Abstract
CD32A, the major phagocytic Fc gamma receptor in humans exhibits a polymorphism in the ligand-binding domain. Individuals homozygous for CD32AR allele are more susceptible to bacterial infections and autoimmune diseases as compared to CD32AH homozygous and CD32AR/H heterozygous individuals. In order to understand the mechanisms behind this differential susceptibility, we have investigated the dynamics of the interaction of these allelic forms of CD32A when they are simultaneously exposed to immune complexes. Binding studies using Ig fusion proteins of CD32A alleles showed that the R allele has significantly lower binding not only to human IgG2, but also to IgG1 and IgG3 subtypes. Competition assays using purified molecules demonstrated that CD32AH-Ig outcompetes CD32AR-Ig for IC binding when both alleles simultaneously compete for the same ligand. CD32AH-Ig blocked the immune-complex (IC) binding mediated by both the allelic forms of cell surface CD32A, whereas CD32AR-Ig blocked only CD32AR and was unable to cross-block IC binding mediated by CD32AH. Two dimensional (2D) affinity measurements also demonstrated that CD32AR has significantly lower affinity towards all three subtypes as compared to CD32AH. Our data suggest that the lower binding of CD32AR not only to IgG2 but also to IgG1 and IgG3 might be responsible for the lack of clearance of IC leading to increased susceptibility to bacterial infections and autoimmune diseases. Our data further suggests that in humans, inflammatory cells from CD32AR/H heterozygous individuals may predominantly use the H allele to mediate antibody coated target cell binding during phagocytosis and ADCC, resulting in a phenotype similar to CD32AH homozygous individuals.
INTRODUCTION
Of the receptors for the Fc domain of immune-complexes (IC), the low affinity Fc gamma receptors (FcγR) play a central role in many types of antibody-dependent cellular cytotoxicity (ADCC) and immunophagocytosis (1–5). In humans, CD32A, the type II FcγR, is the major phagocytic Fc receptor (6). Human CD32A has low affinity for monomeric IgG, but it binds stably to immune-complexes. CD32A has been shown to exhibit a polymorphism in the ligand-binding domain. This single nucleotide polymorphism in the ligand binding domain causes a substitution of amino acid arginine (CD32AR) to histidine (CD32AH) at position 131. Both CD32A alleles binds to human IgG1 and IgG3, but the CD32AR allotype shows a lower binding for human IgG2 when compared to CD32AH (7–9). Evidences suggest that CD32AR allele is associated with increased susceptibility to bacterial infections (10–15). Human IgG2 is the major subclass of antibody elicited by encapsulated bacteria in humans including human pathogens such as Neisseria meningitidis, Haemophilus influenzae and Streptococcus pneumoniae (16–18). Arthur et al. (19) showed that 90% of CD32AR homozygous individuals are more susceptible to S. pneumococcal infection.
Apart from bacterial infections, CD32AR allele is also associated with susceptibility to the development of certain autoimmune disease such as systemic lupus erythematosus (SLE) (16,20–24). Various clinical studies have shown that SLE patients who are CD32AR have a higher likelihood of developing proteinurea, hemolytic anemia, antinuclear RNP antibodies, glomerulonephritis and hypocomplementenia (25). Development of SLE at a younger age was reported in patients with the CD32AR genotype, with an earlier incidence of arthritis, sicca syndrome, nephritis, lymphadenitis, hemotologic abnormalities, lupus anticoagulant, cryoglobulinemia and hypocomplementemia (25).
Taken together, these studies suggest that the CD32A polymorphism plays a pivotal role in certain infectious and autoimmune diseases. The increased susceptibility of CD32AR homozygous individuals for the observed diseases may be due to the poor clearance of IC. Homozygous CD32AH individuals, in contrast, are not susceptible to certain bacterial infections and autoimmune diseases because ICs are cleared efficiently (20,21,26,27). Interestingly, CD32AR/H heterozygous individuals are also resistant to certain bacterial infections even though they express the CD32AR allele. It is not clear why the coexpression of CD32AR in heterozygous individuals is not reducing the efficiency of CD32AH allele. We hypothesize that in heterozygous individuals, CD32AH outcompetes CD32AR for ligand binding when both alleles are expressed on the same cell. To test our hypothesis, we have analyzed the interaction of immune-complexes with cells expressing R and H allelic forms of CD32A and their competition for ligand binding using recombinant dimeric forms of soluble R and H forms of CD32A alleles. The results presented here demonstrate that CD32AH outcompetes CD32AR when they simultaneously compete for the same ligand. Such a dominance of CD32AH allele in heterozygous individuals may be due to the higher affinity of CD32AH for all human IgG isotypes as compared to CD32AR which is demonstrated herein by cell binding assays and 2D affinity measurement studies.
MATERIALS AND METHODS
Cell lines and Reagents
PKH-26 labeling kit, rabbit anti-DNP IgG, HRP-conjugated anti-human Fc antibody, HRP- and FITC-conjugated goat anti-human IgG F(ab′)2 specific for light chain of human IgG, and CNBr activated Sepharose were from Sigma-Aldrich (St. Louis, MO). Human IgG subtypes were from Sigma-Aldrich (St. Louis, MO). As per manufacture’s data sheet the purity of the IgG molecules is more than 95%. To avoid the problems associated with the storage of IgG molecules in solution, they were immediately aliquoted and stored at −20 °C. The ICs were made fresh and used for the experiments. We have also used IgG molecules from 2 different lots and found to have similar binding to human FcγRs. The IgG molecules were purified from human plasma by combination of chromatographic techniques as per Sigma-Aldrich. The catalog numbers are as follows: IgG1 #I5154, IgG2 #I5404, IgG3 #I5654 and IgG4 #4639. FITC- and Cy5-conjugated goat anti-human Fc specific IgG F(ab′)2 from Jackson Immunoresearch Laboratories (West Grove, PA). Lipofectamine2000 was from Invitrogen (Carlsbad, CA). The Micro BCA-protein assay kit was purchased from Pierce (Rockford, IL) and the HRP-substrate from BioRad (Hercules, CA). Quick-change II site-directed mutagenesis kit was from Stratagene (La Jolla, CA). Chinese hamster ovary (CHO) cells (Clone K1) were from ATCC (Manassas, VA). Human red blood cells were isolated from healthy volunteers. Sheep red blood cells were from Colorado Serum Company. Cell culture reagents were from Life Technologies (Gaithersburg, MD).
Construction, expression and purification of recombinant soluble CD32A-Ig molecules
The construction and expression of the dimeric form of CD32AR-Ig was carried out by ligating the extracellular domain of human CD32AR to the Fc domain of the human IgG1 heavy chain as described earlier (28). The mutated IgG1 Fc CH2-CH3 domains were obtained from Dr. Peter Linsley, Bristol-Meyers Squibb. Mutations in the Fc domain are L267F, L268E, G270A, and A363T (numbered as in accession number AAH69020.1). These mutations have been shown to abolish the binding of FcγRs (29–31). The CD32AH-Ig was constructed by Quick-change II site-directed mutagenesis kit (Stratagene) using CD32AR-Ig DNA as a template. The recombinant molecules were purified from CHO cell transfectants using a protein-G (Pharmacia Biotech, NJ) Sepharose column (32). The immunoaffinity purified CD32A-Ig molecules were analyzed using 10% SDS-PAGE under reducing and non-reducing conditions and the protein bands were visualized by silver staining and Western blot. Protein concentration was measured by Micro BCA protein assay kit (Pierce, NJ) using BSA as a standard.
Establishment of CHO stable transfectants expressing cell surface CD32A alleles
CHO cells were transfected with pUB6A plasmid vectors containing CD32AR and CD32AH gene using Lipofectamine. Forty-eight hours after transfection, cell culture medium containing 20 μg/ml Blasticidin (Gibco BRL, Gaithersburg, MD) was used as the selection medium to establish the stable transfectants of CHO-CD32A. Cells expressing high levels of transfected molecules were selected by a panning procedure (33).
