Abstract
Decades after ethanol was first described as a GABA mimetic, the precise mechanisms that produce the acute effects of ethanol and the physiological adaptations that underlie ethanol tolerance and dependence remain unclear. While a substantial body of evidence suggests that ethanol acts on GABAergic neurotransmission to enhance inhibition in the CNS, the precise mechanisms underlying the physiological effects of both acute and chronic ethanol exposure are still under investigation. We have used in vitro ethanol exposure followed by recording of miniature inhibitory postsynaptic currents (mIPSCs) to determine whether acute or chronic ethanol exposure directly alters synaptic GABAA receptor function or GABA release in cultured cortical and hippocampal neurons. Acute ethanol exposure slightly increased the duration of mIPSCs in hippocampal neurons but did not alter mIPSC kinetics in cortical neurons. Acute ethanol exposure did not change mIPSC frequency in either hippocampal or cortical neurons. One day of chronic ethanol exposure produced a transient decrease in mIPSC duration in cortical neurons but did not alter mIPSC kinetics in hippocampal neurons. Chronic ethanol exposure did not change mIPSC frequency in either hippocampal or cortical neurons. Chronic ethanol exposure also did not produce substantial cross-tolerance to a benzodiazepine in either hippocampal or cortical neurons. The results suggest that ethanol exposure in vitro has limited effects on synaptic GABAAR function and action-potential independent GABA release in cultured neurons and suggests that ethanol exposure in cultured cortical and hippocampal neurons may not reproduce all of the effects that occur in vivo and in acute brain slices.
Keywords: GABA, GABAA Receptor, In Vitro, Cerebral Cortex, Hippocampus, Primary Culture
Introduction
The behavioral effects of acute ethanol (EtOH) administration mimic those of benzodiazepines and barbiturates, drugs that are known to act on the GABA system. Furthermore, chronic ethanol exposure in vivo results in cross tolerance to benzodiazepines and barbiturates (Woo and Greenblatt, 1979). These observations suggest that ethanol produces its behavioral effects by altering GABAergic mechanisms, and that changes in GABAergic neurotransmission are involved in the physiological adaptations that produce ethanol tolerance. However, decades after ethanol was first described as a GABA mimetic the precise mechanisms underlying the physiological effects of both acute and chronic ethanol exposure are still under investigation.
Several hypotheses have been advanced to explain ethanol’s acute effects on GABAergic transmission. First, ethanol acts directly on postsynaptic GABAA receptors (GABAARs) to increase their response to GABA (Roberto et al., 2003a; Sanna et al., 2003; Weiner et al., 1997b; Weiner et al., 1994, also vide infra). Second, ethanol acts presynaptically to increase the amount of GABA released (Carta et al., 2003; Carta et al., 2004; Li et al., 2003, 2006; Roberto et al., 2003a; Sanna et al., 2004; Silberman et al., 2009) also see Weiner and Valenzuela (2006) for review. Third, ethanol acts extrasynaptically to enhance tonic inhibition by altering the function of GABAARs present on neuronal membranes outside synapses (Choi et al., 2008; Fleming et al., 2007; Glykys et al., 2007; Wei et al., 2004). Fourth, ethanol acts indirectly to increase local synthesis of GABAergic neuroactive steroids that potentiate GABAAR function (Sanna et al., 2004; VanDoren et al., 2000). Lastly, ethanol increases taurine (De Witte et al., 1994), which enhances tonic inhibition at low concentrations (Jia et al., 2008). Ethanol tolerance could occur due to adaptations in any or all of these mechanisms, a hypothesis that is supported by in vivo studies in rodents. Chronic ethanol exposure produces brain region specific alterations in GABAAR subunit expression, pre and postsynaptic changes in GABAergic neurotransmission and changes in tonic inhibition (Kumar et al., 2009; Roberto et al., 2006; Weiner and Valenzuela, 2006). Previous studies by other laboratories using cultured neurons have shown that some of these effects of acute and chronic ethanol exposure are replicated in vitro (Sanna et al., 2003; Sheela Rani and Ticku, 2006; Tsujiyama et al., 1997), suggesting that cultured neurons may provide an additional, and potentially powerful, model of the effects of ethanol exposure on GABAergic mechanisms in the brain. However, existing studies in cultured neurons do not discriminate between the synaptic and extrasynaptic GABAAR populations, a distinction that may be important because these two populations are affected differently during ethanol exposure (Kumar et al., 2009; Liang et al., 2004; Liang et al., 2006). Furthermore, whether ethanol alters presynaptic release mechanisms in cultured neurons is still unclear. The present study was designed to test the first and second hypotheses for the mechanism of ethanol’s acute action in cultured cortical and hippocampal neurons, and to determine if chronic ethanol exposure produced adaptations in either synaptic GABAAR function or GABA release.
Previous studies have shown that under some experimental conditions ethanol enhances GABAergic neurotransmission in cortex, hippocampus, amygdala, and cerebellum (vide infra). In cultured cortical neurons ethanol potentiates spontaneous action-potential driven inhibitory postsynaptic currents (sIPSCs) (Marszalec et al., 1998) and in acute slice preparations, ethanol enhances action-potential driven (i.e. evoked and / or spontaneous) IPSCs in hippocampus, amygdala, and cerebellum (Ariwodola and Weiner, 2004; Carta et al., 2003; Carta et al., 2004; Li et al., 2003, 2006; Roberto et al., 2003b; Silberman et al., 2009; Weiner et al., 1997a). Because both pre and postsynaptic mechanisms can contribute to changes in action-potential dependent IPSC amplitude, other methods must be used to distinguish between these two possibilities.
On method that is commonly used to test for the postsynaptic mechanism – a direct effect of ethanol on synaptic GABAARs – is direct application of a GABAergic agonist to the postsynaptic neuron. Some electrophysiological studies of this type show that in cultured cortical and hippocampal neurons ethanol increases the Cl− current evoked by applied agonist (Aguayo, 1990; Sanna et al., 2003; Tsujiyama et al., 1997). Sanna et al. (2003) have also shown that exposure to ethanol for 5 days in vitro can reduce the GABAAR modulatory efficacy of both ethanol and benzodiazepines in cultured hippocampal neurons. This observation supports the hypothesis that ethanol tolerance results at least in part from adaptations in GABAAR function. Yet other studies have not found that acute ethanol exposure positively modulates GABAARs (Criswell et al., 2003; Marszalec et al., 1998). Furthermore, applying agonist directly to the cell body can activate both synaptic and extrasynaptic receptors. It is known that these GABAAR populations have different pharmacological and physiological properties, and in particular different sensitivities to ethanol (Kumar et al., 2009), therefore the results of agonist application experiments do not conclusively demonstrate that ethanol acts directly at synaptic GABAARs in cultured neurons.
Action-potential independent miniature inhibitory postsynaptic currents (mIPSCs) are widely used to study neurotransmission at a variety of synapses. Unlike recording of spontaneous IPSCs (sIPSCs), which may include both mIPSCs and action-potential dependent IPSCs, mIPSCs more precisely allow an experimental discrimination between pre and postsynaptic changes in neurotransmission. Changes in the average size and shape of mIPSCs indicate postsynaptic changes in GABAAR function (Jones et al., 1998; Jones and Westbrook, 1995) while a change in mIPSC frequency indicates a change in GABA release probability (Bouron, 2001).
Other laboratories have used acute slice preparations or acutely dissociated neurons and have shown that ethanol increases in mIPSP frequency in hippocampal CA1 pyramidal neurons (Li et al., 2006; Sanna et al., 2004), immature CA3 interneurons (Galindo et al., 2005), neurons from the central nucleus of the amygdala (CeA) (Roberto et al., 2003a) and cerebellar Purkinjie neurons (Criswell and Breese, 2005; Kelm et al., 2007). However, ethanol does not increase mIPSC frequency in cerebellar granule cells (Carta et al., 2004) and the results of studies in cerebrocortical neurons have been mixed. Criswell and Breese (2005) reported no effect of 50 mM ethanol in acutely dissociated neurons, while others have reported decreases in mIPSC frequency in primary cortical cultures (Moriguchi et al., 2007). Furthermore, chronic intermittent ethanol exposure in vivo increases baseline GABA release in CeA neurons (Roberto et al., 2004), but decreases it in CA1 neurons (Cagetti et al., 2003). These findings support the hypotheses that, in some brain regions, acute ethanol exposure enhances synaptic transmission by increasing presynaptic release of GABA and that chronic ethanol exposure alters presynaptic GABAergic mechanisms.
This study, which applies the mIPSC recording technique previously used in brain slices to a cultured neuron model, expands on what is already known about ethanol exposure in in vitro models and allows for further comparison of this model to existing whole animal data. We have used continuous in vitro ethanol exposure, which is effective at producing changes in GABAAR subunit expression in cultured neurons (Sanna et al., 2003; Sheela Rani and Ticku, 2006), followed by recording of mIPSCs to determine if either acute or chronic ethanol exposure alters pre or postsynaptic GABAergic transmission in cultured neurons from cerebral cortex and hippocampus.
