Abstract
Hyaluronan is a prominent component of the micro-environment in most malignant tumors and can be prognostic for tumor progression. Extensive experimental evidence in animal models implicates hyaluronan interactions in tumor growth and metastasis, but it is also evident that a balance of synthesis and turnover by hyaluronidases is critical. CD44, a major hyaluronan receptor, is commonly but not uniformly associated with malignancy, and is frequently used as a marker for cancer stem cells in human carcinomas. Multivalent interactions of hyaluronan with CD44 collaborate in driving numerous tumor-promoting signaling pathways and transporter activities. It is widely accepted that hyaluronan-CD44 interactions are crucial in both malignancy and resistance to therapy, but major challenges for future research in the field are the mechanism of activation of hyaluronan-CD44 signaling in cancer cells, the relative importance of variant forms of CD44 and other hyaluronan receptors, e.g. Rhamm, in different tumor contexts, and the role of stromal versus tumor cell production and turnover of hyaluronan. Despite these caveats, it is clear that hyaluronan-CD44 interactions are an important target for translation into the clinic. Among the approaches that show promise are antibodies and vaccines to specific variants of CD44 that are uniquely expressed at critical stages of progression of a particular cancer, hyaluronidase-mediated reduction of barriers to drug access, and small hyaluronan oligosaccharides that attenuate constitutive hyaluronan-receptor signaling and enhance chemosensitivity. In addition, hyaluronan is being used to tag drugs and delivery vehicles for targeting of anti-cancer agents to CD44-expressing tumor cells.
Background
The importance of the micro-environment in tumor progression is now well established (1-4). Hyaluronan is a prominent component of this micro-environment in most malignant tumors, both in the pericellular milieu immediately surrounding tumor cells and in the tumor stroma; its association with either compartment can be prognostic for tumor progression (5, 6). A major receptor for hyaluronan, CD44, is currently prominent in the cancer literature because it is a common marker for “tumor-initiating cells/cancer stem cells” (CSCs) in human carcinomas. Even though the nature of CSCs is highly controversial, there is a reasonable consensus that CD44-expressing sub-fractions of many human carcinomas are highly malignant and resistant to therapy, properties that are frequently associated with CSCs (7, 8). Surprisingly, however, the functions of CD44 and its hyaluronan ligand in the properties of these particular cells have received little attention in the literature. The functional dynamics of hyaluronan and its receptors, especially CD44, have recently been reviewed in detail with respect to cancer (6, 9-17). In this brief overview, I will summarize my view of the current state of our knowledge of the functions of hyaluronan-CD44 interactions in cancer and the mechanisms whereby these interactions influence a large number of signaling pathways and cellular behaviors. Some of these activities are summarized in Figure 1.
Figure 1. Regulation of signaling cascades by hyaluronan-CD44 interaction.

Hyaluronan synthases produce and extrude hyaluronan, which may be retained by the synthase or released into the pericellular milieu. The extruded hyaluronan interacts multivalently with CD44 to induce and/or stabilize signaling domains within the plasma membrane. These signaling domains contain receptor tyrosine kinases (ErbB2 and EGFR), other signaling receptors (TGFβR1) and non-receptor kinases (Src family) that drive oncogenic pathways, e.g. the MAP kinase and PI3 kinase/Akt cell proliferation and survival pathways, as well as various transporters that participate in drug resistance and malignant cell properties (15, 34). Various adaptor proteins such as Vav2, Grb2 and Gab-1 mediate interaction of CD44 with upstream effectors, e.g. RhoA, Rac1 and Ras, that drive these pathways (11, 34). In other cases carbohydrate side groups on variant regions of CD44, e.g. heparan sulfate chains, bind regulatory factors and co-activate receptor tyrosine kinases, e.g. the c-Met receptor (31). Hyaluronan-CD44 interactions also induce cytoskeletal changes that promote cell motility and invasion. In this case actin filaments are joined to the cytoplasmic tail of CD44 via members of the ezrin-radixin-moiesin (ERM) family or ankyrin (11, 31). Proteoglycans and associated factors attached to pericellular hyaluronan may also influence these activities (13, 23). This diagram emphasizes cell autonomous activities influenced by hyaluronan produced by tumor cells. Hyaluronan produced by stromal cells may have overlapping or different activities but the relative contributions of stromal and tumor-derived hyaluronan are not yet clear (6).
Hyaluronan
Hyaluronan (also hyaluronic acid or hyaluronate) is a very large, linear, negatively charged polysaccharide that is composed of repeating disaccharides of glucuronate and N-acetylglucosamine. Hyaluronan is produced by three hyaluronan synthases (Has1/Has2/Has3), which are integral plasma membrane proteins whose active sites are located at the intracellular face of the membrane (18). Newly synthesized hyaluronan is extruded as it is elongated, and then targeted to the cell surface or to pericellular and extracellular matrices. Hyaluronan is distributed ubiquitously in vertebrate tissues but is especially concentrated in regions of cell division and invasion (19). In adult tissues such as synovial fluid, cartilage and dermis, it clearly plays a structural role that depends on its unique hydrodynamic properties and its interactions with other extracellular matrix components. On the other hand, hyaluronan has an instructive, cell signaling role during dynamic cell processes such as morphogenesis, inflammation, wound repair and cancer, wherein hyaluronan-receptor interactions are activated and collaborate in driving numerous signaling pathways (11, 20, 21). In addition to signal transduction, hyaluronan-receptor interactions participate in at least two other important physiological processes, viz., endocytosis of hyaluronan and assembly of pericellular matrices (19, 22, 23).