Soluble IC binding assay
Soluble immune complexes (sIC) was prepared by mixing with either HRP or FITC conjugated F(ab′)2 fragment of goat anti-human IgG specific for light chain (kappa) with human IgG subtypes (1:1 molar ratio) for 4 h at 4 °C. HRP-conjugated and FITC-conjugated F(ab′)2 fragment of goat anti-human IgG were used for ELISA and cell binding assays, respectively. The complex was centrifuged at 15,000 rpm for 30 min at 4 °C and the supernatant was used for the sIC binding assay. For ELISA, CD32A-Ig molecules (50 μl of 10 μg/ml) were coated on plates overnight and wells were blocked for 1 h with binding buffer (PBS/5 mM EDTA with 1%BSA, pH 7.4). The HRP-IC of human IgG subtypes (50 μl of 10 μg/ml) was then added and incubation continued for 1h at 4 °C. The wells were washed and HRP substrate was added. The reaction was stopped by adding 1 N H2SO4 and read at 450 nm. The HRP-IC of human IgG subtypes bound to IV.3 (a blocking mAb for human CD32A molecule) treated CD32A-Ig coated wells were taken as non-specific binding.
For FITC-IC binding assay, the FcγR positive cells (50 μl of 5 x 106) were incubated with FITC-IC of human IgG subtypes (10 μg/ml) in binding buffer for 1 h at 4°C in the absence or presence of purified CD32A-Ig molecules and/or blocking mAbs. Cells treated with IV.3 mAb for 30 min at 4 °C, served as a specificity control. Cells were then washed and resuspended in 150 μl of binding buffer and fixed by adding 150 μl of 2% formalin in PBS. Binding of FITC-IC to FcγR+ cells was analyzed by flow cytometry.
Particulate IC (EA) binding assay
Sheep red blood cells (SRBC) were coupled with human IgG subtypes (hereafter referred as EA) by the chromium chloride method (34). PKH-labeling of EA was performed using a PKH-labeling kit. Binding of FcγR+ cells to PKH-labeled EA was analyzed by flow cytometry (28,32). Briefly, PKH labeled EA were washed and resuspended in binding buffer. If aggregates of EA were noticed, they were removed using 75 μm nylon filters before the assay. FcγR+ cells (50 μl of 5 × 106/ml) in binding buffer were incubated with PKH labeled EA (50 μl of 1.5 × 108) for 2 h at 4 °C. Binding assays were performed in the absence or continuous presence of CD32A-Ig molecules and/or 10 μg/ml mAbs for CD32A (IV.3) during the incubation with EA. EA bound to cells were analyzed by flow cytometry. PKH-labeled unopsonized erythrocytes were used as a control in all the experiments. Mean fluorescent intensity at FL2 channel and the percentage of FcγR+ cells bound to EA were determined using FACScan flow cytometer (Becton-Dickinson, San Jose). The binding index was calculated using the following formula: % cells bound to EA x mean fluorescence/100.
Competition assay
Saturating concentration of CD32A-Ig molecules for rabbit anti-DNP IgG opsonized sheep erythrocytes (EA) binding was determined using flow cytometry. Briefly, sheep erythrocytes were coated with DNP as described (35) and opsonized with rabbit anti-DNP IgG (EA). EA (50 μl of 1 × 108 cells/ml) were incubated with various concentrations of CD32A-Ig molecules for 1 h at 4°C. After washing twice with binding buffer the FITC-conjugated F(ab′)2 fragment of goat-anti human Fc specific IgG (Ig portion of CD32A-Ig molecules is a human IgG1 Fc domain) was added and incubated for another 1 h at 4 °C. The cells were washed twice and analyzed for CD32A-Ig molecules binding. For the competition assay, EA (50 μl of 1 × 108 cells/ml) were incubated with 50 μl of CD32A-Ig molecules either alone or in combination (equimolar or saturating concentration) for 1 h at 4 °C. The CD32A-Ig molecules were preincubated with F(ab′)2 of goat anti-human Fc specific antibody conjugated with Cy5 (for CD32AH-Ig) or FITC (for CD32AR-Ig) for 1 h at 4 °C. The secondary antibody was titrated and excess dimers were added to avoid the cross binding of secondary antibody during the incubation of both the dimers with EA. The cells were washed and analyzed for CD32A-Ig molecules binding. The cells treated with the secondary antibody alone served as a specificity control.
Micropipette adhesion frequency assay
To determine 2D affinity (the affinity of a receptor-ligand pair expressed on opposing cell surface) of CD32A alleles for the human IgG subtypes, the micropipette adhesion frequency assay was carried out as described (34). Briefly, human red blood cells (HRBCs) were coated with human IgG subtypes using CrCl3 coupling method as described above. The site densities of human IgG subtypes on HRBCs and Fc receptors on CHO cells were determined by flow cytometry using fluorescence coupled standard beads (Quantum™ 25 FITC High Level, Bangs Laboratory, Fishers, IN).
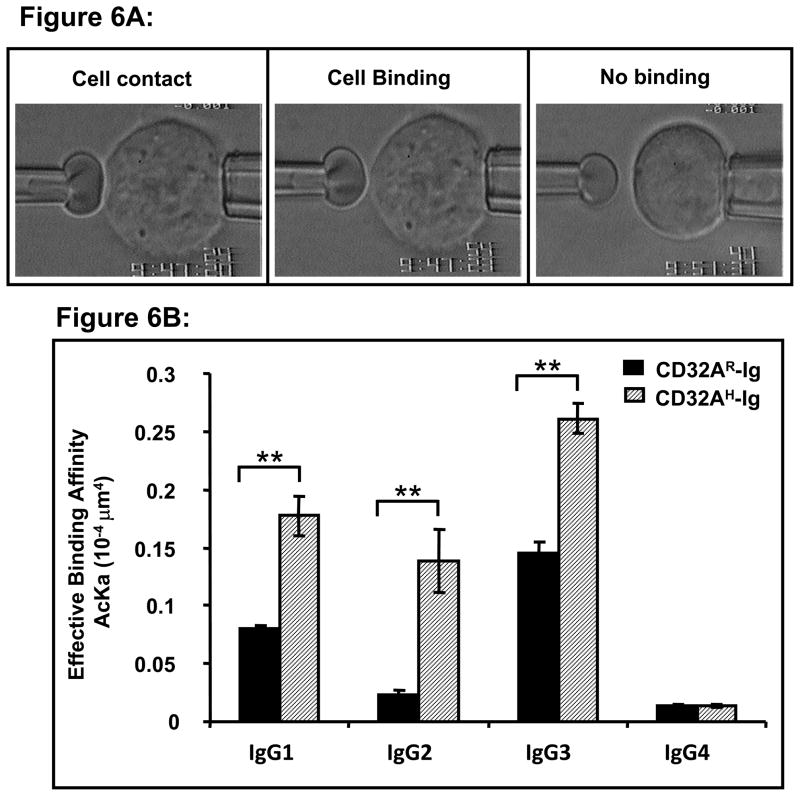
The micropipette apparatus utilized in this experiment has been described previously (34,36,37). Briefly, a micropipette-aspirated red blood cell coated with human IgG subtypes was driven by a piezoelectric translator to make a 10 second contact with a CHO cell expressing CD32A alleles held stationary by another pipette. At the end of the contact duration, the two cells were separated by retracting the red blood cell. Upon retraction, an adhesion event between the two cells was indicated by stretched red blood cell membrane (Fig. 6A). This contact test cycle was repeated 100 times using the same pair of cells, keeping the contact duration (t) and the area (Ac, ~3 μm−2) constant, and the number of adhesion event was counted to obtain an adhesion frequency (Pa). Nonspecific binding is determined by using red blood cells coated with BSA. The effective binding affinity (AcKa) and off-rate (kr) were extracted by fitting the equation,
Figure 6.
