Abstract
Atrial fibrillation (AF) is common in end-stage renal disease (ESRD), but the relationship between more modest decrements in kidney function or albuminuria with AF is uncertain. Among 956 outpatients with coronary heart disease (CHD), we assessed kidney function by 3 methods (cystatin C-based [eGFRcys] and creatinine-based [eGFRCr] estimated glomerular filtration rate, and urinary albumin-to-creatinine ratio [ACR]) and prevalent AF by surface electrocardiogram. Multivariable logistic regression evaluated the associations of each measure of kidney function with AF. The mean eGFRcys was 71 ± 23 ml/min/1.73m2 and median ACR was 10 mg/g (interquartile range 6 – 19 mg/g). Forty subjects (4%) had prevalent AF. Compared to participants with eGFRcys in the highest tertile (eGFRcys > 79), those with eGFRcys in the lowest tertile (eGFRcys < 62) had more than 3-fold greater odds of AF (OR 3.43; 95% CI 1.18 – 9.97) after multivariate adjustment for traditional CVD risk factors. This association remained significant with further adjustment for ACR (OR 3.37; 95% 1.02 – 11.14). Results were similar for eGFRCr, but did not reach statistical significance (OR 1.59; 95% CI 0.57 – 4.40). Participants with ACR in the highest tertile (ACR > 15 mg/g) had more than 4-fold greater odds of AF compared to participants in the lowest ACR tertile (ACR < 7 mg/g); an association that remained significant after adjustment for eGFRcys (OR 4.36; 95% CI 1.45 – 13.05) or eGFRCr (OR 4.61; 95% CI 1.56 – 13.66). In conclusion, among outpatients with CHD, lower eGFRcys and higher ACR are each associated with prevalent AF, independent of one another.
INTRODUCTION
In the present study, we evaluated the association of three measures of kidney function (cystatin C-based [eGFRcys], creatinine-based [eGFRCr] estimated glomerular filtration rate, and urinary albumin-to-creatinine ratio [ACR]) with prevalent AF among 956 outpatients with stable coronary heart disease (CHD). We sought to determine whether these associations were independent of potential confounders including left ventricular ejection fraction (EF), hypertension, and inflammatory biomarkers. We hypothesized that each measure of kidney function would be associated with AF and that these associations would remain significant with adjustment for risk factors common to both conditions.
METHODS
The Heart and Soul Study is a prospective cohort study designed to examine the impact of psychosocial factors on CHD progression. Methods have been described previously.1 Persons with established CHD were recruited from outpatient clinics in the San Francisco Bay Area, and were eligible if they had one or more of the following: (1) a history of coronary revascularization, (2) angiographic evidence of ≥ 50% stenosis in one or more coronary vessels, (3) previous evidence of exercise-induced ischemia by treadmill electrocardiogram (ECG) or stress nuclear perfusion imaging, or (4) a history of non-ST-segment or ST-segment myocardial infarction. Persons were excluded if they were planning to move from the local area within three years, were unable to walk one block, had unstable angina, or had a myocardial infarction in the preceding 6 months. Appropriate institutional review boards approved this protocol and all participants provided written, informed consent.
Between September 2000 and December 2002, a total of 1024 participants provided medical history, physical exam, a comprehensive health status questionnaire, resting transthoracic echocardiograms, and surface 12-lead electrocardiograms at the baseline study visit. Of these, 38 were excluded for either missing creatinine (N=2) or cystatin C (N=36) measurements. Participants with atrial flutter (N=2), paced atrial rhythms (N=3), or paced ventricular rhythms (N=27) were also excluded to allow accurate determination of atrial rhythm on the surface ECG. The remaining 956 participants constituted the study sample for the present analysis.
