Abstract
Bioactive sphingolipids, such as ceramide, sphingosine, and sphingosine-1-phosphate are known bio-effector molecules which play important roles in various aspects of cancer biology including cell proliferation, growth arrest, apoptosis, metastasis, senescence, and inflammation. Therefore, enzymes involved in ceramide metabolism are gaining recognition as being critical regulators of cancer cell growth and/or survival. We previously observed that the ceramide metabolizing enzyme, acid ceramidase (AC), is up-regulated in tumor tissues. Studies have now concluded that this creates a dysfunctional ceramide pathway which is responsible for tumor progression and resistance to chemotherapy and radiation. This suggests that development of small molecule drugs that inhibit AC enzyme activity is a promising approach for improving standard cancer therapy and patient’s clinical outcomes.
Keywords: Prostate cancer, ceramide, acid ceramidase, tumor progression, therapeutic resistance, acid ceramidase inhibition, novel target
1. Introduction
Acid ceramidase (AC) has now been shown to play an important role in ceramide metabolism, in regulating various aspects of tumor pathogenesis, and in resistance to therapy. A previous review has reported on the structure, maturation, secretion, and enzymology of acid ceramidase [1]. Here we emphasize the biology of AC that is directly associated with cancer promotion and resistance to therapy from both oncologic and clinical perspectives. With updated work from our group and others, our current review highlights potential biological consequences of AC over-expression, specifically in prostate cancer, which demonstrates a new level of understanding of AC in prostate cancer progression and its potential clinic application as a therapeutic target.
1.1 Prostate Cancer
Prostate cancer is the most commonly diagnosed noncutaneous cancer in the United States and the second leading cause of cancer-related death in men [2]. Prostate cancer treatment options include surgery, hormone withdrawal, radiation, and/or chemotherapy depending on stage of the disease, patient’s age and other coexisting medical conditions [3, 4]. Advancements in prostate cancer diagnosis combined with current therapeutic options have improved the prognosis for prostate cancer patients [5, 6]. Nevertheless, advanced prostate cancer stages, specifically metastatic castration-resistant prostate cancers, remain unresponsive to all therapies and inevitably lead to patient death.
It is estimated that the lifetime risk for developing prostate cancer, recognizable by histological examination [7], is 42% for a 50-year-old American male. However, the lifetime risk for being diagnosed with clinical cancer is 9.5% and of dying from the disease 2.9% [8]. This large discrepancy between the presence of histopathologically diagnosable tumors and clinical manifestation of the malignancy indicates a missing link in the understanding of prostate cancer pathogenesis and provides impetus for research to develop a better understanding of the biology and development of prostate cancer.
1.2 Sphingolipids and Cancer
Sphingolipids comprise a large number of molecules that share a common sphingoid backbone (reviewed in [9]). Bioactive sphingolipids, such as ceramide, glucosylceramide, sphingosine, and sphingosine-1-phosphate (S1P), act as bio-effector molecules which play important roles in various aspects of cancer biology including cell proliferation, growth arrest, apoptosis, metastasis, senescence, and inflammation [9, 10]. Mounting data indicate aberrant sphingolipid signaling occurs in different types of cancer [11–15] indicating that failure of normal sphingolipid signaling is important in tumor progression. Additionally, sphingolipids influence the response of cancer cells to chemotherapy or radiation, making their biological roles clinically relevant [16–24].
1.3 Aberrant Ceramide Signaling and Cancer Development
Aberrant ceramide signaling has been established in multiple tumor models suggesting that ceramide is an important determinant of tumor development and progression [11–15]. Ceramide is also an important regulator of tumor cell death following exposure to stress stimuli including hypoxia, nutrient deprivation, chemotherapy, or immune attack (reviewed in [9, 25, 26]). This is supported by many studies which observed that defects in ceramide generation or rapid degradation of intracellular ceramide resulted in resistance to cell death signals [27–30]. Conversely, restoration of intracellular ceramide levels re-sensitized cancer cells to stress stimuli providing support for a crucial role of ceramide signaling in cell death [31, 32].
1.4 Ceramide Downstream Signaling
Ceramide induced cell death occurs through two main signaling pathways: Bcl-2-induced mitochondrial depolarization and stress-activated protein kinase (SAPK/p38MAPK). In the mitochondrial pathway, increased ceramide levels cause induction and activation of protein phosphatase 2A (PP2A) [33–35]. Following its activation, PP2A dephosphorylates the pro-apoptotic proteins Bax and Bak, and the anti-apoptotic protein Bcl-2. Importantly, PP2A-induced dephosphorylation of Bax leads to a conformational change and activation [36], while PP2A-induced dephosphorylation of Bcl-2 leads to its proteasomal degradation [37]. These changes induce mitochondrial depolarization resulting in caspase activation and induction of apoptosis [38]. Following radiation and chemotherapy, ceramide generation was also found to facilitate cell death through activation of the pro-apoptotic SAPK signaling while inhibiting the anti-apoptotic MAPK pathway [39] thus resulting in decreased tyrosine kinase receptor-mediated signaling (reviewed in [40]). It is important to note that the effect of ceramide on both mitochondrial and SAPK pathways is required for induction of apoptosis since defects in either pathway leads to resistance to radiation or short chain ceramide administration [39, 41].
Other important direct targets for ceramide have also been identified, including ceramide-activated protein kinase (CAPK) [42], protein kinase C [43], the lysosomal protease cathepsin D [44] and the autophagic signaling molecules beclin-1 and BNIP3 [45, 46].
1.5 Ceramide Metabolism
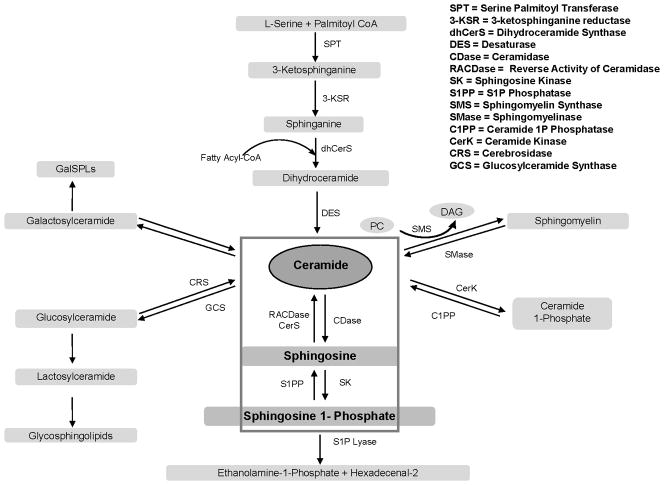
One method for cells to escape ceramide-induced apoptosis is to control stress-induced ceramide accumulation by metabolic routes (reviewed in [47, 48]). Ceramide levels are modulated by interaction of a large number of enzymes in multiple pathways and organelles. Ceramide can be deacylated by ceramidases to form sphingosine, phosphorylated by ceramide kinase yielding the mitogen ceramide-1-phosphate, or converted by sphingomyelin synthase to form sphingomyelin. It can also be glycosylated by glucosylceramide synthase to form glucosylceramide or by galactosylceramide synthase to form galactosylceramide (Figure 1).
Figure 1.