Materials and Methods
Cortical and hippocampal neuron cultures
The University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee approved all protocols involving the use of experimental animals in this report. Rat pups were obtained within the first twenty-four hours after birth. Pups of both sexes were decapitated and the brains were removed from the skull and placed in a petri dish containing CO2-independent medium (Gibco catalog # 18045). The cerebral cortex and hippocampus were pulled away from the brain stem using forceps. The hippocampus was cut away from the cortex and the meninges were removed. Tissue from the desired brain region, either cortex or hippocampus, was transferred to a 15 ml Erlenmeyer flask containing 6 mls of CO2-indpendent medium, 50 U/ml papain (Worthington), 1 mg L-cysteine (Sigma), and 10 U/ml DNAse I (Sigma) and was incubated in a 37° C water bath for 30 minutes. After incubation, the papain solution was removed by aspiration and the tissue was rinsed 3 times with 5 mls of culture medium. Neurons were dissociated into 50 mls of DMEM containing 10% horse serum, 100 U/ml penicillin-streptomycin, and 10 U/ml DNAse I by trituration with a 10 ml serological pipette. The concentration of viable cells was determined by using a hemocytometer to count cells not stained by a 0.4% trypan blue solution. The cells were plated on poly-D-lysine coated glass coverslips in 12-well plates by adding 1.0 × 106 cells in 1 ml of medium to each well. Glass coverslips (Carolina Biological Supply) were cleaned prior to plating by overnight soaking in chromic acid followed by a one hour wash in 6 N HCl and thorough rinsing with distilled H2O. Coverslips were coated overnight in 0.1 mg/ml poly-D-lysine hydrobromide (MW > 300,000, Sigma) in 0.1 M borate buffer, pH 8.0, rinsed with phosphate buffered saline (PBS) and stored until the day of neuron plating, when the PBS was replaced with 0.5 mls of DMEM with GlutaMAX™ I (Gibco catalog # 10566, with high glucose, with pyridoxine-HCl, without sodium pyruvate) containing 10% horse serum and 100 U/ml penicillin-streptomycin. Cells were maintained in 5% CO2/95% air at 37° C in a humidified incubator. Glial cells were not eliminated from this culture in order to encourage neuron survival and synapse formation (Liu et al., 1997). After three days in culture, the cells were fed by adding 0.5 mls of serum-free medium consisting of DMEM with GlutaMAX™ I containing B27 (Gibco catalog # 17504) and 100 U/ml of penicillin-streptomycin. In order to minimize stress to the cultures that can occur after a total medium change, subsequent feedings were done, as needed, by removing ⅓ to ½ of the medium in each well and replacing it with serum-free medium. Cultures were maintained for at least 14 days in vitro before beginning experiments.
Chronic ethanol exposure
Our ethanol exposure method uses a vapor chamber system that allow us to maintain high ethanol concentrations in the tissue culture medium while avoiding total media changes, which can deplete survival factors secreted by both neurons and glia and stress cells in culture (Higgins and Banker, 1998). At the beginning of the ethanol exposure period, cells were fed by replacing ⅓ of the tissue culture medium with fresh medium containing 150 mM ethanol. Stable medium ethanol levels were maintained by placing the cells in a Ziploc® bag containing a beaker with 200 mls of 50 mM ethanol in distilled water. Before sealing the neurons inside, the vapor chamber was allowed to equilibrate with the air inside the incubator. Control cells were fed with medium that did not contain ethanol and were placed in a vapor chamber that held a beaker containing water. Correct medium ethanol levels were verified using an Analox machine (data not shown). On the day of electrophysiological recording, cultures were removed from the vapor chamber and maintained in 50 mM ethanol until a whole cell patch was obtained. Ethanol was then washed out and the neuron was allowed to stabilize for at least 5 minutes before the start of the baseline recording session.
Electrophysiology
Miniature inhibitory postsynaptic currents (mIPSCs) were recorded using whole-cell tight-seal electrophysiology with an Axopatch 200B amplifier (Axon Instruments) in voltage-clamp configuration. Large neurons with smooth cell bodies were selected for recording. All recording was performed at room temperature. The external solution contained (in mM): NaCl 142, HEPES 10, D-glucose 10, KCl 5, CaCl2 4, MgCl2 1, pH 7.4 with 300 nM tetrodotoxin (TTX, Sigma or Molecular Probes) and 1 mM kynurenic acid. Microelectrodes with a resistance of 5-10 MΩ when filled were pulled from borosilicate glass capillaries (Garner Glass Company KG-33) using a Sutter Instrument Co. P-2000 puller. The internal solution contained (in mM): CsCl 130, HEPES 10, EGTA 5, MgATP 4, TrisGTP 0.3, phosphocreatine 10, pH 7.20. The cell membrane potential was clamped at −80 mV. The currents were filtered at 1 kHz and digitized at 10 kHz using in-house software written for MATLAB (version 6; The Mathworks).
Acute drug application
Drugs were dissolved in the external solution and applied either by perfusion in the bath (cortex experiments) or using a U-tube apparatus (hippocampus experiments). After the cell membrane was ruptured, neurons were allowed to stabilize for at least 5 minutes before a baseline was established for each cell by recording mIPSCs for 200 seconds in external solution containing TTX and kynurenic acid. After recording this baseline, cells were exposed to drug for 3 to 5 minutes and mIPSCs were recorded for a second 200-second interval in the presence of drug.
Measurement of neurosteroid 3α,5α-THP levels in cortical neurons
Radioimmunoassays for 3α,5α-THP were conducted as previously described (Janis et al., 1998). Briefly, media and cells were collected from cell culture, added to 2.5 ml of 0.3N NaOH for homogenization with a sonic dismembrator, and extracted three times with 3 ml aliquots of 10% ethyl acetate in heptane (vol/vol). Extraction recovery was monitored by the addition of 2000 cpm of [3H]3α,5α-THP. The extracts were purified using solid phase silica columns (Burdick and Jackson, Muskegon, MI) and subsequently dried. Samples were reconstituted and assayed in duplicate by the addition of [3H]3α,5α-THP and anti-3α,5α-THP antibody. Total binding was determined in the absence of unlabeled 3α,5α-THP and nonspecific binding was determined in the absence of antibody. The antibody binding reaction was allowed to equilibrate for 2 hours and cold dextran coated charcoal was used to separate bound from unbound steroid. Bound radioactivity was determined by liquid scintillation spectroscopy. Steroid levels in the samples were extrapolated from a concurrently run standard curve and corrected for their respective extraction efficiencies.
Data analysis and statistics
Recordings were analyzed using in-house software that implemented a template-matching algorithm for mISPC detection (Clements and Bekkers, 1997). For all recording intervals in each cell, average amplitude, rise time, and interevent interval were calculated. In order to determine the average decay time constant (τd), detected mIPSC events were scaled and averaged and a single exponential was fit to this average mIPSC. Modulation of these mIPSC characteristics by drugs was expressed as percent change: (Id−Ind)/Ind × 100, where Id is the average under the drug condition and Ind is the average under the no-drug baseline condition. All statistical analyses were carried out using SigmaStat (SPSS). Within treatment groups, acute modulation of GABAAR function by exposure to drug was analyzed using the paired t-test to compare the pre-drug baseline to the drug condition. Statistical analysis across groups was done using analysis of variance (ANOVA). If the ANOVA was significant (P<0.05), this was followed by post-hoc multiple comparison testing using the Holm-Sidak procedure (Glantz, 2002). Data are presented as mean ± standard error.
Results
Effect of acute ethanol exposure on mIPSCs in cultured cerebral cortical neurons
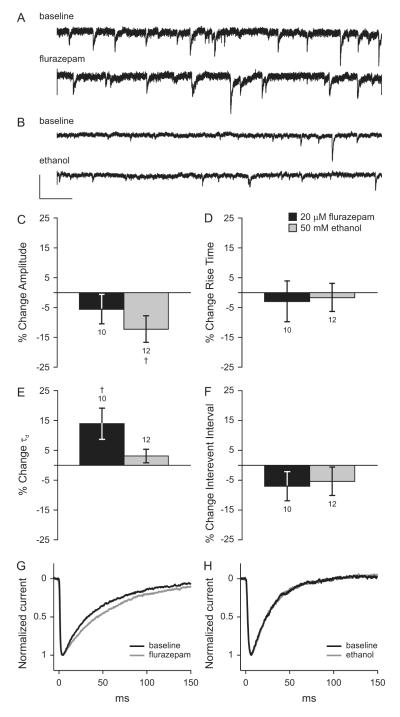
In order to determine the effects of acute exposure to 50 mM ethanol on synaptic GABAergic transmission in cultured cortical neurons, we compared miniature inhibitory postsynaptic currents (mIPSCs) recorded during a baseline interval to those recorded after 5 minutes of exposure to drug. Drug was still present in the bath during the second mIPSC recording interval for a total of 8.3 minutes of exposure. The benzodiazepine flurazepam (20 μM) was used as a positive control. We had previously determined that 20 μM was a saturating concentration of flurazepam in this assay and that the effects were reversible (data not shown). Average mIPSC τd, amplitude, rise time, and interevent interval over the baseline and drug recording periods for each cell were compared using paired t-test. Ethanol treatment for 5 minutes significantly reduced mIPSC amplitude by 12.2 ± 4.5% (Figure 1C). Treatment with flurazepam also decreased mIPSC amplitude although to a smaller degree than ethanol treatment (5.6 ± 4.9%), and this effect was not statistically significant. Five minutes of no drug treatment (negative control) did not alter mIPSC amplitude, kinetics, or frequency. Negative control values, shown as percent change from baseline were: amplitude −12.5 ± 6.1%, rise time 10.2 ± 4.9%, τd 0.6 ± 3.4%, and interevent interval −1.1 ± 4.8%; none of these were statistically significant from baseline by paired t-test. The changes in amplitude for the acute ethanol, positive control, and negative control groups were compared using ANOVA and no significant differences were found. Therefore, we concluded that the decrease in mIPSC amplitude during acute ethanol exposure occurred due to a nonspecific effect of the whole cell patch procedure and did not represent a true effect of ethanol. Miniature IPSC rise time was not altered by exposure to flurazepam or ethanol (Figure 1D). Ethanol exposure did not alter mIPSC τd, but flurazepam significantly increased mIPSC τd by 14.0 ± 5.2% (Figure 1E, representative data is shown in Figure 1 G and H). Interevent interval, the inverse of event frequency, was not altered by 5 minutes of exposure to flurazepam or ethanol (Figure 1F, representative traces are shown in Figure 1 A and B).
