinating diseases (Horsfield and Jones, 2002). FA declines with aging and this decline is positively correlated with average grey matter thickness and negatively correlated with the volume of white matter hyperintensities (Kochunov et al., 2007). Aging related FA decline is due to increased water diffusion (Smith et al., 2006) and has been interpreted to be a result of demyelination and/or gliosis (Mazziotta et al., 1995).
5-HTTLPR polymorphisms may be associated with white matter integrity because 5-HTTLPR influences neurodevelopment across the life span (Gould, 1999). Moreover, the S allele may be related to neuronal vulnerability to cortisol (O'Hara et al., 2007) and is associated with vascular risk factors (Comings et al., 1999) and cardiac events (Nakatani et al., 2005). Thus promoting white matter changes may be one way by which 5-HTTLPR allelic status reduces the remission rate of geriatric depression.
This analysis focuses on the subset of Caucasian depressed elderly patients from our escitalopram treatment trial (Alexopoulos et al., 2008) who also had genotypic characterization. It tests the hypotheses that: 1) Depressed elderly patients have lower FA than psychiatrically normal elderly controls; and 2) the S allele genotype status is associated both with low FA in frontolimbic white matter areas and with low likelihood of remission of geriatric depression.
METHODS
Subjects
Two groups of Caucasian subjects were included in this analysis. The few African Americans of our treatment trial were excluded because they have different distribution of 5-HTTLPR polymorphisms (Lotrich et al., 2003) and higher rates of cerebrovascular risk factors than Caucasians (Cushman et al., 2008). The depressed group (N=27) comprised consecutively recruited individuals aged 60 years and older, who met DSM-IV criteria for unipolar major depression without psychotic features and had a score of 18 or greater on the 24-item Hamilton Depression Rating Scale (HDRS) (Hamilton, 1960). The control group (N=27) consisted of psychiatrically normal subjects (by SCID-R) recruited through advertisement. After complete description of the study to the subjects, written informed consent was obtained.
For the depressed group, exclusion criteria were: 1) history or presence of other axis I psychiatric disorders; 2) presence of delirium, history of stroke, head trauma, multiple sclerosis, and brain degenerative diseases; 3) metastatic cancer, brain tumors, unstable cardiac, hepatic, or renal disease, myocardial infarction, or stroke within the 3 months preceding the study; 4) endocrinopathies other than diabetes, lymphoma, pancreatic cancer; 5) treatment with steroids, alpha-methyl-dopa, clonidine, reserpine, tamoxifen, and cimetidine; 6) Mini-Mental State Examination score < 24 (Folstein et al., 1975), and 7) metal implants. The same criteria were required for the normal control subjects plus absence of the diagnosis of depression and an HDRS score lower than 7.
Assessment
DSM-IV diagnosis was based on the SCID-R, administered at entry to the study. Depressive symptoms were assessed using the 24-item HDRS. Cognitive impairment was rated with the Mini Mental State Examination, disability was quantified with the World Health Organization Disability Assessment Schedule (Epping-Jordan JA, 2000), and vascular risk factors were assessed with the Cerebrovascular Risk Factor Assessment (CRFA) (Wolf et al., 1991). The CRFA score reflects the percentage of individuals expected to have a stroke in the next 10 years.
Treatment
The depressed group had a 2-week, single-blind, placebo drug-wash-out phase. Those who still met DMS-IV criteria for major depression and had HDRS≥18, were genotyped, had a brain MRI and received controlled treatment with escitalopram 10 mg daily for 12 weeks. Subjects received their medication in one-week supply blisters.
Depressed subjects were assessed weekly. Follow-up meetings consisted of a rating session with a research assistant followed by a brief session with a research psychiatrist. Research assistants administered the HDRS, obtained vital signs, questioned the subjects about medication adherence and inspected the escitalopram blister package. No subject received psychotherapy. Remission was defined as HDRS≤7 for two consecutive weeks and absence of DSM-IV diagnosis of depression.
Genotyping
Genomic DNA was isolated from whole blood samples using the QIAamp DNA blood kit (Qiagen) according to the manufacturers standard protocol. Genotyping for the 5HTTLPR was performed as reported (Wendland et al., 2006). Amplified products were resolved on 2.5% agarose gel electrophoresis with ethidium bromide staining. Genotypes were called by visual inspection of the gel. 5-HTTLPR alleles were categorized as S and L. Functional variants of the L allele (LA and LG) were also identified.
MRI
Brain scans were obtained with the 1.5T Siemens Vision Scanner housed at the Nathan Kline Institute Center for Advanced Brain Imaging. Patients received a magnetization prepared rapidly acquired gradient echo (MPRAGE) scan (TR=11.6ms, TE=4.9ms, matrix=256×256, FOV=320mm, NEX=1, slice thickness = 1.25 mm, 172 slices, no gap), as well as a turbo dual spin echo scan (TSE; TR=ms, TE=22/90ms, matrix=256×256, FOV=240mm, slice thickness = 5mm, 26 slices, no gap), and a diffusion tensor imaging scan (TR=6000ms, TE=100ms, matrix=128×128, FOV=320mm, NEX=7, slice thickness = 5mm, 19 slices, no gap). For the DTI scan, eight diffusion sensitization directions were used (with b=1000 s/mm2 along with an image with no diffusion weighting (b=0 s/mm2). The TSE and DTI scans were acquired in an oblique axial plane parallel to the anterior commissure–posterior commissure axis.
DTI were placed into Talairach space using methods described elsewhere (Alexopoulos et al., 2008). FA was computed using AFNI’s nonlinear algorithm (3dDWItoDT). Intersubject registration was carried out using an automatic registration toolkit (ART). The late echo of the TSE scan was used to correct for susceptibility-induced distortion.