Hyaluronan receptors
Hyaluronan interacts with several cell surface receptors, including CD44, Rhamm, LYVE-1, HARE/stabilin-2, and Toll-like receptors-2 and 4 (20, 24, 25). CD44 is widely distributed but particularly important in the immune system and inflammatory processes (24, 26)1 as well as in diseases such as atherosclerosis and cancer (21, 27). Unlike CD44, LYVE-1 and HARE, Rhamm does not belong to the “link module” family of hyaluronan-binding proteins. Rhamm can be present either in the cytoplasm or on the cell surface, and is an important factor in cell motility in wound healing and cancer (10). LYVE-1 is a close relative of CD44 that is mainly restricted to lymphatic vessel and lymph node endothelia but its function is not well established (25). HARE/stabilin-2 is a scavenging receptor that clears hyaluronan and other glycosaminoglycans from the circulation (28). The Toll-like receptors recognize hyaluronan fragments during inflammatory events (24).
The major receptors implicated in cancer are CD44 and Rhamm. I will focus on CD44 in this short review but it is important to note that CD44 and Rhamm can exhibit both cooperative and interchangeable signaling functions. For example, interactions at the plasma membrane between CD44 and Rhamm have been shown to activate CD44 signaling through ERK1/2 and promote cancer cell motility (10). In some cases, e.g. in animal models of autoimmune diseases, Rhamm can compensate for CD44, a very important consideration when interpreting experiments in CD44-null mice (29).
CD44 is a single chain, single-pass, transmembrane glycoprotein that is very widely expressed in physiological and pathological systems. CD44 was first characterized through the confluence of several areas of investigation, including hyaluronan-cell interactions, lymphocyte homing, and cell adhesion (30), and its role in these phenomena is now well-established (26, 31)1,2. Although CD44 arises from a single gene, numerous transcripts are formed by alternative splicing. “Standard” CD44 is comprised of the constant, non-variant exon products, whereas “variant” isoforms arise by splicing of numerous additional exon products into a single site within the membrane-proximal region of the ectodomain (31)2. Carcinoma cells typically produce several variant forms of CD44 as well as standard CD44 whereas some tumor types, e.g. gliomas, produce mainly the standard form (32). All forms of CD44 include an N-terminal, membrane-distal, hyaluronan-binding domain that has significant homology with the hyaluronan-binding region, i.e. link module, of several other proteins and proteoglycans. Hyaluronan is the most widely studied ligand for CD44, but other ligands are clearly important. The best characterized among these are osteopontin and factors such as FGF and selectin ligands that recognize carbohydrate side chains covalently bound to CD44 (31, 33). One of the most puzzling aspects of CD44 physiology is “activation” with respect to hyaluronan binding and consequent signaling. Possible factors contributing to activation include post-translational modifications of CD44 such as glycosylation, CD44-cytoskeletal interactions, localization of CD44 within specialized domains in the plasma membrane, and the mode of pericellular organization and presentation of hyaluronan, but none of these is well established. Nevertheless it is clear that numerous cytokines, growth factors and alterations in cell context can induce the events that result in activation (31)1,3.
Hyaluronan-CD44 signaling
In several types of cancer cells, binding of hyaluronan to CD44 results in direct or indirect interaction of CD44 with signaling receptors, such as ErbB2, EGFR and TGF-beta receptor type I, and influences the activity of these receptors (15, 21, 34). It can also lead to interaction with and altered activity of non-receptor kinases of the Src family or Ras family GTPases (11, 34). Complex formation with adaptor proteins such as Vav2, Grb2 and Gab-1 mediates interaction of CD44 with upstream effectors, e.g. RhoA, Rac1 and Ras, that drive intracellular signaling pathways (11, 34). Thus, hyaluronan-CD44 binding influences the activity of a variety of downstream signaling pathways, especially the MAP kinase and PI3 kinase/Akt pathways, and consequently promotes tumor cell proliferation, survival, motility, invasiveness and chemoresistance (11, 15, 20, 21, 31, 34). In addition, binding of hyaluronan to CD44 stimulates multidrug and metabolic transporters that are important in therapy resistance (15, 35-39) and presentation of proteases that facilitate invasion (31, 40)2. The bulk of current evidence indicates that these interactions involve specific variants of CD44 but the particular variant almost certainly depends on the type of tumor cell and stage of malignant progression, and in some cases standard CD44 rather than variant CD44 is critically involved. The mechanisms of regulation of these various interactions in different tumor cell types and stages are not well understood but the widespread deregulation of many normal pathways in cancer cells most likely includes anomalous involvement of hyaluronan-CD44 interactions that operate normally in other contexts, such as embryonic development (19) and inflammation (24). A related possibility is that deregulated splicing in cancer cells (41) gives rise to CD44 variants that promote oncogenic events such as inappropriate Ras signaling (42) or binding of osteopontin, stromal growth factors or proteases (31).