Concentrations of cystatin C were measured from fasting serum samples using a BNII nephelometer (Dade Behring, Inc., Deerfield, Illinois) and a particle-enhanced immunonephelometric assay (N Latex Cystatin C, Dade Behring Inc.).2 The detection limit of the assay was 0.05 mg/L, the analytic sensitivity is 0.005 mg/L, and the inter- and intra-assay coefficients of variation were < 3.6%. Estimated GFRcys was calculated using the equation (eGFRcys = 76.7*cystatin C concentration−1.19) as has been described and validated previously.3 Serum creatinine concentration was measured by the rate Jaffe method from venous samples taken after an overnight (12-hour) fast. Estimated GFRCr was calculated by the 4-variable Modification of Diet and Renal Disease Study.4 Urinary albumin and creatinine were measured by nephelometry and the rate Jaffe method, respectively. Urinary albumin-to-creatinine ratio (mg albumin/g creatinine) was calculated as described previously.5
Two cardiologists blinded to clinical data reviewed all electrocardiograms. AF was coded if the R-R interval was irregular and no P-waves could be identified in any lead.6 If the 2 reviewers agreed with AF classification, it was binding. If there was disagreement, the reviewers conferred, and when necessary a 3rd cardiologist reviewed the ECG to obtain consensus.
Age, sex, and race/ethnicity were determined by questionnaire. Medical history was determined by self-report. Study personnel measured weight and height and body mass index was calculated (kg/m2). Trained study personnel measured systolic and diastolic blood pressures at rest using a calibrated sphygmomanometer. High sensitivity C-reactive protein (CRP), total cholesterol, and high-density lipoprotein levels were measured from venous samples (12-hour fasting) as has been previously reported.7 A complete resting 2-dimensional echocardiogram, including all standard views, was used to determine left ventricular (LV) ejection fraction (EF) and LV mass. Left ventricular mass was calculated using the truncated ellipsoid method and EF was calculated using the biplane method of disks, as is recommended by the American Society of Echocardiography.8 An experienced cardiologist blinded to clinical data interpreted all echocardiograms.
Differences in baseline demographic and clinical characteristics between participants with and without AF were compared using t tests, Wilcoxon tests, chi square tests, and Fisher’s Exact test, as appropriate. Exploratory analysis evaluated the functional form of the association of each kidney function measure with AF. In each case, there appeared to be a threshold effect at approximately the highest third of the study cohort. Therefore, we elected to model our analyses using tertile groups as the primary predictor variable, treating the tertile with the “best” kidney function (highest eGFRcys or eGFRCr and lowest ACR) as the reference category.
Multivariable logistic regression was used to evaluate the association of each measure of kidney function with AF. All variables listed in Table 1 were considered candidate confounders. Variables were retained in the final model if their inclusion led to a change of > 5% in the β-coefficient describing the association of eGFRcys and AF. To facilitate comparisons, identical covariates were retained for models evaluating eGFRCr and ACR. For each continuous variable, the functional form of the association was evaluated using lowess plots, after transforming the dependent variable (AF) to logits. In these analyses, the form of association of age with AF was curvilinear. We created a quadratic term for age (i.e. age2). When this variable was included in multivariable models, both age and age2 approached statistical significance (P= 0.06 and 0.10, respectively). Thus, both terms were retained in multivariable models to improve model fit. The functional form of other continuous predictors closely approximated linear relationships with AF. Analyses were performed using Stata statistical software (9.2, College Station, TX).
Table 1. Baseline Characteristics in Participants With or Without Atrial Fibrillation.