We used a subject whose intracranial volume was the closest to the mean of the first 11 subjects to derive the T1-weighted template for registration. The volumes were computed in MEDx (Sensor Systems, Sterling, VA) after skull stripping using the FSL’s BET program (http://www.fmrib.ox.ac.uk/fsl/bet/index.html). We placed the case we chose into Talairach space using AFNI. To create a study-specific template, we iteratively registered the T1-weighted images from our subjects to this initial template and used the mean image of the final iteration as our final template.
We computed a white matter mask based on the mean FA map from a larger group of 83 subjects (including the subjects reported here) and a nonparametric histogram-based segmentation. The white matter threshold obtained in this way was applied to the mean FA map, and the resulting mask was applied to each subject’s normalized FA.
Data Analysis
S and SL subjects were grouped together because only one depressed subject was an S homozygote. To test the FA related hypotheses, we conducted voxel-based analysis of FA data with respect to group membership using a general linear model with age and mean diffusivity as the covariates. Mean diffusivity was used as a covariate because it is sensitive to partial volume effects, and possibly to atrophy (Ardekani et al., 2005). Initially, we used the thresholding method described by Baudewig et al. (Baudewig et al., 2003) as in our prior work (Alexopoulos et al., 2008). This approach reduces Type I error by identifying clusters of contiguous voxels (100 mm3) each with significant group differences (p<0.05) and then applies the constraint that one voxel in the cluster must be significant at p<0.005. To verify our observations and further reduce Type 1 error, we used a “false discovery rate” correction (q=.10)(Benjamini et al., 2001) setting an additional threshold of 50 contiguous voxels. The thresholded correlation maps were superimposed onto an MPRAGE image in Talairach space using AFNI . The hypothesis related to remission rates between S carriers vs. LL depressed subjects was tested using Fisher’s Exact Test.
RESULTS
A total of 64 subjects were studied. Of these, 37 met criteria for major depression and 27 were normal control subjects. After the 2-week drug washout placebo phase, 27 continued to meet symptom severity criteria (HDRS≥18) and entered the escitalopram phase. Depressed subjects entering the treatment phase had greater HDRS (t=−32.5, df=52, p<0.0001) and WHODAS scores (t=−6.42, df=45.5, p<0.0001) than control subjects (Table 1). However, there were no statistically significant differences in age, education, and cognitive impairment (MMSE) between depressed and control subjects.
TABLE 1.
Baseline Demographic and Clinical Data on Elderly Patients with Major Depression Treated with Escitalopram 10 mg Daily and on elderly Controls
| Depressed | Controls | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | S Carriers (N=18) |
L/L (N=9) |
S Carriers (N=21) |
L/L (N=6) |
||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Remission (R)/No Remission (NR) | 8R/10NR | 8R/1NR | ||||||
| Age (years) | 72.7 | 6.6 | 67.6 | 5.6 | 70.5 | 7.1 | 73.3 | 5.3 |
| Education (years) | 16.5 | 3 | 17 | 2.5 | 17 | 1.8 | 15.2 | 3.7 |
| Baseline HDRS | 21.2 | 2.8 | 21.1 | 1.8 | 1.8 | 1.8 | 2.3 | 2.1 |
| Age of Onset (years) | 51 | 20.2 | 57.1 | 15.1 | ||||
| Number of Previous Episodes | 2.9 | 1.2 | 3.9 | 4.9 | ||||
| WHODAS II total score | 36.4 | 7.6 | 30.8 | 6.2 | 22.5 | 4.4 | 26.2 | 6.5 |
| CRFA | 10.5 | 9.2 | 10.0 | 9.3 | 9.6 | 10.5 | 11.6 | 5.9 |
| Mini Mental State Exam | 28.2 | 1.4 | 28.8 | 1.2 | 28.5 | 1.2 | 28.8 | 0.8 |
HDRS = 24-item Hamilton Depression Rating Scale; WHODAS II = World Health Organization Disability Assessment Schedule II, CRFA=Cerebrovascular Risk Factor Assessment
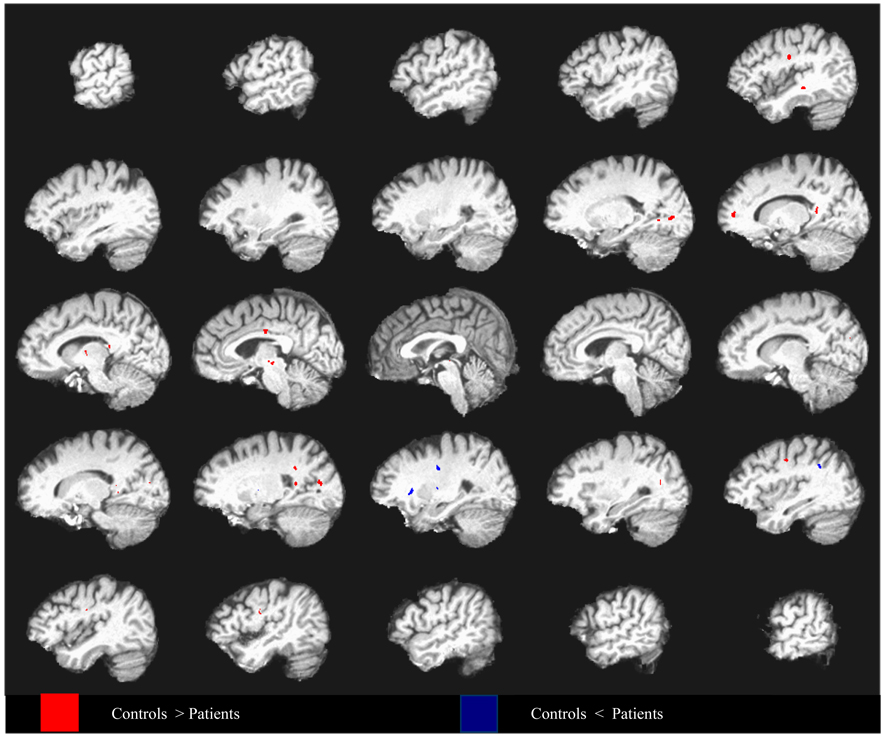
5-HTTLPR genotypes were similarly distributed in depressed and control subjects and were in Hardy-Weinberg equilibrium; allele frequencies were similar to those reported for Caucasians. Depressed patients had lower FA compared to controls in some frontal as well as in some posterior regions identified by the Baudewig et al. method. These findings were confirmed by the false discovery method (Figure 1), which demonstrated lower FA in frontal regions (i.e., dorsolateral, medial frontal, and precentral) along with some posterior regions (i.e., posterior cingulate, superior temporal, precuneus, cuneus, splenium, and visual areas). Additional regions of lower FA in depressed patients were detected in the thalamus and midbrain. Depressed patients had higher FA in the external capsule, lentiform nuclei, and a region of the posterior cingulate.