In addition to its action as a co-receptor or co-activator of membrane-associated signaling molecules, CD44 can influence cellular events such as tumor cell proliferation and motility through cross-linking to the actin cytoskeleton via ankyrin or members of the ezrin-radixin-moiesin family (11, 31, 40). The tumor suppressor, merlin, most likely acts by inhibiting hyaluronan-CD44 interaction as well as displacing ezrin-radixin-moiesin proteins from the cytoplasmic tail of CD44. Release of merlin suppression may trigger activation of hyaluronan-CD44 binding, which in turn leads to the formation of signaling complexes discussed above (40). Another mechanism whereby hyaluronan-CD44 interactions may lead to intracellular signaling is via intracellular cleavage of CD44, translocation of the cytoplasmic product to the nucleus and activation of transcription (43).
Many studies indicate that CD44 is localized at least in part to lipid micro-domains with the properties of lipid rafts, and associates indirectly or directly therein with signaling proteins and transporters. Most of these studies also show that CD44 is recruited into these domains in response to ligand interactions (11, 35, 44, 45). Moreover, endocytosis of hyaluronan and CD44 occurs from these domains (46). Given the large number of pathways affected by CD44, the possibility that indirect and direct interactions with a wide variety of effectors occur within such domains and that these domains are induced and/or stabilized by multivalent interactions of hyaluronan with CD44 provides a compelling postulate to guide current and future investigations, at least from this author's perspective (see Figure 1).
A puzzling aspect of many studies of hyaluronan-induced oncogenic signaling is their basis in experiments in which exogenous hyaluronan is added to cultured tumor cells. Although these studies have resulted in apparently solid data indicative of strong effects on the pathways in question, they are difficult to reconcile with the long history of safe utilization of hyaluronan in numerous reconstructive or regenerative capacities in human patients. For example, hyaluronan is employed widely in eye and knee surgeries and in prevention of adhesions (47, 48). Hyaluronan-based hydrogels are also being developed for a variety of purposes including drug delivery, encapsulation of progenitor cells and tissue engineering (49, 50). Such studies imply that the oncogenic effects of hyaluronan only occur in the context of the tumor micro-environment and that stromal hyaluronan, as well as tumor cell-produced hyaluronan, plays an important role in tumorigenesis, a conclusion supported by correlative studies of numerous human tumor types (6). Strong supporting evidence for the tumor-promoting effects of hyaluronan comes from studies in which tumor hyaluronan levels and interactions with receptors were manipulated in vivo. These studies are discussed briefly below.
Hyaluronan-CD44 Interactions in Malignancy and Resistance to Therapy: the Cancer Stem Cell Phenotype
Extensive experimental evidence implicating hyaluronan in tumor growth and metastasis has been obtained in animal models of several tumor types. The approaches used include manipulation of levels of hyaluronan and perturbation of endogenous hyaluronan-receptor interactions by a number of methods (e.g. see Figure 2) (13, 21). However, it has become evident that turnover of hyaluronan by hyaluronidases is an essential aspect of the promotion of tumor progression by hyaluronan and that the balance of synthesis and degradation is critical (12, 16). Recent work, in which hyaluronan synthesis was up-regulated conditionally in mammary tumors that arise spontaneously in MMTV-Neu mice, highlights the importance of hyaluronan in tumor promotion, especially via recruitment of stromal cells and angiogenesis (13). Numerous studies have demonstrated an important role for hyaluronan-CD44 interactions in recruitment or homing of various cell types, including circulating immune cells and precursor cells (26, 51)1. The MMTV-Neu studies (13) also confirmed the importance of hyaluronan in epithelial-mesenchymal transitions (EMT). A major defect in the Has2-null mouse is failure to undergo EMT during early cardiac development (52). Moreover, up-regulation of Has2 in phenotypically normal epithelium induces the characteristics of EMT, including anchorage-independent growth and invasiveness (53), two of the major properties of malignant cells.
Figure 2. Antagonists of hyaluronan-receptor interactions.
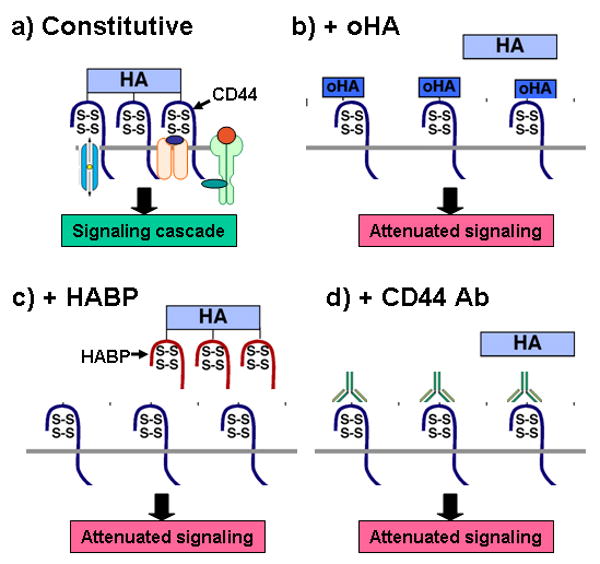
Antagonists of hyaluronan synthesis or hyaluronan-CD44 interaction de-stabilize the signaling domains illustrated in Figure 1, resulting in inhibition of oncogenic pathways and sensitization to drug treatment. Various approaches have been used to interfere with hyaluronan-CD44 interactions in vivo and in vitro (14, 21). Depicted here are: a) a constitutive signaling domain, as illustrated in Figure 1; b) exchange of endogenous, multivalent hyaluronan (HA) with small, monovalent hyaluronan oligomers (oHA); c) displacement of endogenous hyaluronan by a soluble hyaluronan-binding protein (HABP); d) inhibition of hyaluronan-CD44 interaction with blocking antibody. Other approaches such as antisense RNA or siRNA against hyaluronan synthases or CD44 have also been used.