(A) Typical picture of 2D Affinity measurement by micropipette method The typical experimental setup is represented in this figure. Briefly, two glass micropipettes and the chamber medium were filled with binding buffer. A micropipette-aspirated red blood cell coated with human IgG subtypes was driven by a piezoelectric translator to make a 10 second contact with a CHO cell expressing CD32A alleles, held stationary by another pipette (cell contact). At the end of the contact duration, the two cells were separated by retracting the red blood cell. Upon retraction, an adhesion event between the two cells was indicated by stretched red blood cell membrane (cell binding). Nonspecific binding is determined by using red blood cells coated with BSA (no binding). (B) CD32AH allele has a higher affinity for human IgG subtypes than the CD32AR allele as measured by micropipette method. The micropipette adhesion frequency assay was carried out as described under Materials and Methods. The contact test cycle as described in figure 6A was repeated 100 times using the same pair of cells, keeping the contact duration (t) and the area (Ac, ~3 μm−2) constant, and the number of adhesion events was counted to obtain an adhesion frequency (Pa). The effective binding affinity (AcKa) and off-rate (kr) were extracted by fitting the data into the equation as described under Materials and Methods. Data is representative of two individual experiments. *p<0.01, **P<0.001.
| (Eq. 1) |
In the equation, mr and ml are receptor and ligand site density, respectively. For experiments in this study, the adhesion frequency was determined at a sufficiently long contact duration {t =10 s: exp(−krt≈0)} without measuring a full binding curve to simply estimate the effective binding affinity (AcKa) from the equation,
| (Eq. 2) |
which is derived from the steady state version (i.e., t → ∞) of Eq. 1.
Statistical analysis
A statistical comparison of the CD32AR and CD32AH alleles was performed using student t-test; p<0.01 (*) considered as significant and p<0.001 (**) considered as highly significant.
RESULTS
Recombinant Ig fusion proteins of CD32A alleles are secreted as a homodimers
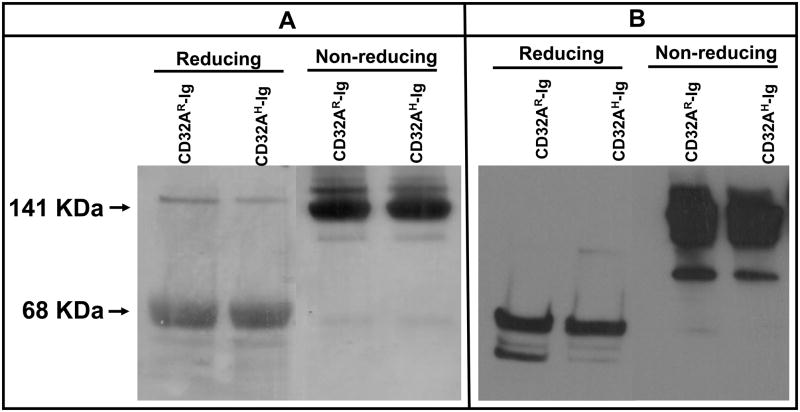
The extracellular domain of CD32AR was ligated to the mutated CH2 and CH3 regions of the Fc domain of human IgG1 molecule using a strategy similar to the construction of CD16A-Ig (28). The CD32AH-Ig was created by site-directed mutagenesis using CD32AR-Ig DNA as a template. The resultant chimeric cDNA was transfected in CHO cells. The culture supernatant obtained from CHO transfectants was analyzed by a sandwich ELISA using mAb IV.3 (anti-CD32A mAb) to confirm expression of CD32A (data not shown). Clones secreting high levels of CD32A-Ig fusion proteins were chosen for further characterization. CD32A-Ig molecules from cell culture supernatant were purified by a single step immunoaffinity chromatography using protein-G-Sepharose. The yield was nearly 1.5 mg for CD32AR-Ig and 2.0 mg for CD32AH-Ig per liter of culture supernatant. SDS-PAGE (Fig. 1A) and Western blot (Fig. 1B) analysis of the purified CD32A-Ig molecules showed a major band of 141 kDa and a minor band of about 120 kDa under non-reducing conditions and 68 kDa under reducing conditions, suggesting that the recombinant CD32A-Ig molecules are secreted as a disulfide-linked homodimer. The minor protein band of 120 kDa (under non-reducing conditions) may be the precursor or degradation product of CD32A-Ig since it reacted with anti-human Fc specific IgG in Western blot analysis (Fig. 1B).
Figure 1. Detection of CD32A-Ig dimers.
SDS-PAGE analysis of immunoaffinity purified dimeric CD32AR-Ig (left lane) and CD32AH-Ig (right lane) alleles. Dimers were purified using Gamma bind plus column from CHO cell culture supernatant. The purified protein (5μg) was subjected to SDS-PAGE under reducing (left panel) and non-reducing (right panel) conditions and the protein bands were visualized using the silver staining (A) method and Western blotting (B).
The IgG binding properties of CD32A-Ig molecules are similar to cell surface expressed CD32A alleles
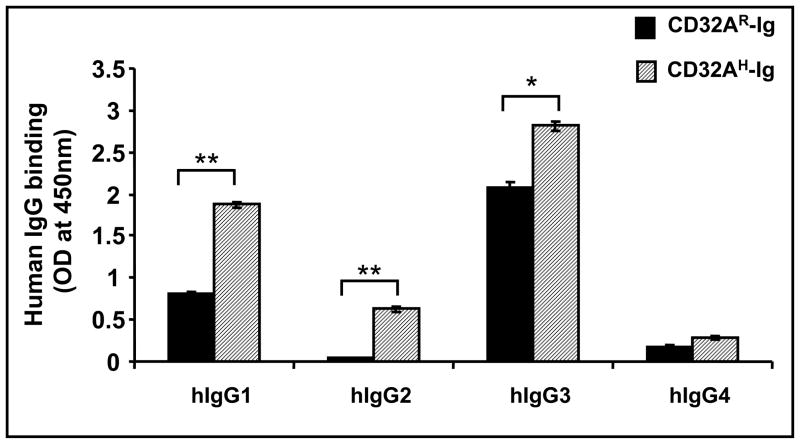
The purified dimeric CD32A-Ig molecules were assayed for their functional ability to bind soluble immune-complexes (sIC) of human IgG subtypes. sIC of human IgG subtypes conjugated with HRP were prepared as described in Materials and Methods and assayed for binding to FcγR-Igs coated on the plates. As expected, CD32AH-Ig bound to human IgG1, IgG2 and IgG3, whereas CD32AR-Ig bound to IgG1 and IgG3 but not to IgG2 (Fig. 2). Both alleles were unable to bind to IgG4. The wells with CD32A-Ig molecules and treated with IV.3 mAb (a mAb that specifically blocks binding of IC to CD32A) before the addition of HRP-F(ab′)2-labeled IC were used as a specificity control. The results show that HRP-F(ab′)2-labeled IC binding to CD32A is specific and can be completely blocked by IV.3 mAb. This also rules out the possibility of uncomplexed HRP-F(ab′)2 binding to CD32A-Ig molecules.
Figure 2. Binding of CD32A-Ig molecules to soluble immune complex (sIC).
A 96 well plate was coated with 50 μl (10 μg/ml) CD32A-Ig molecules overnight at 4°C and wells were blocked with binding buffer (PBS/5 mM EDTA with 1% BSA). After washing the wells three times with binding buffer, 50 μl of HRP conjugated sIC of human IgG subtypes (5 μg/ml) were added and incubated for another 1h at 4 °C. Wells were washed three times; color was developed by adding the HRP substrate and read at 450 nm. IV.3 treated wells served as a specificity control. The ELISA O.D readings after blocking with IV.3 mAb were as follows. For CD32AH-Ig coated wells: IgG1=0.017, IgG2=0.019, IgG3=0.016, IgG4=0.018, and for CD32AR-Ig coated wells: IgG1=0.011, IgG2=0.015, IgG3=0.017, IgG4=0.02. The values are average of triplicate readings and representative of three individual experiments. The values are comparable with BSA coated negative control wells. *p<0.01, **P<0.001.