Figure 1. Effects of flurazepam and ethanol on mIPSCs in cultured cortical neurons.
Representative tracings of voltage-clamp recordings from cultured cortical neurons under baseline conditions and during acute exposure to 20 μM flurazepam (A) or 50 mM ethanol (B) in the extracellular solution. A and B are shown at the same scale. Scale bars: x = 1s, y = 10 pA. Mean changes in mIPSC amplitude (C), rise time (D), decay time constant (E), and interevent interval (F) during exposure to 20 μM flurazepam (black bars) or 50 mM ethanol (gray bars) relative to the no-drug baseline. † p < 0.05 by paired t-test. Scaled and averaged mIPSCs under baseline conditions and during exposure to flurazepam (G) or ethanol (H).
Effect of acute ethanol exposure on mIPSCs in cultured hippocampal neurons
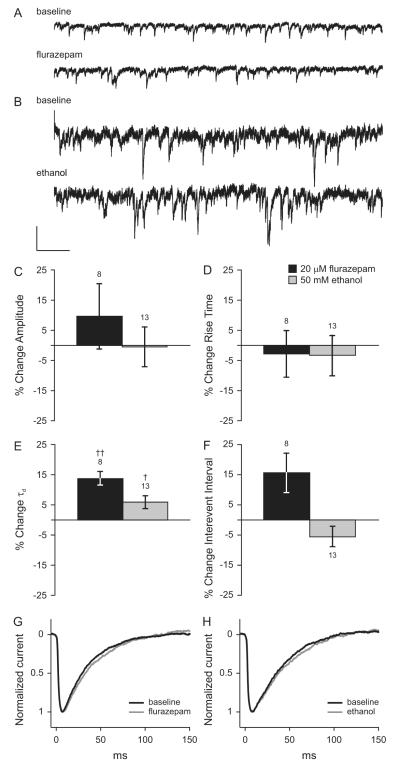
Using methods similar to those described above for cerebral cortex, we tested whether acute exposure to 50 mM ethanol altered synaptic GABAAR function or GABA release in cultured hippocampal neurons. Flurazepam was used again as a positive control. A baseline was obtained by recording mIPSCs in external solution alone. Ethanol (50 mM) was applied for 3 minutes followed by a second mIPSC recording interval in the presence of ethanol (3.3 minutes of total ethanol exposure). Ethanol was washed out for 3 minutes, 20 μM flurazepam was applied, and mIPSCs were recorded in the presence of flurazepam. For each cell, average mIPSC τd, rise time, amplitude, and interevent interval were calculated for each of the three recording periods and the drug conditions were compared to baseline. In cells aged 15-16 days in vitro (DIV), neither acute ethanol exposure nor flurazepam altered mIPSC amplitude or rise time, (Figure 2C, D). However, as shown in Figure 2E, when compared to the baseline value by paired t-test, ethanol (50 mM) significantly increased mIPSC τd by 5.9 ± 2.2% and flurazepam significantly increased mIPSC τd by 13.8 ± 2.3% (see Figure 2 G and H for representative data). Ethanol and flurazepam did not alter mIPSC interevent interval (Figure 2F, representative traces are shown in Figure 2 A and B). This pattern of changes in mIPSC kinetics suggests that the acute effects of ethanol in cultured hippocampal neurons are most likely on postsynaptic GABAARs.
Figure 2. Effects of flurazepam and ethanol on mIPSCs in cultured hippocampal neurons.
Representative tracings of voltage-clamp recordings from cultured hippocampal neurons aged 15-16 days in vitro under baseline conditions and during acute exposure to 20 μM flurazepam (A) or 50 mM ethanol (B) in the extracellular solution. A and B are shown at the same scale. Scale bars: x = 1s, y = 10 pA. Mean changes in average mIPSC amplitude (C), rise time (D), decay time constant (E), and interevent interval (F) during exposure to 20 μM flurazepam (black bars) or 50 mM ethanol (gray bars) relative to the no-drug baseline are shown. † p < 0.05, †† p < 0.01 by paired t-test. Scaled and averaged mIPSCs under baseline conditions and during exposure to flurazepam (G) or ethanol (H).
Chronic ethanol exposure transiently decreases mIPSC duration in cortical neurons
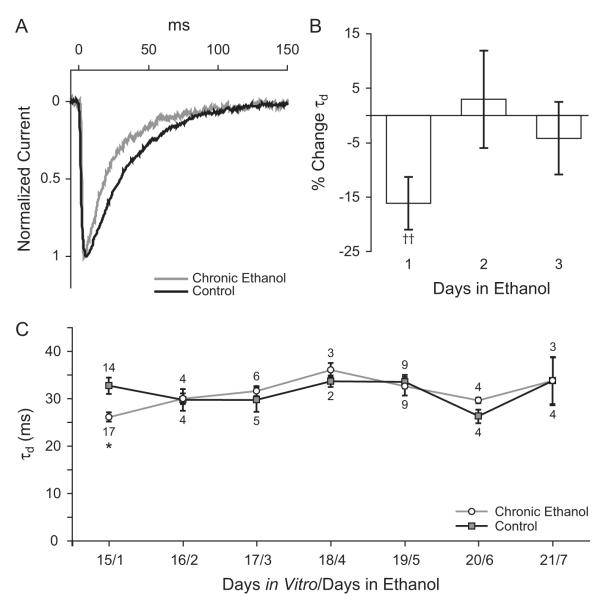
In order to determine the effect of chronic ethanol exposure on synaptic GABAAR function, we recorded mIPSCs in neurons continuously exposed to ethanol for 1 to 7 days and in age-matched controls. Beginning at 14 DIV, cultured cortical neurons were exposed to 50 mM ethanol in the tissue culture medium. After chronic ethanol exposure, cells were removed from the vapor chamber and were maintained in 50 mM ethanol until a whole-cell patch was obtained. Ethanol was washed off and the neuron was allowed to stabilize for at least 5 minutes before the start of the baseline recording session. Miniature IPSCs were recorded and for each cell, average mIPSC rise time, amplitude, τd, and interevent interval were calculated. Data obtained from cells exposed to ethanol were compared to those from control neurons of the same age. Twenty-four hours of ethanol exposure significantly decreased mIPSC duration (Figure 3A-C). When compared to age-matched controls from the same culture, the mIPSC τd in cortical neurons exposed to 50 mM ethanol for 24 hours was decreased by 16.1 ± 4.9% (Figure 3B). Absolute values for τd at this time-point were 32.7 ± 1.7 ms in control neurons to 26.2 ± 1.0 ms in neurons exposed to ethanol (Figure 3A,C). However, after two days of ethanol exposure mIPSC τd in exposed neurons was no longer different from controls (Figure 3C). Chronic ethanol treatment for 1 to 7 days did not significantly alter mIPSC amplitude, rise time, or interevent interval (Table 1).
Figure 3. Effect of chronic in vitro ethanol exposure on mIPSCs in cortical neurons.
Cultured neurons aged 14 days in vitro were exposed to 50 mM ethanol in the tissue culture medium for 1 to 7 days. A. Averaged mIPSCs for a control neuron and a neuron exposed to 50 mM ethanol for 24 hours. B. Percent change in average mIPSC decay time constant calculated relative to age and culture matched controls for neurons exposed to 50 mM ethanol for 1,2, or 3 days. †† p < 0.005 compared to controls by paired t-test. C. Absolute values for mIPSC decay time constants in ethanol treated neurons and controls. One day of ethanol treatment decreased mIPSC τd from 32.7±1.7 ms in control cells to 26.2±1.0 ms in ethanol treated cells. † p < 0.05 by Holm-Sidak posthoc test.
Table 1.