Figure 1.
Differences in fractional anisotropy between depressed elderly patients and non-psychiatric controls covarying for age and mean diffusivity, superimposed on a high resolution T1-weighted template. Data from every fifth sagittal slice are shown going from left hemisphere to right hemisphere. Group differences are thresholded such that 50mm3 clusters of voxels all significant at p < .01 (q = 0.1) are identified.
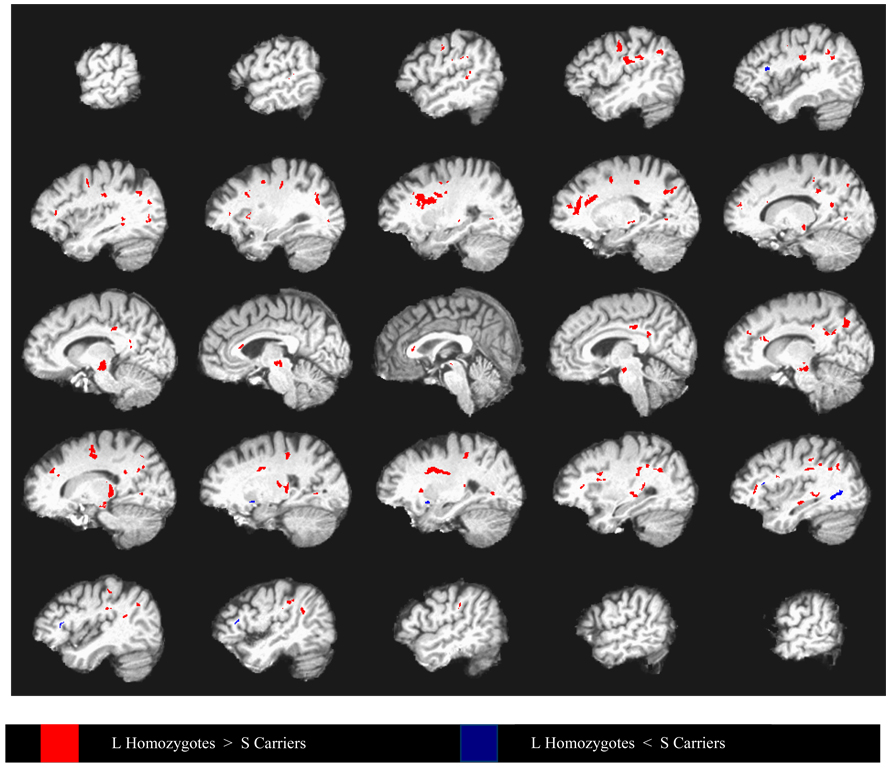
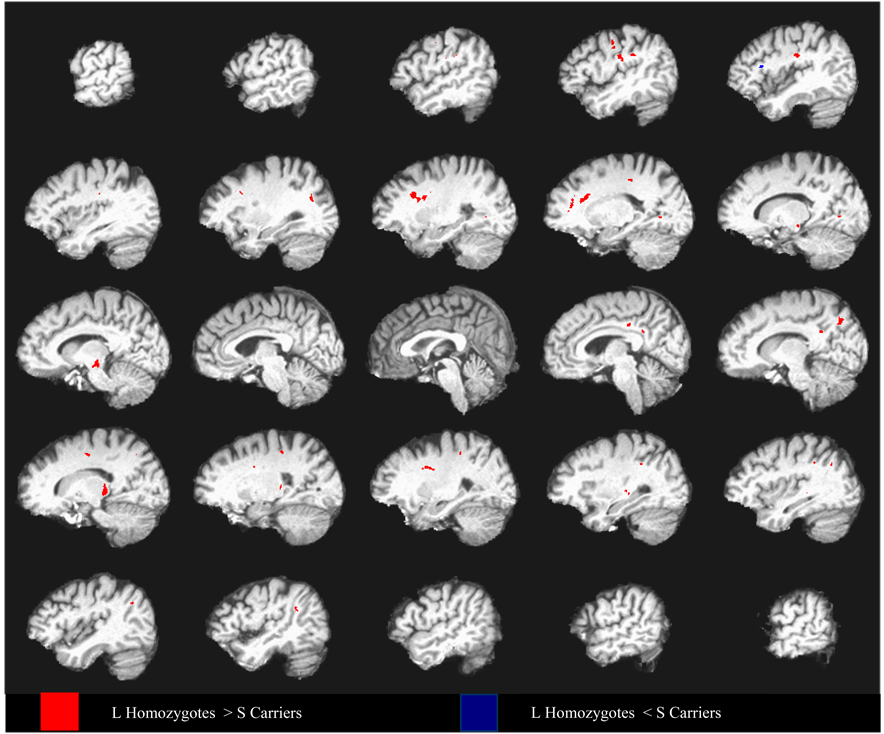
Using the Baudewig et al. method (Baudewig et al., 2003), we observed that depressed S allele carriers had lower FA in multiple frontolimbic and in some subcortical and posterior areas compared to depressed L homozygotes (Figure 2). These differences were confirmed by the false discovery method (Figure 3), which demonstrated clusters of reduced FA in the dorsolateral prefrontal, dorsal and rostral anterior cingulate, posterior cingulate, medial prefrontal regions, and thalamus. In addition, the S allele carriers had lower FA in inferior parietal, cuneus/precuneus, midbrain, external capsule, and visual regions (Table 2).
