Abstract
Purpose
To determine if cycle training of sedentary subjects would increase the expression of the principle muscle glucose transporters, six volunteers completed six weeks of progressively increasing intensity stationary cycle cycling.
Methods
In vastus lateralis muscle biopsies, changes in expression of GLUT1, GLUT4, GLUT5, and GLUT12 were compared using quantitative immunoblots with specific protein standards. Regulatory pathway components were evaluated by immunoblots of muscle homogenates and immunohistochemistry of microscopic sections.
Results
GLUT1 was unchanged, GLUT4 increased 66%, GLUT12 increased 104%, and GLUT5 decreased 72%. A mitochondrial marker (cytochrome c) and regulators of mitochondrial biogenesis (PGC-1α and phospho-AMPK) were unchanged, but the muscle hypertrophy pathway component, phospho-mTOR increased 83% after the exercise program. In baseline biopsies, GLUT4 by immunohistochemical techniques was 37% greater in Type I (slow twitch, red) muscle fibers, but the exercise training increased GLUT4 expression in Type II (fast twitch, white) fibers by 50%, achieving parity with the Type I fibers. Baseline phospho-mTOR expression was 50% higher in Type II fibers and increased more in Type II fibers (62%) with training, but also increased in Type I fibers (34%).
Conclusion
Progressive intensity stationary cycle training of previously sedentary subjects increased muscle insulin-responsive glucose transporters (GLUT4 and GLUT12) and decreased the fructose transporter (GLUT5). The increase in GLUT4 occurred primarily in Type II muscle fibers and this coincided with activation of the mTOR muscle hypertrophy pathway. There was little impact on Type I fiber GLUT4 expression and no evidence of change in mitochondrial biogenesis.
Keywords: fiber type, glucose transporters, GLUT12, GLUT5, AMPK, PGC-1α
Introduction
Exercise is one of the cornerstones of prevention and treatment of diabetes. Exercise training has been shown to improve insulin sensitivity and to improve blood sugar control in patients with type 2 diabetes (26). Both aerobic training and strength training are beneficial (12), even though their effects are apparently brought about via separate pathways in muscle (3).
The muscle response to exercise training can be dramatically different depending on whether the program is for endurance or for strength (3). Not only is the muscle histological response different but the intracellular signaling pathways are discrete (27). Endurance training causes a substantial increase in muscle mitochondrial content, predominately in Type I muscle fibers (slow twitch, red fibers). This adaptation causes increased oxidative capacity and enhanced efficiency of energy generation from fuel (fatty acids and glucose) (3). In contrast, strength training can result in substantial skeletal muscle fiber hypertrophy. The increased cross-sectional area involves primarily Type II fibers (fast twitch, white) and leads to increased maximum strength (5). Strength training normally affects both muscle fiber types but Type II fibers tend to hypertrophy at a faster rate (15). Muscle fiber hypertrophy is energy intensive and involves the net increase in muscle contractile apparatus via increased protein synthesis (4).
Classic studies of one leg endurance training conclusively demonstrated that 5′-AMP-activated protein kinase (AMPK) is increased in amount and activation by a single bout of aerobic exercise and by endurance training (13). Aerobic exercise of long duration results in a rise in the AMP:ATP ratio which allows an upstream kinase, LKB1 to activate AMPK. AMPK and calmodulin-activated protein kinase (CamK) phosphorylate histone deacetylases (HDAC), allowing myocyte-enhancing factor 2 (MEF2) to bind to the promoter of peroxisome proliferator activating receptor γ coactivator 1 α (PGC-1α). PGC-1α coregulates the expression of respiratory genes, mitochondrial transcription factor A (MTF-A), fatty acid oxidation enzymes, GLUT4, and slow myosin heavy chain (33).
Resistance training activates an entirely different signaling pathway. Net increase in muscle protein synthesis appears to involve a muscle protein kinase called mammalian target of rapamycin (mTOR). There are a complex series of upstream and downstream steps that involve PDK1-mediated activation of Akt which eventually results in phosphorylation of mTOR that is part of a protein complex (TORC1) which in turn phosphorylates 70 kDa S6 protein kinase (p70S6K) and decreases phosphorylation of 4E binding proteins (4E-BP), both involved in growth-related protein synthesis (3). Eukaryotic initiation factor 2 (eIF2), a regulator of general protein synthesis, is activated in parallel to mTOR via PDK1 phosphorylation of PKB which phosphorylates and inactivates glycogen synthase kinase (GSK3β), releasing inhibition of eIF2 (3). Animal studies suggest that mTOR is necessary for muscle growth or hypertrophy, but is not needed for maintenance of muscle size in adult animals (4). There is some evidence that activation of the AMPK/PGC-1α pathways by endurance training may suppress the muscle hypertrophy effects of the mTOR pathways (27), providing a potential biochemical mechanism for the earlier clinical observations of Hickson et al (20).
In the current study, sedentary subjects volunteered for six weeks of supervised exercise training on stationary cycles. Gradually increasing intensity stationary cycling was chosen to be a simple activity that untrained subjects would be able to undertake easily. Further, we anticipated that this would be a mixture of strength and endurance training. Changes in the principle glucose transporters in vastus lateralis were quantified. In addition, changes in mitochondrial biogenesis and its principle regulator were measured, as well as a key member of the muscle hypertrophy pathway. Muscle fiber type-specific changes were also quantified for those elements that demonstrated major increases in whole muscle.
Methods
Protocol and subject selection
Eight sedentary normal subjects were recruited to undergo six weeks of supervised exercise training. The research protocol and the consent documents were approved by the East Tennessee State University Institutional Review Board. Each subject gave signed informed consent. Subjects were otherwise well except that they were not engaged in any regular organized or planned exercise. Six subjects whose characteristics are shown in Table 1, successfully completed the training and the planned measurements. The exercise program was performed at the ETSU Exercise and Sports Sciences Laboratory with students from Kinesiology in constant attendance under the supervision of Dr. Diego de Hoyos. This training program was purposely selected to be a realistic one that could be easily undertaken by previously untrained sedentary subjects. The training consisted of a progressive load cycle ride in which the power output was steadily increased over the 6 week period. Pedal rate and loading (watts) were adjusted so that an average target HR of approximately 70–75 % of predicted (220-age) was attained during the last 3 weeks of training. Each cycling session was preceded by a 5 min warm-up at a constant power output. During the first two weeks this warm-up was followed by 30 min at a constant load. During the last four weeks the subjects followed the warm-up by a 15 min constant level load then alternated between 2mins intense and 3 minutes easy pedaling (40 min during week 3, 45 min during week 4 and 50 minutes during weeks 5 and 6). HR’s ranged from 70–85% during the alternating loads. An estimate of the average Volume, intensity and HR over the 6 week training period is shown in Table 2. Body composition, VO2max, plasma insulin, and plasma glucose concentrations were measured prior to and at the end of the training. Quantification of lean mass and fat mass was performed by dual energy x-ray absorptiometry (DEXA) using a Hologic QDR 1000/w (Waltham, MA). An indirect calorimeter (Sensormedics 2400 Metabolic Measurement System Yorba Linda, CA) was used with a treadmill to determine VO2max.
