Abstract
Background
Mutations in the cardiac ryanodine receptor gene (RYR2) have been recently identified in victims of sudden infant death syndrome (SIDS). The aim of this study was to determine whether a gain-of-function mutation in RYR2 increases the propensity to cardiac arrhythmias and sudden death in young mice.
Methods and Results
Incidence of sudden death was monitored prospectively in heterozygous knock-in mice with mutation R176Q in Ryr2 (R176Q/+). Young R176Q/+ mice exhibited a higher incidence of sudden death compared with wild-type (WT) littermates. Optical mapping of membrane potentials and calcium levels in 1-7 day-old R176Q/+ and WT mice revealed an increased incidence of ventricular ectopy and spontaneous calcium releases in neonatal R176Q/+ mice. Surface ECGs in 3-10 day-old mice showed that R176Q/+ mice developed more ventricular arrhythmias following provocation with epinephrine and caffeine. Intracardiac pacing studies in 12-18 day-old mice revealed the presence of an arrhythmogenic substrate in R176Q/+ compared with WT mice. RT-PCR and Western blotting showed that expression levels of other calcium handling proteins were unaltered suggesting that calcium leak through mutant RyR2 underlies arrhythmogenesis and sudden death in young R176Q/+ mice.
Conclusions
Our findings demonstrate that a gain-of-function mutation in RyR2 confers an increased risk of cardiac arrhythmias and sudden death in young mice, and that young R176Q/+ mice may be used as a model for elucidating the complex interplay between genetic and environmental risk factors associated with SIDS.
Keywords: Sudden infant death syndrome, calcium, focal activity, ryanodine receptors, ventricular arrhythmias
Sudden infant death syndrome (SIDS) is a multi-factorial disorder in which newborns die during the first year of life, which is unexpected by history and in which a full postmortem examination fails to demonstrate a cause of death 1, 2. Despite the success of the “Back to Sleep” campaign, the incidence of SIDS remains unacceptably high and efforts have been re-directed from identification of associated factors to determination of the causative mechanisms 3. Maron and Schwartz 4, 5 were first to suggest the possibility of arrhythmogenic factors contributing to SIDS. This theory was confirmed two decades later when mutations in genes linked to inherited arrhythmia syndromes were identified in victims of this syndrome 6. Based on these postmortem molecular analyses, it is now estimated that 10-15% of SIDS cases can be attributed to inherited mutations in ion channels and associated subunits linked to fatal cardiac arrhythmias 7, 8.
Recently, mutations in the intracellular calcium (Ca2+) release channel/ ryanodine receptor gene RYR2 were identified in victims of SIDS 9. Single channel recordings revealed that SIDS-associated mutations in RyR2 result in aberrant channel openings during diastole, when Ca2+ is normally sequestered into the sarcoplasmic reticulum 9, 10. This gain-of-function phenotype observed for SIDS-associated RyR2 mutant channels was similar to that observed for RyR2 channels with mutations identified in older children and adults suffering from an inherited arrhythmia syndrome, catecholaminergic polymorphic ventricular tachycardia (CPVT) 10, 11. CPVT is an inherited disorder characterized by exercise- and stress-induced ventricular tachycardias associated with syncope and sudden cardiac death. It is estimated that >50% of all CPVT cases are caused by genetic mutations in RYR2 11. It has also been suggested that some RYR2 mutations may cause ‘arrhythmogenic right ventricular dysplasia type 2’ (ARVD-2), a condition characterized by fibro-fatty degeneration of the right ventricle and ventricular arrhythmias 12. However, it is currently controversial whether RYR2 mutations actually cause right ventricular structural remodeling and ARVD-2 13, 14.
In this study, we utilized a knock-in mouse model of the R176Q mutation in Ryr2 to examine the hypothesis that a gain-of-function mutation in Ryr2 results in SIDS due to cardiac arrhythmias. This mutation was previously identified in a 15-year old male with sudden arrhythmogenic cardiac death 12. Our results revealed an increased incidence of sudden unexpected death in R176Q/+ knock-in mice during the first weeks of life. Using optical mapping experiments, we found an increased incidence of spontaneous calcium release events and ectopic electrical activity in young R176Q/+ mice hearts. Electrophysiological recordings demonstrated cardiac arrhythmias in R176Q/+ mice associated with sudden cardiac death. Moreover, intracardiac pacing studies revealed the presence of an arrhythmogenic substrate due to the R176Q/+ mutation in RYR2. Together, this study describes the first mouse model for SIDS due to cardiac arrhythmias, and provides evidence for a causal link between mutations in RYR2 and ventricular tachyarrhythmias in young mice.