1.6 Acid Ceramidase, a Central Player in Ceramide Metabolism
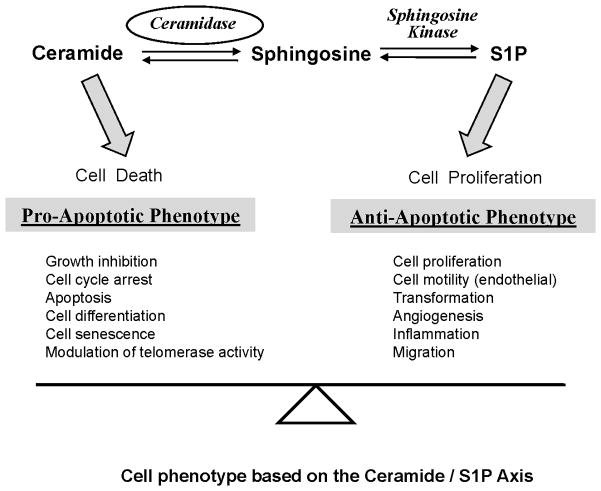
To date, five human ceramidases encoded by 5 distinct genes have been cloned. They are generally categorized by their pH optima as acid, neutral, and three isoforms of alkaline (1–3) species [49–52]. Human AC, whose deficiency is associated with Farber disease, is synthesized as a 53 kDa polypeptide that undergoes autocatalytic processing into 2 subunits (13 and 40 kDa, respectively) [53]. AC is predominantly localized in lysosomes and is considered a major enzyme used by a number of cancers to control ceramide levels (Figure 1). AC mediates ceramide hydrolysis generating sphingosine and a fatty acid [54]. Sphingosine can be phosphorylated by sphingosine kinase (SK) forming S1P. S1P creates a powerful anti-apoptotic phenotype by signaling through five different S1P receptors, several of which counteract ceramide induced pro-apoptotic signaling [55, 56]. Since ceramide metabolism through ceramidase is the major pathway for sphingosine generation [56, 57], AC has been suggested to play a key role in controlling the ceramide-sphingosine-S1P balance that regulates cellular homeostasis (Figure 2).
Figure 2.
2. Up-regulation of Acid Ceramidase in Prostate Tumor Tissues
Seelan et al. [58] first reported that AC is over-expressed in prostate cancer. Their data, obtained using RT-PCR, demonstrated AC over-expression in 40% of primary cancer tissue, with greater frequency in higher grade disease. Similar results were obtained in our lab using Western blot analysis where it was observed that over 60% of Gleason grades 5–6 (n=35) and 80% of Gleason grade 8–10 (n=9) cancers had elevated AC levels compared with patient matched normal tissue [12]. Aberrant AC expression in such a high percentage of primary prostate cancers suggests this ceramide metabolic pathway may contribute to progression of the disease.
The mechanism of AC up-regulation in prostate cancer is unknown. Several hypotheses have been advanced to explain this. One possibility is that the increase in AC is a feedback mechanism to keep levels of ceramide low following cellular exposure to systemic stress. This then selects for a sub-population of cancer cells that exhibit higher AC levels which exhibit a growth-survival advantage. Second, increased AC may be a result of transcriptional control. It is known that the murine AC enhancer-promoter has a KLF6 binding motif embedded within a 43-base pair region that regulates AC expression. KLF6 is a transcription factor identified as a potential candidate tumor suppressor gene in prostate and other cancers [59–65]. Interestingly, Narla et al. [66] demonstrated that a germ line single nucleotide polymorphism creates a splice site resulting in alternatively-spliced KLF6 isoforms, termed KLF6 SV1, SV2, SV3. Studies of these splice variants found that SV1 had a counteracting effect on the wild-type KLF6 tumor suppressor activity, thus permitting tumor progression. However a recent paper [67] would suggest that this mutation has minimal impact in at least in a large cohort of Finnish patients.
Regulation of AC transcription has also been linked to NF-Kβ, AP2, and SP1 [68] and the cAMP response element binding protein (CREB) was shown to regulate expression of AC in H295R human adrenocortical cells [69]. Thus AC is regulated by multiple transcriptional factors; however, it is not yet clear if transcription is the primary mechanism for AC up-regulation. Third, it is well known that AC maps to 8p22 [58], the region of chromosome 8 most frequently deleted in prostate cancer [70]. This has lead us to hypothesize that loss of heterozygosity may cause depletion of a chromosome region containing one or more micro-RNAs or other regulatory controls which lead to increased expression of AC by direct or indirect means. In this regard a related gene, N-acylethanolamine amidase, has been shown to have highly conserved 3′-UTR regulatory elements for miR-1301 and AC has two highly conserved 3′-UTR regulatory elements for miR27a. The relationship of these target elements and regulation of gene expression in AC remains to be defined. However the potential importance of miRNA in prostate cancer is becoming known [71] and would suggest AC up-regulation should be studied as a function of miRNA control of AC mRNA stability or translation.
3. Functional Consequences of AC Up-regulation in Prostate Cancer
3.1 Role of AC in Tumor Progression and Metastasis
The functional consequences of AC up-regulation on tumor cell biology have been studied by our group [17, 72] and others [73]. We demonstrated that higher AC enzymatic activity significantly altered the qualitative expression of ceramide species compared to controls without a significant change in total ceramide. Specifically, the study found lower concentrations of C14, C16, C18 and C20 ceramides (long chain ceramides) and elevated levels of C24, C24:1 ceramides(very long chain ceramides) in DU145 prostate cancer cells that over expressed AC [72]. Furthermore, the study also demonstrated that cells expressing higher AC levels exhibited increased proliferation under nutrient-depleted conditions in cell culture, and displayed augmented tumorigenicity in vivo. AC over expression also enhanced migration rates through collagen coated transwells and increased adhesion to fibronectin or collagen. Administration of AC inhibitors or AC siRNA reversed this aggressive behavior, directly linking AC to this phenotypic change. Although the exact mechanism of increased tumorigenicity and enhanced cell migration induced by AC over-expression still remains unclear, a likely possibility is that very long chain ceramide species (C22, C24, C24:1 and C26), which are elevated in high AC cells, are somehow involved in regulating this activity. This hypothesis is supported by studies that showed very long chain ceramides are linked with cell growth while long chain ceramides are associated with cell apoptosis [14, 74–76]. In addition, AC enzyme activity also promotes formation of S1P, a potent anti-apoptotic agent which may also be responsible for this observed phenotype change since SK inhibition reverses the migration phenotype as describe in Maceyka et al [77] and by our group (unpublished). Collectively, these findings provide additional insight into the multiple mechanisms by which AC over-expression in tumor cells results in improved tumor growth, survival, and resistance to therapy.
Studies in other tumor models reinforce the above observations. Shah et al. [78] found that AC was constitutively over-expressed in leukemic LGLs and that inhibition of AC induced apoptosis. The Kaufmann group [13] used microarrays to analyze the expression of 43 different mRNAs involved in sphingolipid metabolism from different subtypes of breast cancer. They concluded that AC was more often up-regulated in estrogen receptor positive samples. In order to identify candidates for development of new diagnostic tests for cancer detection, Musumarra et al. [79] performed a multivariate analysis of the National Cancer Institute gene expression database and found AC to be among the most important genes up-regulated in melanoma. Taken collectively, these data support the hypothesis that disruption of ceramide metabolism by aberrant AC expression plays a significant role in a diverse group of human cancers and with the development of AC inhibitors by our group [72, 80, 81] makes AC a therapeutic target of importance.