TABLE 1.
Exercise training subjects
| Subject | Gender | Age | BMI (kg·m−2) |
|---|---|---|---|
| EX1 | M | 43 | 27.0 |
| EX2 | F | 24 | 26.8 |
| EX3 | M | 32 | 31.9 |
| EX4 | F | 56 | 28.9 |
| EX5 | F | 22 | 20.8 |
| EX6 | M | 42 | 34.8 |
| Mean ± SEM | 37 ± 5 | 28.3 ±2.0 |
Table 2.
Average Loading and HR Response Over Six Weeks of Cycle Training*
| Weeks | 1 | 2 | 3 | 4 | 5 | 6 | % change |
|---|---|---|---|---|---|---|---|
| Volume | 1364±432 | 2126±333 | 2513±485 | 2877±585 | 3197±824 | 3274±836 | 145±43 |
| Intensity | 49±13 | 61±14 | 62±16 | 64±15 | 65±16 | 66±16 | 36±7 |
| HR | 113±13 | 121±15 | 127±16 | 133±15 | 136±16 | 137±16 | 21±7 |
Volume = Watts × time, Intensity = Volume × time−1, HR beats × min−1
Materials
Affinity-purified rabbit antibodies against hGLUT1 (GT12-A) and hGLUT5 (GT52-A) were purchased from Alpha Diagnostics (San Antonio, TX). GLUT4 antibodies (AB1049, goat anti-human) were purchased from Chemicon (Temecula, CA). Rabbit anti-hGLUT12 antibodies (RDI-GLUT12abrx) were purchased from Research Diagnostics (Flanders, NJ). Rabbit anti-PGC-1α antibody was purchased from Chemicon (AB3242). Rabbit antibodies for phospho-AMPKα1 (Thr172) (#2531) and phospho-mTOR (Ser2448) (#2971) were purchased from Cell Signaling Technology (Danvers, MA). Goat antibodies against actin were purchased from Santa Cruz Biotechnology (sc-1616) and sheep anti-cytochrome c antibodies (AB3547) were purchased from Chemicon. Mouse monoclonal antibody against fast myosin (GTX73432) was purchased from GeneTex (San Antonio, TX). All other chemicals were reagent grade.
Muscle biopsies
Percutaneous needle biopsies of the vastus lateralis were performed after an overnight fast and two hours of quiet recumbency as previously described (35). Biopsies were done in the week prior to commencement of training and repeated from the contralateral leg 40–48 hours after the last exercise session. Briefly, after local lidocaine anesthesia, a 7–10 mm skin incision was made and a Bergstrom-Stille 5 mm muscle biopsy is introduced through the fascia, and under suction, a 50–100 mg specimen is obtained. After quickly blotting, the entire sample was frozen in liquid nitrogen for later analysis. Muscle homogenate was prepared by placing 25–50 mg muscle in 500 μL 0.25 M sucrose, 20 mM HEPES, pH 7.4, containing protease inhibitors (Halt Protease Inhibitor Cocktail Kit from Pierce), and homogenizing with two 30 second bursts of a hand-help homogenizer (Pellet Pestle Motor from Kontes).
Quantification of GLUT4 mRNA
Total RNA was isolated from 25–50 mg of muscle using the RNeasy Mini Kit and subjected to on-column DNase treatment according to the manufacturer’s protocol (Qiagen, Valencia, CA). RNA quality was analyzed using an Agilent 2100e Bioanalyzer (Agilent Technologies, Santa Clara, CA) and only samples with an RNA Integrity Number (RIN) of 7.0 or above was used in subsequent experiments. cDNA was generated by reverse transcription of 300 ng total RNA with oligo dT primers using the GeneAmp RNA PCR Core Kit (Applied Biosystems, Foster City, CA). Quantitative real-time PCR of GLUT4 mRNA was performed on a Bio-Rad iCycler (Hercules, CA) using primers and detection conditions as previously described (35). All samples were amplified in triplicate from three separate biologic replicates. GAPDH mRNA was quantified from each sample as previously described (35) and used as a reference transcript.
Glucose transporter protein standards
The construct pOva-GLUT1 was generated to express a soluble chimeric protein comprised of ovalbumin and a GLUT1 epitope tag. Chicken ovalbumin (nt 66–68, 81–1223; Genbank accession V00383) was amplified from pOV2 (generously provided by Dr. Michel Sanders of the University of Minnesota) using the primers ova F1 and ova-Glut1R (Table 3) and TA-cloned into pCR T7 TOPO (Invitrogen, Carlsbad, CA). As the ovalbumin coding region in pOV2 was incomplete, missing the first five codons and first bp of the sixth codon, a sense primer (ova F1) was designed to restore the initiator ATG and correct the sixth codon, while excluding the second through fifth codons. The antisense primer (ova Glut1R) was complimentary to ovalbumin nt 1201–1222 and included sequence encoding the C-terminal 12 amino acids of human GLUT1 (aa 481–492; SwissProt accession P11166). Codons were optimized for E. coli usage. Chimeric protein was expressed using a coupled in vitro transcription/translation system (Active Pro In Vitro Translation kit, Ambion, Inc.) according to the manufacturer’s protocol. The amount of chimeric protein generated was then quantified using the Agilent Bioanalyzer 2100e. The ova-GLUT1 fusion protein had a deduced molecular weight of 44,244 daltons and migrated as a discrete band, which represented 3.5% of the total protein present. Further purification was not necessary when the product was used as a standard in gel electrophoresis and immunoblotting.
Table 3.
Ovalbumin Antigen Chimeric Protein Primer Sequences
| construct | primer name | primer sequence | |
|---|---|---|---|
| 1 | ova 1F | 5′-ATG GCA GCA AGC ATG GAA TTT TG | |
| 2 | ova-GLUT1 | ova glut1R | 5′-TTA CAC TTG AGA ATC AGC GCC CAG TGG ATG GAA CAG CTC AGG GGA AAC ACA TCT GCC AAA G |
| 3 | ova-GLUT4 | ova glut4R | 5′-TCA ATC GTT CTC ATC CGG GCC TAA ATA TTC AAG TTC GGT AGG GGA AAC ACA TCT GCC AAA G |
| 4 | ova-GLUT5 | ova glut5R | 5′-TTA CTG CTC TGA AGT GAC AGG TGG AAG CTC TTT CAG TTC AGG GGA AAC ACA TCT GCC AAA G |
| 5 | ova-GLUT12 | ova glut12R | 5′-TTA GGT CTC TGG AGA AAG CTG CCT GGA TTG ACC CCT ACC AGG GGA AAC ACA TCT GCC AAA G |
The chimeric constructs used in this study (ova-GLUT4, ova-GLUT5 and ova-GLUT12) as well as those for GLUT3, mGlut3, GLUT6 and GLUT8 were generated using the same protocol as for ova-GLUT1, with the exception that the anti-sense primer was changed to correspond to the appropriate GLUT carboxy terminus sequence (Table 2). Figure 1 displays Immunoblots of serial dilutions of ova-GLUT1 and ova-GLUT4.