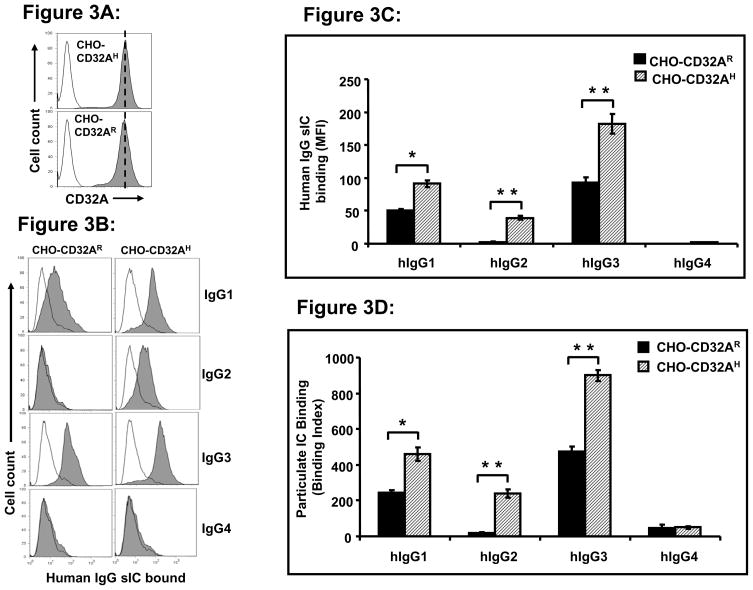
Next, we determined whether the dimerized CD32A alleles had a ligand binding pattern similar to cell surface CD32A alleles. To test this we assayed the CHO transfectants expressing the CD32AR and CD32AH alleles separately. Flow cytometry analysis using IV.3 mAb confirmed that both alleles of CD32A were expressed to almost similar levels on CHO cell surface (Fig. 3A). sIC of human IgG subtypes conjugated with FITC were prepared as described under Materials and Methods. Flow cytometry analysis of FITC-conjugated IC showed that CHO cells expressing CD32AH allele (CHO-CD32AH) bound to human IgG1, IgG2 and IgG3, whereas CHO-CD32AR bound to IgG1 and IgG3 but not to IgG2 (Fig. 3B, closed histogram). As a specificity control for FITC F(ab′)2 binding, the CHO-CD32A cells were treated with IV.3 antibody before the addition of FITC labeled-IC. Results show that the FITC labeled-IC was completely blocked by IV.3 suggesting that the uncomplexed FITC F(ab′)2 does not bind non-specifically to CD32A expressed on CHO cells (Fig. 3B; open histogram). Figure 3C shows the graphical representation of the level of human IgG subtypes ICs bound to CHO-CD32A alleles. Thus, as we observed in the previous experiment with dimerized CD32A-Ig (Fig. 2), both of the cell surface CD32A alleles bind to IgG1 and IgG3, but CD32AH binds better than CD32AR (Fig. 3C).
Figure 3.
(A) Flow cytometry analysis of expression of CD32A alleles in CHO cells: Stable transfectants of CHO cells expressing CD32A alleles were stained with anti-hCD32A mAb (IV.3). Open histogram shows the isotype control, solid histogram indicates the binding of IV.3 antibody to CD32A alleles. Dotted line indicates the expression level of CD32A alleles. (B) Binding of cell surface CD32A alleles to soluble immune complex (sIC) of human IgG subtypes. CHO cells expressing CD32A alleles were incubated with FITC conjugated sIC of human IgG subtypes (10 μg/ml) in the presence and absence of IV.3 (10 μg/ml) and analyzed for FITC-IC binding to CHO-CD32A alleles using flow cytometry (solid histogram). IV.3 treated cells served as a negative control (open histogram). Data is representative of three individual experiments. (C). Graphical representation of human IgG subtypes sIC bound to CHO-CD32A alleles. Values are mean ± SD of data from three experiments. *p<0.01, **P<0.001. (D) Binding of cell surface CD32A alleles to human IgG subtypes coated with sheep erythrocytes (EA). CHO cells expressing CD32A alleles were incubated with human IgG subtype coated, PKH-labeled-EA (50 μl of 1.5 x 108 cells) for 2 h at 4°C. Values are mean ± SD of data from three experiments. EA bound to the cells were analyzed by flow cytometry. Cells incubated with PKH-labeled unopsonized-E and IV.3 treated cells served as specificity control. The binding index was calculated using the following formula: % cells bound to EA x mean fluorescence/100. Values are mean ± SD of data from three experiments. *p<0.01, **P<0.001.
The ICs formed between autoantibodies and target cells are particulate in nature. Therefore, we determined the binding pattern of CD32A alleles to particulate IC of hIgG subtypes using CHO cells expressing CD32AR and CD32AH forms. As shown in figure 3D, CHO cells expressing CD32AH alleles bound to human IgG1, IgG2 and IgG3 whereas CHO-CD32AR cells bound to IgG1 and IgG3 but not to IgG2. Neither of the alleles bound to IgG4. These results show that CD32AR and CD32AH alleles have a similar binding pattern for both soluble and particulate ICs of human IgG subtypes. Soluble and particulate IC binding to CD32A alleles was completely blocked by IV.3 mAb (a monoclonal antibody to CD32A) and served as a specificity control. These results suggest that dimeric recombinant CD32A-Ig molecules and cell surface CD32A alleles interact similarly with human IgG subtypes. Furthermore, dimerization of the recombinant CD32A-Ig molecules did not change its binding specificity to human IgG subtypes. Interestingly, though both CD32A alleles bound to IgG1 and IgG3, CD32AH allele binding was better than the CD32AR allele. Taken together, these results suggest that CD32A alleles not only differ in binding to IgG2 but also to IgG1 and IgG3.
CD32AH allele outcompetes CD32AR allele for ligand binding when both alleles are exposed to IC simultaneously
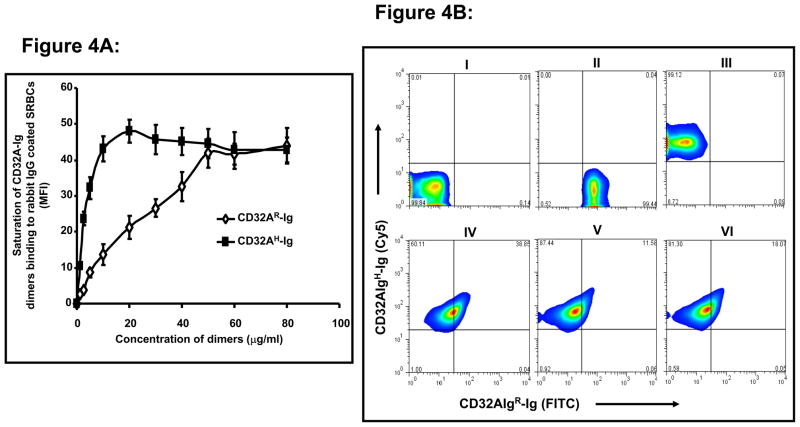
Since previous ligand binding experiments suggest that CD32AH binds to IC more efficiently than CD32AR allele, next we studied whether CD32AH allele competes with CD32AR allele for ligand binding when both the alleles are exposed to IC simultaneously. We studied the competition between these alleles for ligand binding using purified CD32A-Ig molecules. A simple in vitro competition assay was performed to directly determine whether CD32AH competes with CD32AR for ligand binding. In this experiment, we used rabbit IgG coated SRBCs (EA) as a model IC and anti-human Fc specific antibody for detection. First, we determined the saturating concentration of the dimers binding to rabbit IgG coated SRBCs by incubating rabbit IgG coated SRBCs with various concentration of CD32AR-Ig and CD32AH-Ig separately. FITC-conjugated F(ab′)2 goat anti-human Fc specific antibody was used to detect binding via flow cytometry. As shown in figure 4A, CD32AH-Ig reaches saturation around 25 μg/ml, whereas CD32AR-Ig requires 50 μg/ml. These results suggest that these molecules differ significantly (p<0.001) in their affinity for rabbit IgG binding, which is consistent with our previous report (38). Then we determined the competition between these molecules for binding to rabbit IgG when both the alleles are exposed simultaneously to IC. In this experiment, we incubated the EA with either equimolar (25 μg/ml of each CD32A-Ig molecules) or saturating concentrations (CD32AR-Ig: 50 μg/ml, CD32AH-Ig: 25 μg/ml) of CD32A-Ig molecules. CD32AH-Ig was pre-complexed with Cy5-conjugated goat anti-human Fc specific F(ab′)2 antibody, whereas CD32AR-Ig was pre-complexed with FITC-conjugated goat anti-human Fc specific F(ab′)2 antibody. Both molecules (either equimolar or saturating concentration) were then added to the EA to study the competition of these molecules for ligand binding. The secondary antibody was titrated and excess dimers were added to avoid cross-binding of the secondary antibody to the dimers during the incubation of the dimers with EA. As shown in figure 4B, both CD32AR-Ig (Panel-II) and CD32AH-Ig (Panel-III) molecules bind to rabbit IgG coated SRBCs when incubated separately. When both molecules were incubated together at their saturation concentrations (panel-IV, CD32AR-Ig: 50 μg/ml, CD32AH-Ig: 25 μg/ml), 60% of the EA were Cy5 positive, indicating that 60% of EA bound only to CD32AH-Ig. Only 40% of the EA were both Cy5 and FITC positive, suggesting that 40% of EA bound to both the dimers. At equimolar concentrations (panel-V, 25 μg/ml of both molecules; panel-VI, 50 μg/ml of both the molecules) of the dimers, more than 80% of EA were Cy5 positive, indicating that more than 80% of EA was bound only to CD32AH-Ig. Only less than 20% of the EA were both Cy5 and FITC positive, suggesting that less than 20% of EA bound to both the dimers. The EA treated with secondary antibody alone served as a specificity control (panel-I). These results suggest that CD32AH allele has a higher binding efficiency for IC and thus outcompetes the R allelic form for ligand binding under situations where both alleles are exposed to the same ligand simultaneously, as may happen in R/H heterozygous individuals.
