Abstract
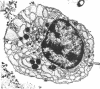
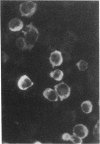
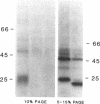
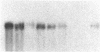
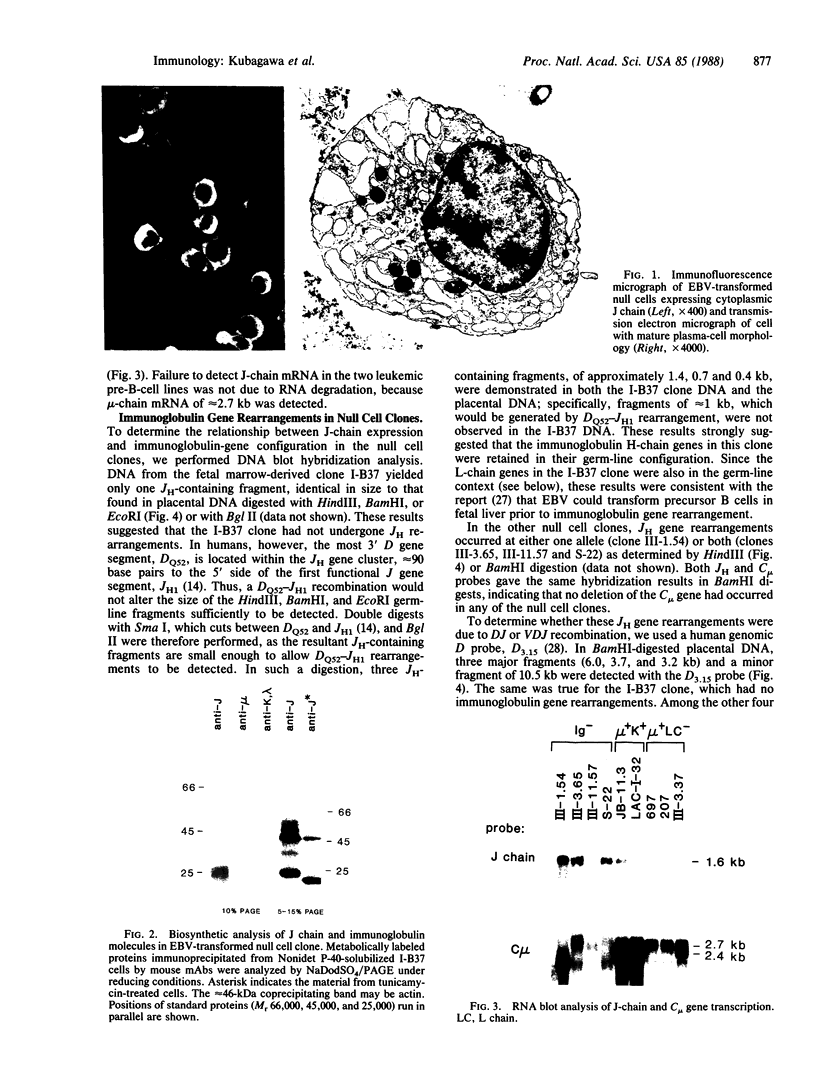
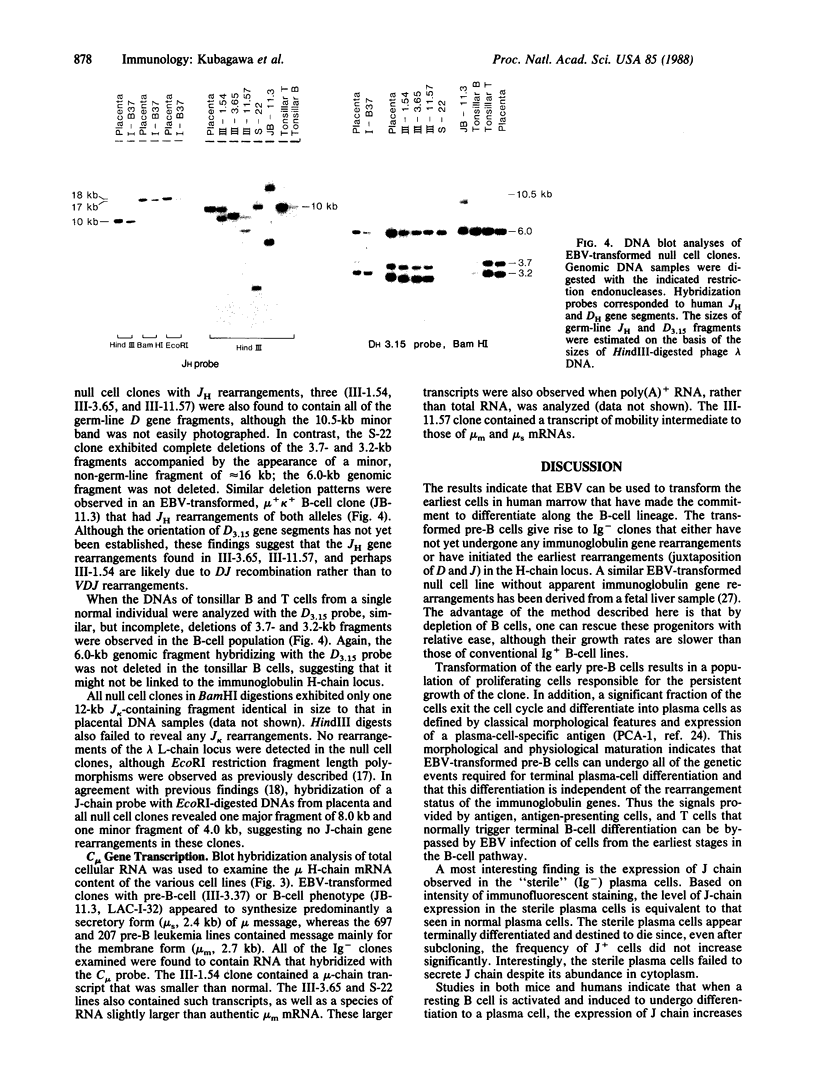
Human bone marrow cells were depleted of B lymphocytes to enrich for precursor B cells that could be transformed with Epstein-Barr virus. Transformed immunoglobulin-negative precursors either maintained their immunoglobulin genes in the germ-line configuration or had undergone DJ or abortive VDJ rearrangements (V, D, and J represent variable, diversity, and joining gene segments). All cell lines and their derivative clones, even those with no detectable immunoglobulin gene rearrangements, generated subpopulations of cells that produced high levels of joining (J) chain when analyzed by immunoprecipitation after biosynthetic labeling and by blot hybridization of cytoplasmic RNA. Morphologic and immunofluorescence analyses revealed that J-chain production was confined to clonal progeny that had exited the cell cycle to undergo plasma-cell differentiation. Analysis of cell surface antigens revealed expression of several B-cell maturational markers, including complement receptor type 2 (CR2) and plasma cell antigen 1 (PCA-1). Epstein-Barr virus can thus transform B-cell progenitors, allowing them to proliferate and undergo terminal B-cell differentiation coupled with J-chain expression. These events appear to occur independently of the immunoglobulin gene status of the transformed cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Landay A., Balch C. M., Cooper M. D. A human B cell differentiation antigen selectively expressed on mature B cells identified by a monoclonal antibody (HB-2). Hum Immunol. 1985 Aug;13(4):253–264. doi: 10.1016/0198-8859(85)90004-7. [DOI] [PubMed] [Google Scholar]
- Anderson K. C., Park E. K., Bates M. P., Leonard R. C., Hardy R., Schlossman S. F., Nadler L. M. Antigens on human plasma cells identified by monoclonal antibodies. J Immunol. 1983 Mar;130(3):1132–1138. [PubMed] [Google Scholar]
- Borzillo G. V., Cooper M. D., Kubagawa H., Landay A., Burrows P. D. Isotype switching in human B lymphocyte malignancies occurs by DNA deletion: evidence for nonspecific switch recombination. J Immunol. 1987 Aug 15;139(4):1326–1335. [PubMed] [Google Scholar]