Chronic ethanol exposure in vitro does not alter mIPSC amplitude, rise time, or interevent interval in cultured cortical neurons. Statistical analysis across groups was done using ANOVA followed by post-hoc multiple comparison testing if the ANOVA was significant; only the data for τd met this criterion.
| Days in Vitro / Days in Ethanol | ||||||||
|---|---|---|---|---|---|---|---|---|
| 15 / 1 | 16 / 2 | 17 / 3 | 18 / 4 | 19 / 5 | 20 / 6 | 21 / 7 | ||
| Control | Amplitude (pA) |
28.0 ± 2.4 (14) |
23.8 ± 2.2 (4) |
29.9 ± 5.7 (5) |
30.0 ± 0.3 (2) |
33.1 ± 3.4 (9) |
25.6 ± 4.4 (4) |
34.0 ± 4.1 (4) |
| Rise Time (ms) |
1.7 ± 0.1 (14) |
1.6 ± 0.2 (4) |
1.6 ± 0.3 (5) |
2.1 ± 0.1 (2) |
1.9 ± 0.2 (9) |
2.1 ± 0.3 (4) |
2.2 ± 0.3 (4) |
|
| τd (ms) |
32.7 ± 1.7 (14) |
29.7 ± 2.3 (4) |
29.7 ± 2.5 (5) |
33.7 ± 1.2 (2) |
33.6 ± 1.5 (9) |
26.3 ± 1.4 (4) |
33.8 ± 5.1 (4) |
|
| Interevent Interval (ms) |
1007.7 ± 73.6 (14) |
1246.1 ± 174.8 (4) |
1063.0 ± 222.6 (5) |
1252.7 ± 412.9 (2) |
683.3 ± 113.6 (9) |
886.9 ± 420.4 (4) |
688.6 ± 166.4 (4) |
|
| Ethanol treated | Amplitude (pA) |
26.4 ± 2.7 (17) |
21.8 ± 3.3 (4) |
22.4 ± 2.8 (6) |
26.6 ± 5.6 (3) |
25.1 ± 2.6 (9) |
36.3 ± 6.2 (4) |
43.7 ± 13.7 (3) |
| Rise Time (ms) |
1.5 ± 0.6 (17) |
2.0 ± 0.2 (4) |
1.8 ± 0.2 (6) |
2.1 ± 0.2 (3) |
1.9 ± 0.2 (9) |
1.6 ± 0.3 (4) |
1.5 ± 0.3 (3) |
|
| τd (ms) |
26.2 † ± 1.0 (17) |
30.0 ± 1.2 (4) |
31.6 ± 1.1 (6) |
36.1 ± 1.5 (3) |
32.6 ± 1.9 (9) |
29.7 ± 0.6 (4) |
33.8 ± 4.9 (3) |
|
| Interevent Interval (ms) |
1237.0 ± 125.2 (17) |
1008.9 ± 40.8 (4) |
1466.4 ± 223.1 (6) |
1225.6 ± 387.9 (3) |
1342.7 ± 125.1 (9) |
850.0 ± 228.1 (4) |
1166.2 ± 353.9 (3) |
|
p < 0.05 by Holm-Sidak posthoc test.
Chronic ethanol exposure does not alter mIPSCs in hippocampal neurons
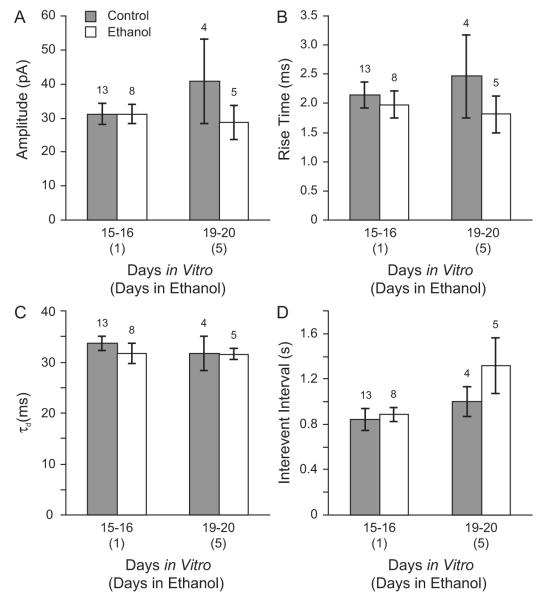
The data presented so far suggest that acute ethanol exposure does not alter GABAAR-mediated synaptic transmission in cultured cortical neurons, while chronic ethanol exposure produces a transient change in mIPSC duration. However, as discussed above, previous studies have shown that exposing cultured hippocampal neurons to ethanol for 5 days changes the gene expression of GABAAR subunits that can localize at synapses (Sanna et al., 2003; Sheela Rani and Ticku, 2006). Sanna et al (2003) also reported that these changes in gene expression were associated with overall changes in GABAAR pharmacology. Therefore, in order to determine whether cultured cortical and hippocampal neurons respond differently to chronic ethanol exposure in vitro, and to determine the effect of chronic ethanol exposure on synaptic GABAAR function specifically in cultured hippocampal neurons, we recorded mIPSCs in neurons chronically exposed to ethanol and in controls. Beginning at 14 or 15 DIV, cultured hippocampal neurons were exposed to 50 mM ethanol in the tissue culture medium. After 1 or 5 days of ethanol exposure, cells were removed from the vapor chamber and were maintained in 50 mM ethanol until a whole-cell patch was obtained. Ethanol was washed off and the neuron was allowed to stabilize before the start of baseline recording. Miniature IPSCs were recorded and for each cell, average mIPSC rise time, amplitude, τd, and interevent interval were calculated. Data obtained from cells exposed to ethanol at 14 and 15 DIV were grouped together, as were data from age-matched controls. Each ethanol exposed group was compared to the control group of the same age, i.e. cells treated with ethanol for 1 day were compared to controls 15-16 DIV and cells exposed to ethanol for 5 days were compared to controls 19-20 DIV. Chronic ethanol exposure for either 1 or 5 days did not alter mIPSC amplitude. Mean peak amplitude was 31.2 ± 3.2 pA in neurons 15-16 DIV and 40.8 ± 12.3 pA in control neurons 19-20 DIV. After 1 and 5 days of ethanol exposure mIPSC amplitude was 31.1 ± 2.8 pA and 28.5 ± 5.1 pA, respectively (Figure 4A). Ethanol treatment for 1 or 5 days did not alter average mIPSC rise times (in ms): 2.1 ± 0.2 controls 15-16 DIV, 2.0 ± 0.2 1 DIE, 2.5 ± 0.7 controls 19-20 DIV, 1.8 ± 0.3 5 DIE (Figure 4B). In addition, in contrast to the data shown for cortical neurons, 1 day of ethanol exposure did not reduce mIPSC duration in hippocampal neurons (τd in ms): controls 33.6 ± 1.4, 1 day in ethanol (DIE) 31.7 ± 1.9. Five days of ethanol exposure also did not alter mIPSC τd (in ms) controls 31.7 ± 3.4, 5 DIE 31.5 ± 1.1 (Figure 4C). Ethanol treatment also did not change the frequency of mIPSCs, as shown by measuring the interevent interval (in ms): 842.5 ± 97.1 controls 15-16 DIV, 885.6 ± 59.2 1 DIE, 1001.0 ± 130.5 controls 19-20 DIV, 1317.7 ± 242.5 5 DIE (Figure 4D).
Figure 4. Chronic ethanol treatment does not alter synaptic GABAAR function in cultured hippocampal neurons.
Beginning at 14 or 15 days in vitro, hippocampal neurons were cultured in the absence (gray bars) or presence (open bars) of 50 mM ethanol in the tissue culture medium. Average mIPSC amplitude (A), rise time (B), decay time constant (C), and interevent interval (D) are shown for age-matched controls and neurons treated with ethanol for 1 to 5 days. Ethanol treatment did not alter mIPSC amplitude, kinetics, or frequency.
Chronic ethanol exposure does not produce cross-tolerance to benzodiazepines in mIPSCs from cortical and hippocampal neurons
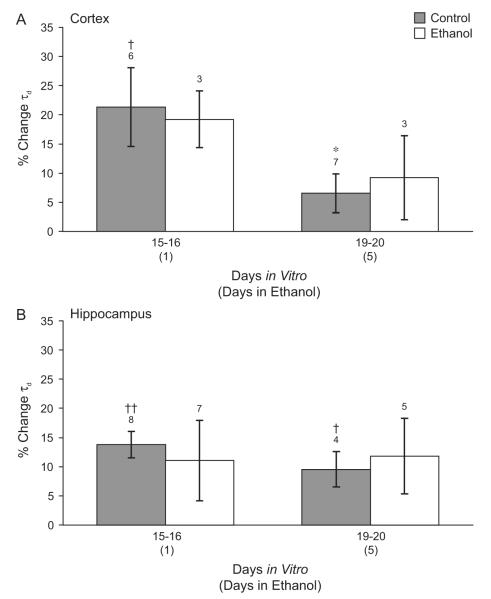
We tested whether chronic ethanol exposure produced cross-tolerance of synaptic GABAARs to benzodiazepines in our system. Cortical neurons aged 14 or 15 DIV were exposed to 50 mM ethanol in the tissue culture medium for 1 or 5 days. The effects of acute flurazepam exposure on mIPSC duration, amplitude, rise time, and interevent interval were determined using the methods described above. Data obtained from neurons exposed to ethanol at 14 and 15 DIV were grouped together, as were data from the age-matched controls. Each ethanol exposed group was compared to the untreated group of the same age. Flurazepam significantly increased mIPSC τd by 21.3 ± 6.7% in control cortical neurons 15-16 DIV when the flurazepam condition was compared to baseline by paired t-test (Figure 5A). Flurazepam increased mIPSC τd by 6.6 ± 3.3% in control neurons 19-20 DIV but this increase was not significant. In neurons exposed to ethanol, flurazepam did not significantly increase τd in cells at 1 or 5 DIV (% change 19.3 ± 4.8 and 9.3 ± 7.2, respectively). When ANOVA was used to compare the effects of flurazepam on τd among these groups, the mean increase in τd at 19-20 DIV was significantly smaller than at 15-16 DIV (% change 6.6 ± 3.3 and 21.3 ± 6.7, respectively, P < 0.05 by Holm-Sidak posthoc test). However, the effect of ethanol on flurazepam enhancement of τd after 1 or 5 days of ethanol treatment was not statistically significant by post-hoc test. Flurazepam did not alter mIPSC amplitude, rise time, or interevent interval in either control or ethanol-treated neurons (data not shown).
Figure 5. Effect of chronic ethanol treatment on the acute effect of benzodiazepine.