Figure 4. Competition of CD32AR-Ig and CD32AH-Ig for binding to rabbit IgG opsonized SRBCs.
(A) To determine the saturating concentration of CD32A-Ig molecules binding to rabbit IgG coated erythrocytes (EA), varying concentrations of CD32A-Ig molecules were incubated separately with 50 μl of EA (2 × 108 cells/ml) for 1h at 4 °C. After washing three times with binding buffer, the incubation was continued with F(ab′)2 of goat anti human Fc specific antibody conjugated with FITC for 1 h at 4 °C. Then Cells were analyzed for CD32A-Ig molecules binding by flow cytometry. (B) For competition assay, an equal amount or saturating concentration of both the molecules were mixed and incubated with EA. The CD32A-Ig molecules were preincubated with F(ab′)2 of goat anti human Fc specific antibody conjugated with Cy5 (for CD32AH-Ig) or FITC (for CD32AR-Ig) for 1 h at 4 °C and then added to EA to determine the competitive binding of both the alleles to EA. The cells were washed and analyzed for CD32A-Ig binding by flow cytometry. The cells treated with secondary antibody alone served as a specificity control. (I) EA treated with secondary antibody alone, (II) EA incubated with 50 μg/ml of FITC-CD32AR-Ig, (III) EA incubated with 50 μg/ml of Cy5-CD32AH-Ig, (IV) EA incubated with CD32AR-Ig (50 μg/ml) and CD32AH-Ig (25 μg/ml), (V) EA incubated with CD32AR-Ig (25 μg/ml) and CD32AH-Ig (25 μg/ml), (VI) EA incubated with CD32AR-Ig (50 μg/ml) and CD32AH-Ig (50 μg/ml). Lower left quadrant: unstained cells, lower right: CD32AR-Ig bound EA, upper left: CD32AH-Ig bound EA, upper right: EA bound to both the CD32A-Ig molecules. Data is representative of three individual experiments.
CD32AH-Ig is able to cross-block IC binding mediated by cell surface CD32AR
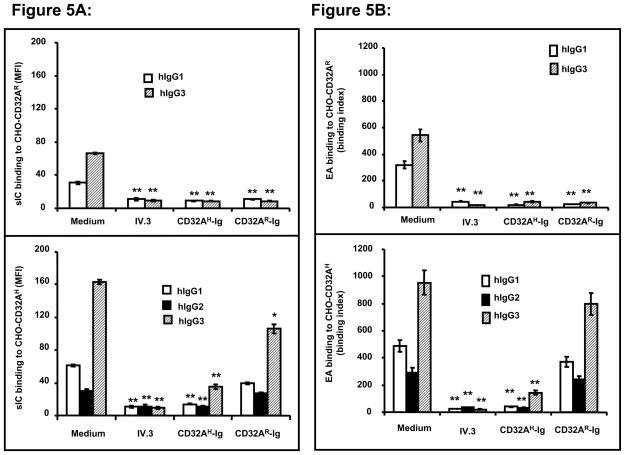
Next we determined whether the CD32AH-Ig molecules can cross-block the ligand binding mediated by the cell surface CD32AR alleles. The cross-blocking studies were carried out using CHO transfectants expressing CD32AR and CD32AH alleles separately. In this experiment we have used human IgG1 and IgG3 for CHO-CD32AR, whereas IgG1, IgG2 and IgG3 were used for CHO-CD32AH for both soluble and particulate IC. As shown in figure 5A, at 25 μg/ml, CD32AH-Ig was able to block more than 90% of human IgG subtype binding to both the cell surface CD32A alleles. At the same concentration, CD32AR-Ig was able to block up to 90% of human IgG binding to CHO-CD32AR, but only cross-blocked 10% of human IgG binding to CHO-CD32AH (Fig. 5A; compare lower and upper panel). Similar results were observed when EA was used as an IC (Fig. 5B). These data suggest that the CD32AH-Ig cross-blocks the ligand binding mediated by cell surface CD32AR alleles, whereas CD32AR-Ig is unable to cross-block the ligand binding mediated by cell surface CD32AH allele.
Figure 5.
(A) Soluble CD32A-Ig molecules compete with CD32A alleles expressed on CHO cell surface and block binding of soluble immune-complex of human IgG subtypes. A: CHO-CD32AR (upper panel) or CHO-CD32AH (lower panel) cells were incubated with FITC-IC (sIC; 10 μg/ml) in the presence and absence of CD32A-Ig molecules (50 μg/ml) and analyzed. IV.3 antibody treated cells served as a specificity control. Blocking reagents are represented on the Y-axis. Values are mean ± SD of data from three experiments. *p<0.01, **P<0.001. (B) Soluble CD32A-Ig molecules compete with cell surface CHO-CD32A alleles and block binding of erythrocytes coated with human IgG subtypes. CHO-CD32AR (upper panel) or CHO-CD32AH (lower panel) cells were incubated human IgG subtype coated, PKH-labeled-EA (50 μl of 1.5 × 108 cells) for 2 h at 4 °C in the presence and absence of purified CD32A-Ig molecules (50 μg/ml). The cells were analyzed for binding of EA using flow cytometry. IV.3 treated cells and cells incubated with PKH-labeled unopsonized SRBCs served as a specificity control. Blocking reagents are represented on the Y-axis. The binding index was calculated using the following formula: % cells bound to EA × mean fluorescence/100. Values are mean ± SD of data from three experiments. *p<0.01, **P<0.001.
CD32AH allele has higher affinity for all human IgG subtypes than CD32AR allele
The above ligand binding studies and cross-blocking studies suggest that the CD32A alleles might differ in their affinity not only for human IgG2 but also for IgG1 and IgG3. Using a micropipette adhesion frequency assay, we measured the 2D binding affinity of the two CD32A alleles (CHO-CD32AR and CHO-CD32AH) for four human IgG subtypes (IgG1, 2, 3, and 4). The adhesion frequencies of CHO cells expressing either CD32AR or CD32AH to red blood cells coated with one of the four subtypes of human IgG were measured and the effective binding affinities were calculated using Equation-2 as described under Materials and Methods. The typical binding is represented in figure 6A. The results showed that CD32AH has higher binding affinity than CD32AR for IgG1, IgG2, and IgG3 (Fig. 6B). Both alleles have a similarly low binding affinity for IgG4. We observed that CD32AH has 1.8, 5.8 and 2.22 fold higher affinity for IgG1, IgG2 and IgG3 when compared to CD32AR (Table I). For both CD32A alleles, the binding affinity for human IgG subtypes ranked as IgG3>IgG1>IgG2>IgG4. These results indicate that the CD32A alleles not only differ in human IgG2 binding but also differ substantially in their affinity for IgG1 and IgG3.