Evidence for involvement of CD44 in tumor progression is also strong but very complex. Studies of tumorigenesis in CD44-null mice and manipulation of CD44 levels in various tumor systems have provided contradictory results, but treatments with CD44 antibodies and vaccines have demonstrated the importance of CD44 in tumor growth and metastasis in mouse models of leukemias and carcinomas (14, 54-56). Many studies have implicated variants of CD44 rather than standard CD44 in tumor progression but this depends on the stage of progression and type of tumor (14, 31). A striking development in recent years is the emergence of CD44 as a marker for sub-populations of several types of human carcinomas, often termed CSCs, that exhibit highly malignant and chemoresistant properties (7, 8). Interestingly, the characteristics of EMT have recently been linked to the properties of these cell sub-populations. A CD44+/CD24- sub-population exhibiting CSC properties is induced by up-regulation of EMT-associated transcription factors in primary human breast epithelium, and a similar sub-population with both EMT and CSC properties can be isolated from transformed epithelial cells (8, 57). Notably, these cells exhibit anchorage-independent growth of colonies in soft agar, a property that usually reflects resistance to apoptosis, which in turn is linked to chemoresistance. Numerous studies have shown that the CSC sub-population of carcinomas and other tumor types is resistant to chemotherapeutic agents, most likely due to increased anti-apoptotic pathway activity and enrichment of multidrug transporters (8, 15, 57). Another important outcome of EMT is invasiveness (4, 58) and, accordingly, CSCs have been linked to invasiveness and metastasis (7, 8, 59). As noted above, hyaluronan is closely associated with EMT, and these same properties of anchorage-independent growth, resistance to apoptosis, drug resistance and invasiveness are induced or increased by up-regulation of hyaluronan synthesis and reversed by antagonists of hyaluronan-CD44 interactions (15, 21). In particular, strong evidence has been published showing hyaluronan-dependent association of CD44 with receptor kinases (21, 31, 34) and transporters (15, 35-39) that are important in drug resistance and malignancy. Recently, we have examined hyaluronan-CD44 interactions in a CSC-like sub-population of cells isolated from human patient ovarian carcinoma ascites. We found that the CSCs are enriched in receptor tyrosine kinases and ABC-family drug transporters, that these proteins are present in close association with CD44 in the plasma membrane of the CSCs, and that this association depends on constitutive hyaluronan interactions (M. Slomiany, L. Dai, L. Tolliver, D. Grass, Y. Zeng, B. Toole, unpublished data).
Clinical-Translational Advances
Although the published literature on hyaluronan-CD44 interactions in cancer is riddled with paradoxes and apparent contradictions, most investigators in the field agree that these interactions offer an important target for translation into the clinic. A frequently expressed concern is the widespread expression and functions of hyaluronan and CD44 in normal physiology. However, two observations provide promise that therapeutic interventions can be developed that target oncogenic events with some degree of specificity or differential sensitivity. First is the finding that many of the interactions described above involve variants of CD44 that are amplified greatly in many tumor types in comparison to normal processes (14, 32, 41). Second is the nature of “activation” of hyaluronan-CD44 interactions in malignant tumor cells. Although these processes may have overlapping features with immune and inflammatory pathways, there are also clear differences that may be possible to exploit. Some of the studies described above have utilized antagonists that may ultimately have therapeutic value (e.g. see Figure 2) but these have not yet reached the clinic. Below I have summarized some approaches that appear to show promise in approaching this important objective.
CD44 antibodies and vaccines
Several studies have shown that administration of antibodies against CD44 inhibits tumor growth and progression. For example, injection of monoclonal antibodies against CD44 that block binding of hyaluronan inhibit invasion of mouse lymphoma cells into lymph nodes (14). Antibodies against CD44 have also been shown to block homing and promote differentiation of acute myeloid leukemic stem cells, and consequently to eliminate tumor initiating cells (54). Prolonged survival also occurred in mice with leukemic stem cells expressing BCR-ABL after treatment with CD44 antibody (55). Recently, a CD44 variant vaccine was shown to reduce mouse mammary carcinoma tumor growth and metastases (56). It is recognized in this field that these approaches will be greatly improved by tailoring antibodies and vaccines to specific variants of CD44 that are uniquely expressed at critical stages of progression of the cancer in question (14, 31). However, Phase I trials in breast and head and neck carcinoma patients with an antibody against CD44v6 have been discontinued due to toxicity (60, 61).