Methods
Mouse strains, animal care and genotyping
All animal studies were performed according to protocols approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine conforming the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). R176Q/+ mice were created as described, and mated with wildtype (WT) littermates in a C57Bl6 background 15. Mating cages were surveyed and all dead pups between postnatal days 1-24 were collected daily, and genotyped for gender 16, and the R176Q mutation as described in the online supplement 15. The postnatal developmental stages of mice are not as clearly defined as in human. Because weaning occurs in mice by the age of about 21 days, we defined infancy in mice as postnatal days 1-21.
Optical mapping of neonatal mouse hearts
Hearts were dissected from anesthetized young mice (day 1-7 postnatal), and bathed in modified oxygenated Tyrode's solution containing (mmol/l) 136 NaCl, 5.4 KCl, 1.8 CaCl2, 0.33 NaH2PO4, 1 MgCl2, 10 HEPES, and 10 glucose, pH 7.3. The same solution was used as the bath solution during optical mapping experiments. Blebbistatin (Sigma, St. Louis, MO) was used as an excitation-contraction uncoupler to minimize motion artifact. Isolated mouse hearts were strained with the Ca2+ indicator Rhod-2-acetoxymethyl ester (Rhod-2-AM; Invitrogen, Carlsbad, CA) by incubation with 10 μmol/l dye solution at 37°C for 45 min in the presence of 0.1% pluronic F-127 (Invitrogen, Carlsbad, CA) as described 17, 18. Hearts were transferred to the experimental chamber and superfused with the voltage-sensitive dye RH237 (12.5 μmol/l; Invitrogen, Carlsbad, CA) dissolved in Tyrode's solution for 15 min. Fluorescence was excited with a 532 nm laser source (B&W TEK Inc., Neward, DE). Emitted fluorescence was split with a dichroic mirror and collected with two aligned electron-multiplying CCD cameras (Cascade 128+, Photometrics, Tucson, AZ) from a field of 3 mm × 3 mm, acquiring at 0.6 ms per frame with a spatial resolution of 32 × 32 pixels (∼0.09 mm/pixel). Simultaneous intracellular Ca2+ and membrane voltage signals were collected as previously described 17. Bipolar stimuli were delivered at the base of the heart using platinum electrodes and a Grass stimulator triggered by computer-controlled pacing sequences as described 17. Hearts were paced at baseline at 3 Hz, followed by a incremental pacing protocol, during which the cycle length was decreased by 20 ms every 10 beats from 400 to 200 ms. Data were analyzed using custom software as described previously 17. See the online supplement for additional information about this setup.
Cardiac electrophysiology studies in neonatal mice
Surface electrocardiograms were recorded in 2-10 day-old mice anesthetized with 1.5% isoflurane in 100% oxygen. For some experiments, surface ECGs were recorded in 1-day-old mice that were restrained but not anesthetized. Body temperature of the young mice was continuously maintained at 36-37°C using a heated pad connected to a controller that received feedback from a temperature sensor attached to the mouse. A computer-based data acquisition system (Emka Technologies, Falls Church, VA) recorded simultaneous six-lead surface ECG using platinum needle electrodes inserted in each of the four limbs. The ECG signals were amplified and filtered between 0.5 and 200Hz. QT intervals were corrected according to: QTc=QT/[SQRT*(RR/100)] 19. After baseline recordings, an arrhythmia provocation test was performed by injection of epinephrine (2 mg/kg of body weight i.p.) and caffeine (120 mg/kg of body weight i.p.) once or twice if no cardiac arrhythmia were induced, as previously reported 15.
Invasive intracardiac electrophysiology studies were performed in young mice at 12-18 day of age, the youngest mice we could reliably perform these studies. A 1.1F octapolar catheter (Scisense Inc, London, Canada) was advanced via the internal jugular vein into the right ventricle. After baseline recordings, pacing thresholds were determined using a programmed electrical stimulator (STG3008, Multi Channel Systems, Reutlingen, Germany) and stimulation was delivered at 0.2 ms pulse width, at twice the capture threshold. A drive cycle length of 90 ms followed by a decrementing single extra stimulus was used to determine inducibility of VT 15. Episodes of premature ventricular contractions (PVCs) were defined as 1 to 3 QRS complexes not preceded by atrial activity. Non-sustained ventricular tachycardia was defined as 4-9 QRS complexes not preceded by atrial activity, whereas ventricular tachycardia was defined as more than 9 QRS complexes not preceded by atrial activity 20. The pacing protocols were repeated after administration of 0.5 mg/kg isoproterenol intraperitoneally (i.p.) 10. In all of the above studies, mice body temperature was monitored using a temperature probe and maintained between 36-37 °C.