3.2 Role of AC in Androgen Depletion Therapy
Over the course of prostate cancer therapy for metastatic disease, there is a shift from androgen-dependent to androgen-independent cell growth. The mechanism for this transition remains unclear. However it is currently the leading problem in prostate cancer treatment ultimately leading to patient death. Data generated by Eto’s group [82] demonstrated a specific increase in C16 ceramide during androgen ablation in androgen-dependent LNCaP prostate cancer cells followed by G0/G1 arrest and apoptosis. More recently, they observed enhanced intracellular ceramide levels induced by serum deprivation when they co-treated cells with the AC inhibitor N-oleoylethanolamine (NOE) [83]. This was accompanied by an increase in apoptosis. Although there is no established link between androgen depletion and AC elevation, the above data demonstrate that androgen depletion induces ceramide production. However, if AC is elevated in cancer by androgen depletion therapy, this would negate ceramide signaling and prevent the cell from undergoing apoptosis. Thus AC may be an important contributor to development of hormone refractory disease.
3.3 Role of AC in Resistance to Radiation Therapy
Ceramide has been identified as both a necessary and sufficient mediator of radiation-induced cell death [31, 32]. Most studies indicate that defects in ceramide generation are related to increased resistance to radiation-induced apoptosis. Samsel et al. [32] first demonstrated that modulating ceramide generation by using the AC inhibitor, B13, sensitized tumors to radiation. In a recent study, our group [17] revealed that radiation therapy (RT) caused up-regulation of AC. This suggests a possible mechanism allowing cancer cells to establish radio-resistance. Although increased ceramide may be helpful in mediating apoptosis, AC up-regulation upon radiation exposure was shown to enhance rapid hydrolysis of ceramide which resulted in formation of sphingosine and S1P. The antagonistic effects of S1P on ceramide function result in minimizing the pro-apoptotic effect of ceramide with the paradoxical result of elevating the anti-apoptotic and angiogenic properties of S1P which leads to enhanced tumor survival.
3.4 Role of AC in Resistance to Chemotherapy
AC has been shown to protect L929 cells from TNF-induced apoptosis by modification of ceramide generation [84]. Published findings by our group [72] suggest that in prostate cancer with high levels of AC, deregulation of sphingolipid balance promoted chemo-resistance. This study demonstrated that resistance to cell death was increased when cells were subjected to a broad range of therapies including doxorubicin, etoposide, cisplatin, gemcitabine, or C6 ceramide. Importantly, resistance to these drugs was reversed if AC was down-regulated by siRNA. Cells treated with doxorubicin or etoposide exhibited a 5-fold increase in long chain ceramides in control cells compared to AC over-expressing cells where very long chain ceramides were elevated. Similar results were obtained by others [85] using daunorubicin treatment of hepatoma cells following AC silencing with siRNA or by pharmacological inhibition using N-oleoylethanolamine (NOE). This again suggests that AC enzymatic activity prevents accumulation of ceramides and favors sphingosine/S1P formation which uncouples the pro-apoptotic from the anti-apoptotic pathway. More importantly, from a clinic perspective, correlation of AC levels with patient response to chemotherapy might demonstrate that the level of AC is a predictor for decreased chemo-sensitivity. Therefore combining an AC inhibitor with chemotherapy during treatment might improve therapeutic outcomes in those patients with tumors that up-regulate AC.
3.5 Radiation-Induced AC Up-regulation Confers Resistance to Taxane
Interest in the efficacy of taxanes for treating prostate cancer was sparked by the TAX 327 [86] and SWOG 99-16 [87] studies which demonstrated that docetaxel increased survival of patients with advanced prostatic disease. As a result, taxane chemotherapy has been examined in combination with standard treatment modalities including ionizing radiation [88, 89]. Recently, Sanfilippo reported a Phase I/II study of biweekly paclitaxel and radiation in androgen-ablated locally advanced prostate cancer, with encouraging but modest results [89]. Another trial (RTOG-99-02) hypothesized that adding cytotoxic chemotherapy to long term androgen suppression plus an RT regimen improved survival rates. Unfortunately, this study was closed early because of concerns associated with toxicity related to the chemotherapy. It is uncertain why the outcome of these trials was not as positive as expected but one possibility is that radiation may evoke a resistant feature in treated cells as described above. Previous reports demonstrated an antagonism between RT and taxane-induced cell death [90–94], and suggested gamma-radiation was able to inhibit paclitaxel-induced IkB-alpha degradation and Bcl-2 phosphorylation by arresting target cells in G2 [90]. Our data point out another pathway that involves AC activation induced by RT as a means for developing resistance [17]. This was observed in irradiated prostate cancer cells that were subsequently treated with taxol resulting in decreased sensitivity to the drug. This study [17] confirmed, using both siRNA and AC inhibitors, that AC up-regulation accounted for the insensitivity of irradiated cells to taxol and provides a potential explanation for failure of radiation/chemotherapy combinations.
3.6 Regulation of Gene Expression by AC in Prostate Cancer Cells
In multiple primary cancer specimens, as discussed in this review, AC was shown to be up-regulated at both protein and mRNA levels. The known major function of AC is to hydrolyze ceramide. Ceramides are involved in regulating multiple signaling pathways associated with gene expression [9]. Since AC enzymatic activity regulates the level of ceramide, this suggests AC up-regulation might also exert effects on gene expression by altering ceramide metabolism. To examine this, we performed microarray analysis using the Affymetrix GeneChip U133-Plus-2.0-array on 12 prostate cancer cell lines, engineered to over-express AC, and 8 control cell lines. In our preliminary analysis, we find that AC over-expression regulates the expression of many important genes that generally clustered into the following functional categories: transcription factors, ion channels/transporters, apoptosis regulators, cell adhesion molecules, inflammatory response, cytokine-cytokine receptor interaction, cell proliferation, growth factor binding and tumor antigens. These findings provide further insight into understanding the multiple functions of AC and ceramide metabolism in prostate cancer.
4. AC Inhibition, a New Target for the Improvement of Prostate Cancer Therapy
Over the past few years, there has been a growing interest in exploring the role of ceramide and its metabolites in cancer pathology and therapy. Since levels of ceramide can be manipulated by various drugs and treatments, adjustment of sphingolipid metabolism to allow accumulation of ceramide is becoming a strategy used for arresting growth or promoting apoptosis. These strategies include using ceramide analogs [95, 96], increasing ceramide generation, and/or blocking ceramide metabolism [97, 98]. In this review we have implicated AC as being important in cancer biology because of its pivotal role in regulating inter-conversion of ceramide, sphingosine, and S1P. Specific inhibition of AC leads to enhanced pro-apoptotic signaling through ceramide accumulation, and prevents conversion of ceramide to sphingosine, which decreases formation of S1P and anti-apoptotic signaling. Compared with other inhibitors involved in sphingolipid metabolism, AC inhibitors have become more attractive because of AC’s central regulatory role.
Our group has developed a class of drugs that are AC enzyme inhibitors (LCL204, LCL385) that were rationally designed based on the structure of B13 [32] [17, 81]. LCL204 and LCL385 specifically target the lysosome where AC is localized [75, 76, 81, 96, 99]. Studies revealed that application of these AC inhibitors augment radiation therapy in PPC1 prostate xenograft model [17] and enhance Ad-FasL and Ad-Apoptin gene therapy of prostate cancer and head and neck squamous cell carcinoma models [30, 80]. These data suggest a clinical potential for these inhibitors, as agents that inhibit AC enzymatic activity and promote improved therapeutic outcomes by overcoming resistance to both chemo- and radiation therapy.