Hyaluronidases
Although constitutive hyaluronidase may promote the pro-oncogenic functions of hyaluronan, over-expression or exogenous administration of large amounts of hyaluronidase is usually inhibitory (12, 16, 17). Hyaluronidase has been used in the clinic for many years as an adjunct to chemotherapy in which it was believed to improve access of drugs to cancer cells through effects on cell adhesion and matrix barriers (12, 62). Highly purified recombinant hyaluronidase (63) is currently in Phase I trial for patients with advanced solid tumors. Interestingly, hyaluronidase was also shown to sensitize mouse mammary carcinoma cells to chemotherapeutic drugs when the cells are cultured as drug-resistant spheroids (64), a technique now known to enrich for CSCs. Although hyaluronidase may act in part by reducing barriers to drug diffusion, it may also act via its oligosaccharide products, which have been found to inhibit constitutive hyaluronan-CD44 signaling, resulting in decreased cell survival and chemoresistance (15) (see below).
Small hyaluronan oligosaccharides
Small oligomers of hyaluronan suppress anti-apoptotic signaling pathways in tumor cells and inhibit the activity of transporters that enhance resistance to therapeutic agents (15). Initially, the use of these oligomers was based on previous findings that oligomers consisting of 3-9 disaccharides bind CD44 monovalently (65) and displace hyaluronan polymer from membrane-bound receptors (66), but recent work has shown that they also inhibit hyaluronan synthesis (38). Treatment of tumor cells with these oligomers causes disassembly of CD44-transporter and CD44-receptor tyrosine kinase complexes, internalization of the disassembled components, and attenuation of function (38, 39, 44). Treatment in vivo with small hyaluronan oligomers suppresses tumor growth and/or induces tumor regression in experiments using xenografts of various tumor types, viz., melanoma, carcinomas, glioma, osteosarcoma, and malignant peripheral nerve sheath tumors (15, 21, 38, 67, 68). Notably, one of these studies showed significant effects on metastasis (67). Also, significant effects on tumor growth and invasion were seen when CSC-like sub-populations obtained from a glioma cell line (68) or from human patient ovarian carcinoma ascites (M. Slomiany, L. Dai, L. Tolliver, D. Grass, Y. Zeng, B. Toole, unpublished data) were used. Moreover, we have shown that systemic administration of sub-optimal doses of hyaluronan oligomers sensitizes highly resistant, malignant peripheral nerve sheath tumors to doxorubicin treatment in vivo (38). Although this approach might be expected to interfere with all activated hyaluronan-CD44 interactions, malignant tumors appear to be far more sensitive than normal physiological processes.
Targeting drugs to tumor cell CD44
In addition to targeting of hyaluronan-CD44 interactions themselves, these interactions are being exploited for delivery of chemotherapeutic drugs and other anti-cancer agents to tumor cells. Many investigators have shown increased efficacy in cell and animal tumor models by conjugating drugs to hyaluronan or CD44 antibody or by incorporating drugs or siRNAs into vehicles such as liposomes, hydrogels and nanoparticles that have been decorated with hyaluronan or antibodies against CD44 (69). Initial trials in human patients with drugs conjugated to CD44 antibody have shown some promise, although complicated by various toxicities (69). Clearly, targeting to relevant variants of CD44 is a crucial aspect of this approach. It has also been found, however, that the enormous hydrodynamic domain encompassed by hyaluronan can be used to entrap drugs, without the need for chemical conjugation, and deliver them to CD44-expressing tumors (70). Increased safety and efficacy of irinotecan, when combined with hyaluronan using this approach, have been shown in a pilot trial with colorectal carcinoma patients (71).
Acknowledgments
The author acknowledges the excellent work performed by numerous investigators in the field. Due to restricted space, I have referred mostly to more comprehensive reviews that provide further details regarding primary sources of information and the various paradoxes and controversies in the field. I thank Drs. Eva Turley and Cornelia Tolg for critical reading of the manuscript.
Grant support: NIH grants CA073839 and CA082867; DOD OC050368
Footnotes
Disclosure of Potential Conflicts of Interest: The author is an inventor on a patent related to some of the contents of this article.
Ruffell B and Johnson P. The regulation and function of hyaluronan binding by CD44 in the immune system. www.glycoforum.gr.jp/science/hyaluronan/HA32/HA32E.html. 2009.
Knudson W and Knudson CB. The hyaluronan receptor, CD44 - an update. www.glycoforum.gr.jp/science/hyaluronan/HA10a/HA10aE.html. 2005.
Heldin P. Growth factor regulation of hyaluronan metabolism in tumor progression. www.glycoforum.gr.jp/science/hyaluronan/HA33/HA33E.html. 2009.