Histological procedures
Mouse hearts were fixed with 10% buffered formalin, sectioned longitudinally (5 μm) and stained with either hematoxylin-eosin (H&E) for cell morphology, or Masson's Trichrome for interstitial fibrosis.
Reverse transcription polymerase chain reaction
Total RNA was extracted from 10-days old neonatal hearts using TRIzol (Invitrogen Life Technologies, Carlsbad, CA). Frozen hearts were mechanically homogenized in 1.0 ml TRIzol reagent. Total RNA was dissolved in 30 μl RNAse-free H2O and RNA concentration was determined by measuring absorbance at 260 nm using a DU 530 UV/Vis spectrophotometer (Beckman Coulter, Fullerton, CA). For each sample, 1.0 μg was used as a template for reverse transcription using oligo(dT)12-18 primer and SuperScript II reverse transcriptase (Invitrogen Life Technologies, Carlsbad, CA) in a 50 μl total volume, to generate first-strand cDNA. The cDNA (2.5 μl for each sample) was then amplified by PCR in a 50 μl total volume, using Taq DNA polymerase (Invitrogen Life Technologies, Carlsbad, CA) and primers specific for RyR2, the alpha subunit of the cardiac L-type voltage-dependent Ca2+ channel (Cav1.2), Na+/Ca2+ exchanger (NCX1), sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA2a) and phospholamban (PLB). Ribosomal protein L7 mRNA levels were determined as control for equal sample input and amplification 21. As a control for genomic DNA contamination, the same RT-PCR amplification was performed in the absence of reverse transcriptase.
Western blotting
Western blotting was performed as previously described 18, 21. Mouse ventricular lysates were subjected to electrophoresis on 6% (for Cav1.2, RyR2, SERCA2a, NCX1, and GAPDH) and 20% (for PLN) acrylamide gels, and transferred onto polyvinyl difluoride (PVDF) membranes. Proteins were detected using antibodies against Cav1.2 (1:200; Sigma), RyR2 (1:5,000; Affinity BioReagents), SERCA2a (1: 500; Santa Cruz Biotechnology), PLN (1:5,000, Affinity BioReagents), NCX1 (1:500; Swant), and GAPDH (1:5000; Millipore). Membranes were incubated with secondary antibodies conjugated to Alexa-Fluor 680 (Invitrogen Molecular Probes) or IR800Dye (Rockland Immunochemicals), and bands were quantified using densitometry (Odyssey System).
Statistical analysis
Results are expressed as mean ± SEM. Student's t test, χ2, or Fisher's exact test was applied when appropriate. A Kaplan-Meier survival analysis was done to estimate the incidence of sudden death. P<0.05 was considered statistically significant.