However, the LCL204 family, which specifically targets the lysosome, also causes induction of lysosomal destabilization, and proteolytic degradation of AC. This raises the concern of potential side effects on normal tissues [75, 81]. To circumvent this, a second group of drugs was designed based on the structures of the parent compound, B13 [75, 76]. These new compounds have the key structural elements from the parent compound necessary for effective molecular recognition and lysosomal targeting. However, they do not induce lysosome dysfunction. LCL464, the representative analog of this group [100], exhibits early inhibition of AC corresponding to a decrease of sphingosine and specific increases in C14 and C16 ceramide. Using MCF7 cells, this compound showed dose dependent cell growth inhibition with an increased IC50 value suggesting an improved in vivo safety profile which is now under investigation.
5. Conclusion and Expert Opinion
To better achieve improvements in cancer therapy, a better understanding is needed not only of the mechanisms involved in tumor progression but also of the biological mechanisms used by such therapies to attack the disease. It is of note but frequently not mentioned that common and most often used cancer therapies including radiation and many chemotherapeutic drugs, act finally, to kill cancer by elevating ceramide which then acts to propagate death signals. Cancer cells that are treated with these approaches typically develop resistance to ceramide elevation. Based on our studies one such resistance mechanism is through elevation of acid ceramidase. AC elevation thus becomes a negative prognostic factor for achieving therapeutic success by virtue of its ability to deacylate ceramide. While previous studies have focused on developing ways to intrinsically elevate ceramide levels to enhance cancer cell response to therapies (radiation and chemotherapy), AC over-expression in tumor tissues raises the concern that such tumors are less likely to be responsive to these approaches. As discussed in this review, unless AC enzyme activity is inhibited, elevating ceramide levels in tumor tissues can result in the paradoxical situation where its activity leads to increased production of the anti-apoptotic and angiogenic sphingolipid S1P, and enhanced tumor survival. Therefore, it is our considered opinion that targeting AC is a new promising translational avenue in cancer therapy which can be combined with current standards of care therapy to enhance patient outcomes. Future studies will translate this knowledge into the clinic.
Acknowledgments
We apologize to those investigators whose important work was not included due to space limitations. Our work was supported by NIH/NCI PO1 CA97132, Division of Laboratory Animal Research, NIH, C06 RR015455 and the MUSC Lipidomics Core, NIH, C06 RR018823.
Abbreviations
- AC
Acid ceramidase
- Gy
Gray
- RT
Radiation therapy
- S1P
Sphingosine-1-phosphate
- SK1
Sphingosine kinase-1
Contributor Information
Xiang Liu, Email: liux@musc.edu, Assistant Professor, Division of Basic Sciences, Departments of: Biochemistry & Molecular Biology, Cell and Molecular Pharmacology & Experimental Therapeutics, Microbiology & Immunology, MUSC, 173 Ashley Avenue, MSC 504, Charleston, South Carolina 29425-5040, FAX: 843.792.4882, Phone: 843.792.7412.
Joseph C. Cheng, Email: chengj@musc.edu, MD/PhD Student, Division of Basic Sciences, Departments of: Biochemistry & Molecular Biology, Cell and Molecular Pharmacology & Experimental Therapeutics, Microbiology & Immunology, MUSC, 173 Ashley Avenue, MSC 504, Charleston, South Carolina 29425-5040, FAX: 843.792.4882, Phone: 843.792.8499
Lorianne S. Turner, Email: turnerloj@musc.edu, Postdoctoral Fellow, Division of Basic Sciences, Departments of: Biochemistry & Molecular Biology, Cell and Molecular Pharmacology & Experimental Therapeutics, Microbiology & Immunology, MUSC, 173 Ashley Avenue, MSC 504, Charleston, South Carolina 29425-5040, FAX: 843.792.4882, Phone: 843.792.8499.
Saeed Elojeimy, Email: elojeim@gmail.com, Division of Basic Sciences, Departments of: Biochemistry & Molecular Biology, Cell and Molecular Pharmacology & Experimental Therapeutics, Microbiology & Immunology, MUSC, 173 Ashley Avenue, MSC 504, Charleston, South Carolina 29425-5040, FAX: 843.792.4882, Phone: 843.814.7010.
Thomas H. Beckham, Email: beckham@musc.edu, MD/PhD Student, Departments of: Biochemistry & Molecular Biology, Cell and Molecular Pharmacology & Experimental Therapeutics, Microbiology & Immunology, MUSC, 173 Ashley Avenue, MSC 504, Charleston, South Carolina 29425-5040, FAX: 843.792.4882, Phone: 843.792.8499
Alicja Bielawska, Email: bielawsk@musc.edu, Professor, Departments of: Biochemistry & Molecular Biology, Cell and Molecular Pharmacology & Experimental Therapeutics, Microbiology & Immunology, MUSC, 173 Ashley Avenue, MSC 504, Charleston, South Carolina 29425-5040, FAX: 843.792.1627, Phone: 843.792.0273.
Thomas E. Keane, Email: keanet@musc.edu, Professor and Chair, Department of Urology, MUSC, 96 Jonathan Lucas Street, Room 644, Clinical Science Building, Phone: 843.792.1666.
Yusuf A. Hannun, Email: hannun@musc.edu, Senior Associate Dean for Basic Sciences, Director, Division of Basic Sciences, Distinguished University Professor, Chair, Department of Biochemistry & Molecular Biology, Cell and Molecular Pharmacology & Experimental Therapeutics, Microbiology & Immunology, MUSC, 173 Ashley Avenue, MSC 509, Charleston, South Carolina 29425-5090, FAX: 843.792.4322, Phone: 843.792.9318.
James S. Norris, Email: norrisjs@musc.edu, Professor and Chair, Department of Microbiology & Immunology, MUSC, 173 Ashley Avenue, MSC 504, Charleston, South Carolina 29425-5040, FAX: 843.792.4882, Phone: 843.792.7915.