- Brandtzaeg P. Presence of J chain in human immunocytes containing various immunoglobulin classes. Nature. 1974 Nov 29;252(5482):418–420. doi: 10.1038/252418a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Findley H. W., Jr, Cooper M. D., Kim T. H., Alvarado C., Ragab A. H. Two new acute lymphoblastic leukemia cell lines with early B-cell phenotypes. Blood. 1982 Dec;60(6):1305–1309. [PubMed] [Google Scholar]
- Fingeroth J. D., Weis J. J., Tedder T. F., Strominger J. L., Biro P. A., Fearon D. T. Epstein-Barr virus receptor of human B lymphocytes is the C3d receptor CR2. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4510–4514. doi: 10.1073/pnas.81.14.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdu I., Moldoveanu Z., Cooper M. D., Mestecky J. Ultrastructural studies of human lymphoid cells. mu and J chain expression as a function of B cell differentiation. J Exp Med. 1983 Dec 1;158(6):1993–2006. doi: 10.1084/jem.158.6.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieter P. A., Hollis G. F., Korsmeyer S. J., Waldmann T. A., Leder P. Clustered arrangement of immunoglobulin lambda constant region genes in man. Nature. 1981 Dec 10;294(5841):536–540. doi: 10.1038/294536a0. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., Maizel J. V., Jr, Leder P. Evolution of human immunoglobulin kappa J region genes. J Biol Chem. 1982 Feb 10;257(3):1516–1522. [PubMed] [Google Scholar]
- Kaji H., Parkhouse R. M. Intracellular J chain in mouse plasmacytomas secreting IgA, IgM and IgG. Nature. 1974 May 3;249(452):45–47. doi: 10.1038/249045a0. [DOI] [PubMed] [Google Scholar]
- Katamine S., Otsu M., Tada K., Tsuchiya S., Sato T., Ishida N., Honjo T., Ono Y. Epstein-Barr virus transforms precursor B cells even before immunoglobulin gene rearrangements. Nature. 1984 May 24;309(5966):369–372. doi: 10.1038/309369a0. [DOI] [PubMed] [Google Scholar]
- Kincade P. W. Formation of B lymphocytes in fetal and adult life. Adv Immunol. 1981;31:177–245. doi: 10.1016/s0065-2776(08)60921-9. [DOI] [PubMed] [Google Scholar]
- Kiyotaki M., Cooper M. D., Bertoli L. F., Kearney J. F., Kubagawa H. Monoclonal anti-Id antibodies react with varying proportions of human B lineage cells. J Immunol. 1987 Jun 15;138(12):4150–4158. [PubMed] [Google Scholar]
- Koshland M. E. Structure and function of the J chain. Adv Immunol. 1975;20:41–69. doi: 10.1016/s0065-2776(08)60206-0. [DOI] [PubMed] [Google Scholar]
- Koshland M. E. The coming of age of the immunoglobulin J chain. Annu Rev Immunol. 1985;3:425–453. doi: 10.1146/annurev.iy.03.040185.002233. [DOI] [PubMed] [Google Scholar]