Figure 2.
Differences in fractional anisotropy between depressed elderly 5HTTLPR L- allele homozygotes and S carriers covarying for age and mean diffusivity, superimposed on a high resolution T1-weighted template. Data from every fifth sagittal slice are shown going from left hemisphere to right hemisphere. Group differences are thresholded such that 100mm3 clusters of voxels all significant at p < .05 are identified. Within each cluster, at least one voxel is significant at p< .005.
Figure 3. LL vs S Carriers : Patients Only – False discovery correction.
Differences in fractional anisotropy between depressed elderly 5HTTLPR L- allele homozygotes and S carriers covarying for age and mean diffusivity, superimposed on a high resolution T1-weighted template. Data from every fifth sagittal slice are shown going from left hemisphere to right hemisphere. Group differences are thresholded such that 50mm3 clusters of voxels all significant at p < .01 (FDR q =0.1) are identified.
Table 2.
Clusters of Significant Fractional Anisotropy Differences Between Depressed Elderly S Allele Carriers and Depressed Elderly L Homozygotes after Covarying for Age and Mean Diffusivity.
| Talairach Coordinatesa | ||||||
|---|---|---|---|---|---|---|
| Anatomical Region | Laterality | Cluster Sizeb |
Mean t- valuec |
X (+ Right) |
Y (+Anterior) |
Z (+Superior) |
| Dorsolateral Prefrontal | Left | 597 | −3.48 | −25 | 16 | 25 |
| Dorsal Anterior Cingulate | Right | 185 | −3.16 | 23 | −1 | 30 |
| L Dorsal/Rostral Anterior | Left | 174 | −3.08 | −18 | 36 | 25 |
| Cingulate/Medial Prefrontal | ||||||
| Dorsal Anterior/Posterior Cingulate | Left | 101 | −3.19 | −23 | −14 | 31 |
| Posterior Cingulate | Left | 57 | −3.11 | −18 | −31 | 41 |
| Posterior Cingulate | Right | 76 | −3.09 | 14 | −9 | 46 |
| Posterior Cingulate | Right | 53 | −3.20 | 5 | −27 | 35 |
| Posterior Cingulate | Right | 66 | −3.23 | 10 | −47 | 28 |
| Thalamus | Right | 293 | −3.83 | 14 | −29 | 5 |
| Inferior Parietal | Left | 97 | −3.72 | −48 | −29 | 27 |
| Inferior Parietal | Right | 50 | −3.07 | 31 | −41 | 35 |
| Inferior Parietal | Right | 66 | −3.30 | 43 | −45 | 28 |
| Inferior Parietal/Post Central | Left | 261 | −3.50 | −39 | −21 | 27 |
| Inferior Parietal/Precuneus | Right | 171 | −3.52 | 13 | −64 | 35 |
| Precuneus/Angular | Right | 54 | −3.29 | 37 | −59 | 35 |
| Postcentral | Right | 67 | −3.12 | 21 | −31 | 47 |
| Midbrain | Left | 229 | −3.25 | −10 | −21 | −7 |
| External Capsule | Right | 69 | −3.30 | 31 | −25 | 5 |
| Precentral | Left | 150 | −3.57 | −47 | −12 | 36 |
| Middle Temporal/Occipital | Left | 78 | −3.25 | −31 | −65 | 19 |
| Lingual | Left | 73 | −3.55 | −17 | −63 | 3 |
| Inferior Frontald | Left | 51 | 3.16 | −41 | 20 | 16 |
Reflect peak of cluster
Cluster size is in number of voxels
Mean t-value per cluster
Higher FA in S allele carriers than L homozygotes
The relationship between FA and 5-HTTLPR was attenuated in controls. Controls who were S-allele carriers had lower FA than L homozygotes in a limited number of white matter regions, i.e. lateral to the middle frontal gyrus and at the right posterior cingulate. Depressed S-allele carriers had lower FA than S-allele controls in regions including the medial prefrontal, posterior cingulate, parahippocampal, cuneus, and cerebellum.
S allele carrier status has been associated with vascular risk factors (Comings et al., 1999) and vascular disorders (Nakatani et al., 2005). However, introducing the Cerebrovascular Risk Factor Assessment score as a covariate in comparisons of fractional anisotropy between S carriers and L homozygotes did not substantially change our findings.
LG and S alleles have comparable levels of serotonin transporter expression, both of which are lower than that of LA (Nakamura et al., 2000; Hu et al., 2005). For this reason, we reclassified the alleles on the basis of lower and higher levels of expression. LG and S were reclassified as S', and LA was reclassified as L'. Reclassification of depressed subjects resulted in S'=21 and L'=6. None of the subjects of the control group was reclassified (S'=21 and L'=6). Repeating the comparisons between S' and L' depressed subjects resulted in almost identical findings to those shown in Figure 2 and Figure 3.
Of the 27 depressed subjects who entered the escitalopram phase, 24 completed the 12-week trial. Of the remaining 3, one had 7 weeks of treatment (withdrew because she developed hyponatremia but met criteria for remission), one had 9 weeks of treatment and exited because of worsening depression and one had 11 weeks of treatment with escitalopram (exited because he found the treatment ineffective). Thus two of the three subjects who exited prior to 12 weeks had failed to achieve remission.
Depressed S carriers had a lower remission rate (44.4%) than LL depressed subjects (88.9%), (Fisher’s Exact Test p<0.0417). At study exit, S carriers had higher HDRS scores than LL carriers (t=2.91, df=25, p<0.00075). There were no statistically significant differences between depressed S carriers and depressed L homozygotes in variables likely to influence antidepressant response, including age, gender, education, age of depression first onset, number of previous episodes, severity of depression or cognitive impairment at baseline (Table 1). Similar results were obtained when treatment outcomes were compared between S' and L' depressed subjects based on triallelic classification. Specifically, S' subjects had a lower remission rate than L' subjects (Fisher’s Exact Test p<0.024) and higher HDRS scores at study exit (t=4.17, df=24.7, p<0.0003).