References
- 1.Zeidan YH, Jenkins RW, Korman JB, Liu X, Obeid LM, Norris JS, et al. Molecular targeting of acid ceramidase: implications to cancer therapy. Current drug targets. 2008 Aug;9(8):653–61. doi: 10.2174/138945008785132358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelucci A, Gravina GL, Rucci N, Millimaggi D, Festuccia C, Muzi P, et al. Suppression of EGF-R signaling reduces the incidence of prostate cancer metastasis in nude mice. Endocr Relat Cancer. 2006 March 1;13(1):197–210. doi: 10.1677/erc.1.01100. [DOI] [PubMed] [Google Scholar]
- 3.Canil CM, Tannock IF. Is there a role for chemotherapy in prostate cancer? British journal of cancer. 2004 Sep 13;91(6):1005–11. doi: 10.1038/sj.bjc.6601850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward JF, Slezak JM, Blute ML, Bergstralh EJ, Zincke H. Radical prostatectomy for clinically advanced (cT3) prostate cancer since the advent of prostate-specific antigen testing: 15-year outcome. BJU international. 2005 Apr;95(6):751–6. doi: 10.1111/j.1464-410X.2005.05394.x. [DOI] [PubMed] [Google Scholar]
- 5.Zelefsky MJ, Fuks Z, Hunt M, Lee HJ, Lombardi D, Ling CC, et al. High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer. J Urol. 2001 Sep;166(3):876–81. [PubMed] [Google Scholar]
- 6.Peschel RE, Colberg JW. Surgery, brachytherapy, and external-beam radiotherapy for early prostate cancer. Lancet Oncol. 2003 Apr;4(4):233–41. doi: 10.1016/s1470-2045(03)01035-0. [DOI] [PubMed] [Google Scholar]
- 7.Scardino PT. Early detection of prostate cancer. Urologic Clinics of North America. 1989;16(4):635–55. [PubMed] [Google Scholar]
- 8.Seidman H, Mushinski MH, Gelb SK, Silverberg E. Probabilities of eventually developing or dying of cancer--United States, 1985. Ca: a Cancer Journal for Clinicians. 1985;35(1):36–56. doi: 10.3322/canjclin.35.1.36. [DOI] [PubMed] [Google Scholar]
- 9.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nature Reviews of Cancer. 2004;4(8):604–16. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 10.Futerman AH, Hannun YA. The complex life of simple sphingolipids. EMBO reports. 2004 Aug;5(8):777–82. doi: 10.1038/sj.embor.7400208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim WJ, Okimoto RA, Purton LE, Goodwin M, Haserlat SM, Dayyani F, et al. Mutations in the neutral sphingomyelinase gene SMPD3 implicate the ceramide pathway in human leukemias. Blood. 2008 May 1;111(9):4716–22. doi: 10.1182/blood-2007-10-113068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norris JS, Bielawska A, Day T, El-Zawahri A, Elojeimy S, Hannun Y, et al. Combined therapeutic use of AdGFPFasL and small molecule inhibitors of ceramide metabolism in prostate and head and neck cancers: a status report. Cancer Gene Ther. 2006 Dec;13(12):1045–51. doi: 10.1038/sj.cgt.7700965. [DOI] [PubMed] [Google Scholar]
- 13.Ruckhaberle E, Rody A, Engels K, Gaetje R, von Minckwitz G, Schiffmann S, et al. Microarray analysis of altered sphingolipid metabolism reveals prognostic significance of sphingosine kinase 1 in breast cancer. Breast cancer research and treatment. 2008 Nov;112(1):41–52. doi: 10.1007/s10549-007-9836-9. [DOI] [PubMed] [Google Scholar]
- 14.Senkal CE, Ponnusamy S, Rossi MJ, Bialewski J, Sinha D, Jiang JC, et al. Role of human longevity assurance gene 1 and C18-ceramide in chemotherapy-induced cell death in human head and neck squamous cell carcinomas. Mol Cancer Ther. 2007 Feb;6(2):712–22. doi: 10.1158/1535-7163.MCT-06-0558. [DOI] [PubMed] [Google Scholar]
- 15.Tilly JL, Kolesnick RN. Sphingolipids, apoptosis, cancer treatments and the ovary: investigating a crime against female fertility. Biochimica et Biophysica Acta (BBA) -Molecular and Cell Biology of Lipids. 2002;1585(2–3):135–8. doi: 10.1016/s1388-1981(02)00333-5. [DOI] [PubMed] [Google Scholar]
- 16.Wang X-Z, Beebe JR, Pwiti L, Bielawska A, Smyth MJ. Aberrant Sphingolipid Signaling Is Involved in the Resistance of Prostate Cancer Cell Lines to Chemotherapy. Cancer Res. 1999 November 1;59(22):5842–8. [PubMed] [Google Scholar]
- 17.Mahdy AEM, Cheng JC, Li J, Elojeimy S, Meacham WD, Turner LS, et al. Acid Ceramidase Upregulation in Prostate Cancer Cells Confers Resistance to Radiation: AC Inhibition, a Potential Radiosensitizer. Mol Ther. 2008;17(3):430–8. doi: 10.1038/mt.2008.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pchejetski D, Doumerc N, Golzio M, Naymark M, Teissie J, Kohama T, et al. Chemosensitizing effects of sphingosine kinase-1 inhibition in prostate cancer cell and animal models. Mol Cancer Ther. 2008 July 1;7(7):1836–45. doi: 10.1158/1535-7163.MCT-07-2322. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y-Y, Han T-Y, Giuliano AE, Cabot MC. Expression of Glucosylceramide Synthase, Converting Ceramide to Glucosylceramide, Confers Adriamycin Resistance in Human Breast Cancer Cells. J Biol Chem. 1999 January 8;274(2):1140–6. doi: 10.1074/jbc.274.2.1140. [DOI] [PubMed] [Google Scholar]
- 20.Gouaze V, Yu JY, Bleicher RJ, Han TY, Liu YY, Wang H, et al. Overexpression of glucosylceramide synthase and P-glycoprotein in cancer cells selected for resistance to natural product chemotherapy. Mol Cancer Ther. 2004 May;3(5):633–9. [PubMed] [Google Scholar]
- 21.van Vlerken LE, Duan Z, Seiden MV, Amiji MM. Modulation of Intracellular Ceramide Using Polymeric Nanoparticles to Overcome Multidrug Resistance in Cancer. Cancer Res. 2007 May 15;67(10):4843–50. doi: 10.1158/0008-5472.CAN-06-1648. [DOI] [PubMed] [Google Scholar]
- 22.Veldman RJ, Klappe K, Hinrichs J, Hummel INA, Van Der Schaaf G, Sietsma H, et al. Altered sphingolipid metabolism in multidrug-resistant ovarian cancer cells is due to uncoupling of glycolipid biosynthesis in the Golgi apparatus. FASEB J. 2002 July 1;16(9):1111–3. doi: 10.1096/fj.01-0863fje. [DOI] [PubMed] [Google Scholar]
- 23.Pchejetski D, Golzio M, Bonhoure E, Calvet C, Doumerc N, Garcia V, et al. Sphingosine Kinase-1 as a Chemotherapy Sensor in Prostate Adenocarcinoma Cell and Mouse Models. Cancer Res. 2005 December 15;65(24):11667–75. doi: 10.1158/0008-5472.CAN-05-2702. [DOI] [PubMed] [Google Scholar]
- 24.Garzotto M, Haimovitz-Friedman A, Liao W-C, White-Jones M, Huryk R, Heston WDW, et al. Reversal of Radiation Resistance in LNCaP Cells by Targeting Apoptosis through Ceramide Synthase. Cancer Res. 1999 October 1;59(20):5194–201. [PubMed] [Google Scholar]
- 25.Spiegel S, Milstien S. Sphingosine-1-Phosphate: An enigmatic signalling lipid. Nature Reviews Molecular Cell Biology. 2003;4(5):397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 26.Spiegel S, Milstien S. Functions of the Multifaceted Family of Sphingosine Kinases and Some Close Relatives. J Biol Chem. 2007 January 26;282(4):2125–9. doi: 10.1074/jbc.R600028200. [DOI] [PubMed] [Google Scholar]