Table I.
Affinity values of cell surface CD32A alleles by 2D affinity measurement.
| Human IgG subtypes | hIgG1 | hIgG2 | hIgG3 | hIgG4 |
|---|---|---|---|---|
| CHO-CD32AR | 7.98×10−06 (μm4) | 2.34×10−06 (μm4) | 1.45×10−05 (μm4) | 1.31×10−06 (μm4) |
| CHO-CD32AH | 1.77×10−05 (μm4) | 1.38×10−05 (μm4) | 2.61×10−05 (μm4) | 1.39×10−06 (μm4) |
DISCUSSION
The importance of low affinity FcγRs in the development of autoimmune diseases and defense against bacterial infections has recently been well documented (16,22,23,25,27). The consequences of polymorphisms in CD32A, particularly their different ligand binding characteristics, are being intensely investigated due to their role in certain infectious and autoimmune diseases. CD32AH has been shown to bind to IgG2 with high affinity whereas CD32AR shows little or no binding to IgG2 (16–18,39–41). Several studies have documented that CD32AR homozygous individuals are more susceptible to bacterial infections when compared to CD32AH homozygous or CD32AR/H heterozygous individuals (7,8). Most of the bacterial infections elicit predominantly IgG2 antibody response (16–18). Because CD32A is the only known FcγR that binds to IgG2 with high affinity, individuals homozygous for CD32AR might be more susceptible to bacterial infections when compared to CD32AH homozygous individuals due to the lack of CD32A-IgG2 interaction.
In addition to the IgG2 mediated response, there are several studies that document the pivotal role of IgG1 and IgG3 subtypes in protective immunity against bacterial infections (42,43). In this report, we have shown that CD32A R and H alleles not only differ in their binding to human IgG2, which is in agreement with earlier reports, but also differ significantly in their affinity for IgG1 and IgG3. These results suggest that reduced binding of CD32AR to IgG1 and IgG3 may also be a contributing factor to the higher incidence of bacterial infections in CD32AR homozygous individuals. Our observation with IgG1 binding contrasts but agrees on IgG3 binding with a previous report by Bredius et al. (44) which shows that CD32A alleles differ in binding to IgG2 and IgG3 but not IgG1. A recent report by Bruhns et al. (9) has shown that CD32A alleles do not differ in binding to IgG1 and IgG3. At present, the reason for these discrepancies is not clear but it may be due to the use of different cell types and molecules. The report by Bredius et al. used neutrophils treated with anti-CD16 monoclonal antibody, whereas Bruhns at al. used CD32A fused with FLAG peptide either as expressed on CHO cells or purified molecules. In this report we have used CHO cells expressing the unmodified CD32A alleles and purified dimeric CD32A-Igs. Furthermore, we have used a 2D affinity measurement technique (33) which directly measures the affinity of a receptor-ligand pair expressed on cell membranes when they are mediating cell-cell adhesion as happens during FcγR expressing cells binding to antibody coated target cells.
Interestingly, CD32AR/H heterozygous individuals are not susceptible to bacterial infections and have a phenotype similar to CD32AH individuals. To determine whether this is due to the dominant effect of CD32AH binding to ICs, we studied the competition between these alleles for ligand binding using purified molecules. The results of the competition assay using rabbit IgG coated SRBCs suggest that when both alleles are exposed to the same ligand simultaneously, CD32AH outcompetes CD32AR for ligand binding. Furthermore, our results show that purified CD32AH-Ig is capable of blocking both the cell surface CD32A R and H alleles from binding to human IgG subtypes, whereas purified CD32AR-Ig was unable to cross-block the binding mediated by cell surface CD32AH allele. These differences in the ability to compete for the ligand and cross-block each other may be due to differences in the strength of the cell-cell adhesion mediated by these molecules, as evidenced by differences in their 2D affinity. For instance, CD32AH has a two fold higher affinity for IgG1 and IgG3 and a five fold higher affinity for IgG2 compared to the CD32AR allele. The results from binding and affinity studies suggest that individuals homozygous for the CD32AR allele have lower binding affinity for all human IgG subtypes and therefore may not be able to bind to antibody-coated bacteria effectively, becoming susceptible to infection as a result. In individuals homozygous for CD32AH allele, the H allele has a higher affinity for all human IgG subtypes and binds more effectively to antibody-coated bacteria leading to quicker clearance of infection. In heterozygous individuals, as the CD32AH allele has a higher affinity for all human IgG subtypes, it is able to outcompete the CD32AR allele for binding to antibody-coated bacteria. Consequently, the heterozygous individuals display a phenotype similar to the CD32AH homozygous individuals.
Apart from bacterial infections, studies have shown an association between CD32AR and immune-complex mediated autoimmune diseases such as SLE (20,21). In humans, individuals who express only CD32AR are more susceptible to antibody-mediated autoimmune diseases than individuals expressing the CD32AH allele, which binds to human IgG with higher affinity (16–18). This increased susceptibility in CD32AR individuals is suggested to be due to the inability of CD32AR to bind and clear IC from circulation leading to the accumulation and deposition of ICs on tissues (26,27,45,46). In humans, the ICs formed are not only with IgG2 but also with other IgG subtypes and therefore the accumulation of ICs in CD32AR individuals cannot be attributed to the inefficient binding of IgG2 alone. Our findings suggest that CD32AR individuals have a lower affinity not only for IgG2 but also for human IgG1 and IgG3 subtypes, and this might explain why ICs from the circulation are not cleared efficiently. The ICs deposited on tissues in CD32AR homozygous individuals may lead to the development and/or acceleration of the disease via complement activation and engagement of other activating FcγRs such as CD16. Several studies suggest the involvement of complement proteins (47–52) and CD16 (16,53,54) in the development of autoimmune diseases.
In conclusion, our results demonstrate that the H allelic form of CD32A has a higher affinity for human IgG1, IgG2, and IgG3 subtypes and outcompetes the R allelic form for ligand binding when both alleles are co-expressed on the same cell surface. As a result of this, the inflammatory cells from CD32AR/H heterozygous individuals may predominantly use the H allele to mediate antibody-coated target cell binding during phagocytosis and ADCC, resulting in a phenotype similar to CD32AH homozygous individuals. Furthermore, our data also suggest that CD32AR has lower affinity not only for the IgG2 subtype but also for IgG1 and IgG3 subtypes, which results in the lack of clearance of ICs from circulation leading to IC-mediated autoimmune disease susceptibility.
Acknowledgments
The authors thank Saranya Selvaraj, Archana Boopathy, Erica Bozeman, and Jaina Patel for critical reading of the manuscript.
This study was supported by a NIH grant RO1 AI049400 (P.S), a grant from Emory University seed grant program (P.S) and a NIH grant RO1 AI38282 (C.Z).