Figure 1. Serial dilutions of ova-GLUT1 and ova-GLUT4 on immunoblots.

Shown here are serial dilution immunoblots of ova-GLUT1 and ova-GLUT4. The first lane of each blot contains 10 μL of the cell free translation protein production as described in Methods, diluted 1:1,000. The fmoles/lane as indicated was calculated based on the protein content of the specific band determined by the Agilent Bioanalyzer 2100e and a deduced molecular weight of 44,244.
Immunoblots
The techniques used for performing immunoblots were described previously (34). Quantitative immunoblots were performed for GLUT4, GLUT5, and GLUT12 using image analysis (Quantity One version 4.5.2, BioRad) of blots that included known amounts of the corresponding ovalbumin-antigen chimeric protein.
Immunohistochemistry
Confocal microscopic assessments of specific fluorescent labeling of phospho-mTOR protein in human muscle sections were performed using methods previously described for glucose transporters (34). Muscle fiber type composition was determined using an anti-fast myosin heavy chain monoclonal antibody and methods previously described (34). GLUT4 is expressed predominantly in Type I muscle fibers with the ratio of GLUT4 in Type I to its expression in Type II fibers approaching 3 to 1 in some reports (17, 35) whereas GLUT5 and mTOR are expressed at higher concentrations in Type II fibers (4, 34, 35). Muscle fiber type-specific protein expression was quantified as previously described (34). Briefly, all sections were digitized, coded, and then signal intensity was quantified using Quantity One software by an investigator who was unaware of which subject or treatment each of the images represented. To accurately compare the expression of GLUT4 in the pre-training and post-training biopsy material, the two specimens from each subject were cut and mounted at the same time on the same glass slide. The pre- and post-training samples were then probed together simultaneously in a single incubation on that slide. Images of each slide were made with the same magnification and settings of the confocal microscope so that image analysis program settings were also identical and directly comparable.
Muscle fiber size cross-sectional area
Frozen muscle specimens were cut perpendicular to the fiber direction using a Leica CM3050 S cryostat and pre-training and post-training sections were placed on the same slide in the same order for each subject. Sections were evaluated for fiber cross-sectional area determination after they were treated with a fluorescent antibody that distinguished between Type I and Type II fibers. Digital images were obtained that included a key of a known dimension which were coded and submitted to the technician for quantification. In each image, ten Type I fibers and ten Type II fibers had their two major diameters measured (d1 and d2) and the area estimated by the formula for an ellipse (π·d1/2·d2/2).
Statistics
All data are displayed as mean ± standard error. Paired t test was used for comparisons before and after training. Effect size correlations were calculated using Cohen’s d (19). Statistical procedures were performed using SigmaStat version 3.11 from Systat Software (San Jose, California).
Results
Effects of six weeks of stationary cycle training on muscle expression of glucose transporters GLUT1, GLUT4, GLUT5, and GLUT12
Eight previously sedentary subjects volunteered to undergo six weeks of training on a stationary cycle, and six completed the protocol. The subject characteristics are shown in Table 1. Two subjects were obese (BMI>30), three were overweight (BMI 25 to 30), and one was normal in weight. All subjects had normal fasting plasma glucose concentrations and there was no diabetes in parents or siblings. The training followed the protocol describe in Methods and was individually supervised by students in the Kinesiology, Leisure, and Sports Science Department acting as a “personal trainers”. Table 4 displays the pre- and post- training VO2max, body composition, and fasting glucose and insulin data. Body composition, fasting glucose and insulin concentrations were not changed by the training program. Body mass index appears to have increased slightly with a “p value” of 0.031 by paired t-test. However, the effect size was quite small indicating little training effect on BMI. The small change in VO2max did not achieve statistical significance because the data failed the normality test and significance was tested using the Wilcoxon Signed Rank Test. However, an effect size of 0.56 suggests a moderate positive training effect on this measure of cardio-respiratory related endurance.
Table 4.
Exercise Training Impact on VO2max, Body Composition, Glucose, and Insulin
| VO2max 1 | VO2max 2 | BMI 1 | BMI 2 | fat 1 | fat 2 | glucose 1 | glucose 2 | Insulin 1 | Insulin 2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Subject | mL/kg.min | mL/kg.min | kg/m2 | kg/m2 | percent | percent | mg/dL | mg/dL | μU/mL | μU/mL |
| EX1 | 35.3 | 37.0 | 27.0 | 27.5 | 27 | 27 | 88 | 91 | 5.7 | 5.4 |
| EX2 | 26.8 | 27.5 | 26.8 | 27.5 | 41 | 40 | 78 | 82 | 7.0 | 8.7 |
| EX3 | 29.4 | 31.1 | 31.9 | 31.9 | 39 | 39 | 98 | 86 | 31.7 | 29.1 |
| EX4 | 22.6 | 22.6 | 28.9 | 29.4 | 42 | 44 | 93 | 91 | 8.2 | 16.1 |
| EX5 | 36.5 | 38.6 | 20.8 | 21.2 | 30 | 29 | 77 | 72 | 9.1 | 10.4 |
| EX6 | 25.7 | 27.5 | 34.8 | 34.8 | 42 | 44 | 74 | 77 | 6.8 | 8.3 |
| mean±sem | 29.4±2.3 | 30.7±2.5 | 28.3±2.0 | 28.7±1.9 | 37±3 | 37±3 | 85±4 | 83±3 | 11.4±4.1 | 13.0±3.5 |
| paired t-test | p=0.063 | p=0.031 | p=0.576 | p=0.580 | p=0.318 | |||||
| Effect Size | 0.56 | 0.21 | 0 | 0.056 | 0.21 |
We evaluated by immunohistochemistry which fiber accounted for the bulk of the increase in GLUT4 that we documented by immunoblotting of muscle homogenates. Figure 2C and 2D shows the results of these studies. Panel C displays one subject’s images that manifest an exercise training-related reversal of the GLUT4 fiber type dominance. The baseline sample image shows GLUT4 expressed at higher levels in the Type I fibers, whereas the post-training image shows GLUT4 actually expressed at higher concentrations in the Type II fibers. Panel D displays the individual image analysis-generated GLUT4 signal intensity data for all six subjects. In baseline samples, Type I fibers expressed 37% more GLUT4 than did Type II fibers. After exercise training, Type II fiber GLUT4 increased by 50% (p=0.001) above Type II baseline, whereas the 9% GLUT4 increase in Type I fibers was not statistically significant (p=0.510). The post-training GLUT4 signal intensity in Type II fibers was 101% of that in Type I muscle fibers.
Figure 2. Change in muscle Glut4 expression by stationary cycle training.