- 27.Bonnaud S, Niaudet C, Pottier G, Gaugler MH, Millour J, Barbet J, et al. Sphingosine-1-phosphate protects proliferating endothelial cells from ceramide-induced apoptosis but not from DNA damage-induced mitotic death. Cancer Res. 2007 Feb 15;67(4):1803–11. doi: 10.1158/0008-5472.CAN-06-2802. [DOI] [PubMed] [Google Scholar]
- 28.Chmura SJ, Nodzenski E, Kharbanda S, Pandey P, Quintans J, Kufe DW, et al. Down-regulation of ceramide production abrogates ionizing radiation-induced cytochrome c release and apoptosis. Mol Pharmacol. 2000 Apr;57(4):792–6. doi: 10.1124/mol.57.4.792. [DOI] [PubMed] [Google Scholar]
- 29.Santana P, Pena LA, Haimovitz-Friedman A, Martin S, Green D, McLoughlin M, et al. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell. 1996 Jul 26;86(2):189–99. doi: 10.1016/s0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- 30.Litvak DA, Bilchik AJ, Cabot MC. Modulators of ceramide metabolism sensitize colorectal cancer cells to chemotherapy: a novel treatment strategy. J Gastrointest Surg. 2003 Jan 7;1:140–8. doi: 10.1016/S1091-255X(02)00126-9. discussion 8. [DOI] [PubMed] [Google Scholar]
- 31.Alphonse G, Bionda C, Aloy MT, Ardail D, Rousson R, Rodriguez-Lafrasse C. Overcoming resistance to gamma-rays in squamous carcinoma cells by poly-drug elevation of ceramide levels. Oncogene. 2004 Apr 8;23(15):2703–15. doi: 10.1038/sj.onc.1207357. [DOI] [PubMed] [Google Scholar]
- 32.Samsel L, Zaidel G, Drumgoole HM, Jelovac D, Drachenberg C, Rhee JG, et al. The ceramide analog, B13, induces apoptosis in prostate cancer cell lines and inhibits tumor growth in prostate cancer xenografts. Prostate. 2004;58(4):382–93. doi: 10.1002/pros.10350. [DOI] [PubMed] [Google Scholar]
- 33.Chalfant CE, Rathman K, Pinkerman RL, Wood RE, Obeid LM, Ogretmen B, et al. De novo ceramide regulates the alternative splicing of caspase 9 and Bcl-x in A549 lung adenocarcinoma cells. Dependence on protein phosphatase-1. J Biol Chem. 2002 Apr 12;277(15):12587–95. doi: 10.1074/jbc.M112010200. [DOI] [PubMed] [Google Scholar]
- 34.Dobrowsky RT, Hannun YA. Ceramide stimulates a cytosolic protein phosphatase. J Biol Chem. 1992 Mar 15;267(8):5048–51. [PubMed] [Google Scholar]
- 35.Chalfant CE, Kishikawa K, Mumby MC, Kamibayashi C, Bielawska A, Hannun YA. Long chain ceramides activate protein phosphatase-1 and protein phosphatase-2A. Activation is stereospecific and regulated by phosphatidic acid. Journal of Biological Chemistry. 1999;274(29):20313–7. doi: 10.1074/jbc.274.29.20313. [DOI] [PubMed] [Google Scholar]
- 36.Xin M, Deng X. Protein phosphatase 2A enhances the proapoptotic function of Bax through dephosphorylation. J Biol Chem. 2006 Jul 7;281(27):18859–67. doi: 10.1074/jbc.M512543200. [DOI] [PubMed] [Google Scholar]
- 37.Lin SS, Bassik MC, Suh H, Nishino M, Arroyo JD, Hahn WC, et al. PP2A regulates BCL-2 phosphorylation and proteasome-mediated degradation at the endoplasmic reticulum. J Biol Chem. 2006 Aug 11;281(32):23003–12. doi: 10.1074/jbc.M602648200. [DOI] [PubMed] [Google Scholar]
- 38.Pastorino JG, Tafani M, Rothman RJ, Marcinkeviciute A, Hoek JB, Farber JL. Functional consequences of the sustained or transient activation by Bax of the mitochondrial permeability transition pore. The Journal of biological chemistry. 1999 Oct 29;274(44):31734–9. doi: 10.1074/jbc.274.44.31734. [DOI] [PubMed] [Google Scholar]
- 39.Verheij M, van Blitterswijk WJ, Bartelink H. Radiation-induced apoptosis--the ceramide-SAPK signaling pathway and clinical aspects. Acta Oncol. 1998;37(6):575–81. doi: 10.1080/028418698430287. [DOI] [PubMed] [Google Scholar]
- 40.Gulbins E. Regulation of death receptor signaling and apoptosis by ceramide. Pharmacological Research. 2003;47(5):393–9. doi: 10.1016/s1043-6618(03)00052-5. [DOI] [PubMed] [Google Scholar]
- 41.Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, et al. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996 Mar 7;380(6569):75–9. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 42.Yao B, Zhang Y, Delikat S, Mathias S, Basu S, Kolesnick R. Phosphorylation of Raf by ceramide-activated protein kinase. Nature. 1995 Nov 16;378(6554):307–10. doi: 10.1038/378307a0. [DOI] [PubMed] [Google Scholar]
- 43.Kashiwagi K, Shirai Y, Kuriyama M, Sakai N, Saito N. Importance of C1B domain for lipid messenger-induced targeting of protein kinase C. J Biol Chem. 2002 May 17;277(20):18037–45. doi: 10.1074/jbc.M111761200. [DOI] [PubMed] [Google Scholar]
- 44.Deiss LP, Galinka H, Berissi H, Cohen O, Kimchi A. Cathepsin D protease mediates programmed cell death induced by interferon-gamma, Fas/APO-1 and TNF-alpha. EMBO Journal. 1996;15(15):3861–70. [PMC free article] [PubMed] [Google Scholar]
- 45.Pattingre S, Bauvy C, Carpentier S, Levade T, Levine B, Codogno P. Role of JNK1-dependent Bcl-2 Phosphorylation in Ceramide-induced Macroautophagy. J Biol Chem. 2009 January 30;284(5):2719–28. doi: 10.1074/jbc.M805920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daido S, Kanzawa T, Yamamoto A, Takeuchi H, Kondo Y, Kondo S. Pivotal Role of the Cell Death Factor BNIP3 in Ceramide-Induced Autophagic Cell Death in Malignant Glioma Cells. Cancer Res. 2004 June 15;64(12):4286–93. doi: 10.1158/0008-5472.CAN-03-3084. [DOI] [PubMed] [Google Scholar]
- 47.Reynolds CP, Maurer BJ, Kolesnick RN. Ceramide synthesis and metabolism as a target for cancer therapy. Cancer Lett. 2004 Apr 8;206(2):169–80. doi: 10.1016/j.canlet.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 48.Hannun YA, Luberto C, Argraves KM. Enzymes of sphingolipid metabolism: from modular to integrative signaling. Biochemistry. 2001 Apr 24;40(16):4893–903. doi: 10.1021/bi002836k. [DOI] [PubMed] [Google Scholar]
- 49.Koch J, Gartner S, Li CM, Quintern LE, Bernardo K, Levran O, et al. Molecular cloning and characterization of a full-length complementary DNA encoding human acid ceramidase. Identification Of the first molecular lesion causing Farber disease. Journal of Biological Chemistry. 1996;271(51):33110–5. doi: 10.1074/jbc.271.51.33110. [DOI] [PubMed] [Google Scholar]
- 50.Mao C, Xu R, Szulc ZM, Bielawska A, Galadari SH, Obeid LM. Cloning and characterization of a novel human alkaline ceramidase. A mammalian enzyme that hydrolyzes phytoceramide. Journal of Biological Chemistry. 2001;276(28):26577–88. doi: 10.1074/jbc.M102818200. [DOI] [PubMed] [Google Scholar]