References
- 1.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment. Trends Genet. 2009;25:30–8. doi: 10.1016/j.tig.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knudson W, Biswas C, Li XQ, Nemec RE, Toole BP. The role and regulation of tumour-associated hyaluronan. Ciba Found Symp. 1989;143:150–9. doi: 10.1002/9780470513774.ch10. [DOI] [PubMed] [Google Scholar]
- 6.Tammi RH, Kultti A, Kosma VM, et al. Hyaluronan in human tumors: Pathobiological and prognostic messages from cell-associated and stromal hyaluronan. Semin Cancer Biol. 2008;18:288–95. doi: 10.1016/j.semcancer.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 8.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–73. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 9.Stern R, editor. Hyaluronan in Cancer Biology. San Diego: Academic Press; 2009. This volume contains 21 articles covering numerous aspects of hyaluronan in cancer. [Google Scholar]
- 10.Maxwell CA, McCarthy J, Turley E. Cell-surface and mitotic-spindle RHAMM: moonlighting or dual oncogenic functions? J Cell Sci. 2008;121:925–32. doi: 10.1242/jcs.022038. [DOI] [PubMed] [Google Scholar]
- 11.Bourguignon LY. Hyaluronan-mediated CD44 activation of RhoGTPase signaling and cytoskeleton function promotes tumor progression. Semin Cancer Biol. 2008;18:251–9. doi: 10.1016/j.semcancer.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lokeshwar VB, Selzer MG. Hyaluronidase: both a tumor promoter and suppressor. Semin Cancer Biol. 2008;18:281–7. doi: 10.1016/j.semcancer.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itano N, Kimata K. Altered hyaluronan biosynthesis in cancer progression. Semin Cancer Biol. 2008;18:268–74. doi: 10.1016/j.semcancer.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Naor D, Wallach-Dayan SB, Zahalka MA, Sionov RV. Involvement of CD44, a molecule with a thousand faces, in cancer dissemination. Semin Cancer Biol. 2008;18:260–7. doi: 10.1016/j.semcancer.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Toole BP, Slomiany MG. Hyaluronan, CD44 and Emmprin: partners in cancer cell chemoresistance. Drug Resist Updat. 2008;11:110–21. doi: 10.1016/j.drup.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simpson MA, Lokeshwar VB. Hyaluronan and hyaluronidase in genitourinary tumors. Front Biosci. 2008;13:5664–80. doi: 10.2741/3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stern R. Hyaluronidases in cancer biology. Semin Cancer Biol. 2008;18:275–80. doi: 10.1016/j.semcancer.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Weigel PH, DeAngelis PL. Hyaluronan synthases: a decade-plus of novel glycosyltransferases. J Biol Chem. 2007;282:36777–81. doi: 10.1074/jbc.R700036200. [DOI] [PubMed] [Google Scholar]
- 19.Toole BP. Hyaluronan in morphogenesis. Semin Cell Dev Biol. 2001;12:79–87. doi: 10.1006/scdb.2000.0244. [DOI] [PubMed] [Google Scholar]
- 20.Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277:4589–92. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- 21.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–39. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 22.Knudson W, Chow G, Knudson CB. CD44-mediated uptake and degradation of hyaluronan. Matrix Biol. 2002;21:15–23. doi: 10.1016/s0945-053x(01)00186-x. [DOI] [PubMed] [Google Scholar]
- 23.Evanko SP, Tammi MI, Tammi RH, Wight TN. Hyaluronan-dependent pericellular matrix. Adv Drug Deliv Rev. 2007;59:1351–65. doi: 10.1016/j.addr.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–61. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 25.Jackson DG. Immunological functions of hyaluronan and its receptors in the lymphatics. Immunol Rev. 2009;230:216–31. doi: 10.1111/j.1600-065X.2009.00803.x. [DOI] [PubMed] [Google Scholar]
- 26.Johnson P, Ruffell B. CD44 and its role in inflammation and inflammatory diseases. Inflamm Allergy Drug Targets. 2009;8:208–20. doi: 10.2174/187152809788680994. [DOI] [PubMed] [Google Scholar]
- 27.Toole BP, Wight TN, Tammi M. Hyaluronan-cell interactions in cancer and vascular disease. J Biol Chem. 2002;277:4593–6. doi: 10.1074/jbc.R100039200. [DOI] [PubMed] [Google Scholar]
- 28.Pandey MS, Harris EN, Weigel JA, Weigel PH. The cytoplasmic domain of the hyaluronan receptor for endocytosis (HARE) contains multiple endocytic motifs targeting coated pit-mediated internalization. J Biol Chem. 2008;283:21453–61. doi: 10.1074/jbc.M800886200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naor D, Nedvetzki S, Walmsley M, et al. CD44 involvement in autoimmune inflammations: the lesson to be learned from CD44-targeting by antibody or from knockout mice. Ann N Y Acad Sci. 2007;1110:233–47. doi: 10.1196/annals.1423.025. [DOI] [PubMed] [Google Scholar]