Six subjects underwent muscle biopsy before and after six weeks of supervised, increasing intensity training on a stationary bike. Panel A. RNA was isolated and mRNA was quantified using real-time quantitative PCR as described. One sample was degraded and not usable. The data shown here are means of three separate assays for each sample. The 22% increase was significant at p=0.05 by paired t-test. Panel B. Immunoblots of muscle homogenates and GLUT4 standards were quantified by image analysis in four separate experiments. The amount of muscle homogenate applied to each lane for GLUT4 measurements was 10 μg membrane protein. Each subject is indicated by his/her subject code (EX01 through EX06). The baseline sample is indicated by “A” and the post-training sample by “B” for each subject. The blot shown is typical and the graph shows the means of four studies for each sample. The 66% increase was significant at the p<0.01 level with paired t-test. Panel C. Panel C displays images from one subject. The top two images are from the pre-training biopsy and the bottom two are from the post-training biopsy. The red image is tagged using anti-hGLUT4 as the primary antibody and the blue image from the same section was probed with the anti-human fast myosin monoclonal antibody. The most intense staining in the top GLUT4 image is in the Type I fibers (unstained in the blue image) and the most intense GLUT4 signal in the post-training image is in the Type II fibers (blue positive fibers). The images for each subject were from two corresponding sections prepared and incubated simultaneously on the same slide. The confocal microscope settings were identical for all twelve images shown here. Panel D. This panel shows the results of image analysis assessment of the intensity of the fluorescent signal in Type I and Type II fibers. Ten fibers of each type in each image were assessed for average intensity using the Quantity One software and the average intensity from those ten fibers was plotted for each data point shown in Panel D. Paired t-tests gave p < 0.01 for both Type I and Type II changes.
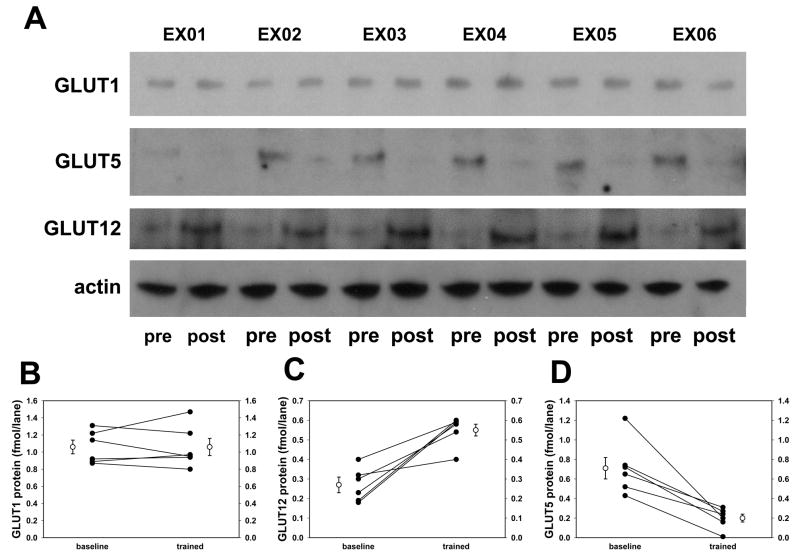
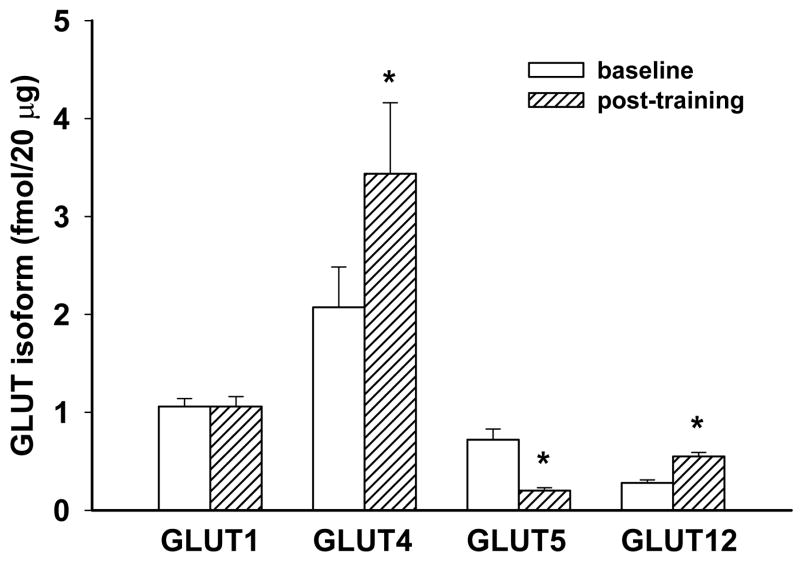
Percutaneous biopsies of the vastus lateralis were performed before training and 2 days after the last training bout. Both GLUT4 mRNA and protein increased significantly (22% and 66%, respectively) as shown in Figures 2A and 2B. Figure 3 shows the immunoblots for GLUT1, GLUT5 and GLUT12. GLUT1 did not change, but GLUT12 from muscle homogenate increased 104% and GLUT5 declined 72%. The mean data from quantification of each of the four principle muscle GLUT’s are displayed in Figure 4. GLUT4 represented half of hexose transporters in the baseline muscle biopsies and demonstrated the largest absolute training-related increment in transporters, making up 65% of the glucose transporters in the trained muscle. These data suggest that the 0.5 fmol/20 μg decline in the fructose transporter, GLUT5, was more than offset by increased GLUT4 and GLUT12, totaling 1.6 fmol/20 μg.
Figure 3. Changes in GLUT5 and GLUT12 induced by six weeks of stationary cycle training.
Similar to the studies of GLUT4 expression, GLUT1, GLUT5, and GLUT12 were quantified on immunoblots with specific protein standards. The blots shown are typical of several studies. Each subject is indicated by his/her subject code (EX01 through EX06). These blots included 20 μg membrane protein in each lane. The graphs (Panels B, C, and D) represent means of four separate analyses for GLUT5 and GLUT12, and three for GLUT1. The 104% increase in GLUT12 expression and 72% decrease in GLUT5 were each significant at p<0.01 by paired t-test.
Figure 4. Comparison of changes in muscle glucose transporter expression.
Shown here are the quantification of the glucose transporter protein as fmoles per 20 μg membrane protein applied to the polyacrylamide gel electrophoresis system for immunoblotting. Since each lane for GLUT4 measurement as shown in Figure 3 contained 10 μg, the GLUT4 data in this figure are adjusted to allow direct comparison. Each of these three hexose transporters were significantly different in expression after the training protocol at p<0.01 as indicated by the asterisk. The data presented are compiled from at least three separate determinations for each glucose transporter.