Results
Higher incidence of SIDS in R176Q/+ knock-in mice
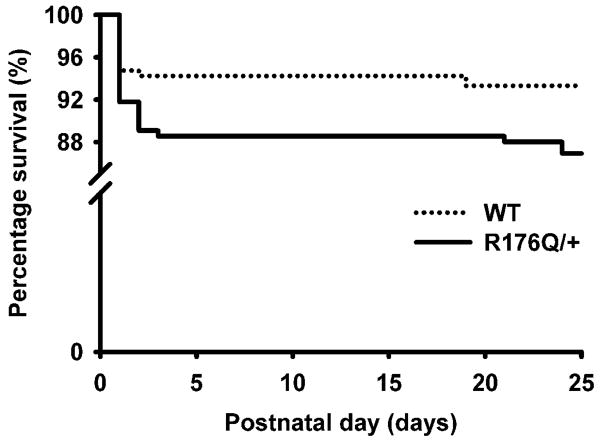
Sudden infant death syndrome (SIDS) is defined clinically as the death of an infant under the age of one year. To determine the effects of the inherited R176Q mutation in Ryr2 on postnatal survival, we prospectively assessed the incidence of sudden death in live births generated in mating cages containing one wildtype (WT) and one R176Q/+ knock-in mouse. Mating schemes included similar numbers of male (n=12) and female (n=9) R176Q/+ breeders, mated with WT littermates in each case, respectively. During the 24-month study, 48 (out of 487) live-born neonates died during postnatal days 1-24. The total number of R176Q/+ mice (n=248) born was similar to that of WT mice (n=239) (P=0.798), suggesting that the R176Q mutation does not cause embryonic lethality. Following PCR genotyping of deceased pups (Supplemental Fig. 1), we found a greater incidence of sudden death during postnatal days 1-24 in R176Q/+ neonates (31/248; 12.5 %) compared with WT littermates (17/239; 7.1%) (P=0.047) (Fig. 1). Interestingly, most deaths in both R176Q/+ and WT pups occurred during the first 4 neonatal days, while fewer mice died during the subsequent three weeks. Between month 1 and 6, mortality incidence in a different cohort of mice was 1 out of 72 in WT mice, and 0 out of 102 for sedentary R176Q/+ mice. Thus, there was no increased mortality rate in R176Q/+ mice beyond the infant period.
Fig. 1. Increased incidence of SIDS in neonatal mice with mutation R176Q in RYR2.
Kaplan-Meier survival curve demonstrate that SIDS occurs preponderantly in the first 4 postnatal days in both R176Q/+ (n=248) and WT (n=239) neonates.
Absence of structural heart disease and ARVD in R176Q/+ mice
A detailed post-mortem examination was performed on deceased 1-day-old R176Q/+ (n=8) and WT (n=4) mice. In addition, we electively sacrificed two whole litters of 1 day-old mice born to WT and R176Q/+ parents (9 R176Q/+ and 7 WT neonatal mice) for post-mortem and histological analysis. The autopsy findings confirmed that all dissected mice (both sudden death victims and electively sacrificed mice) were born alive, as they all had milk in their digestive system. Micro-dissection revealed the absence of macroscopic abnormalities, such as organ malformation, cleft palate, incomplete sternum fusion, diaphragmatic hernia, spina bifida, lymph node hyperplasia, or tumors. In particular, no abnormalities were noted in the hearts and great vessels in any of the autopsied mice. There were no differences between the heart weight to body weight ratios between R176Q/+ mice (0.0089 ± 0.0020, n=8) and WT mice (0.0091 ± 0.0028, n=4; P=0.935) that died suddenly on postnatal day 1. Moreover, those heart weight to body weight ratios were similar to those of WT and R176Q/+ mice electively sacrificed on day 1 after birth. Thus, a macroscopic pathological examination revealed no obvious morphological abnormalities in the pups that died suddenly or mice that were electively autopsied at the same age.
To determine if the R176Q mutation in Ryr2 causes remodeling of the neonatal heart, we performed a detailed histological examination of electively sacrificed 1-day-old WT and R176Q/+ mice (n=4 per genotype). Sections stained with hematoxylin-eosin revealed the absence of ventricular enlargement and myofiber disorganization (data not shown). Because some patients with the R176Q mutation in RyR2 exhibited right ventricular abnormalities believed to be associated with ARVD 12, a Masson's trichrome staining was performed which revealed the absence of fibrosis in the right ventricle of R176Q/+ mice (Fig. 2A-B). To exclude structural heart disease as a cause of sudden unexpected death in R176Q/+ mice, we also performed a histological analysis of the hearts dissected from deceased neonatal mice (8 R176Q/+ and 4 WT mice). Although the quality of these sections was lower because the dead mice had been frozen prior to processing for histology, Masson's trichrome staining appeared to exclude right ventricular dysplasia and/or interstitial fibrosis as a cause of death (Fig. 2C-D).
Fig. 2. Absence of structural heart disease and ARVC/D in 1-day-old R176Q/+ mice.
A-B, Photo micrographs of sections stained with Masson's trichrome of WT and R176Q/+ mouse hearts electively sacrificed at postnatal day 1 revealing the absence of structural heart disease and interstitial fibrosis in the right ventricle. C-D, Masson's trichrome staining of hearts from WT and R176Q/+ mice that died unexpectedly on postnatal day 1 also reveal the absence of interstitial fibrosis or right ventricular dysplasia. Open spaces between the myocytes are caused by freezing artifacts.