| Atrial Fibrillation |
|||
|---|---|---|---|
| Variable | No (n=916 [96%]) |
Yes (n=40 [4%]) |
P-Value |
| Age (years) ± SD | 66 ± 10 | 73 ± 9 | < 0.001 |
| Men | 742 (81%) | 37 (93%) | 0.07 |
| Caucasian | 539 (59%) | 35 (88%) | |
| African-American | 155 (17%) | 0 | 0.01 |
| Other | 221 (24%) | 5 (13%) | |
| Hypertension | 649 (71%) | 23 (59%) | 0.10 |
| Myocardial Infarction | 493 (54%) | 19 (50%) | 0.61 |
| Coronary Revascularization | 536 (59%) | 23 (58%) | 0.90 |
| Heart Failure | 153 (17%) | 10 (26%) | 0.15 |
| Diabetes mellitus | 248 (27%) | 8 (21%) | 0.41 |
| Body mass index (kg/m2) ± SD | 28 ± 5 | 30 ± 8 | 0.22 |
| Ejection Fraction (%) ± SD | 62 ± 9 | 59 ±9 | 0.06 |
| Left Ventricular Mass Index (g/m2) ± SD | 97 ± 25 | 96 ± 25 | 0.78 |
| C-reactive protein (mg/dL)** | 2.1 [0.9, 4.9] | 2.8 [1.8, 5.3] | 0.11 |
| Systolic blood pressure (mmHg) ± SD | 133 ± 21 | 129 ± 21 | 0.10 |
| Diastolic blood pressure (mmHg) ± SD | 74 ± 11 | 74 ± 11 | 0.63 |
| Total cholesterol (mg/dL) | 179 ± 43 | 164 ± 42 | 0.03 |
| High-density lipoprotein (mg/dL) | 46 ± 14 | 43 ± 10 | 0.20 |
| eGFRcys** (ml/min/1.73m2) ± SD | 72 ± 23 | 57± 20 | < 0.01 |
| eGFRCr** (ml/min/1.73m2) ± SD | 77 ± 23 | 66 ± 19 | < 0.001 |
| Albumin to Creatinine Ratio (mg/g)*** | 10 [6, 19] | 16 [9, 46] | < 0.01 |
p value for trend
eGFRCys = estimated glomerular filtration rate based on serum cystatin C; eGFRCr = estimated glomerular filtration rate based on serum creatinine
Median [Interquartile Range]
RESULTS
The mean age of the 956 study participants was 67 years, 81% were male, and 60% were Caucasian. The mean eGFRcys was 71 ± 23 ml/min/1.73m2, the mean eGFRCr was 76 ± 23 ml/min/1.73m2, and the median ACR was 10 mg/g (interquartile range 6 to 19 mg/g). One hundred eighty-nine (20%) participants had stage 3 CKD, 11 (1%) had stage 4 CKD, and 8 (1%) participants had Stage 5 CKD, as defined by the National Kidney Foundation criterion. None of the participants was receiving maintenance dialysis. Forty subjects (4%) had prevalent AF. Compared to participants without AF, those with AF were older, had a lower EF, had lower total cholesterol levels, and were more frequently Caucasian and male (Table 1).
Subjects with more advanced kidney disease by each kidney function measure had a higher prevalence of AF (Figure 1). In each case, however, there appeared to be a threshold effect. Compared to subjects with the most preserved kidney function (higher eGFR or lower ACR), those in the middle tertile had a modestly higher prevalence of AF, and those with kidney disease in the most advanced tertile had substantially greater prevalence of AF. This association was minimally attenuated with adjustment for age, sex, and race, and remained significant in the fully adjusted model, such that participants with eGFRcys in the lowest tertile had greater than 3-fold odds of AF in the fully adjusted model (Table 2). When this model was further adjusted for ACR, the association of eGFRcys with AF was essentially unaltered.
Figure 1.
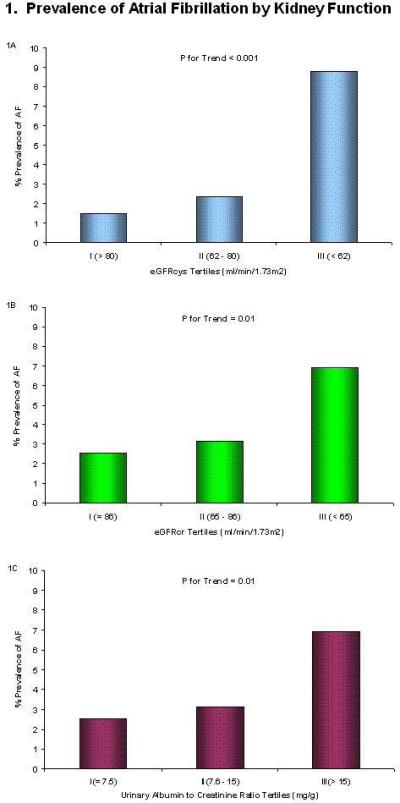
AF, atrial fibrillation; eGFRcys, estimated glomerular filtration rate based on serum cystatin C; eGFRcr, estimated glomerular filtration rate based on serum creatinine
Table 2. Association of Kidney Function with Atrial Fibrillation in Outpatients with Stable Coronary Heart Disease.