Effects of six weeks of stationary cycle training on muscle expression of cytochrome c and PGC-1α
Mitochondrial expression should be increased if the training sessions described above were primarily aerobic. We therefore quantified changes in a mitochondrial marker, cytochrome c. Immunoblots of the six subjects’ homogenates from pre-and post-training muscle biopsies were quantified by image analysis. Figure 5 displays a typical cytochrome c immunoblot. There was a mean 6% decrease that was not statistically significant. Since PGC-1α is the principle regulator of mitochondrial biogenesis, we quantified changes in its expression in the muscle biopsies from this study. Figure 5 also displays a representative PGC-1α immunoblot. PGC-1α expression was not significantly altered by the training program.
Figure 5. Changes in cytochrome c and PGC-1α by an exercise training program.
Immunoblots of cytochrome c and PGC-1α are shown along with a blot showing actin expression as a housekeeper protein. Each subject is indicated by his/her subject code (EX01 through EX06). The baseline sample is indicated by “A” and the post-training sample by “B” for each subject. Cytochrome c expression was not altered by the stationary cycle training program, nor was the mitochondrial biogenesis coactivator, PGC-1α, suggesting that there was little or no aerobic training-related muscle adaptation.
Effects of six weeks of stationary cycle training on muscle expression of phospho-AMPK and phospho-mTOR
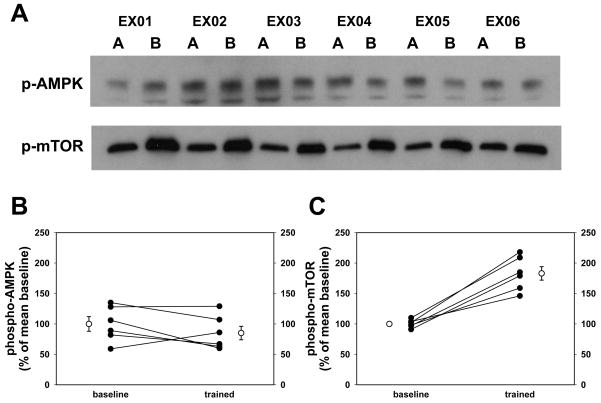
We quantified the change in phospho-AMPK in immunoblots as shown in Figure 6. We also quantified phospho-mTOR in immunoblots as shown in Figure 6, Panels A and C. The exercise training program that we employed resulted in a near doubling of the phospho-mTOR and a 12% decrease in phospho-AMPK that was not statistically significant. These data suggest that the stationary cycle training program did not produce typical aerobic training effects, but was more characteristic of strength training effects for the quadriceps muscles that were biopsied.
Figure 6. Changes in phospho-AMPK and phospho-mTOR induced by six weeks of cycle training of sedentary volunteers.
Immunoblots of muscle homogenate from vastus lateralis biopsies were probed with antibodies against phospho-AMPK and phospho-mTOR to determine which of these two protein kinase systems were activated by six weeks of progressive training on stationary cycles. Each subject is indicated by his/her subject code (EX01 through EX06). The baseline sample is indicated by “A” and the post-training sample by “B” for each subject. Image analysis showed a non-significant 12% drop in AMPK phosphorylation (Panel B), but phospho-mTOR increased by 83% (Panel C). The data shown in Panel B are means from three separate experiments and those in Panel C represent the mean of two separate studies for each data point. The increase in phospho-mTOR was significant at p<0.01 by paired t-test.
Fiber-specific changes in phospho-mTOR associated with six weeks of stationary cycle training
Activation of mTOR by phosphorylation is associated with protein synthesis regulation (3), and resistance exercise training is usually manifested histologically by increased size of Type II fibers (4). We evaluated the change in expression of phospho-mTOR in Type I and Type II muscle fibers between the baseline and post-training muscle biopsies from our six subjects. Figure 7 shows the results of these immunohistochemical studies. In the baseline biopsies, the specific phospho-mTOR intensity was 50% higher in the Type II fibers. The exercise training was associated with increased phospho-mTOR in both Type I and Type II fibers, 34% more in Type I fibers and 62% more in Type II fibers. In four subjects, both fiber types increased in cross-sectional area, but in the other two both fiber types decreased, and as a group, fiber size was not statistically different after training (Type I baseline 6180±900 μm2, Type II baseline 5940±1050, Type I trained 6480±823, Type II trained 5820±1040).
Figure 7. Muscle fiber-specific changes in phospho-mTOR from cycle training.
Panel A is similar to Panel C of Figure 2 above, except that the primary antibody in the red image was anti-phospho-mTOR. Panel B shows the results of quantitative image analysis assessment of the intensity of the fluorescent signal in Type I and Type II fibers. The image analysis revealed increased signal in both fiber types, but the increase was greater in the Type II fibers (+62%) than in the Type I fibers (+34%). Paired t-tests gave p < 0.01 for both Type I and Type II changes.
Discussion
Persons who previously had a sedentary life style but wanted to engage in an exercise program to improve their overall fitness were recruited. They underwent regular supervised exercise training using a stationary cycle with effort gradually increasing over a six week period. Needle biopsies were obtained from the vastus lateralis and muscle glucose transporters were quantified. GLUT4 and GLUT12 were increased by the exercise training, but GLUT1 did not change, and GLUT5 decreased in response to training. Mitochondrial markers, phospho-AMPK, and PGC-1α did not change, but phospho-mTOR increased significantly in muscle homogenates. Immunohistochemical studies showed that the increase in GLUT4 expression occurred in Type II fibers (fast twitch, white fibers), bringing the amount of GLUT4 in those fibers to levels equivalent to the amount found in Type I fibers (slow twitch, red fibers) at baseline. Analysis of digital images of phospho-mTOR expression demonstrated this exercise training protocol induced increases in both Type I and Type II fibers, but the changes were much more in Type II fibers (increased 62% vs 34%). There have been no prior reports of exercise-related changes in GLUT5 or GLUT12. Even though resistance training in humans has been shown to increase muscle GLUT4 (9, 22) this report is the first to demonstrate that the increase occurs in Type II (fast-twitch) fibers and that it is independent of any increase in mitochondria.
Regular exercise is beneficial to the management of type 2 diabetes (12). Whether the exercise consists predominantly of endurance training or strength training, glycemic control improves (11), as does insulin sensitivity (26). While, over a short-term neither type of exercise converts one muscle fiber type to another and no new fibers are formed, both can be associated with an increase in the size of the corresponding fibers (1, 22). However, long-term aerobic exercise is associated with a decrease in cross sectional area (14). In addition, both types of exercise are associated with an increase in GLUT4, the insulin-responsive glucose transporter (22), and an increase in glycogen synthase (22) and muscle glycogen content (6).
Endurance training causes an increase in mitochondrial biogenesis and a coincident increase in oxidative enzymes and fatty acid oxidation. These effects are predominantly in red fibers (23). In contrast, strength training does not typically increase mitochondrial production or oxidative enzymes (7, 22). Hosten and coworkers showed that strength training can increase insulin receptors (22), but two key components of the insulin intracellular pathway, IRS-1 and PI3-K, were not increased (22).