- 30.Toole BP. Hyaluronan and its binding proteins, the hyaladherins. Curr Opin Cell Biol. 1990;2:839–44. doi: 10.1016/0955-0674(90)90081-o. [DOI] [PubMed] [Google Scholar]
- 31.Ponta H, Sherman L, Herrlich P. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 32.Heider KH, Kuthan H, Stehle G, Munzert G. CD44v6: a target for antibody-based cancer therapy. Cancer Immunol Immunother. 2004;53:567–79. doi: 10.1007/s00262-003-0494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sackstein R. Glycosyltransferase-programmed stereosubstitution (GPS) to create HCELL: engineering a roadmap for cell migration. Immunol Rev. 2009;230:51–74. doi: 10.1111/j.1600-065X.2009.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bourguignon LY. Hyaluronan-mediated CD44 interaction with receptor and non-receptor kinases promotes oncogenic signaling, cytoskeleton activation and tumor progression. In: Stern R, editor. Hyaluronan in Cancer Biology. San Diego: Academic Press; 2009. pp. 89–107. [Google Scholar]
- 35.Bourguignon LY, Singleton PA, Diedrich F, Stern R, Gilad E. CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell invasion. J Biol Chem. 2004;279:26991–7007. doi: 10.1074/jbc.M311838200. [DOI] [PubMed] [Google Scholar]
- 36.Miletti-Gonzalez KE, Chen S, Muthukumaran N, et al. The CD44 receptor interacts with P-glycoprotein to promote cell migration and invasion in cancer. Cancer Res. 2005;65:6660–7. doi: 10.1158/0008-5472.CAN-04-3478. [DOI] [PubMed] [Google Scholar]
- 37.Colone M, Calcabrini A, Toccacieli L, et al. The multidrug transporter P-glycoprotein: a mediator of melanoma invasion? J Invest Dermatol. 2008;128:957–71. doi: 10.1038/sj.jid.5701082. [DOI] [PubMed] [Google Scholar]
- 38.Slomiany MG, Dai L, Bomar PA, et al. Abrogating drug resistance in malignant peripheral nerve sheath tumors by disrupting hyaluronan-CD44 interactions with small hyaluronan oligosaccharides. Cancer Res. 2009;69:4992–8. doi: 10.1158/0008-5472.CAN-09-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slomiany MG, Grass GD, Robertson AD, et al. Hyaluronan, CD44 and emmprin regulate lactate efflux and membrane localization of monocarboxylate transporters in human breast carcinoma cells. Cancer Res. 2009;69:1293–301. doi: 10.1158/0008-5472.CAN-08-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stamenkovic I, Yu Q. CD44 meets merlin and ezrin: Their interplay mediates the pro-tumor activity of CD44 and tumor-suppressing effect of merlin. In: Stern R, editor. Hyaluronan in Cancer Biology. San Diego: Academic Press; 2009. pp. 71–87. [Google Scholar]
- 41.Skotheim RI, Nees M. Alternative splicing in cancer: noise, functional, or systematic? Int J Biochem Cell Biol. 2007;39:1432–49. doi: 10.1016/j.biocel.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 42.Cheng C, Yaffe MB, Sharp PA. A positive feedback loop couples Ras activation and CD44 alternative splicing. Genes Dev. 2006;20:1715–20. doi: 10.1101/gad.1430906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagano O, Saya H. Mechanism and biological significance of CD44 cleavage. Cancer Sci. 2004;95:930–5. doi: 10.1111/j.1349-7006.2004.tb03179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghatak S, Misra S, Toole BP. Hyaluronan regulates constitutive ErbB2 phosphorylation and signal complex formation in carcinoma cells. J Biol Chem. 2005;280:8875–83. doi: 10.1074/jbc.M410882200. [DOI] [PubMed] [Google Scholar]
- 45.Lee JL, Wang MJ, Sudhir PR, Chen JY. CD44 engagement promotes matrix-derived survival through the CD44-SRC-integrin axis in lipid rafts. Mol Cell Biol. 2008;28:5710–23. doi: 10.1128/MCB.00186-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thankamony SP, Knudson W. Acylation of CD44 and its association with lipid rafts are required for receptor and hyaluronan endocytosis. J Biol Chem. 2006;281:34601–9. doi: 10.1074/jbc.M601530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balazs EA, Denlinger JL. Clinical uses of hyaluronan. Ciba Found Symp. 1989;143:265–80. doi: 10.1002/9780470513774.ch16. [DOI] [PubMed] [Google Scholar]
- 48.Prestwich GD, Kuo JW. Chemically-modified HA for therapy and regenerative medicine. Curr Pharm Biotechnol. 2008;9:242–5. doi: 10.2174/138920108785161523. [DOI] [PubMed] [Google Scholar]
- 49.Allison DD, Grande-Allen KJ. Review. Hyaluronan: a powerful tissue engineering tool Tissue Eng. 2006;12:2131–40. doi: 10.1089/ten.2006.12.2131. [DOI] [PubMed] [Google Scholar]
- 50.Prestwich GD. Engineering a clinically-useful matrix for cell therapy. Organogenesis. 2008;4:42–7. doi: 10.4161/org.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haylock DN, Nilsson SK. The role of hyaluronic acid in hemopoietic stem cell biology. Regen Med. 2006;1:437–45. doi: 10.2217/17460751.1.4.437. [DOI] [PubMed] [Google Scholar]