DISCUSSION
The principal finding of this study is that depressed elderly individuals with low serotonin transporter expressing 5-HTTLPR alleles (S carriers or S') have more microstructural white matter abnormalities in frontolimbic and other regions and are less likely to achieve remission of depression than L homozygotes. To our knowledge, this is the first study to demonstrate an association between 5-HTTLPR allele status and microstructural frontolimbic white matter abnormalities in depression. The depressed subjects of this study had more microstructural white matter frontolimbic abnormalities than normal controls. This observation suggests that our sample was similar to those of studies that documented microstructural white matter abnormalities in depressed patients (Nobuhara et al., 2006).
The mechanisms by which low transcribing 5-HTTLPR allele status may lead to low remission rate are unclear. However, some abnormalities in serotonin-related functions have been documented in S carriers compared to L homozygotes. These include blunted neuroendocrine response to serotonin function probes (Reist et al., 2001), lower platelet serotonin uptake (Greenberg et al., 1999) lower concentrations of the serotonin metabolite 5-hydroxy-indolo-acetic acid (Williams et al., 2003) and lesser decrease in right and greater decrease in left cortical metabolism in response to citalopram administration in S carriers compared to the LL subjects (Smith et al., 2004). These findings parallel the differences found in comparing depressed elderly patients to age-matched controls (Smith et al., 2002).
5-HTTLPR polymorphisms may influence brain structures. Steffens et al observed that depressed elderly patients with SL genotype have more total brain lesions and white matter lesions than depressed L and S homozygotes (Steffens et al., 2008b). In our sample, only one of the S allele carriers was an S homozygote. Thus the association between S allele status and reduced fractional anisotropy was mainly due to our SL subjects and thus consistent with the Steffens et al report. Moreover, S-allele elderly carriers with major depression had lower caudate nucleus volume than L homozygotes (Hickie et al., 2007). Finally, non-depressed S carriers had reduced gray matter in the perigenual cingulate and amygdala and increased sensitivity in processing fearful stimuli (Pezawas et al., 2005).
The relationship of low serotonin transporter expressing alleles to microstructural white matter abnormalities may be multi-determined. Serotonin signaling is a regulator of adult neurogenesis (Gould, 1999) and is influenced by the serotonin transporter. Low serotonin transporter expressing 5-HTTLPR alleles account for more than 30% of the variance in depression severity directly and by increasing the impact of life events (Zalsman et al., 2006). The S allele increases the likelihood of developing depression in the presence of stress (Caspi et al., 2003). S-allele carrier status interacts with waking cortisol and is associated with impaired memory and lower hippocampal volume (O'Hara et al., 2007). These observations suggest that the S allele confers increased vulnerability to hyperactivity of the hypothalamic-pituitary-adrenal axis. Finally, some 5-HTTLPR polymorphisms are associated with increased risk for vascular disease. SL-allele status increased cholesterol, triglycerides and risk for heart disease, angina, and heart attacks in individuals aged 50–70 years (Comings et al., 1999). Moreover, cardiac events including cardiac death, revascularization, heart failure, reinfarction, arrhythmia, and unstable angina were more frequent after acute myocardial infarction in S-allele carriers than in L homozygotes (Nakatani et al., 2005). This effect was in part mediated by depressive symptoms.
In our sample, taking vascular risk factors into consideration did not change the relationship of S carrier status to FA abnormalities. Vascular risk factors have an indirect relationship with cerebrovascular damage (Wolf et al., 1991). Therefore, the association between S carrier status and FA abnormalities may be due to factors other than vascular. Alternatively, S carrier status may promote vascular damage regardless of presence or absence of vascular risk factors.
FA differences between S-allele careers and L homozygotes were most pronounced in depressed patients compared to controls. This finding suggests that the S allele may interact with factors associated with geriatric depression to promote FA abnormalities above and beyond those of normal subjects. These interactions are likely complex. First, low transporter expressing alleles may increase neuronal vulnerability to hypothalamic-pituitary-adrenal axis (HPA) hyperactivity or to vascular changes occurring during depression, thus leading to microstructural white matter abnormalities. Second, S carriers are more likely to have compromised cerebrovascular integrity than L homozygotes (Comings et al., 1999; Nakatani et al., 2005) and thus present more microstructural white matter abnormalities. Finally, microstructural white matter abnormalities in S-allele carriers may result from a combination of cerebrovascular compromise (a state predisposing to depression) (Alexopoulos, 2005) and increased sensitivity to hypercortesolemia of the S allele carriers during depressive episodes.
The small number of subjects does not permit an examination of whether the association between S-allele status and low remission rate is mediated by frontolimbic white matter abnormalities. However, similar structural brain abnormalities have been associated with poor outcomes of late life depression. We reported that low FA in several corticolimbic white matter areas predict low remission rates in depressed elderly patients treated with citalopram (Alexopoulos et al., 2002) and escitalopram (Alexopoulos et al., 2008). Similarly, white matter hyperintensities have been associated with chronicity of geriatric depression (Simpson et al., 1998) although negative findings also exist (Salloway et al., 2002). Basal ganglia lesions predicted failure to respond to antidepressants (Patankar et al., 2007). Finally, lesions in subcortical grey matter predict poor outcome of geriatric depression over a 5 year period (Steffens et al., 2005).