Reports of exercise training of subjects with type 2 diabetes have consistently shown effects that are qualitatively similar to the adaptations found in non-diabetic subjects. These data suggest that the enhanced insulin action seen after exercise training (either endurance or strength training) is due to increased GLUT4 expression and may be related to the consequent increased glycogen content of the contracting fiber. The role of mitochondrial biogenesis and fatty acid oxidation may be supplemental in endurance training but do not play a significant role in strength training.
The molecular mediators of muscle adaptation appear to be quite different between endurance training and strength training (3). Endurance training causes an increase in the amount and activity of AMP-activated protein kinase (AMPK) which in turn activates a cascade of intramuscular pathways that increase energy generation (13). Peroxisome proliferator-activated receptor-gamma co-activator 1 alpha (PGC-1α) is a key subsequent step in the stimulation of mitochondrial biogenesis (2). In contrast, resistance training does not cause an increase in mitochondrial biogenesis, but stimulates increased protein synthesis in muscle fibers, resulting in increased size of the fibers (4). Substantial evidence suggests that resistance exercise-induced adaptive muscle growth is dependent on the activation of a serine-threonine protein kinase, mammalian target of rapamycin (mTOR) and its downstream targets (4). The AMPK pathway and the mTOR signaling pathways may interact such that the AMPK/PGC-1α cascade suppresses the high energy consuming mTOR protein synthetic pathway (27). Combining endurance training with resistance training may suppress the muscle growth and strength enhancement seen with resistance training alone, but it does not appear that resistance training inhibits endurance training-related mitochondrial biogenesis (27). Baar has suggested that even though the mTOR pathway may also reciprocally block the AMPK pathway, the clinical observations do not support strength training suppressing endurance adaptation (3).
High-intensity interval training (HIT) has been a popular approach intended to improve performance in already aerobically trained athletes (16). HIT is short-duration, low volume, high intensity training, alternating with less intense intervals. Gibala and others have used 30 second “all-out” sprints alternating with 1–4 minutes of rest or low intensity running or cycling (16). Gibala’s group has demonstrated increased endurance without increased VO2max. They found increased mitochondrial markers and enzyme activity associated with increased glycogen content, decreased glycogen oxidation, and increased fat oxidation. GLUT4 protein was increased in muscle homogenate. They did not find any change in muscle fiber size or fiber composition. In acute, single day training, they have shown increased AMPK activation and increased PGC-1α mRNA. Even though there is potential for cross talk between the pathways of muscle hypertrophy and mitochondrial biogenesis, they have not reported whether mTOR/p70S6K is activated by this protocol. The mechanism for the muscle adaptation to HIT is still unclear, but the changes appear indistinguishable from those associated with traditional aerobic low intensity-long duration training (16).
GLUT4 protein in muscle is increased by exercise training (10). Houmard and coworkers found that GLUT4 content of vastus lateralis muscle biopsies nearly doubled after 14 weeks of training (25) and more recently showed a more than doubling of the GLUT4 content of vastus lateralis homogenate after only 7 days of intense cycle ergometer training (24). Daugaard and coworkers evaluated individual fiber type expression of GLUT4 and found that two weeks of primarily endurance training resulted in increased GLUT4 in Type I fibers only (8). The increased GLUT4 induced by training is rapidly lost with detraining. McCoy and coworkers prevented six distance runners from exercising for 10 days and measured a one third drop in GLUT4 in vastus lateralis homogenate (30).
These studies summarized here give strong evidence that endurance exercise training has a direct effect on intramuscular PGC-1α through AMPK. PGC-1α activates cassettes of genes, both nuclear and mitochondrial, resulting in increased mitochondrial biogenesis. At least some of the effects of PGC-1α are mediated through PPARδ and these may include increased GLUT4 expression and suppression of GLUT5 expression. Any impact of exercise on muscle fiber capillaries is likely mediated through other nuclear factors such as hypoxia-inducible-factor-1 (HIF-1) (38).
Activation of AMPK occurs transiently with each exercise bout and, in humans, baseline phospho-AMPK is increased chronically in response to exercise training (13, 32). Muscle contraction acutely results in phosphorylation of the α subunit of 5′-AMP-activated protein kinase (AMPK) presumably primarily via AMPK-kinase (AMPKK) (21). In rat treadmill training studies from Winder’s group, neither AMPK or basal phospho-AMPK are increased by the endurance exercise (21, 37). However, the reports of endurance training in humans demonstrated some key differences. Nielsen and coworkers in Copenhagen evaluated basal and exercise-stimulated phospho-AMPK by western blots in vastus lateralis muscle from seven endurance- trained athletes and seven sedentary subjects (32). Biopsies were performed after an overnight fast, before and after a 20 minute bout of exercise on a cycle ergometer at 80% VO2max. In the trained subjects, phosphorylation of AMPK was nearly twice that of the sedentary subjects and the activity of AMPK was 34% higher than the sedentary subjects, even though neither of these differences reached statistical significance.
Frosig and coworkers also of Copenhagen performed a classic study where they had eight healthy young men train only one leg for three weeks (13). The contralateral leg became the control leg. These biopsies were performed at least 15 hours after the last training session. The α1 subunit protein was 41% greater, basal phospho-AMPK (α1subunit) was 74% higher, and basal AMPK activity was 180% more than that of the contralateral control muscle. They felt that the increased basal AMPK phosphorylation and activity were training effects rather than a residual effect of the last exercise bout (13). They repeated their observations at 55 hours post-exercise and found that the increased basal AMPK activity persisted, suggesting that the AMPK activity is chronically elevated in skeletal muscle during periods of regularly repeated endurance exercise.
Laurie Goodyear and coworkers trained rats for six weeks and showed that muscle GLUT4 protein was increased 30% but noted that virtually all of the increase was at the plasma membrane in the basal state (18). Similar to our results in human muscle, she found GLUT1 content and subcellular distribution was not altered by training. Exercise training studies of mice and rats have generally used models of endurance training. These studies have shown that a cascade of mitochondrial biogenesis events in both nuclear and mitochondrial genes are activated via peroxisome proliferator-activated receptor-gamma co-activator 1 alpha (PGC-1α) (36). Activation of peroxisome proliferator-activated receptor- delta (PPARδ) may be a key step in this process (39). Even a single bout of exhaustive exercise can activate an array of mitochondria-related genes (28). Using microarrays, Mahoney reported the genes stimulated included a number of mitochondrial enzymes, PPARγ, PPARδ, metalothionines, and nuclear factor subfamily 4A family members (28).