| Variable | Tertile I (Reference) |
Tertile II OR (95% CI) |
Tertile III OR (95% CI) |
P-Value for Trend |
|---|---|---|---|---|
| eGFRCys* | ||||
| Range (ml/min/1.73m2) | > 79 | 62–79 | < 62 | |
| Unadjusted | -- | 1.59 (0.50–5.07) | 6.43 (2.45–16.87) | <0.001 |
| Age, sex, race adjusted | -- | 1.11 (0.34–3.61) | 3.72 (1.34–10.34) | 0.01 |
| Fully adjusted** | -- | 1.00 (0.29–3.45) | 3.43 (1.18–9.97) | 0.02 |
| Fully adjusted + ACR | -- | 1.21 (0.32–4.56) | 3.37 (1.02–11.14) | 0.05 |
| eGFRCr* | ||||
| Range (ml/min/1.73m2) | > 85 | 66–85 | <66 | |
| Unadjusted | -- | 1.26 (0.49–3.23) | 2.89 (1.27–6.59) | 0.01 |
| Age, sex, race adjusted | -- | 0.83 (0.21–2.20) | 1.60 (0.67–3.84) | 0.28 |
| Fully adjusted** | -- | 0.85 (0.30–2.41) | 1.69 (0.67–4.27) | 0.27 |
| Fully adjusted + ACR | -- | 1.00 (0.33–3.01) | 1.59 (0.57–4.40) | 0.37 |
| Urinary ACR* | ||||
| Range (mg/g) | <7 | 7–15 | >15 | |
| Unadjusted | -- | 2.27 (0.78–6.63) | 3.88 (1.42–10.61) | 0.01 |
| Age, sex, race adjusted | -- | 2.14 (0.72–6.34) | 4.09 (1.46–11.5) | 0.01 |
| Fully adjusted** | -- | 2.43 (0.79–7.42) | 4.89 (1.67–14.32) | 0.004 |
| Fully adjusted + eGFRcys | -- | 2.40 (0.78–7.41) | 4.36 (1.45–13.05) | 0.01 |
| Fully adjusted + eGFRCr | -- | 2.55 (0.83–7.83) | 4.61 (1.56–13.66) | 0.01 |
eGFRCys = estimated glomerular filtration rate based on serum cystatin C; eGFRCr = estimated glomerular filtration rate based on serum creatinine; ACR = albumin to creatinine ratio
Adjusted for age, age2, sex, race (white/non-white), body mass index, systolic blood pressure, diastolic blood pressure, history of hypertension, and left ventricular ejection fraction.
Results were qualitatively similar for models evaluating eGFRCr, however these analyses did not reach statistical significance. In each case, subjects with eGFRCr in the lowest tertile had higher odds of AF, but the point estimates for these associations were weaker than for eGFRcys and the differences did not reach statistical significance (Table 2).
Participants with ACR in the highest tertile (ACR > 15 mg/g) had approximately 4-fold greater odds of AF compared to those in the lowest tertile in the unadjusted model as well as in the age, sex, and race adjusted, and fully adjusted models. Further adjustment for eGFR, whether by eGFRcys or eGFRCr, had little effect on this association (Table 2).