Some of the metabolic changes in corticolimbic systems occurring during depression are restored with improvement of depression. Remission of depression is associated with metabolic increases in dorsal cortical regions (Drevets, 2000). In contrast, decreases in ventral limbic and paralimbic structures, i.e. subgenual cingulate, ventral mid- and posterior insula, hippocampus and hypothalamus occur during remission (Mayberg et al., 1999). ACC metabolic abnormalities occurring during depressive episodes are normalized during remission (Mayberg et al., 1999). Rostral ACC hypermetabolism subsides after response to antidepressants (Mayberg et al., 1997), ECT (Nobler et al., 2001), or sleep deprivation (Smith et al., 1999), and cognitive behavioral therapy (Goldapple et al., 2004). A potential explanation of our findings is that some of the corticolimbic abnormalities of S-allele carriers interfere with the reciprocal neocortical-subcortical regulation and contribute to poor antidepressant response. Another contributor to non-remission may be the increased amygdala activation in response to negative stimuli reported in S allele carriers (Munafo et al., 2008). Amygdalar hyper-reactivity may be another source of neocortical-subcortical imbalance (Hariri et al., 2006) added to microstructural abnormalities of S allele carriers and complicating re-equilibration.
The findings of this study should be viewed in the context of its limitations. These include the small number of subjects, the lack of random sampling, the subject selection bias inherent in imaging studies (healthier patients are likely favored), the fixed dose of the antidepressant, and the absence of long-term follow-up. It is likely that some subjects may have remitted if treated with higher dosages of escitalopram or longer treatment was offered. However, the only two non-remitted subjects who exited the trial prematurely received escitalopram for 9 and 11 weeks. Moreover, there were no significant differences at baseline between remitters and non-remitters in variables associated with prediction of treatment resistance. The absence of population stratification is also a limitation of the present study but the small sample size of this study prevents a meaningful application of currently available methods for the identification of and correction for population substructure. Finally, our observations may be influenced by the large number of comparisons, as voxelwise analysis increases the risk of Type I error. To address this potential problem, we used a white matter mask to reduce the number of comparisons. We also applied a rather conservative threshold and cluster size requirement for additional protection over Type I error.
While this study found a relationship between S allele carriers and low remission rate following treatment with an SSRI, the literature suggests that the effect size of this relationship is small (Munafo et al., 2008). Overall, SSRIs are well tolerated and effective in the elderly (Solai et al., 2001), including patients with post-stroke depression (Starkstein et al., 2008). Furthermore, a recent study has shown that prophylactic use of the SSRI escitalopram in stroke patients reduced the incidence of depression over 12 months of treatment compared to placebo (Robinson et al., 2008). A theoretically, appealing observation is that paroxetine was shown to decrease beta-thromboglobulin and platelet factor 4 suggesting that it may reduce platelet aggregation and perhaps exert a protective effect on vasculature (Pollock et al., 2000b). Moreover, paroxetine was shown to decrease platelet serotonin, an effect most pronounced in S allele carriers (Abdelmalik et al., 2008). Nonetheless, use of SSRIs does not reduce the progression of white matter lesions in older adults over a period of 5 years (Steffens et al., 2008a). These findings suggest that the risk-benefit of using SSRIs in depressed elderly S allele carriers needs further evaluation.
This study suggests that elderly S-allele carriers with major depression have both a low remission rate and microstructural white matter abnormalities in several areas including areas of frontolimbic networks. Microstructural abnormalities in these areas have been associated with low remission rates of geriatric depression (Alexopoulos et al., 2008). It remains unclear whether the risk for chronicity of geriatric depression in S-allele carriers is mediated by frontolimbic compromise along with other mechanisms. Nonetheless, these observations can be used as the basis of studies aiming to identify the relationship of the S allele to impairment in specific frontolimbic functions interfering with response of geriatric depression to antidepressants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abdelmalik N, Ruhe HG, Barwari K, Van Den Dool EJ, Meijers JC, Middeldorp S, Buller HR, Schene AH, Kamphuisen PW. Effect of the selective serotonin reuptake inhibitor paroxetine on platelet function is modified by a SLC6A4 serotonin transporter polymorphism. J Thromb Haemost. 2008;6:2168–2174. doi: 10.1111/j.1538-7836.2008.03196.x. [DOI] [PubMed] [Google Scholar]
- Alessandro S, Kato M. The serotonin transporter gene and effectiveness of SSRIs. Expert Rev Neurother. 2008;8:111–120. doi: 10.1586/14737175.8.1.111. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961–1970. doi: 10.1016/S0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Kiosses DN, Choi SJ, Murphy CF, Lim KO. Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. Am J Psychiatry. 2002;159:1929–1932. doi: 10.1176/appi.ajp.159.11.1929. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Latoussakis V, Kanellopoulos D, Klimstra S, Lim KO, Hoptman MJ. Microstructural white matter abnormalities and remission of geriatric depression. Am J Psychiatry. 2008;165:238–244. doi: 10.1176/appi.ajp.2007.07050744. [DOI] [PubMed] [Google Scholar]