- 51.El Bawab S, Cungui M, Obeid LM, Hannun YA. Ceramidases in the Regulation of Ceramide Levels and Function. In: Quinn, Kagan C, editors. Subcellular Biochemistry:Phospholipid Metabolism in Apoptosis. New York: Kluwer Academic/Plenum Publishers; 2002. pp. 187–205. [DOI] [PubMed] [Google Scholar]
- 52.Mao C, Obeid LM. Ceramidases: regulators of cellular responses mediated by ceramide, sphingosine, and sphingosine-1-phosphate. Biochimica et Biophysica Acta (BBA) -Molecular and Cell Biology of Lipids. 2008;1781(9):424–34. doi: 10.1016/j.bbalip.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li CM, Park JH, He XX, Levy B, Chen F, Arai K, et al. The human acid ceramidase gene (ASAH): Structure, chromosomal location, mutation analysis, and expression. Genomics. 1999 Dec 1;62(2):223–31. doi: 10.1006/geno.1999.5940. [DOI] [PubMed] [Google Scholar]
- 54.Park J-H, Schuchman EH. Acid ceramidase and human disease. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2006;1758(12):2133–8. doi: 10.1016/j.bbamem.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 55.Hannun YA, Bell RM. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science. 1989;243(4890):500–7. doi: 10.1126/science.2643164. [comment] [DOI] [PubMed] [Google Scholar]
- 56.Spiegel S, Cuvillier O, Edsall LC, Kohama T, Menzeleev R, Olah Z, et al. Sphingosine-1-phosphate in cell growth and cell death. Annals of the New York Academy of Sciences. 1998;845:11–8. doi: 10.1111/j.1749-6632.1998.tb09658.x. [DOI] [PubMed] [Google Scholar]
- 57.Spiegel S, Merrill AH., Jr Sphingolipid metabolism and cell growth regulation. FASEB Journal. 1996;10(12):1388–97. doi: 10.1096/fasebj.10.12.8903509. [DOI] [PubMed] [Google Scholar]
- 58.Seelan RS, Qian C, Yokomizo A, Bostwick DG, Smith DI, Liu W. Human acid ceramidase is overexpressed but not mutated in prostate cancer. Genes, Chromosomes & Cancer. 2000 Oct;29(2):137–46. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1018>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 59.Narla G, Heath KE, Reeves HL, Li D, Giono LE, Kimmelman AC, et al. KLF6, a Candidate Tumor Suppressor Gene Mutated in Prostate Cancer. Science. 2001 December 21;294(5551):2563–6. doi: 10.1126/science.1066326. [DOI] [PubMed] [Google Scholar]
- 60.Chen C, Hyytinen E-R, Sun X, Helin HJ, Koivisto PA, Frierson HF, Jr, et al. Deletion, Mutation, and Loss of Expression of KLF6 in Human Prostate Cancer. Am J Pathol. 2003 April 1;162(4):1349–54. doi: 10.1016/S0002-9440(10)63930-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ito G, Uchiyama M, Kondo M, Mori S, Usami N, Maeda O, et al. Kruppel-Like Factor 6 Is Frequently Down-Regulated and Induces Apoptosis in Non-Small Cell Lung Cancer Cells. Cancer Res. 2004 June 1;64(11):3838–43. doi: 10.1158/0008-5472.CAN-04-0185. [DOI] [PubMed] [Google Scholar]
- 62.Kremer-Tal S, Reeves HL, Narla G, Thung SN, Schwartz M, Difeo A, et al. Frequent inactivation of the tumor suppressor Kruppel-like factor 6 (KLF6) in hepatocellular carcinoma. Hepatology. 2004 Nov;40(5):1047–52. doi: 10.1002/hep.20460. [see comment] [DOI] [PubMed] [Google Scholar]
- 63.Reeves HL, Narla G, Ogunbiyi O, Haq AI, Katz A, Benzeno S, et al. Kruppel-Like Factor 6 (KLF6) is a tumor-suppressor gene frequently inactivated in colorectal cancer. Gasteroenterology. 2004;126:1090–103. doi: 10.1053/j.gastro.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 64.Wang S, Chen S, Zhang W, Qiu F. kLF6mRNA expression in primary hepatocellular carcinoma. J Huazhong Univ Sci Technol Med Sci. 2004;24:585–7. doi: 10.1007/BF02911362. [DOI] [PubMed] [Google Scholar]
- 65.Yung-Ming Jeng H-CH. KLF6, a putative tumor suppressor gene, is mutated in astrocytic gliomas. International Journal of Cancer. 2003;105(5):625–9. doi: 10.1002/ijc.11123. [DOI] [PubMed] [Google Scholar]
- 66.Narla G, DiFeo A, Yao S, Banno A, Hod E, Reeves HL, et al. Targeted Inhibition of the KLF6 Splice Variant, KLF6 SV1, Suppresses Prostate Cancer Cell Growth and Spread. Cancer Res. 2005 July 1;65(13):5761–8. doi: 10.1158/0008-5472.CAN-05-0217. [DOI] [PubMed] [Google Scholar]
- 67.Seppala EH, Autio V, Duggal P, Ikonen T, Stenman UH, Auvinen A, et al. KLF6 IVS1 -27G>A variant and the risk of prostate cancer in Finland. European urology. 2007 Oct;52(4):1076–81. doi: 10.1016/j.eururo.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 68.Park J-H, Eliyahu E, Narla G, DiFeo A, Martignetti JA, Schuchman EH. KLF6 is one transcription factor involved in regulating acid ceramidase gene expression. Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 2005;1732(1–3):82–7. doi: 10.1016/j.bbaexp.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 69.Lucki N, Sewer MB. The cAMP responsive element binding protein (CREB) regulates the expression of acid ceramidase (ASAH1) in H295R human adrenocortical cells. Biochim Biophys Acta. 2009 Mar 16; doi: 10.1016/j.bbalip.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dong JT. Chromosomal deletions and tumor suppressor genes in prostate cancer. Cancer & Metastasis Reviews. 2001;20(3–4):173–93. doi: 10.1023/a:1015575125780. [DOI] [PubMed] [Google Scholar]
- 71.Gandellini P, Folini M, Zaffaroni N. Towards the definition of prostate cancer-related microRNAs: where are we now? Trends in Molecular Medicine. 2009;15(9):381–90. doi: 10.1016/j.molmed.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 72.Saad AF, Meacham WD, Bai A, Anelli V, Mahdy EM, Turner LS, et al. The Functional Effects of Acid Ceramidase Over-Expression in Prostate Cancer Progression and Resistance to Chemotherapy. Cancer Biology & Therapy. 2007;6(9):1455–60. doi: 10.4161/cbt.6.9.4623. [DOI] [PubMed] [Google Scholar]
- 73.Bedia C, Canals D, Matabosch X, Harrak Y, Casas J, Llebaria A, et al. Cytotoxicity and acid ceramidase inhibitory activity of 2-substituted aminoethanol amides. Chemistry and physics of lipids. 2008 Nov;156(1–2):33–40. doi: 10.1016/j.chemphyslip.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 74.Karahatay S, Thomas K, Koybasi S, Senkal CE, Elojeimy S, Liu X, et al. Clinical relevance of ceramide metabolism in the pathogenesis of human head and neck squamous cell carcinoma (HNSCC): attenuation of C(18)-ceramide in HNSCC tumors correlates with lymphovascular invasion and nodal metastasis. Cancer Lett. 2007 Oct 18;256(1):101–11. doi: 10.1016/j.canlet.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bielawska A, Bielawski J, Szulc ZM, Mayroo N, Liu X, Bai A, et al. Novel analogs of D-e-MAPP and B13. Part 2: Signature effects on bioactive sphingolipids. Bioorganic & Medicinal Chemistry. 2008;16(2):1032–45. doi: 10.1016/j.bmc.2007.