Both cortical (A) and hippocampal neurons (B) were cultured in the absence (gray bars) or presence (open bars) of 50 mM ethanol in the tissue culture medium. The increase in mIPSC τd during acute application of 20 μM flurazepam in the external solution is shown. (A) Cultured cortical cells 19-20 days in vitro were less sensitive to flurazepam than control neurons 15-16 days in vitro. Chronic ethanol exposure did not alter mIPSC sensitivity to flurazepam at either timepoint. p < 0.05 ANOVA. * p < 0.05 compared to control 15-16 DIV by Holm-Sidak post-hoc test, † p < 0.05 compared to pre-drug baseline by paired t-test. (B) In cultured hippocampal neurons, flurazepam significantly increased mIPSC τd in control cells 15-16 and 19-20 days in vitro. Ethanol treatment for 1 or 5 days did not alter mIPSC flurazepam sensitivity. † p < 0.05, †† p < 0.01 compared to pre-drug baseline by paired t-test.
We also investigated whether chronic ethanol exposure in vitro produced benzodiazepine cross-tolerance in synaptic GABAARs in hippocampal neurons. The effects of acute flurazepam exposure on mIPSC duration, amplitude, rise time, and interevent interval were determined using methods described above. Data obtained from neurons chronically exposed to ethanol for 1 or 5 days were compared to those from untreated controls of the same age. Figure 5B shows that chronic ethanol exposure for 1 or 5 days did not alter the effect of 20 μM flurazepam on mIPSC τd. Flurazepam increased τd by 13.8 ± 2.7% and 9.5 ± 3.0% in control neurons 15-16 DIV and 19-20 DIV. Similar increases in τd were measured in neurons treated with 50 mM ethanol for 1 or 5 days (11.1 ± 6.9%, 1 DIE and 11.8 ± 6.5%, 5 DIE). These data indicate that up to 5 days of 50 mM ethanol exposure does not produce benzodiazepine cross-tolerance in synaptic GABAARs in cultured hippocampal neurons.
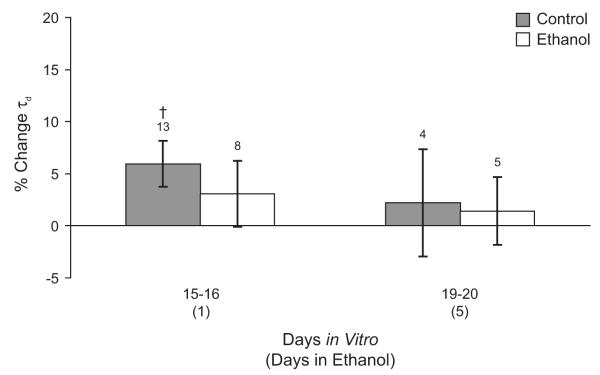
Chronic ethanol exposure does not produce tolerance to acute ethanol exposure in mIPSCs from hippocampal neurons
Acute ethanol exposure increased mIPSC τd in hippocampal (Figure 2E), but not cortical neurons (Figure 1E). Therefore, we tested whether chronic ethanol exposure in vitro produced tolerance to the acute effects of ethanol in hippocampal neurons, because they were initially ethanol sensitive, but not in cortical neurons, which were ethanol-insensitive. As shown in Figure 6, acute application of 50 mM ethanol increased mIPSC τd by 5.9 ± 2.2% in control neurons 15-16 DIV (P< 0.05 by paired t-test comparing the ethanol condition to the no-ethanol baseline), but not in control neurons 19-20 DIV (2.2 ± 5.1%). Acute application of 50 mM ethanol did not increase mIPSC τd in cells treated with 50 mM ethanol for 1 or 5 days (3.1 ± 3.2% 1 DIE, 1.4 ± 3.3% 5 DIE). However, because the acute effect of ethanol in neurons 15-16 DIV is small, it is not statistically significant from the day 1 ethanol group when the groups are compared by ANOVA. Therefore, it appears that the signal-to-noise ratio was too low to detect ethanol tolerance in this system.
Figure 6. Effect of chronic ethanol treatment on acute effects of ethanol in cultured hippocampal neurons.
Changes in mIPSC τd during acute application of 50 mM ethanol are shown for age-matched control neurons (gray bars) and for hippocampal neurons cultured in the presence of 50 mM ethanol for 1 or 5 days (open bars). Acute application of ethanol increased mIPSC τd in control neurons 15-16 days in vitro but not in older control neurons or ethanol treated neurons. † p < 0.05 by paired t-test comparing acute ethanol exposure to pre-drug baseline.
Ethanol exposure does not induce neurosteroid production in cultured neurons
The work of multiple laboratories has produced a large body of evidence that suggests that ethanol-induced elevations of GABAergic neurosteroids contribute to several behavioral effects of ethanol, including ethanol’s memory-impairing effect (Kumar et al., 2009; Matthews et al., 2002; Morrow et al., 2001). Sanna et al. (2004) have shown that in the hippocampal slice ethanol-induced de novo synthesis of the neurosteroid 3α,5α-THP mediates some of the ethanol enhancement of miniature and evoked IPSC amplitude that has been reported in CA1 neurons. We hypothesized that lack of ethanol-induced neurosteroid synthesis in our neuron culture model could partially explain differences between our results and the results of experiments done in vivo or in vitro with an acute slice preparation that allows for neurosteroid synthesis. Therefore, we measured levels of the GABAergic neurosteroid 3α,5α-THP in the tissue and media of neuron cultures acutely exposed to ethanol and in ethanol-naïve cultures. Cultured cortical neurons 18 DIV were exposed to 50 mM ethanol in the tissue culture medium for 8 minutes. Cell scrapings and tissue culture medium from ethanol exposed neurons, age-matched controls, and a positive control with 1.25 ng 3α,5α-THP added to the culture medium were collected and assayed for 3α,5α-THP. 3α,5α-THP was undetectable in the cell scrapings from both ethanol exposed and control neurons. Levels of 3α,5α-THP measured in the tissue culture medium were 0.06 ± 0.003 in controls and 0.06 ± 0.003 in ethanol-exposed neurons (n=7/grp). Detectable levels of 3α,5α-THP were recovered from the tissue and medium of the positive control as expected. In hippocampal slices, baseline levels of 3α,5α-THP have been reported to be 0.50 ± 0.04 with significant increases of 70 ± 8.3% after the addition of 50 mM ethanol (Sanna et al., 2004). Therefore, we conclude that ethanol does not induce de novo synthesis of neurosteroid in neuron cultures as it does in the slice preparation and in vivo.
Discussion
The ethanol exposure conditions in this study were chosen to model our lab’s in vivo chronic ethanol administration protocol, which uses continuous access ethanol feeding in a liquid diet. This regimen produces changes in GABA-mediated Cl− uptake, GABAAR subunit mRNA levels, GABAAR protein levels, and receptor surface expression in the absence of withdrawal (Devaud et al., 1997; Devaud et al., 1995; Kumar et al., 2003; Matthews et al., 1998). The 50 mM (230 mg/dl) ethanol concentration is in the range of mean blood ethanol concentrations of 150-250 mg/dl produced by our in vivo feeding protocol (Matthews et al., 1998). We have found that acute exposure to 50 mM ethanol slightly increased mIPSC duration in cultured hippocampal neurons aged 15-16 days in vitro, but not in cultured cortical neurons of similar age. The benzodiazepine flurazepam increased mIPSC duration in both cortical and hippocampal neurons. In cortical neurons, the GABAAR modulatory effects of flurazepam decrease the longer neurons are maintained in culture. Acute ethanol exposure did not increase GABA release probability as measured by changes in mIPSC frequency in either cortical or hippocampal neurons.
Chronic ethanol exposure in vitro transiently decreased mIPSC duration in cortical neurons. Faster mIPSC decay results in less charge transfer across the membrane during an IPSC and reduced inhibition at these synapses. Therefore, this change in mIPSC kinetics is consistent with the increased CNS excitability seen during ethanol tolerance (Woo and Greenblatt, 1979). In contrast, chronic ethanol exposure had no effect on mIPSC kinetics in hippocampal neurons. Chronic ethanol exposure did not alter GABA release probability in either cortical or hippocampal neurons. Neither did it produce benzodiazepine cross-tolerance in either cortical or hippocampal neurons as measured by the increase in mIPSC duration during acute exposure to flurazepam. In hippocampal neurons exposed to ethanol, acute ethanol exposure had less of an effect on mIPSC duration than in controls, but this trend was nonsignificant. The effect of ethanol on mIPSC τd in control neurons was already small, and was limited to younger neurons, so this adaptation is unlikely to be a major factor in the development of ethanol tolerance in cultured neurons.
Acute ethanol exposure and GABAergic transmission
Physiological concentrations of ethanol have been shown to alter GABAAergic neurotransmission in cortical and hippocampal slices and in cultured cortical neurons (Carta et al., 2004; Li et al., 2003, 2006; Marszalec et al., 1998; Proctor et al., 1992; Sanna et al., 2004; Weiner et al., 1997a). These alterations in GABAergic transmission could be due to either presynaptic effects of ethanol on GABA release or to postsynaptic effects of ethanol on GABAAR function (or both). Our experiments were designed to detect both presynaptic and postsynaptic effects of ethanol on the GABA system in cultured neurons.