DISCUSSION
We found that lower estimated GFRcys and higher urinary ACR were strongly associated with prevalent AF in outpatients with stable CHD. Although results were qualitatively similar with eGFRCr, this association did not reach statistical significance. The associations of eGFR with AF were independent of ACR, and vice versa. Among patients with end-stage renal disease (ESRD), the prevalence of AF is 13–27%, or 10- to 20-fold higher than in the general population.9-12 Iguchi and colleagues recently reported an association of CKD with AF in a community-living Japanese cohort predominantly free of CHD; however, neither cystatin C nor ACR data were presented in this manuscript.13 Our investigation confirms the findings of this study, extends them to persons with prevalent CHD, and demonstrates a novel, independent association of ACR with AF. If confirmed, and if future longitudinal studies demonstrate that kidney dysfunction precedes onset of AF, albuminuria and moderate CKD may represent previously unrecognized risk factors for AF that are highly prevalent in the general population.
Several possible mechanisms may account for these associations. First, lower GFR or greater ACR might lead to AF. Subtle decrements in kidney function may activate the renin-angiotensin-aldosterone system (RAAS). Up-regulation of the RAAS may lead to AF by promoting salt and water retention and, in turn, atrial distension. RAAS activation is also associated, independent of its hemodynamic effects, with pathological atrial structural and electrophysiological remodeling that may create a pro-fibrillatory substrate.14 Either of these mechanisms in isolation, or in concert, may account for these associations. Second, we have previously demonstrated that kidney dysfunction is associated with lower heart rate recovery after exercise testing, and heart rate recovery is an established marker of cardiovascular autonomic function.15 Since autonomic signaling in the left atrium and pulmonary veins is thought to be a key factor in the initiation and maintenance of AF, kidney dysfunction may lead to AF through its association with altered autonomic tone.16 Third, our study was cross-sectional, and therefore it is possible that AF may have led to kidney dysfunction. We believe this possibility is less likely. Although AF may lead to decreased cardiac output and pre-renal azotemia, this mechanism is unlikely to have led to albuminuria, which requires injury to the glomerulus.
Finally kidney dysfunction may be a manifestation of cumulative exposure to cardiovascular risk factors over the life course and shared risk factors may have mediated the observed association between CKD and AF. Although we have adjusted for risk factors at one point in time, differences over the life course may not have been captured. Future studies with longitudinal measures of kidney function, atrial rhythm, markers of RAAS activation, and volume status are required to evaluate these potential mechanisms. Such studies should be a high priority. Mild to moderate kidney dysfunction and albuminuria are highly prevalent and can be readily measured in most clinical settings. If kidney dysfunction precedes onset of AF, measurement of kidney function may represent a relatively inexpensive and efficient way to identify individuals at higher risk of AF.
We observed that the association of eGFRcys with AF was stronger than that of eGFRCr. Recent studies have suggested that eGFRcys may be a more accurate measure of kidney function than creatinine-based measures among persons with normal to near-normal kidney function.9-12 Thus, the greater strength of association with eGFRcys might be the result of more accurate measurement of kidney function. Point estimates for the association of the lowest tertile of eGFRCr with AF were in the same direction, but did not reach statistical significance. Since our study included only 40 subjects with prevalent AF, it remains possible that we had insufficient power to detect an association between eGFRCr and AF, as has been reported elsewhere.13
This study has several limitations. First, although the associations between kidney dysfunction and AF were relatively strong and persisted with statistical adjustment for potential confounders, the low number of subjects with prevalent AF provided limited precision, and these findings should be confirmed elsewhere. Second, the study population consisted of predominantly older men, and all had CHD. Future studies are required to determine if results generalize to younger persons, women, and subjects without CHD. Last, AF was classified on the basis of ECG at one point in time, so it is possible that subjects with paroxysmal AF were missed. However, any missed paroxysmal AF would have biased our results toward the null hypothesis.