Unaltered expression levels of calcium handling proteins in R176Q/+ mice
We also examined the possibility that the R176Q/+ mutation in Ryr2 results in compensatory changes in other major Ca2+-handling proteins that could be pro-arrhythmogenic. RT-PCR analysis of L-type Ca2+ channel (Cav1.2), RyR2, SR Ca2+-ATPase (SERCA2a), phospholamban (PLB), and Na+/Ca2+-exchanger (NCX1) revealed unaltered expression mRNA levels when normalized to loading control L7 in R176Q/+ compared with WT mouse hearts (n=4 and P=N.S. in each group) (Fig. 3A). Additionally, Western blot analysis revealed equal protein expression levels of these Ca2+-handling channels and exchangers in hearts of WT and R176Q/+ mice on postnatal day 1 (Fig. 3B). These data suggest that compensatory remodeling of Ca2+ handling proteins may be excluded as a cause of sudden unexplained death in neonatal R176Q/+ mice.
Fig. 3. Expression levels of calcium handling proteins are unaltered in neonatal R176Q/+ mice.
A, Representative examples of RT-PCR products for the genes encoding Cav1.2, RyR2, SERCA2a, PLB, and NCX1, and the loading control (60S ribosomal protein L7). Summary bar graphs show that mRNA expression levels of these Ca2+-handling proteins are unaltered in neonatal R176Q/+ knock-in mice after normalization to the L7 housekeeping gene (n=4, each genotype). B, Representative examples of Western blots for proteins Cav1.2, RyR2, SERCA2a, PLB, NCX1, and the loading control GAPDH. Quantification of protein levels normalized to GAPDH expression reveals no significant differences in the levels of these major Ca2+-handling proteins in 1-day old WT and R176Q/+ knock-in mice.
Spontaneous electrical activity and calcium releases in neonatal R176Q/+ hearts
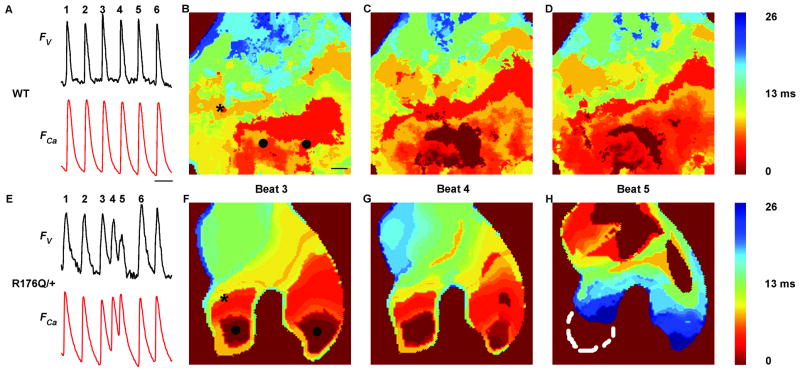
To better understand the mechanisms underlying sudden death in young R176Q/+ mice (1-7-day old), we developed a system to simultaneously measure cardiac action potentials and calcium transients using optical mapping in isolated neonatal mouse hearts (see Supplemental Fig. 2). Isolated neonatal mouse hearts, loaded with the Ca2+ indicator Rhod-2-AM and the voltage-sensitive dye RH237, were superfused with Tyrode solution in an experimental chamber. The cardiac preparations were paced using field stimulation, and ventricular action potentials and Ca2+ transients were recorded simultaneously (Fig. 4). Under control conditions during pacing at 3 Hz, the ventricular action potential duration (APD80) averaged 101 ± 3 ms in WT and 145 ± 8 ms in R176Q/+ mice (P=0.004). The Ca2+ transient duration was longer in R176Q/+ hearts (276 ± 5 ms, n=8) than in WT hearts (211 ± 19 ms, n=4) (P=0.001). The Ca2+ transient decay (tau) was prolonged in neonatal R176Q/+ hearts (112 ± 2 ms, n=8) compared to WT hearts (86 ± 2 ms; P<0.001), consistent with diastolic leakage of Ca2+ from the sarcoplasmic reticulum via R176Q/+ mutant RyR2 channels prolongs the Ca2+ transient (Fig. 4A-B).
Fig. 4. Abnormal calcium release and ectopic electrical activity in R176Q/+ neonatal hearts.