Our results show that in cultured cortical neurons, acute ethanol exposure does not alter mIPSC amplitude, rise time, or decay time. Changes in the average size and shape of mIPSCs usually reflect postsynaptic changes in GABAAR function (Jones et al., 1998; Jones and Westbrook, 1995). Therefore, our results suggest that acute ethanol exposure has no effect on postsynaptic GABAAR function in cultured cortical neurons, a finding that is consistent with the current state of the literature. While early studies investigating the effects of ethanol on GABAAR function suggested that synaptic GABAARs were the primary targets for ethanol (Criswell et al., 1995; McKernan and Whiting, 1996; Wafford et al., 1991), it is now known that most synaptic GABAARs are relatively ethanol insensitive and are potentiated only by very high anesthetic or lethal ethanol concentrations (Eckardt et al., 1998; Wallner et al., 2003; Whitten et al., 1996). Therefore, in most cases, ethanol enhancement of synaptic GABAergic neurotransmission is probably not mediated by direct effects of ethanol on synaptic GABAARs.
In cultured hippocampal neurons 50 mM ethanol slightly increased mIPSC τd by 5.9% in neurons 15-16 DIV. GABAARs that contain the δ subunit are expressed in some neurons of the hippocampus (Pirker et al., 2000; Wisden et al., 1992) and in some experimental paradigms these δ-type receptors are potentiated by ethanol concentrations that are as low as 3 mM (Sundstrom-Poromaa et al., 2002; Wallner et al., 2003; Wallner et al., 2006). These receptors are located extrasynaptically or perisynaptically, where they can be activated by GABA spillover (Fritschy and Brunig, 2003; Saxena and Macdonald, 1994; Wei et al., 2003). Therefore, the effect of ethanol on mIPSCs in hippocampal neurons could occur due to potentiation of perisynaptic δ-type GABAARs. However, ethanol did not increase mIPSC τd in hippocampal neurons 19–20 DIV. This suggests that there may be maturational changes in GABAAR function that affect ethanol sensitivity in these neurons.
The fact that most synaptic GABAARs are ethanol insensitive suggests that many of ethanol’s effects on GABAergic neurotransmission may be mediated by presynaptic mechanisms, such as increases in the probability of quantal GABA release from the presynaptic terminal. However, when we measured the frequency of spontaneous mIPSCs in cultured neurons from cortex and hippocampus we did not find evidence that ethanol increased release probability at GABAergic synapses. This finding in cultured cortical neurons is consistent with the findings of (Criswell and Breese, 2005) who showed that ethanol does not increase mIPSC frequency in acutely dissociated cerebrocortical neurons, but inconsistent with the findings of Moriguchi et al. (2007), which show that ethanol decreases mIPSC frequency in cultured cortical neurons. In the hippocampus, studies using the acute slice preparation have shown that ethanol increases mIPSC frequency in CA1 neurons, a brain region where it also potentiates evoked IPSCs (Ariwodola and Weiner, 2004; Li et al., 2003, 2006; Sanna et al., 2004; Weiner et al., 1997b), and in immature CA3 interneurons (Galindo et al., 2005). However, ethanol’s effect on GABAergic neurotransmission varies not only from one brain region to another, but also among different classes of neurons within the same brain region. For example, in the hippocampus, ethanol increases the frequency of both spontaneous (action-potential dependent) and miniature (action-potential independent) IPSCs in CA1 pyramidal cells but does not increase spontaneous IPSC frequency in granule cells from the dentate gyrus (Fleming et al., 2007; Li et al., 2006; Wei et al., 2004). Therefore it is not clear what results we should expect from hippocampal cultures, which are composed of mixed populations of neurons. Our results suggest that cultured hippocampal and cortical neurons do not support the ethanol enhancement of GABA release probability, as indicated by increases in mIPSP frequency, that has been found in hippocampal (Galindo et al., 2005; Li et al., 2006; Sanna et al., 2004), amygdalar (Roberto et al., 2003a) and cerebellar (Criswell and Breese, 2005; Kelm et al., 2007) slice preparations. We discuss possible explanations for this difference below.
First, GABAB receptors were not blocked during our experiments, and these receptors, when activated by ambient or synaptically-released GABA, can regulate both evoked release and spontaneous TTX-resistant release. Several studies have shown that GABAB receptors blockade unmasks presynaptic ethanol effects in hippocampal slices (Ariwodola and Weiner, 2004; Wan et al., 1996). However, these studies were conducted using measurement of evoked and/or spontaneous IPSCs, in the absence of TTX, and thus do not discriminate presynaptic terminal effects from changes in somatic excitability or action potential shape. Furthermore, ethanol has effects on mIPSC frequency in hippocampal slices in the absence of GABAB blockers (Li et al., 2006). In amygdala and cerebellum, pharmacological block of GABAB receptors is not necessary to observe an ethanol-induced increase the rate of miniature (not spontaneous) release at GABAergic synapses, although pharmacological activation of the receptors does prevent ethanol’s effects (Kelm et al., 2008)(Silberman et al., 2009). These different results may depend on the free GABA concentrations in the different preparations, or on regional variability in GABAB regulation of preterminal release, which can be different between cortical layers (Bailey et al., 2001). While it is not clear whether differences in GABABR activation can explain the difference between our results and what is shown in slices, block of ethanol effects due to elevated GABAB tone could have been a contributing factor.
Second, the work of Swartzwelder and colleagues has shown that ethanol’s effect on GABAergic synaptic transmission is developmentally regulated in the hippocampus. Ethanol enhancement of evoked and spontaneous (action-potential driven) IPSCs in CA1 is greater in slices from adult rats than adolescent rats (Li et al., 2003, 2006), which raises the possibility that the lack of ethanol enhancement of GABA release in our system is due to the study of relatively immature synapses. However, Li et al. (2006) found no age-dependent difference in the effect of ethanol on miniature (TTX-independent) IPSCs. Ethanol increased mIPSC frequency similarly in slices from both adolescent and adult animals and had no effect on mIPSC amplitude and kinetics in both age groups. This suggests that the age-dependent effects of ethanol on GABAergic inhibition in slices are largely due to changes in presynaptic neuron excitability or presynaptic terminal mechanisms activated during evoked release. Nevertheless, our neurons are younger than those used in the Li et al. (2003, 2006) studies. Thus, we cannot eliminate the possibility that synapse immaturity may have been a factor. This possible explanation is supported by the work of Moriguchi et al. (2007) who found that ethanol decreased mIPSC frequency in older cultured cortical neurons, although reports of ethanol effects on mIPSCs in cortical neurons are inconsistent (Criswell and Breese, 2005).
Chronic ethanol exposure transiently alters synaptic GABAAR function in cortical but not hippocampal neurons
As opposed to the acute effects just discussed, twenty-four hours of exposure to 50 mM ethanol in the tissue culture medium significantly reduced τd of mIPSCs in cultured cortical, but not hippocampal, neurons. In cortical neurons the mIPSC τd had returned to control within 48 hours of ethanol treatment and remained unchanged after 7 days of ethanol exposure. This result is similar to the report of a transient decrease in mIPSC area in CA1 pyramidal cells following acute ethanol administration to rats (Liang et al., 2007), and suggests that downregulation of synaptic GABAAR function may be an early adaptation to ethanol exposure. However, longer ethanol exposure did not sustain this effect in cultured neurons whereas repeated exposure and withdrawal cycles in vivo produce changes in the pharmacology of both synaptic and extrasynaptic GABAARs in neurons from CA1 and the dentate gyrus (Liang et al., 2004; Liang et al., 2006). The differences between the effects of ethanol in cultured neurons reported here and the work of Liang and colleagues suggest that repeated ethanol exposure and withdrawals in vivo involve mechanisms that are not modeled by continuous ethanol exposure in vitro.
Chronic ethanol exposure does not produce benzodiazepine cross-tolerance in cultured cortical or hippocampal neurons
Cross-tolerance to benzodiazepines is found in humans and animal models of ethanol tolerance (Woo and Greenblatt, 1979). Chronic ethanol exposure in vivo produces benzodiazepine tolerance and alters the levels of many GABAAR subunit mRNAs and proteins in the cerebral cortex and hippocampus (see Kumar et al., 2009 for review). In general, benzodiazepine sensitive GABAAR subtypes, such as α1, are decreased while benzodiazepine insensitive subunits, such as α4, are increased. However, the specific changes in GABAAR subunit expression that are observed after ethanol exposure in vivo differ according to the method of ethanol administration and the peak blood ethanol levels achieved. In the cortex, GABAAR mRNA and protein for the α1 subunit decrease in cerebral cortex after 14 days of ethanol consumption, while α4 subunit mRNA and protein increase (Devaud et al., 1997; Devaud et al., 1995). In the hippocampus, in vivo ethanol exposure for 40 days increases α4 subunit protein, but does not change α1 subunit levels (Matthews et al., 1998). In contrast, (Cagetti et al., 2003) reported decreases in α1 protein in the hippocampus after chronic intermittent exposure in vivo for 60 days using high doses of ethanol. Thus, we were surprised to find no evidence for benzodiazepine tolerance following ethanol exposure in cortical or hippocampal cultured neurons.