Acknowledgments
This study was supported by grants from the Department of Veterans Affairs, Washington, DC; the Robert Wood Johnson Foundation (Faculty Scholars Program), Princeton, New Jersey; the American Federation for Aging Research (Paul Beeson Scholars Program), New York, New York; the Ischemia Research and Education Foundation, South San Francisco, California; and the National Institutes of Health (R01 HL079235). J.H.I was supported by an American Heart Association Fellow to Faculty Transition Award (0575021N), Dallas, Texas. Dade Behring supported the cystatin C measurements in the Heart and Soul cohort.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290:215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest. 1999;59:1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 3.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levey AS. Measurement of renal function in chronic renal disease. Kidney Int. 1990;38:167–184. doi: 10.1038/ki.1990.182. [DOI] [PubMed] [Google Scholar]
- 5.Toto RD. Microalbuminuria: definition, detection, and clinical significance. J Clin Hypertens (Greenwich) 2004;6:2–7. doi: 10.1111/j.1524-6175.2004.4064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olgin J, Zipes D. Heart Disease: A Textbook of Cardiovascular Medicine. In: Braunwald E, Zipes D, Libby P, editors. Heart Disease: A Textbook of Cardiovascular Medicine. Elsevier and Sanders; Philadelphia: 2005. p. 2. [Google Scholar]
- 7.Ix JH, Shlipak MG, Brandenburg VM, Ali S, Ketteler M, Whooley MA. Association between human fetuin-A and the metabolic syndrome: data from the Heart and Soul Study. Circulation. 2006;113:1760–1767. doi: 10.1161/CIRCULATIONAHA.105.588723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, et al. American Society of Echocardiography Committee on Standards. Subcommittee on Quantitation of Two-Dimensional Echocardiograms Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 9.Collins AJ, Kasiske B, Herzog C, Chavers B. US Renal Data System: USRDS 2005 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. American Journal of Kidney Diseases. 2006;47:A4. [Google Scholar]
- 10.Bozbas H, Atar I, Yildirir A, Ozgul A, Uyar M, Ozdemir N, Muderrisoglu H, Ozin B. Prevalence and predictors of arrhythmia in end stage renal disease patients on hemodialysis. Ren Fail. 2007;29:331–339. doi: 10.1080/08860220701191237. [DOI] [PubMed] [Google Scholar]
- 11.Genovesi S, Pogliani D, Faini A, Valsecchi MG, Riva A, Stefani F, Acquistapace I, Stella A, Bonforte G, DeVecchi A, DeCristofaro V, Buccianti G, Vincenti A. Prevalence of atrial fibrillation and associated factors in a population of long-term hemodialysis patients. Am J Kidney Dis. 2005;46:897–902. doi: 10.1053/j.ajkd.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 12.Vazquez E, Sanchez-Perales C, Borrego F, Garcia-Cortes MJ, Lozano C, Guzman M, Gil JM, Borrego MJ, Perez V. Influence of atrial fibrillation on the morbido-mortality of patients on hemodialysis. Am Heart J. 2000;140:886–890. doi: 10.1067/mhj.2000.111111. [DOI] [PubMed] [Google Scholar]
- 13.Iguchi Y, Kimura K, Kobayashi K, Aoki J, Terasawa Y, Sakai K, Uemura J, Shibazaki K. Relation of atrial fibrillation to glomerular filtration rate. Am J Cardiol. 2008;102:1056–1059. doi: 10.1016/j.amjcard.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Ehrlich JR, Hohnloser SH, Nattel S. Role of angiotensin system and effects of its inhibition in atrial fibrillation: clinical and experimental evidence. Eur Heart J. 2006;27:512–518. doi: 10.1093/eurheartj/ehi668. [DOI] [PubMed] [Google Scholar]
- 15.McManus D, Shlipak M, Ix JH, Ali S, Whooley MA. Association of cystatin C with poor exercise capacity and heart rate recovery: data from the heart and soul study. Am J Kidney Dis. 2007;49:365–372. doi: 10.1053/j.ajkd.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharifov OF, Fedorov VV, Beloshapko GG, Glukhov AV, Yushmanova AV, Rosenshtraukh LV. Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation in dogs. J Am Coll Cardiol. 2004;43:483–490. doi: 10.1016/j.jacc.2003.09.030. [DOI] [PubMed] [Google Scholar]