- Kubagawa H., Mayumi M., Kearney J. F., Cooper M. D. Immunoglobulin VH determinants defined by monoclonal antibodies. J Exp Med. 1982 Oct 1;156(4):1010–1024. doi: 10.1084/jem.156.4.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuritani T., Cooper M. D. Human b-cell differentiation. I. Analysis of immunoglobulin heavy chain switching using monoclonal anti-immunoglobulin M, G, and A antibodies and pokeweed mitogen-induced plasma cell differentiation. J Exp Med. 1982 Mar 1;155(3):839–851. doi: 10.1084/jem.155.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutteh W. H., Moldoveanu Z., Prince S. J., Kulhavy R., Alonso F., Mestecky J. Biosynthesis of J-chain in human lymphoid cells producing immunoglobulins of various isotypes. Mol Immunol. 1983 Sep;20(9):967–976. doi: 10.1016/0161-5890(83)90037-8. [DOI] [PubMed] [Google Scholar]
- Lamson G., Koshland M. E. Changes in J chain and mu chain RNA expression as a function of B cell differentiation. J Exp Med. 1984 Sep 1;160(3):877–892. doi: 10.1084/jem.160.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon G. G., Perry R. P. C mu-containing transcripts initiate heterogeneously within the IgH enhancer region and contain a novel 5'-nontranslatable exon. Nature. 1985 Dec 5;318(6045):475–478. doi: 10.1038/318475a0. [DOI] [PubMed] [Google Scholar]
- Mason D. Y., Stein H. Reactive and neoplastic human lymphoid cells producing J chain in the absence of immunoglobulin: evidence for the existence of 'J chain disease'? Clin Exp Immunol. 1981 Nov;46(2):305–312. [PMC free article] [PubMed] [Google Scholar]
- Matsuuchi L., Cann G. M., Koshland M. E. Immunoglobulin J chain gene from the mouse. Proc Natl Acad Sci U S A. 1986 Jan;83(2):456–460. doi: 10.1073/pnas.83.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max E. E., Korsmeyer S. J. Human J chain gene. Structure and expression in B lymphoid cells. J Exp Med. 1985 Apr 1;161(4):832–849. doi: 10.1084/jem.161.4.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune J. M., Fu S. M., Kunkel H. G. J chain biosynthesis in pre-B cells and other possible precursor B cells. J Exp Med. 1981 Jul 1;154(1):138–145. doi: 10.1084/jem.154.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- Mestecky J., Winchester R. J., Hoffman T., Kunkel H. G. Parallel synthesis of immunoglobulins and J chain in pokeweed mitogen-stimulated normal cells and in lymphoblastoid cell lines. J Exp Med. 1977 Mar 1;145(3):760–765. doi: 10.1084/jem.145.3.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Lipman M. Comparison of the yield of infectious virus from clones of human and simian lymphoblastoid lines transformed by Epstein-Barr virus. J Exp Med. 1973 Dec 1;138(6):1398–1412. doi: 10.1084/jem.138.6.