Changes in GABAAR subunit expression and function similar to those that occur after chronic in vivo ethanol exposure have previously been reported in cultured hippocampal and cortical neurons after chronic ethanol exposure in vitro (Sanna et al., 2003; Sheela Rani and Ticku, 2006). Sanna et al. (2003) found that 5 days of exposure to 100 mM ethanol decreased benzodiazepine sensitivity and levels of mRNA for benzodiazepine sensitive subunits. Increases in α4 subunit mRNA and protein levels and corresponding changes in the GABA modulatory effects of Ro15-4513 occurred after ethanol withdrawal. Sheela Rani and Ticku (2006) found that 5 days of continuous exposure to 75 mM ethanol decreased mRNA and protein levels for the α1 and α2 subunits and increased mRNA and protein levels for the α4 subunits. In hippocampal slices, simultaneous decreases in α1 and increases in α4 expression following chronic ethanol exposure in vivo are associated with decreases in mIPSC duration and loss of synaptic benzodiazepine sensitivity (Cagetti et al., 2003). Therefore, we expected to see similar changes in mIPSC characteristics in cultured neurons, especially in cultures from the hippocampus. There are several possible explanations for the discrepancy between our findings and those reported previously in cultured neurons. First, the ethanol concentration that we used (50 mM), which was lower than that used by both Sanna et al. (2003) and Sheela Rani and Ticku (2006) may have been too low to produce similar changes in GABAAR subunit expression. However, previous studies in our lab have demonstrated numerous alterations in GABAAR subunit expression and GABA mediated Cl− uptake in cerebral cortex using comparable ethanol doses in vivo (Devaud et al., 1997; Devaud et al., 1995; Kumar et al., 2003; Matthews et al., 1998). In vivo ethanol exposure at these doses did not produce changes in the α1 subunit in the hippocampus, although increases in α4 subunit expression were found (Matthews et al., 1998). Second, the experiments reported by Sanna et al. (2003) differed from ours in the method of assaying GABAAR function. Sanna et al. (2003) measured currents evoked by applying GABA directly to the cell body and, as discussed above, this method does not discriminate between extrasynaptic and synaptic receptors. In contrast, our method, recording of mIPSCs, selectively assays synaptic GABAAR function. These comparisons suggest that the changes in GABAAR subunit expression reported by Sanna et al. (2003) and Sheela Rani and Ticku (2006) could occur primarily in an extrasynaptic GABAAR population. More experiments are needed to determine if extrasynaptic GABAAR function is altered in our model system and if higher ethanol concentrations are necessary to produce synaptic changes similar to those reported by in vivo studies.
In cortical neurons, the decrease in the GABAAR modulatory effect of flurazepam between days 15-16 and days 19-20, was unexpected and could have obscured losses of benzodiazepine sensitivity in these neurons after 5 days of ethanol exposure. It seems unlikely that this change reflects decreased viability of cortical neurons in culture because baseline mIPSC amplitude, kinetics, and frequency did not change during this period (Table 1). Rather, it appears that GABAAR sensitivity to allosteric modulation decreases over time. It is known that brief GABA exposure in cultured neurons causes functional uncoupling of GABA and benzodiazepine allosteric modulation without loss of either GABA or benzodiazepine binding (Gravielle et al., 2005) and we propose that a similar process may have occurred during our study.
Overall, the results of the present study show that under some experimental conditions the effects of ethanol in cultured neurons differ from those reported in acute brain slices. The only direct effect of 50 mM ethanol on synaptic GABAARs in cultured neurons is an increase in mIPSC duration in hippocampal neurons and there is no effect on GABA release probability, as indicated by changes in mIPSC frequency, in cultured cortical or hippocampal neurons. While the lack of effects of acute ethanol on mIPSC frequency in cortical neurons is consistent with some previous reports (Criswell and Breese, 2005), the finding in hippocampal neurons is inconsistent with data obtained in slices (Galindo et al., 2005; Li et al., 2006; Sanna et al., 2004).
Ethanol exposure for one day produced a transient decrease in mIPSC duration in cortical, but not hippocampal neurons. Furthermore, functional adaptations to chronic ethanol exposure that have been observed in vivo and in slice preparations (vide supra) are not detectable in synaptic GABAARs in cultured cortical or hippocampal neurons. While further studies on extrasynaptic GABAARs in these cultures are warranted, the absence of ethanol-induced changes in GABAergic synaptic transmission suggests that chronic ethanol exposure in cultured cortical and hippocampal neurons may not reproduce all of the effects of ethanol that occur in vivo and in acute brain slices.
Acknowledgements
This research was supported by NIAAA grant AA11605 to ALM and NIDCD grant DC000425 to PBM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguayo LG. Ethanol potentiates the GABAA-activated Cl− current in mouse hippocampal and cortical neurons. Eur. J. Pharmacol. 1990;187:127–130. doi: 10.1016/0014-2999(90)90349-b. [DOI] [PubMed] [Google Scholar]
- Ariwodola OJ, Weiner JL. Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABA-B receptors. J. Neurosci. 2004;24:10679–10686. doi: 10.1523/JNEUROSCI.1768-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CD, Brien JF, Reynolds JN. Chronic prenatal ethanol exposure increases GABA(A) receptor subunit protein expression in the adult guinea pig cerebral cortex. J. Neurosci. 2001;21:4381–4389. doi: 10.1523/JNEUROSCI.21-12-04381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouron A. Modulation of spontaneous quantal release of neurotransmitters in the hippocampus. Prog. Neurobiol. 2001;63:613–635. doi: 10.1016/s0301-0082(00)00053-8. [DOI] [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol. Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- Carta M, Ariwodola OJ, Weiner JL, Valenzuela CF. Alcohol potently inhibits the kainate receptor-dependent excitatory drive of hippocampal interneurons. Proc. Natl. Acad. Sci. USA. 2003;100:6813–6818. doi: 10.1073/pnas.1137276100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF. Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J. Neurosci. 2004;24:3746–3751. doi: 10.1523/JNEUROSCI.0067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DS, Wei W, Deitchman JK, Kharazia VN, Lesscher HM, McMahon T, Wang D, Qi ZH, Sieghart W, Zhang C, et al. Protein kinase Cdelta regulates ethanol intoxication and enhancement of GABA-stimulated tonic current. J. Neurosci. 2008;28:11890–11899. doi: 10.1523/JNEUROSCI.3156-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JD, Bekkers JM. Detection of spontaneous synaptic events with an optimally scaled template. Biophys. J. 1997;73:220–229. doi: 10.1016/S0006-3495(97)78062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell HE, Breese GR. A conceptualization of integrated actions of ethanol contributing to its GABAmimetic profile: a commentary. Neuropsychopharmacology. 2005;30:1407–1425. doi: 10.1038/sj.npp.1300750. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Ming Z, Griffith BL, Breese GR. Comparison of effect of ethanol on N-methyl-D-aspartate- and GABA-gated currents from acutely dissociated neurons: absence of regional differences in sensitivity to ethanol. J. Pharmacol. Exp. Ther. 2003;304:192–199. doi: 10.1124/jpet.102.041590. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Simson PE, Knapp DJ, Devaud LL, McCown TJ, Duncan GE, Morrow AL, Breese GR. Effect of zolpidem on γ-aminobutyric acid (GABA)-induced inhibition predicts the interaction of ethanol with GABA on individual neurons in several rat brain regions. J. Pharmacol. Exp. Ther. 1995;273:526–536. [PubMed] [Google Scholar]
- De Witte P, Dahchour A, Quertemont E. Acute and chronic alcohol injections increase taurine in the nucleus accumbens. Alcohol Alcohol. (Oxford, Oxfordshire) 1994;2:229–233. [PubMed] [Google Scholar]
- Devaud LL, Fritschy J-M, Sieghart W, Morrow AL. Bidirectional alterations of GABAA receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. J. Neurochem. 1997;69:126–130. doi: 10.1046/j.1471-4159.1997.69010126.x. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Smith FD, Grayson DR, Morrow AL. Chronic ethanol consumption differentially alters the expression of γ-aminobutyric acid type A receptor subunit mRNAs in rat cerebral cortex: Competitive, quantitative reverse transcriptase-polymerase chain reaction analysis. Mol. Pharmacol. 1995;48:861–868. [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, Kalant H, Koob GF, Li TK, Tabakoff B. Effects of moderate alcohol consumption on the central nervous system. Alcohol. Clin. Exp. Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Fleming RL, Wilson WA, Swartzwelder HS. Magnitude and ethanol sensitivity of tonic GABAA receptor-mediated inhibition in dentate gyrus changes from adolescence to adulthood. J. Neurophysiol. 2007;97:3806–3811. doi: 10.1152/jn.00101.2007. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Brunig I. Formation and plasticity of GABAergic synapses: physiological mechanisms and pathophysiological implications. Pharmacol. Ther. 2003;98:299–323. doi: 10.1016/s0163-7258(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Galindo R, Zamudio PA, Valenzuela CF. Alcohol is a potent stimulant of immature neuronal networks: implications for fetal alcohol spectrum disorder. J. Neurochem. 2005;94:1500–1511. doi: 10.1111/j.1471-4159.2005.03294.x. [DOI] [PubMed] [Google Scholar]