- Ardekani BA, Bappal A, D'angelo D, Ashtari M, Lencz T, Szeszko PR, Butler PD, Javitt DC, Lim KO, Hrabe J, Nierenberg J, Branch CA, Hoptman MJ. Brain morphometry using diffusion-weighted magnetic resonance imaging: application to schizophrenia. Neuroreport. 2005;16:1455–1459. doi: 10.1097/01.wnr.0000177001.27569.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias B, Catalan R, Gasto C, Gutierrez B, Fananas L. 5-HTTLPR polymorphism of the serotonin transporter gene predicts non-remission in major depression patients treated with citalopram in a 12-weeks follow up study. J Clin Psychopharmacol. 2003;23:563–567. doi: 10.1097/01.jcp.0000095350.32154.73. [DOI] [PubMed] [Google Scholar]
- Baudewig J, Dechent P, Merboldt KD, Frahm J. Thresholding in correlation analyses of magnetic resonance functional neuroimaging. Magn Reson Imaging. 2003;21:1121–1130. doi: 10.1016/j.mri.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, Mcclay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Comings DE, Macmurray JP, Gonzalez N, Ferry L, Peters WR. Association of the serotonin transporter gene with serum cholesterol levels and heart disease. Mol Genet Metab. 1999;67:248–253. doi: 10.1006/mgme.1999.2870. [DOI] [PubMed] [Google Scholar]
- Cushman M, Cantrell RA, Mcclure LA, Howard G, Prineas RJ, Moy CS, Temple EM, Howard VJ. Estimated 10-year stroke risk by region and race in the United States: geographic and racial differences in stroke risk. Ann Neurol. 2008;64:507–513. doi: 10.1002/ana.21493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan Ja ÜT. The WHODAS-II: leveling the playing field for all disorders. WHO Mental Health Bulletin. 2000;6:5–6. [Google Scholar]
- Folstein MF, Folstein SE, Mchugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, Mayberg H. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry. 2004;61:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- Gould E. Serotonin and hippocampal neurogenesis. Neuropsychopharmacology. 1999;21:46S–51S. doi: 10.1016/S0893-133X(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Tolliver TJ, Huang SJ, Li Q, Bengel D, Murphy DL. Genetic variation in the serotonin transporter promoter region affects serotonin uptake in human blood platelets. Am J Med Genet. 1999;88:83–87. [PubMed] [Google Scholar]
- Gunning-Dixon FM, Hoptman MJ, Lim KO, Murphy CF, Klimstra S, Latoussakis V, Majcher-Tascio M, Hrabe J, Ardekani BA, Alexopoulos GS. Macromolecular white matter abnormalities in geriatric depression: a magnetization transfer imaging study. Am J Geriatr Psychiatry. 2008;16:255–262. doi: 10.1097/JGP.0b013e3181602a66. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biol Psychiatry. 2006;59:888–897. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Hickie IB, Naismith SL, Ward PB, Scott EM, Mitchell PB, Schofield PR, Scimone A, Wilhelm K, Parker G. Serotonin transporter gene status predicts caudate nucleus but not amygdala or hippocampal volumes in older persons with major depression. J Affect Disord. 2007;98:137–142. doi: 10.1016/j.jad.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Horsfield MA, Jones DK. Applications of diffusion-weighted and diffusion tensor MRI to white matter diseases - a review. NMR Biomed. 2002;15:570–577. doi: 10.1002/nbm.787. [DOI] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL, Goldman D. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, Paulus MP, Kunovac JL, Leon AC, Mueller TI, Rice JA, Keller MB. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord. 1998;50:97–108. doi: 10.1016/s0165-0327(98)00138-4. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Thompson PM, Lancaster JL, Bartzokis G, Smith S, Coyle T, Royall DR, Laird A, Fox PT. Relationship between white matter fractional anisotropy and other indices of cerebral health in normal aging: tract-based spatial statistics study of aging. Neuroimage. 2007;35:478–487. doi: 10.1016/j.neuroimage.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Kraft JB, Peters EJ, Slager SL, Jenkins GD, Reinalda MS, Mcgrath PJ, Hamilton SP. Analysis of association between the serotonin transporter and antidepressant response in a large clinical sample. Biol Psychiatry. 2007;61:734–742. doi: 10.1016/j.biopsych.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Kumar A, Gupta RC, Albert Thomas M, Alger J, Wyckoff N, Hwang S. Biophysical changes in normal-appearing white matter and subcortical nuclei in late-life major depression detected using magnetization transfer. Psychiatry Res. 2004;130:131–140. doi: 10.1016/j.pscychresns.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lotrich FE, Pollock BG, Ferrell RE. Serotonin transporter promoter polymorphism in African Americans : allele frequencies and implications for treatment. Am J Pharmacogenomics. 2003;3:145–147. doi: 10.2165/00129785-200303020-00007. [DOI] [PubMed] [Google Scholar]
- Mann JJ. Role of the serotonergic system in the pathogenesis of major depression and suicidal behavior. Neuropsychopharmacology. 1999;21:99S–105S. doi: 10.1016/S0893-133X(99)00040-8. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, Mcginnis S, Glass TG, Martin CC, Fox PT. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, Mcginnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development. The International Consortium for Brain Mapping (ICBM) Neuroimage. 1995;2:89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DL, Fox MA, Timpano KR, Moya PR, Ren-Patterson R, Andrews AM, Holmes A, Lesch KP, Wendland JR. How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology. 2008;55:932–960. doi: 10.1016/j.neuropharm.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Ueno S, Sano A, Tanabe H. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Mol Psychiatry. 2000;5:32–38. doi: 10.1038/sj.mp.4000698. [DOI] [PubMed] [Google Scholar]
- Nakatani D, Sato H, Sakata Y, Shiotani I, Kinjo K, Mizuno H, Shimizu M, Ito H, Koretsune Y, Hirayama A, Hori M. Influence of serotonin transporter gene polymorphism on depressive symptoms and new cardiac events after acute myocardial infarction. Am Heart J. 2005;150:652–658. doi: 10.1016/j.ahj.2005.03.062. [DOI] [PubMed] [Google Scholar]
- Nobler MS, Oquendo MA, Kegeles LS, Malone KM, Campbell CC, Sackeim HA, Mann JJ. Decreased regional brain metabolism after ect. Am J Psychiatry. 2001;158:305–308. doi: 10.1176/appi.ajp.158.2.305. [DOI] [PubMed] [Google Scholar]