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson K. J., Haimovich J., Perry R. P. Characterization of productive and sterile transcripts from the immunoglobulin heavy-chain locus: processing of micron and muS mRNA. Mol Cell Biol. 1983 Jul;3(7):1317–1332. doi: 10.1128/mcb.3.7.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl E. R., Vogler L. B., Okos A. J., Crist W. M., Lawton A. R., 3rd, Cooper M. D. B lymphocyte precursors in human bone marrow: an analysis of normal individuals and patients with antibody-deficiency states. J Immunol. 1978 Apr;120(4):1169–1175. [PubMed] [Google Scholar]
- Ravetch J. V., Siebenlist U., Korsmeyer S., Waldmann T., Leder P. Structure of the human immunoglobulin mu locus: characterization of embryonic and rearranged J and D genes. Cell. 1981 Dec;27(3 Pt 2):583–591. doi: 10.1016/0092-8674(81)90400-1. [DOI] [PubMed] [Google Scholar]
- Reth M. G., Alt F. W. Novel immunoglobulin heavy chains are produced from DJH gene segment rearrangements in lymphoid cells. 1984 Nov 29-Dec 5Nature. 312(5993):418–423. doi: 10.1038/312418a0. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Ravetch J. V., Korsmeyer S., Waldmann T., Leder P. Human immunoglobulin D segments encoded in tandem multigenic families. Nature. 1981 Dec 17;294(5842):631–635. doi: 10.1038/294631a0. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Sanders S. K., Butler J. L., Gartland G. L., Komiyama K., Cooper M. D. Identification of an early activation antigen (Bac-1) on human B cells. J Immunol. 1986 Aug 15;137(4):1208–1213. [PubMed] [Google Scholar]
- Tedder T. F., Clement L. T., Cooper M. D. Discontinuous expression of a membrane antigen (HB-7) during B lymphocyte differentiation. Tissue Antigens. 1984 Sep;24(3):140–149. doi: 10.1111/j.1399-0039.1984.tb02118.x. [DOI] [PubMed] [Google Scholar]
- Wall R., Kuehl M. Biosynthesis and regulation of immunoglobulins. Annu Rev Immunol. 1983;1:393–422. doi: 10.1146/annurev.iy.01.040183.002141. [DOI] [PubMed] [Google Scholar]
- Webb C. F., Cooper M. D., Burrows P. D., Griffin J. A. Immunoglobulin gene rearrangements and deletions in human Epstein-Barr virus-transformed cell lines producing different IgG and IgA subclasses. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5495–5499. doi: 10.1073/pnas.82.16.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Regulation of the assembly and expression of variable-region genes. Annu Rev Immunol. 1986;4:339–368. doi: 10.1146/annurev.iy.04.040186.002011. [DOI] [PubMed] [Google Scholar]