- Glantz SA. Primer of Biostatistics. 5 edn McGraw-Hill; New York: 2002. [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABA(A) receptor subunit partnership with high sensitivity to ethanol. Nat. Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Gravielle MC, Faris R, Russek SJ, Farb DH. GABA induces activity dependent delayed-onset uncoupling of GABA/benzodiazepine site interactions in neocortical neurons. J. Biol. Chem. 2005;280:20954–20960. doi: 10.1074/jbc.M500131200. [DOI] [PubMed] [Google Scholar]
- Higgins D, Banker G. Primary Dissociated Cell Cultures. In: Banker G, Goslin K, editors. Culturing Nerve Cells. The MIT Press; Cambridge, MA: 1998. pp. 37–78. [Google Scholar]
- Janis GC, Devaud LL, Mitsuyama H, Morrow AL. Effects of chronic ethanol consumption and withdrawal on the neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one in male and female rats. Alcohol. Clin. Exp. Res. 1998;22:2055–2061. [PubMed] [Google Scholar]
- Jia F, Yue M, Chandra D, Keramidas A, Goldstein PA, Homanics GE, Harrison NL. Taurine is a potent activator of extrasynaptic GABA(A) receptors in the thalamus. J. Neurosci. 2008;28:106–115. doi: 10.1523/JNEUROSCI.3996-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MV, Sahara Y, Dzubay JA, Westbrook GL. Defining affinity with the GABAA receptor. J.Neurosci. 1998;18:8590–8604. doi: 10.1523/JNEUROSCI.18-21-08590.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995;15:181–191. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR. Calcium release from presynaptic internal stores is required for ethanol to increase spontaneous gamma-aminobutyric acid release onto cerebellum Purkinje neurons. J. Pharmacol. Exp. Ther. 2007;323:356–364. doi: 10.1124/jpet.107.126144. [DOI] [PubMed] [Google Scholar]
- Kumar S, Kralic JE, O’Buckley TK, Grobin AC, Morrow AL. Chronic ethanol consumption enhances internalization of alpha1 subunit-containing GABAA receptors in cerebral cortex. J. Neurochem. 2003;86:700–708. doi: 10.1046/j.1471-4159.2003.01894.x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wilson WA, Swartzwelder HS. Developmental differences in the sensitivity of hippocampal GABAA receptor-mediated IPSCS to ethanol. Alcohol. Clin. Exp. Res. 2003;27:2017–2022. doi: 10.1097/01.ALC.0000108390.62394.71. [DOI] [PubMed] [Google Scholar]
- Li Q, Wilson WA, Swartzwelder HS. Developmental differences in the sensitivity of spontaneous and miniature IPSCs to ethanol. Alcohol. Clin. Exp. Res. 2006;30:119–126. doi: 10.1111/j.1530-0277.2006.00006.x. [DOI] [PubMed] [Google Scholar]
- Liang J, Cagetti E, Olsen RW, Spigelman I. Altered pharmacology of synaptic and extrasynaptic GABAA receptors on CA1 hippocampal neurons is consistent with subunit changes in a model of alcohol withdrawal and dependence. J. Pharmacol. Exp. Ther. 2004;310:1234–1245. doi: 10.1124/jpet.104.067983. [DOI] [PubMed] [Google Scholar]
- Liang J, Suryanarayanan A, Abriam A, Snyder B, Olsen RW, Spigelman I. Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J. Neurosci. 2007;27:12367–12377. doi: 10.1523/JNEUROSCI.2786-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Zhang N, Cagetti E, Houser CR, Olsen RW, Spigelman I. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J. Neurosci. 2006;26:1749–1758. doi: 10.1523/JNEUROSCI.4702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QY, Schaffner AE, Chang YH, Vaszil K, Barker JL. Astrocytes regulate amino acid receptor current densities in embryonic rat hippocampal neurons. J.Neurobiol. 1997;33:848–864. [PubMed] [Google Scholar]
- Marszalec W, Aistrup AL, Narahashi T. Ethanol modulation of excitatory and inhibitory synaptic interactions in cultured cortical neurons. Alcohol. Clin. Exp. Res. 1998;22:1516–1524. [PubMed] [Google Scholar]
- Matthews DB, Devaud LL, Fritschy J-M, Sieghart W, Morrow AL. Differential regulation of GABAA receptor gene expression by ethanol in the rat hippocampus vs. cerebral cortex. J. Neurochem. 1998;70:1160–1166. doi: 10.1046/j.1471-4159.1998.70031160.x. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Morrow AL, Tokunaga, McDaniel JR. Acute ethanol administration and acute allopregnanolone administration impair spatial memory in the morris water task. Alcohol. Clin. Exp. Res. 2002;26:1747–1751. doi: 10.1097/01.ALC.0000037219.79257.17. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Moriguchi S, Zhao X, Marszalec W, Yeh JZ, Narahashi T. Effects of ethanol on excitatory and inhibitory synaptic transmission in rat cortical neurons. Alcohol. Clin. Exp. Res. 2007;31:89–99. doi: 10.1111/j.1530-0277.2006.00266.x. [DOI] [PubMed] [Google Scholar]
- Morrow AL, VanDoren MJ, Penland SN, Matthews DB. The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Res. Brain Res. Rev. 2001;37:98–109. doi: 10.1016/s0165-0173(01)00127-8. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Proctor WR, Soldo BL, Allan AM, Dunwiddie TV. Ethanol enhances synaptically evoked GABAA receptor-mediated responses in cerebral cortical neurons in rat brain slices. Brain Res. 1992;595:220–227. doi: 10.1016/0006-8993(92)91053-h. [DOI] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc. Natl. Acad. Sci. USA. 2003a;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J. Neurosci. 2004;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Nelson TE, Ur CL, Brunelli M, Sanna PP, Gruol DL. The transient depression of hippocampal CA1 LTP induced by chronic intermittent ethanol exposure is associated with an inhibition of the MAP kinase pathway. Eur. J. Neurosci. 2003b;17:1646–1654. doi: 10.1046/j.1460-9568.2003.02614.x. [DOI] [PubMed] [Google Scholar]
- Roberto M, Treistman SN, Pietrzykowski AZ, Weiner J, Galindo R, Mameli M, Valenzuela F, Zhu PJ, Lovinger D, Zhang TA, et al. Actions of acute and chronic ethanol on presynaptic terminals. Alcohol. Clin. Exp. Res. 2006;30:222–232. doi: 10.1111/j.1530-0277.2006.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Mostallino MC, Busonero F, Talani G, Tranquilli S, Mameli M, Spiga S, Follesa P, Biggio G. Changes in GABA(A) receptor gene expression associated with selective alterations in receptor function and pharmacology after ethanol withdrawal. J. Neurosci. 2003;23:11711–11724. doi: 10.1523/JNEUROSCI.23-37-11711.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J. Neurosci. 2004;24:6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Assembly of GABAA receptor subunits: Role of the δ subunit. J.Neurosci. 1994;14:7077–7086. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheela Rani, C.S., Ticku MK. Comparison of chronic ethanol and chronic intermittent ethanol treatments on the expression of GABA(A) and NMDA receptor subunits. Alcohol. 2006;38:89–97. doi: 10.1016/j.alcohol.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Ariwodola OJ, Weiner JL. Differential effects of GABAB autoreceptor activation on ethanol potentiation of local and lateral paracapsular GABAergic synapses in the rat basolateral amygdala. Neuropharmacology. 2009;56:886–895. doi: 10.1016/j.neuropharm.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated alpha(4)beta(2)delta GABA(A) receptors are a target for alcohol. Nat. Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujiyama S, Akaike A, Ujihara H, Sasa M. Potentiation by ethanol of GABA-induced current and facilitation of its desensitization in cultured rat cortical neurons. Gen. Pharmacol. 1997;28:375–380. doi: 10.1016/s0306-3623(96)00164-4. [DOI] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J. Neurosci. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford KA, Burnett DM, Leidenheimer NJ, Burt DR, Wang JB, Kofuji P, Dunwiddie TV, Harris RA, Sikela JM. Ethanol sensitivity of the GABAA receptor expressed in xenopus oocytes requires 8 amino acids contained in the gamma2L subunit. Neuron. 1991;7:27–33. doi: 10.1016/0896-6273(91)90071-7. [DOI] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen R. Ethanol enhances α4β3δ and α6β3δ γ-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc. Natl. Acad. Sci USA. 2003 doi: 10.1073/pnas.2435171100. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Low dose acute alcohol effects on GABAA receptor subtypes. Pharmacol. Ther. 2006;112:513–528. doi: 10.1016/j.pharmthera.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan F-J, Berton F, Madamba SG, Francesconi W, Siggins GR. Low ethanol concentrations enhance GABAergic inhibitory postsynaptic potentials in hippocampal pyramidal neurons only after block of GABAB receptors. Proc. Natl. Acad. Sci. USA. 1996;93:5049–5054. doi: 10.1073/pnas.93.10.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABAA receptors in hippocampal neurons. J. Neurosci. 2004;24:8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of delta subunit-containing GABA(A) receptors and their activation by GABA spillover in the mouse dentate gyrus. J. Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Gu C, Dunwiddie TV. Differential ethanol sensitivity of subpopulations of GABAA synapses onto rat hippocampal CA1 pyramidal neurons. J. Neurophysiol. 1997a;77:1306–1312. doi: 10.1152/jn.1997.77.3.1306. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol. Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF, Watson PL, Frazier CJ, Dunwiddie TV. Elevation of basal protein kinase C activity increases ethanol sensitivity of GABAA receptors in rat hippocampal CA1 pyramidal neurons. J. Neurochem. 1997b;68:1949–1959. doi: 10.1046/j.1471-4159.1997.68051949.x. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Zhang L, Carlen PL. Potentiation of GABAA-mediated synaptic current by ethanol in hippocampal CA1 neurons: Possible role of protein kinase C. J. Pharmacol. Exp. Ther. 1994;268:1388–1395. [PubMed] [Google Scholar]
- Whitten RJ, Maitra R, Reynolds JN. Modulation of GABAA receptor function by alcohols: Effects of subunit composition and differential effects of ethanol. Alcohol. Clin. Exp. Res. 1996;20:1313–1319. doi: 10.1111/j.1530-0277.1996.tb01128.x. [DOI] [PubMed] [Google Scholar]
- Wisden W, Laurie DH, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J. Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo E, Greenblatt DJ. Massive benzodiazepine requirements during acute alcohol withdrawal. Am. J. Psychiatry. 1979;136:821–823. doi: 10.1176/ajp.136.6.821. [DOI] [PubMed] [Google Scholar]