- Nobuhara K, Okugawa G, Sugimoto T, Minami T, Tamagaki C, Takase K, Saito Y, Sawada S, Kinoshita T. Frontal white matter anisotropy and symptom severity of late-life depression: a magnetic resonance diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2006;77:120–122. doi: 10.1136/jnnp.2004.055129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'hara R, Schroder CM, Mahadevan R, Schatzberg AF, Lindley S, Fox S, Weiner M, Kraemer HC, Noda A, Lin X, Gray HL, Hallmayer JF. Serotonin transporter polymorphism, memory and hippocampal volume in the elderly: association and interaction with cortisol. Mol Psychiatry. 2007;12:544–555. doi: 10.1038/sj.mp.4001978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie AD, Battersby S, Bubb VJ, Fink G, Harmar AJ, Goodwim GM, Smith CA. Polymorphism in serotonin transporter gene associated with susceptibility to major depression. Lancet. 1996;347:731–733. doi: 10.1016/s0140-6736(96)90079-3. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Hastings RS, Oquendo MA, Huang YY, Simpson N, Arcement J, Huang Y, Ogden RT, Van Heertum RL, Arango V, Mann JJ. Lower serotonin transporter binding potential in the human brain during major depressive episodes. Am J Psychiatry. 2006;163:52–58. doi: 10.1176/appi.ajp.163.1.52. [DOI] [PubMed] [Google Scholar]
- Patankar TF, Baldwin R, Mitra D, Jeffries S, Sutcliffe C, Burns A, Jackson A. Virchow-Robin space dilatation may predict resistance to antidepressant monotherapy in elderly patients with depression. J Affect Disord. 2007;97:265–270. doi: 10.1016/j.jad.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Pollock BG, Ferrell RE, Mulsant BH, Mazumdar S, Miller M, Sweet RA, Davis S, Kirshner MA, Houck PR, Stack JA, Reynolds CF, Kupfer DJ. Allelic variation in the serotonin transporter promoter affects onset of paroxetine treatment response in late-life depression. Neuropsychopharmacology. 2000a;23:587–590. doi: 10.1016/S0893-133X(00)00132-9. [DOI] [PubMed] [Google Scholar]
- Pollock BG, Laghrissi-Thode F, Wagner WR. Evaluation of platelet activation in depressed patients with ischemic heart disease after paroxetine or nortriptyline treatment. J Clin Psychopharmacol. 2000b;20:137–140. doi: 10.1097/00004714-200004000-00004. [DOI] [PubMed] [Google Scholar]
- Reist C, Mazzanti C, Vu R, Tran D, Goldman D. Serotonin transporter promoter polymorphism is associated with attenuated prolactin response to fenfluramine. Am J Med Genet. 2001;105:363–368. doi: 10.1002/ajmg.1360. [DOI] [PubMed] [Google Scholar]
- Robinson RG, Jorge RE, Moser DJ, Acion L, Solodkin A, Small SL, Fonzetti P, Hegel M, Arndt S. Escitalopram and problem-solving therapy for prevention of poststroke depression: a randomized controlled trial. Jama. 2008;299:2391–2400. doi: 10.1001/jama.299.20.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloway S, Boyle PA, Correia S, Malloy PF, Cahn-Weiner DA, Schneider L, Krishnan KR, Nakra R. The relationship of MRI subcortical hyperintensities to treatment response in a trial of sertraline in geriatric depressed outpatients. Am J Geriatr Psychiatry. 2002;10:107–111. [PubMed] [Google Scholar]
- Simpson S, Baldwin RC, Jackson A, Burns AS. Is subcortical disease associated with a poor response to antidepressants? Neurological, neuropsychological and neuroradiological findings in late-life depression. Psychol Med. 1998;28:1015–1026. doi: 10.1017/s003329179800693x. [DOI] [PubMed] [Google Scholar]
- Smith GS, Kramer E, Hermann CR, Goldberg S, Ma Y, Dhawan V, Barnes A, Chaly T, Belakhleff A, Laghrissi-Thode F, Greenwald B, Eidelberg D, Pollock BG. Acute and chronic effects of citalopram on cerebral glucose metabolism in geriatric depression. Am J Geriatr Psychiatry. 2002;10:715–723. [PubMed] [Google Scholar]
- Smith GS, Lotrich FE, Malhotra AK, Lee AT, Ma Y, Kramer E, Gregersen PK, Eidelberg D, Pollock BG. Effects of serotonin transporter promoter polymorphisms on serotonin function. Neuropsychopharmacology. 2004;29:2226–2234. doi: 10.1038/sj.npp.1300552. [DOI] [PubMed] [Google Scholar]
- Smith GS, Reynolds CF, 3rd, Pollock B, Derbyshire S, Nofzinger E, Dew MA, Houck PR, Milko D, Meltzer CC, Kupfer DJ. Cerebral glucose metabolic response to combined total sleep deprivation and antidepressant treatment in geriatric depression. Am J Psychiatry. 1999;156:683–689. doi: 10.1176/ajp.156.5.683. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Solai LK, Mulsant BH, Pollock BG. Selective serotonin reuptake inhibitors for late-life depression: a comparative review. Drugs Aging. 2001;18:355–368. doi: 10.2165/00002512-200118050-00006. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Mizrahi R, Power BD. Antidepressant therapy in post-stroke depression. Expert Opin Pharmacother. 2008;9:1291–1298. doi: 10.1517/14656566.9.8.1291. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Chung H, Krishnan KR, Longstreth WT, Jr, Carlson M, Burke GL. Antidepressant treatment and worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2008a;39:857–862. doi: 10.1161/STROKEAHA.107.498097. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Pieper CF, Bosworth HB, Macfall JR, Provenzale JM, Payne ME, Carroll BJ, George LK, Krishnan KR. Biological and social predictors of long-term geriatric depression outcome. Int Psychogeriatr. 2005;17:41–56. doi: 10.1017/s1041610205000979. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Taylor WD, Mcquoid DR, Krishnan KR. Short/long heterozygotes at 5HTTLPR and white matter lesions in geriatric depression. Int J Geriatr Psychiatry. 2008b;23:244–248. doi: 10.1002/gps.1869. [DOI] [PubMed] [Google Scholar]
- Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ, Kuhn CM, Lewis JG, Schanberg SM, Stafford-Smith M, Suarez EC, Clary GL, Svenson IK, Siegler IC. Serotonin-related gene polymorphisms and central nervous system serotonin function. Neuropsychopharmacology. 2003;28:533–541. doi: 10.1038/sj.npp.1300054. [DOI] [PubMed] [Google Scholar]
- Wolf PA, D'agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- Zalsman G, Huang YY, Oquendo MA, Burke AK, Hu XZ, Brent DA, Ellis SP, Goldman D, Mann JJ. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am J Psychiatry. 2006;163:1588–1593. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]