- 52.Camenisch TD, Spicer AP, Brehm-Gibson T, et al. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–60. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zoltan-Jones A, Huang L, Ghatak S, Toole BP. Elevated hyaluronan production induces mesenchymal and transformed properties in epithelial cells. J Biol Chem. 2003;278:45801–10. doi: 10.1074/jbc.M308168200. [DOI] [PubMed] [Google Scholar]
- 54.Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–74. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 55.Krause DS, Lazarides K, von Andrian UH, Van Etten RA. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat Med. 2006;12:1175–80. doi: 10.1038/nm1489. [DOI] [PubMed] [Google Scholar]
- 56.Wallach-Dayan SB, Rubinstein AM, Hand C, Breuer R, Naor D. DNA vaccination with CD44 variant isoform reduces mammary tumor local growth and lung metastasis. Mol Cancer Ther. 2008;7:1615–23. doi: 10.1158/1535-7163.MCT-07-2383. [DOI] [PubMed] [Google Scholar]
- 57.Hollier BG, Evans K, Mani SA. The epithelial-to-mesenchymal transition and cancer stem cells: a coalition against cancer therapies. J Mammary Gland Biol Neoplasia. 2009;14:29–43. doi: 10.1007/s10911-009-9110-3. [DOI] [PubMed] [Google Scholar]
- 58.Turley EA, Veiseh M, Radisky DC, Bissell MJ. Mechanisms of disease: epithelial-mesenchymal transition--does cellular plasticity fuel neoplastic progression? Nat Clin Pract Oncol. 2008;5:280–90. doi: 10.1038/ncponc1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sleeman JP, Cremers N. New concepts in breast cancer metastasis: tumor initiating cells and the microenvironment. Clin Exp Metastasis. 2007;24:707–15. doi: 10.1007/s10585-007-9122-6. [DOI] [PubMed] [Google Scholar]
- 60.Rupp U, Schoendorf-Holland E, Eichbaum M, et al. Safety and pharmacokinetics of bivatuzumab mertansine in patients with CD44v6-positive metastatic breast cancer: final results of a phase I study. Anticancer Drugs. 2007;18:477–85. doi: 10.1097/CAD.0b013e32801403f4. [DOI] [PubMed] [Google Scholar]
- 61.Riechelmann H, Sauter A, Golze W, et al. Phase I trial with the CD44v6-targeting immunoconjugate bivatuzumab mertansine in head and neck squamous cell carcinoma. Oral Oncol. 2008;44:823–9. doi: 10.1016/j.oraloncology.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 62.Baumgartner G, Gomar-Hoss C, Sakr L, Ulsperger E, Wogritsch C. The impact of extracellular matrix on the chemoresistance of solid tumors--experimental and clinical results of hyaluronidase as additive to cytostatic chemotherapy. Cancer Lett. 1998;131:85–99. [PubMed] [Google Scholar]
- 63.Frost GI. Recombinant human hyaluronidase (rHuPH20): an enabling platform for subcutaneous drug and fluid administration. Expert Opin Drug Deliv. 2007;4:427–40. doi: 10.1517/17425247.4.4.427. [DOI] [PubMed] [Google Scholar]
- 64.St Croix B, Man S, Kerbel RS. Reversal of intrinsic and acquired forms of drug resistance by hyaluronidase treatment of solid tumors. Cancer Lett. 1998;131:35–44. doi: 10.1016/s0304-3835(98)00199-2. [DOI] [PubMed] [Google Scholar]
- 65.Lesley J, Hascall VC, Tammi M, Hyman R. Hyaluronan binding by cell surface CD44. J Biol Chem. 2000;275:26967–75. doi: 10.1074/jbc.M002527200. [DOI] [PubMed] [Google Scholar]
- 66.Underhill CB, Toole BP. Binding of hyaluronate to the surface of cultured cells. J Cell Biol. 1979;82:475–84. doi: 10.1083/jcb.82.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hosono K, Nishida Y, Knudson W, et al. Hyaluronan oligosaccharides inhibit tumorigenicity of osteosarcoma cell lines MG-63 and LM-8 in vitro and in vivo via perturbation of hyaluronan-rich pericellular matrix of the cells. Am J Pathol. 2007;171:274–86. doi: 10.2353/ajpath.2007.060828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gilg AG, Tye SL, Tolliver LB, et al. Targeting hyaluronan interactions in malignant gliomas and their drug-resistant multipotent progenitors. Clin Cancer Res. 2008;14:1804–13. doi: 10.1158/1078-0432.CCR-07-1228. [DOI] [PubMed] [Google Scholar]
- 69.Platt VM, Szoka FC., Jr Anticancer therapeutics: targeting macromolecules and nanocarriers to hyaluronan or CD44, a hyaluronan receptor. Mol Pharm. 2008;5:474–86. doi: 10.1021/mp800024g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown TJ. The development of hyaluronan as a drug transporter and excipient for chemotherapeutic drugs. Curr Pharm Biotechnol. 2008;9:253–60. doi: 10.2174/138920108785161514. [DOI] [PubMed] [Google Scholar]
- 71.Gibbs P, Brown TJ, Ng R, et al. A pilot human evaluation of a formulation of irinotecan and hyaluronic acid in 5-fluorouracil-refractory metastatic colorectal cancer patients. Chemotherapy. 2009;55:49–59. doi: 10.1159/000180339. [DOI] [PubMed] [Google Scholar]