References
- 1.Hulett MD, Hogarth PM. Molecular basis of Fc receptor function. Adv Immunol. 1994;57:1–127. doi: 10.1016/s0065-2776(08)60671-9. [DOI] [PubMed] [Google Scholar]
- 2.Kimberly RP, Salmon JE, Edberg JC. Receptors for immunoglobulin G. Molecular diversity and implications for disease. Arthritis Rheum. 1995;38:306–314. doi: 10.1002/art.1780380303. [DOI] [PubMed] [Google Scholar]
- 3.Ravetch JV. Fc receptors. Curr Opin Immunol. 1997;9:121–125. doi: 10.1016/s0952-7915(97)80168-9. [DOI] [PubMed] [Google Scholar]
- 4.Unkeless JC, Scigliano E, Freedman VH. Structure and function of human and murine receptors for IgG. Annu Rev Immunol. 1988;6:251–281. doi: 10.1146/annurev.iy.06.040188.001343. [DOI] [PubMed] [Google Scholar]
- 5.Selvaraj P, Fifadara N, Nagarajan S, Cimino A, Wang G. Functional regulation of human neutrophil Fcgamma receptors. Immunol Res. 2004;29:219–230. doi: 10.1385/IR:29:1-3:219. [DOI] [PubMed] [Google Scholar]
- 6.Selvaraj P, Rosse WF, Silber R, Springer TA. The major Fc receptor in blood has a phosphatidylinositol anchor and is deficient in paroxysmal nocturnal hemoglobinuria. Nature. 1988;333:565–567. doi: 10.1038/333565a0. [DOI] [PubMed] [Google Scholar]
- 7.Parren PW, Warmerdam PA, Boeije LC, Arts J, Westerdaal NA, Vlug A, Capel PJ, Aarden LA, van de Winkel JG. On the interaction of IgG subclasses with the low affinity Fcgamma RIIa (CD32) on human monocytes, neutrophils, and platelets. Analysis of a functional polymorphism to human IgG2. J Clin Invest. 1992;90:1537–1546. doi: 10.1172/JCI116022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salmon JE, Edberg JC, Brogle N, Kimberly RP. Allelic Polymorphisms of Human Fcgamma receptor IIA and Fcgamma receptor IIIB. Independent mechanism for differences in human phagocyte function. J Clin Invest. 1992;89:1274–1281. doi: 10.1172/JCI115712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daëron M. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716–3725. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 10.Sanders LA, van de Winkel JG, Rijkers GT, Voorhorst-Ogink MM, de Haas M, Capel PJ, Zegers BJ. Fcgamma receptor IIa (CD32) heterogeneity in patients with recurrent bacterial respiratory tract infections. J Infect Dis. 1994;170:854–861. doi: 10.1093/infdis/170.4.854. [DOI] [PubMed] [Google Scholar]
- 11.Yee AM, Phan HM, Zuniga R, Salmon JE, Musher DM. Association between Fcgamma RIIa-R131 allotype and bacteremic pneumococcal pneumonia. Clin Infect Dis. 2000;30:25–28. doi: 10.1086/313588. [DOI] [PubMed] [Google Scholar]
- 12.Domingo P, Muniz-Diaz E, Baraldes MA, Arilla M, Barquet N, Pericas R, Juarez C, Madoz P, Vázquez G. Association between Fcgamma receptor IIa polymorphisms and the risk and prognosis of meningococcal disease. Am J Med. 2002;112:19–25. doi: 10.1016/s0002-9343(01)01047-6. [DOI] [PubMed] [Google Scholar]
- 13.Platonov AE, Shipulin GA, Vershinina IV, Dankert J, van de Winkel JG, Kuijper EJ. Association of human Fcgamma RIIa (CD32) polymorphism with susceptibility to and severity of meningococcal disease. Clin Infect Dis. 1998;27:746–750. doi: 10.1086/514935. [DOI] [PubMed] [Google Scholar]
- 14.Moens L, Van Hoeyveld E, Vrhaegen J, De Boeck K, Peetermans WE, Bossuyt X. Fcgamma receptorIIA genotype and invasive pneumococcal infection. Clin Immunol. 2006;118:20–23. doi: 10.1016/j.clim.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Endeman H, Cornips MC, Grutters JC, van der Bosch JM, Ruven HJ, van Velzen-Blad H, Rijkers GT, Biesma DH. The Fcgamma receptor IIA-R/R131 genotype is associated with sever sepsis in community acquired pneumonia. Clin Vaccine Immunolo. 2009;16:1087–1090. doi: 10.1128/CVI.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Sorge NM, van der Pol WL, Van de Winkel JGJ. FcgammaR Polymorphisms: Implications for function, disease susceptibility and immunotherapy. Tis Ant. 2003;61:189–202. doi: 10.1034/j.1399-0039.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- 17.Sanders LA, Feldman RG, Voorhorst-Ogink MM, de Haas M, Rijkers GT, Capel PJ, Zegers BJ, van de Winkel JG. Human immunoglobulin G (IgG) Fc receptor IIA (CD32) polymorphism and IgG2-mediated bacterial phagocytosis by neutrophils. Pediatr Infect Dis J. 1995;63:73–81. doi: 10.1128/iai.63.1.73-81.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrante A, Beard LJ, Feldman RG. IgG subclass distribution of antibodies to bacterial and viral antigens. Pediatr Infect Dis J. 1990;9:S16–S24. [PubMed] [Google Scholar]
- 19.Yee AMF, Ng SC, Sobel RE, Salmon JE. Fc gamma RIIA polymorphism as a risk factor for invasive pneumococcal infections in systemic lupus erythematosus. Arthritis and Rheum. 1997;40:1180–1182. doi: 10.1002/art.1780400626. [DOI] [PubMed] [Google Scholar]
- 20.Karassa FB, Trikalions TA, Ioannidis JP. Role of Fc gamma receptor IIa polymorphism in susceptibility to systemic lupus erythematosus and lupus nephritis: a meta-analysis. Arthritis Rheum. 2002;46:1563–1571. doi: 10.1002/art.10306. [DOI] [PubMed] [Google Scholar]
- 21.Dijstelbloem HM, Scheepers RH, Oost WW, Stegeman CA, van Der Pol WL, Sluiter WJ, Kallenberg CG, van de Winkel JG, Tervaert JW. Fcgamma receptor polymorphisms in Wegener’s granulomatosis: risk factors for disease relapse. Arthritis and Rheum. 1999;42:1823–1827. doi: 10.1002/1529-0131(199909)42:9<1823::AID-ANR5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 22.Ludo van der Pol W, van de winkel GJ. IgG receptor polymorphism: risk factor for disease. Immunogen. 1998;48:222–232. doi: 10.1007/s002510050426. [DOI] [PubMed] [Google Scholar]
- 23.Tan SY. Fcgamma RIIa polymorphism in systemic lupus erythematosus. Kidney Blood Press Res. 2000;23:138–142. doi: 10.1159/000025967. [DOI] [PubMed] [Google Scholar]
- 24.Edberg JC, Wainstein E, Wu J, Csernok E, Sneller MC, Hoffman GS, Keystone EC, Gross WL, Kimberly RP. Analysis of Fcgamma RII gene polymorphisms in Wegener’s granulomatosis. Exp Clin Immunogenet. 1997;14:183–195. [PubMed] [Google Scholar]
- 25.Manger K, Repp R, Spriewald BM, Rascu A, Geiger A, Wassmuth R, Westerdaal NA, Wentz B, Manger B, Kalden JR, van de Winkel JG. Fcgamma receptor IIa polymorphism in caucasian patients with systemic lupus erythromatosus: association with clinical symptoms. Arthritis Rheum. 1998;41:1181–1189. doi: 10.1002/1529-0131(199807)41:7<1181::AID-ART6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 26.Carlsson LE, Satoso S, Baurichter G, Kroll H, Papenberg S, Eichler P, Westerdaal NA, Kiefel V, van de Winkel JG, Greinacher A. Heparin-induced thrombocytopenia: new insights into the impact of the FcgammaRIIa-R-H131 polymorphims. Blood. 1998;92:1526–1531. [PubMed] [Google Scholar]
- 27.Dijstelbloem HM, Bijl M, Fijnheer R, Scheepers RH, Oost WW, Jansen MD, Sluiter WJ, Limburg PC, Derksen RH, van de Winkel JG. Fcgamma receptor polymorphisms in systemic lupus erythematosus: association with disease and in vivo clearance of immune complexes. Arthritis and Rheum. 2000;43:2793–2800. doi: 10.1002/1529-0131(200012)43:12<2793::AID-ANR20>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 28.Li P, Nagarajan S, Zhu C, Selvaraj P. Recombinant CD16A-Ig forms a homodimer and cross-blocks the ligand binding functions of neutrophil and monocyte Fcgamma receptors. Mol Immunol. 2001;38:527–538. doi: 10.1016/s0161-5890(01)00088-8. [DOI] [PubMed] [Google Scholar]