The subjects of this study were sedentary prior to this training and most were overweight or obese. This form of increasing intensity cycle training may be more typical of the initial training in subjects desiring to improve their fitness status than more often studied daily jogging or running protocols of previous reports. Activities that are considered aerobic for long term training in acute exercise may be mixed strength and endurance for untrained subjects. Mascher and coworkers evaluated the time course of phosphorylation in the protein synthesis signaling pathways in untrained men undergoing 60 minutes of ergometer cycling exercise at 75% of VO2max (29). They found that phosphorylation of mTOR was three-fold elevated immediately after the exercise period and the increased phosphorylation continued, although declining, throughout the 3-hour recovery period. The immediate post-mTOR factor, p70S6k was phosphorylated on Ser424 five to eight-fold greater in the first 30 minutes of recovery. The time course of activation of Akt showed that its phosphorylation occurred later than the peak activation of mTOR and p70S6k, suggesting a major Akt-independent activation of mTOR (29). Studies in untrained rats by Morrison et al, demonstrated 3 hours of swimming was associated with mTOR activation in both red and white quadriceps muscle during the post-exercise recovery period (31). Consistent with phosphorylation-mediated activation of mTOR, these investigators demonstrated in the recovery period increased phosphorylation of p70S6k and ribosomal protein rpS6 and decreased phosphorylation of 4E-BP1 (31). These data point out the complexity of the regulation of the muscle protein synthetic systems. The activation of components in response to a given training program may be largely dependent on the training status of the subjects.
The stationary cycle training program that we used was anticipated to have a mixed strength-endurance impact, but the muscle adaptation in our subjects appears to have been predominantly protein synthetic with little or no effect on mitochondrial numbers. The results show increased GLUT4 and GLUT12 expression and a coincident decrease in GLUT5. These glucose transporter changes occurred with no change in mitochondria as quantified by cytochrome c and no alteration in either phospho-AMPK or PGC-1α, the major regulators of mitochondrial biogenesis. Instead, the muscle hypertrophy-related protein synthetic pathway was stimulated as indicated by a training-induced near doubling of phospho-mTOR. The increase in phospho-mTOR occurred in both Type I and Type II muscle fibers, but was relatively more in Type II fibers. The GLUT4 increase was also predominantly in Type II fibers. We conclude that our stationary cycle exercise program resulted in increased insulin-responsive glucose transporter expression in trained muscles via resistance exercise-responsive pathways predominantly in Type II muscle fibers and there was no involvement of the mitochondrial biogenesis system in the muscle adaptation to the program. These data suggest that the beneficial effect of cycle training in insulin resistance and diabetes management may be more closely tied to GLUT4 expression and the consequent increased glycogen storage, and less related to changes in mitochondrial glucose and fatty acid oxidation. In summary, the muscle adaptation observed in this study represent a unique finding in that this type of program may be more acceptable as initial exercise training among very sedentary subjects.
Acknowledgments
This study was funded in part by grants from Takeda Pharmaceuticals and the National Institutes of Health DK080488.
The authors wish to thank research nurse Mary Ward for her key contribution in recruiting subjects and coordinating measurements and muscle biopsies. Special thanks goes to Dr. Diego De Hoyos for his help in coordinating the exercise training sessions. This study was funded in part grants from Takeda Pharmaceuticals and the National Institutes of Health DK080488. There was no potential conflict of interest. The results of the present study do not constitute endorsement by the ACSM.
References
- 1.Allenberg K, Johansen K, Saltin B. Skeletal muscle adaptations to physical training in type II (non-insulin-dependent) diabetes mellitus. Acta Med Scand. 1988;223(4):365–373. doi: 10.1111/j.0954-6820.1988.tb15886.x. [DOI] [PubMed] [Google Scholar]
- 2.Baar K. Involvement of PPAR gamma co-activator-1, nuclear respiratory factors 1 and 2, and PPAR alpha in the adaptive response to endurance exercise. Proc Nutr Soc. 2004;63(2):269–273. doi: 10.1079/PNS2004334. [DOI] [PubMed] [Google Scholar]
- 3.Baar K. Training for endurance and strength: lessons from cell signaling. Med Sci Sports Exerc. 2006;38(11):1939–1944. doi: 10.1249/01.mss.0000233799.62153.19. [DOI] [PubMed] [Google Scholar]
- 4.Bodine SC. mTOR signaling and the molecular adaptation to resistance exercise. Med Sci Sports Exerc. 2006;38(11):1950–1957. doi: 10.1249/01.mss.0000233797.24035.35. [DOI] [PubMed] [Google Scholar]
- 5.Campos GE, Luecke TJ, Wendeln HK, et al. Muscular adaptations in response to three different resistance-training regimens: specificity of repetition maximum training zones. Eur J Appl Physiol. 2002;88(1–2):50–60. doi: 10.1007/s00421-002-0681-6. [DOI] [PubMed] [Google Scholar]
- 6.Castaneda C, Layne JE, Munoz-Orians L, et al. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care. 2002;25(12):2335–2341. doi: 10.2337/diacare.25.12.2335. [DOI] [PubMed] [Google Scholar]
- 7.Chilibeck PD, Syrotuik DG, Bell GJ. The effect of strength training on estimates of mitochondrial density and distribution throughout muscle fibres. Eur J Appl Physiol Occup Physiol. 1999;80(6):604–609. doi: 10.1007/s004210050641. [DOI] [PubMed] [Google Scholar]
- 8.Daugaard JR, Nielsen JN, Kristiansen S, Andersen JL, Hargreaves M, Richter EA. Fiber type-specific expression of GLUT4 in human skeletal muscle: influence of exercise training. Diabetes. 2000;49(7):1092–1095. doi: 10.2337/diabetes.49.7.1092. [DOI] [PubMed] [Google Scholar]
- 9.Derave W, Eijnde BO, Verbessem P, et al. Combined creatine and protein supplementation in conjunction with resistance training promotes muscle GLUT-4 content and glucose tolerance in humans. J Appl Physiol. 2003;94(5):1910–1916. doi: 10.1152/japplphysiol.00977.2002. [DOI] [PubMed] [Google Scholar]
- 10.Dohm GL. Invited review: Regulation of skeletal muscle GLUT-4 expression by exercise. J Appl Physiol. 2002;93(2):782–787. doi: 10.1152/japplphysiol.01266.2001. [DOI] [PubMed] [Google Scholar]