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Szulc ZM, Mayroo N, Bai A, Bielawski J, Liu X, Norris JS, et al. Novel analogs of De-MAPP and B13. Part 1: Synthesis and evaluation as potential anticancer agents. Bioorganic & Medicinal Chemistry. 2008;16(2):1015–31. doi: 10.1016/j.bmc.2007.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maceyka M, Alvarez SE, Milstien S, Spiegel S. Filamin A links sphingosine kinase 1 and sphingosine-1-phosphate receptor 1 at lamellipodia to orchestrate cell migration. Molecular and cellular biology. 2008 Sep;28(18):5687–97. doi: 10.1128/MCB.00465-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shah MV, Zhang R, Irby R, Kothapalli R, Liu X, Arrington T, et al. Molecular profiling of LGL leukemia reveals role of sphingolipid signaling in survival of cytotoxic lymphocytes. Blood. 2008 Aug 1;112(3):770–81. doi: 10.1182/blood-2007-11-121871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Musumarra G, Barresi V, Condorelli DF, ScirÃ̈ S. A Bioinformatic Approach to the Identification of Candidate Genes for the Development of New Cancer Diagnostics. Biological Chemistry. 2003;384(2):321–7. doi: 10.1515/BC.2003.037. [DOI] [PubMed] [Google Scholar]
- 80.Elojeimy S, Liu X, McKillop JC, El-Zawahry AM, Holman DH, Cheng JY, et al. Role of Acid Ceramidase in Resistance to FasL: Therapeutic Approaches Based on Acid Ceramidase Inhibitors and FasL Gene Therapy. Mol Ther. 2007;15(7):1259–63. doi: 10.1038/sj.mt.6300167. [DOI] [PubMed] [Google Scholar]
- 81.Holman D, Turner L, El-Zawahry A, Elojeimy S, Liu X, Bielawski J, et al. Lysosomotropic acid ceramidase inhibitor induces apoptosis in prostate cancer cells. Cancer Chemotherapy and Pharmacology. 2008;61(2):231–42. doi: 10.1007/s00280-007-0465-0. [DOI] [PubMed] [Google Scholar]
- 82.Eto M, Bennouna J, Hunter OC, Hershberger PA, Kanto T, Johnson CS, et al. C16 ceramide accumulates following androgen ablation in LNCaP prostate cancer cells. Prostate. 2003 Sep 15;57(1):66–79. doi: 10.1002/pros.10275. [DOI] [PubMed] [Google Scholar]
- 83.Eto M, Bennouna J, Hunter OC, Lotze MT, Amoscato AA. Importance of C16 ceramide accumulation during apoptosis in prostate cancer cells. International Journal of Urology. 2006;13(2):148–56. doi: 10.1111/j.1442-2042.2006.01249.x. [DOI] [PubMed] [Google Scholar]
- 84.Strelow A, Bernardo K, Adam-Klages S, Linke T, Sandhoff K, Kronke M, et al. Overexpression of acid ceramidase protects from tumor necrosis factor-induced cell death. Journal of Experimental Medicine. 2000;192(5):601–12. doi: 10.1084/jem.192.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morales A, Paris R, Villanueva A, Llacuna L, Garcia-Ruiz C, Fernandez-Checa JC. Pharmacological inhibition or small interfering RNA targeting acid ceramidase sensitizes hepatoma cells to chemotherapy and reduces tumor growth in vivo. Oncogene. 2006;26(6):905–16. doi: 10.1038/sj.onc.1209834. [DOI] [PubMed] [Google Scholar]
- 86.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004 Oct 7;351(15):1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 87.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004 Oct 7;351(15):1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 88.Kumar P, Perrotti M, Weiss R, Todd M, Goodin S, Cummings K, et al. Phase I trial of weekly docetaxel with concurrent three-dimensional conformal radiation therapy in the treatment of unfavorable localized adenocarcinoma of the prostate. J Clin Oncol. 2004 May 15;22(10):1909–15. doi: 10.1200/JCO.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 89.Sanfilippo NJ, Taneja SS, Chachoua A, Lepor H, Formenti SC. Phase I/II study of biweekly paclitaxel and radiation in androgen-ablated locally advanced prostate cancer. J Clin Oncol. 2008 Jun 20;26(18):2973–8. doi: 10.1200/JCO.2007.14.4105. [DOI] [PubMed] [Google Scholar]
- 90.Sui M, Dziadyk JM, Zhu X, Fan W. Cell cycle-dependent antagonistic interactions between paclitaxel and gamma-radiation in combination therapy. Clin Cancer Res. 2004 Jul 15;10(14):4848–57. doi: 10.1158/1078-0432.CCR-03-0707. [DOI] [PubMed] [Google Scholar]
- 91.Liebmann J, Cook JA, Fisher J, Teague D, Mitchell JB. Changes in radiation survival curve parameters in human tumor and rodent cells exposed to paclitaxel (Taxol) Int J Radiat Oncol Biol Phys. 1994 Jun 15;29(3):559–64. doi: 10.1016/0360-3016(94)90456-1. [DOI] [PubMed] [Google Scholar]
- 92.Ingram ML, Redpath JL. Subadditive interaction of radiation and Taxol in vitro. Int J Radiat Oncol Biol Phys. 1997 Mar 15;37(5):1139–44. doi: 10.1016/s0360-3016(96)00629-3. [DOI] [PubMed] [Google Scholar]
- 93.Stromberg JS, Lee YJ, Armour EP, Martinez AA, Corry PM. Lack of radiosensitization after paclitaxel treatment of three human carcinoma cell lines. Cancer. 1995 May 1;75(9):2262–8. doi: 10.1002/1097-0142(19950501)75:9<2262::aid-cncr2820750912>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 94.Geard CR, Jones JM. Radiation and taxol effects on synchronized human cervical carcinoma cells. Int J Radiat Oncol Biol Phys. 1994 Jun 15;29(3):565–9. doi: 10.1016/0360-3016(94)90457-x. [DOI] [PubMed] [Google Scholar]
- 95.Mehta S, Blackinton D, Omar I, Kouttab N, Myrick D, Klostergaard J, et al. Combined cytotoxic action of paclitaxel and ceramide against the human Tu138 head and neck squamous carcinoma cell line. Cancer Chemother Pharmacol. 2000;46(2):85–92. doi: 10.1007/s002800000140. [DOI] [PubMed] [Google Scholar]
- 96.Selzner M, Bielawska A, Morse MA, Rudiger HA, Sindram D, Hannun YA, et al. Induction of apoptotic cell death and prevention of tumor growth by ceramide analogues in metastatic human colon cancer. Cancer Res. 2001 Feb 1;61(3):1233–40. [PubMed] [Google Scholar]
- 97.Saddoughi SA, Song P, Ogretmen B. Roles of bioactive sphingolipids in cancer biology and therapeutics. Sub-cellular biochemistry. 2008;49:413–40. doi: 10.1007/978-1-4020-8831-5_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Senchenkov A, Litvak DA, Cabot MC. Targeting Ceramide Metabolism--a Strategy for Overcoming Drug Resistance. J Natl Cancer Inst. 2001 March 7;93(5):347–57. doi: 10.1093/jnci/93.5.347. [DOI] [PubMed] [Google Scholar]
- 99.Raisova M, Goltz G, Bektas M, Bielawska A, Riebeling C, Hossini AM, et al. Bcl-2 overexpression prevents apoptosis induced by ceramidase inhibitors in malignant melanoma and HaCaT keratinocytes. FEBS Letters. 2002;516(1–3):47–52. doi: 10.1016/s0014-5793(02)02472-9. [DOI] [PubMed] [Google Scholar]
- 100.Bai A, Szulc ZM, Bielawski J, Mayroo N, Liu X, Norris J, et al. Synthesis and bioevaluation of [omega]-N-amino analogs of B13. Bioorganic & Medicinal Chemistry. 2009;17(5):1840–8. doi: 10.1016/j.bmc.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]