- 29.Chappel MS, Isenman DE, Everett M, Xu YY, Dorrington KJ, Klein MH. Identification of the Fcgamma receptor class I binding site in human IgG through the use of recombinant IgG1/IgG2 hybrid and point-mutated antibodies. Proc Natl Acad Sci USA. 1991;88:9036–9040. doi: 10.1073/pnas.88.20.9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chappel MS, Isenman DE, Oomen R, Xu YY, Klein MH. Identification of a secondary Fcgamma RI binding site within a genetically engineered human IgG antibody. J Biol Chem. 1993;268:25124–25131. [PubMed] [Google Scholar]
- 31.Ledbetter JA, Gilliand LK, Hayden MS, Linsley PS, Bajorath J, Fell HP. Expression vectors encoding bispecific fusion proteins and methods of producing biologically active bispecific fusion proteins in mammalian cells. 6132992 United States Patent. 2000
- 32.Shashidharamurthy R, Hennigar RA, Fuchs S, Palaniswami P, Sherman M, Selvaraj P. Extravasations and emigration of neutrophils to the inflammatory site depend on the interaction of immune-complex with Fcgamma receptors and can be effectively blocked by decoy Fcgamma receptors. Blood. 2008;111:894–904. doi: 10.1182/blood-2007-04-085944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McHugh RS, Ahmed SN, Wang YC, Sell KW, Selvaraj P. Construction, purification and functional reconstitution on tumor cells of glycolipid-anchored human B7-1 (CD80) Proc Natl Acad Sci USA. 1995;92:8059–8063. doi: 10.1073/pnas.92.17.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chesla SE, Selvaraj P, Zhu C. Measuring two-dimensional receptor-ligand binding kinetics by micropipette. Biophys J. 1998;75:1553–1572. doi: 10.1016/S0006-3495(98)74074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pricop L, Salmon JE, Edberg JC, Beavis AJ. Flow cytometric quantitation of attachment and phagocytosis in phenotypically-defined subpopulations of cells using PKH26-labeled Fcgamma R-specific probes. J Immunol Methods. 1997;205:55–65. doi: 10.1016/s0022-1759(97)00053-7. [DOI] [PubMed] [Google Scholar]
- 36.Williams TE, Selvaraj P, Zhu C. Concurrent binding to multiple ligands: kinetic rates of CD16b for membrane-bound IgG1 and IgG2. Biophys J. 2000;79:1858–1866. doi: 10.1016/S0006-3495(00)76435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams TE, Nagarajan S, Selvaraj P, Zhu C. Concurrent and independent binding of Fcgamma receptors IIa and IIIb to surface-bound IgG. Biophys J. 2000;79:1867–1875. doi: 10.1016/S0006-3495(00)76436-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shashidharamurthy R, Amano A, Ezekwudo D, Selvaraj P. Analysis of competitive interaction of H and R allelic forms of CD32A with rabbit IgG immune-complex. Proc 13th Intl. Cong. Immunol; 2007. pp. 513–522. [Google Scholar]
- 39.Clark MR, Clarkson SB, Ory PA, Stollman N, Goldstein IM. Molecular basis for a polymorphism involving Fc receptor II on human monocytes. J Immunol. 1989;143:1731–1734. [PubMed] [Google Scholar]
- 40.Clark MR, Stuart S, Kimberly RP, Ory PA, Goldstein IM. A single amino acid distinguishes the high-responder from the low-responder form of Fc receptor II on human monocytes. Eur J Immunol. 1991;21:1911–1916. doi: 10.1002/eji.1830210820. [DOI] [PubMed] [Google Scholar]
- 41.Warmerdam PAM, VanDeWinkel JGJ, Vlug A, Westerdaal NAC, Capel PJA. A Single Amino Acid in the Second Ig-Like Domain of the Human Fc-gamma Receptor II is Critical for Human IgG2 Binding. J Immunol. 1991;147:1338–1343. [PubMed] [Google Scholar]
- 42.Falconer AE, Carson R, Johnstone R, Bird P, Kehoe M, Calvert JE. Distinct IgG1 and IgG3 subclass responses to two streptococcal protein antigens in man: analysis of antibodies to streptolysin O and M protein using standardized subclass-specific enzyme-linked immunosorbent assays. Immunology. 1993;79:89–94. [PMC free article] [PubMed] [Google Scholar]
- 43.Hussain R, Kifayet A, Chiang TJ. Immunoglobulin G1 (IgG1) and IgG3 antibodies are markers of progressive disease in leprosy. Pediatr Infect Dis J. 1995;63:410–415. doi: 10.1128/iai.63.2.410-415.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bredius RG, Fijen CA, de Haas M, Kuijper EJ, Weening RS, van de Winkel JG, Out TA. Role of neutrophil Fcgamma RIIa (CD32) and Fcgamma RIIIb (CD16) polymorphic forms in phagocytosis of human IgG1- and IgG3-opsonized bacteria and erythrocytes. Immunology. 1994;83:624–630. [PMC free article] [PubMed] [Google Scholar]
- 45.Miescher S, Spycher MO, Amstutz H, de Haas M, Kleijer M, Kalus UJ, Radtke H, Hubsch A, Andresen I, Martin RM, Bichler J. A single recombinant anti-RhD IgG prevents RhD immunization: association of RhD-positive red blood cell clearance rate with polymorphisms in the Fcgamma RIIA and Fcgamma IIIA genes. Blood. 2004;103:4028–4035. doi: 10.1182/blood-2003-11-3929. [DOI] [PubMed] [Google Scholar]
- 46.Zuniga R, Markowitz GS, Arkachaisri T, Imperatore EA, D’Agati VD, Salmon JE. Identification of IgG subclasses and C-reactive protein in lupus nephritis: the relationship between the composition of immune deposits and Fcgamma receptor type IIA alleles. Arthritis and Rheum. 2003;48:460–470. doi: 10.1002/art.10930. [DOI] [PubMed] [Google Scholar]
- 47.Ingram G, Hakobyan S, Robertson NP, Morgan BP. Complement in multiple sclerosis: its role in disease and potential as biomarker. Clin Exp Immunol. 2009;155:128–139. doi: 10.1111/j.1365-2249.2008.03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lessey E, Li N, Diaz L, Liu Z. Complement and cutaneous autoimmune blistering diseases. Immunol Res. 2008;41:223–232. doi: 10.1007/s12026-008-8028-y. [DOI] [PubMed] [Google Scholar]
- 49.Holers VM. The spectrum of complement alternative pathway-mediated diseases. Immunol Rev. 2008;223:300–316. doi: 10.1111/j.1600-065X.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- 50.Ruiz-Arguelles A, Llorente L. The role of complement regulatory proteins (CD55 and CD59) in the pathogenesis of autoimmune hemocytopenias. Autoimmun Rev. 2007;6:155–161. doi: 10.1016/j.autrev.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 51.Seelen MA, Daha MR. The role of complement in autoimmune renal disease. Autoimmunity. 2006;39:411–415. doi: 10.1080/08916930600739688. [DOI] [PubMed] [Google Scholar]
- 52.Lemieux R, Bazin R. Autoantibody-induced formation of immune complexes in normal human serum. curr Pharm des. 2006;12:173–179. doi: 10.2174/138161206775193055. [DOI] [PubMed] [Google Scholar]
- 53.Meyer D, Schiller C, Westermann J, Izui S, Hazenbos WLW, Verbeek JS, Schmidt RE, Gessner JE. Fcgamma RIII (CD16)-deficient mice show IgG isotype-dependent protection to experimental autoimmune hemolytic anemia. Blood. 1998;92:3997–4002. [PubMed] [Google Scholar]
- 54.Wu J, Edberg JC, Redecha PB, Bansal V, Guyre PM, Coleman K, Salmon JE, Kimberly RP. A novel polymorphism of Fcgamma RIIIa (CD16) alters receptor function and predisposes to autoimmune disease. J Clin Invest. 1997;100:1059–1070. doi: 10.1172/JCI119616. [DOI] [PMC free article] [PubMed] [Google Scholar]