- 11.Dunstan DW, Daly RM, Owen N, et al. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care. 2002;25(10):1729–1736. doi: 10.2337/diacare.25.10.1729. [DOI] [PubMed] [Google Scholar]
- 12.Eriksson JG. Exercise and the treatment of type 2 diabetes mellitus. An update. Sports Med. 1999;27(6):381–391. doi: 10.2165/00007256-199927060-00003. [DOI] [PubMed] [Google Scholar]
- 13.Frosig C, Jorgensen SB, Hardie DG, Richter EA, Wojtaszewski JF. 5′-AMP-activated protein kinase activity and protein expression are regulated by endurance training in human skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286(3):E411–E417. doi: 10.1152/ajpendo.00317.2003. [DOI] [PubMed] [Google Scholar]
- 14.Gardiner PF. Endurance Training. In: Gardiner PF, editor. Neuromuscular Aspects of Physical Activity. Champaign, IL: Human Kinetics; 2001. pp. 111–142. [Google Scholar]
- 15.Gardiner PF. Strength Training. In: Gardiner PF, editor. Neuromuscular Aspects of Physical Activity. Champaign, IL: Human Kinetics; 2001. pp. 143–170. [Google Scholar]
- 16.Gibala MJ, McGee SL. Metabolic adaptations to short-term high-intensity interval training: a little pain for a lot of gain? Exerc Sport Sci Rev. 2008;36(2):58–63. doi: 10.1097/JES.0b013e318168ec1f. [DOI] [PubMed] [Google Scholar]
- 17.Goodyear LJ, Hirshman MF, Smith RJ, Horton ES. Glucose transporter number, activity, and isoform content in plasma membranes of red and white skeletal muscle. Am J Physiol Endocrinol Metab. 1991;261:E556–E561. doi: 10.1152/ajpendo.1991.261.5.E556. [DOI] [PubMed] [Google Scholar]
- 18.Goodyear LJ, Hirshman MF, Valyou PM, Horton ES. Glucose transporter number, function, and subcellular distribution in rat skeletal muscle after exercise training. Diabetes. 1992;41:1091–1099. doi: 10.2337/diab.41.9.1091. [DOI] [PubMed] [Google Scholar]
- 19.Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. Journal of Educational Statistics. 1981;6(2):107–128. [Google Scholar]
- 20.Hickson RC. Interference of strength development by simultaneously training for strength and endurance. Eur J Appl Physiol Occup Physiol. 1980;45(2–3):255–263. doi: 10.1007/BF00421333. [DOI] [PubMed] [Google Scholar]
- 21.Holmes BF, Kurth-Kraczek EJ, Winder WW. Chronic activation of 5′-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J Appl Physiol. 1999;87(5):1990–1995. doi: 10.1152/jappl.1999.87.5.1990. [DOI] [PubMed] [Google Scholar]
- 22.Holten MK, Zacho M, Gaster M, Juel C, Wojtaszewski JF, Dela F. Strength training increases insulin-mediated glucose uptake, GLUT4 content, and insulin signaling in skeletal muscle in patients with type 2 diabetes. Diabetes. 2004;53(2):294–305. doi: 10.2337/diabetes.53.2.294. [DOI] [PubMed] [Google Scholar]
- 23.Hoppeler H, Fluck M. Plasticity of skeletal muscle mitochondria: structure and function. Med Sci Sports Exerc. 2003;35(1):95–104. doi: 10.1249/01.MSS.0000043292.99104.12. [DOI] [PubMed] [Google Scholar]
- 24.Houmard JA, Hickey MS, Tyndall GL, Gavigan KE, Dohm GL. Seven days on exercise increase GLUT-4 protein content in human skeletal muscle. J Appl Physiol. 1995;79(6):1936–1938. doi: 10.1152/jappl.1995.79.6.1936. [DOI] [PubMed] [Google Scholar]
- 25.Houmard JA, Shinebarger MH, Dolan PL, et al. Exercise training increases GLUT-4 protein concentration in previously sedentary middle-aged men. Am J Physiol. 1993;264(6 Pt 1):E896–E901. doi: 10.1152/ajpendo.1993.264.6.E896. [DOI] [PubMed] [Google Scholar]
- 26.Ishii T, Yamakita T, Sato T, Tanaka S, Fujii S. Resistance training improves insulin sensitivity in NIDDM subjects without altering maximal oxygen uptake. Diabetes Care. 1998;21(8):1353–1355. doi: 10.2337/diacare.21.8.1353. [DOI] [PubMed] [Google Scholar]
- 27.Kimball SR. Interaction between the AMP-activated protein kinase and mTOR signaling pathways. Med Sci Sports Exerc. 2006;38(11):1958–1964. doi: 10.1249/01.mss.0000233796.16411.13. [DOI] [PubMed] [Google Scholar]
- 28.Mahoney DJ, Parise G, Melov S, Safdar A, Tarnopolsky MA. Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. FASEB J. 2005;19(11):1498–1500. doi: 10.1096/fj.04-3149fje. [DOI] [PubMed] [Google Scholar]
- 29.Mascher H, Andersson H, Nilsson PA, Ekblom B, Blomstrand E. Changes in signalling pathways regulating protein synthesis in human muscle in the recovery period after endurance exercise. Acta Physiol (Oxf) 2007;191(1):67–75. doi: 10.1111/j.1748-1716.2007.01712.x. [DOI] [PubMed] [Google Scholar]
- 30.McCoy M, Proietto J, Hargreaves M. Effect of detraining on GLUT-4 protein in human skeletal muscle. J Appl Physiol. 1994;77:1532–1536. doi: 10.1152/jappl.1994.77.3.1532. [DOI] [PubMed] [Google Scholar]
- 31.Morrison PJ, Hara D, Ding Z, Ivy JL. Adding protein to a carbohydrate supplement provided after endurance exercise enhances 4E-BP1 and RPS6 signaling in skeletal muscle. J Appl Physiol. 2008;104(4):1029–1036. doi: 10.1152/japplphysiol.01173.2007. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen JN, Mustard KJ, Graham DA, et al. 5′-AMP-activated protein kinase activity and subunit expression in exercise-trained human skeletal muscle. J Appl Physiol. 2003;94(2):631–641. doi: 10.1152/japplphysiol.00642.2002. [DOI] [PubMed] [Google Scholar]
- 33.Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24(1):78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 34.Stuart CA, Howell MEA, Yin D. Overexpression of GLUT5 in Diabetic Muscle Is Reversed by Pioglitazone. Diabetes Care. 2007;30(4):925–931. doi: 10.2337/dc06-1788. [DOI] [PubMed] [Google Scholar]
- 35.Stuart CA, Yin D, Howell MEA, Dykes RJ, Laffan JJ, Ferrando AA. Hexose transporter mRNAs for GLUT4, GLUT5, and GLUT12 predominate in human muscle. Am J Physiol Endocrinol Metab. 2006;291(5):E1067–E1073. doi: 10.1152/ajpendo.00250.2006. [DOI] [PubMed] [Google Scholar]
- 36.Suwa M, Nakano H, Kumagai S. Effects of chronic AICAR treatment on fiber composition, enzyme activity, UCP3, and PGC-1 in rat muscles. J Appl Physiol. 2003;95(3):960–968. doi: 10.1152/japplphysiol.00349.2003. [DOI] [PubMed] [Google Scholar]
- 37.Taylor EB, Hurst D, Greenwood LJ, et al. Endurance training increases LKB1 and MO25 protein but not AMP-activated protein kinase kinase activity in skeletal muscle. Am J Physiol Endocrinol Metab. 2004;287(6):E1082–E1089. doi: 10.1152/ajpendo.00179.2004. [DOI] [PubMed] [Google Scholar]
- 38.Vogt M, Puntschart A, Geiser J, Zuleger C, Billeter R, Hoppeler H. Molecular adaptations in human skeletal muscle to endurance training under simulated hypoxic conditions. J Appl Physiol. 2001;91(1):173–182. doi: 10.1152/jappl.2001.91.1.173. [DOI] [PubMed] [Google Scholar]
- 39.Wang YX, Zhang CL, Yu RT, et al. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol. 2004;2(10):e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]