(A) Simultaneous recordings of voltage (Fv) and Ca2+ fluorescence (FCa) traces in WT neonatal hearts at pacing cycle length of 360 ms sampled at the pixel indicated by an asterisk in B. Scale represents 350 ms. (B, C, D) Isochronal calcium maps corresponding to the paced beat 3, 4, and 5 from A. in WT neonatal hearts shows propagation pattern across the ventricle from pacing electrode at the bottom (represented by 2 black solid circles in B). Scale in B represents 100 μm and is the same for all isochronal maps. (E) Simultaneous recordings of voltage (Fv) and Ca2+ fluorescence (FCa) traces in R176Q/+ neonatal hearts at pacing CL of 360 ms at the pixel indicated by the asterisk in F. R176Q/+ neonatal heart display 2 ectopic beats after beat 3 (beats 4 and 5). (F, G, H) Isochronal calcium maps corresponding to the paced beat 3 and ectopic beats 4 and 5 from E in R176Q/+ neonatal hearts shows propagation pattern across the ventricle. Paced beat 3 propagates from the electrode at the base of the heart (represented by 2 black solid circles in C) toward the apex (F). Ectopic beat 4 propagates with a slower velocity from the base of the heart to the apex (G) while ectopic beat 5 originates near the apex of the heart (H).
Mouse hearts could be paced to a cycle length of 200 ± 20 ms before losing 1:1 capture, and there was no significant difference comparing R176Q/+ and WT. However, there was a trend towards increased incidence of ectopic depolarizations in R176Q/+ paced hearts (3 out of 8 mice) compared with WT (0 out of 10 mice; P=0.069). The average number of ectopic beats in R176Q/+ preparations with spontaneous activity was 8 ± 3 beats/min. By comparing voltage and calcium mapping data, we observed that ventricular ectopy occurred in R176Q/+ neonatal hearts as early afterdepolarizations arising as a second depolarization during the preceding triggering Ca2+ transient (Fig. 4F-H). These experiments suggest that abnormal Ca2+ releases caused by mutant RyR2 channels led to ventricular ectopy in R176Q/+ neonatal hearts, which might result in the formation of ventricular arrhythmias in vivo.
Ventricular tachycardias in R176Q/+ knock-in mice
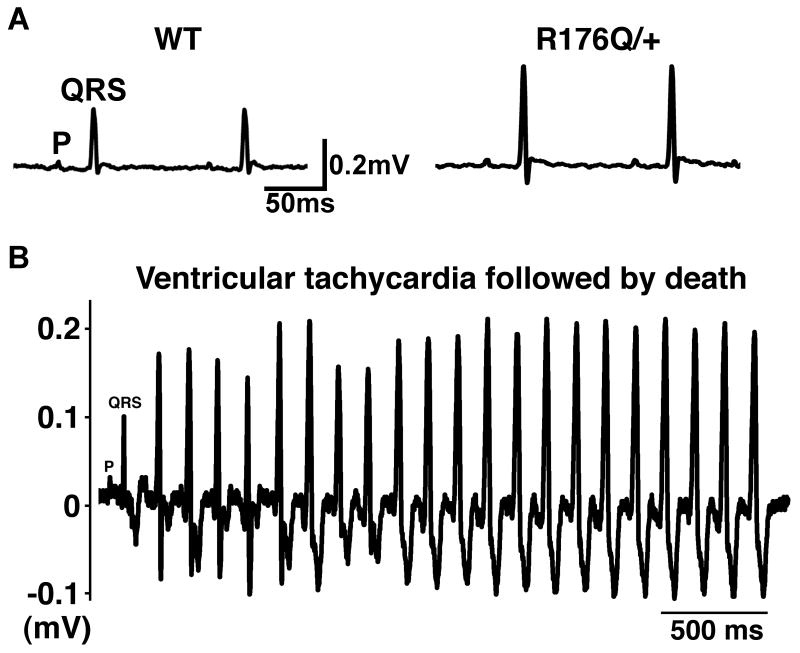
To determine whether sudden death in neonatal R176Q/+ mice might be caused by cardiac arrhythmias as suggested by optical mapping studies, we recorded surface electrocardiograms in 3-10 day-old mice. At baseline, there were no significant differences in heart rate, PR interval, QRS or QTc duration, comparing R176Q/+ and WT littermates (Fig. 5A, Table 1), suggesting the absence of conduction disease or repolarization abnormalities. Moreover, ectopic ventricular beats were not observed in any of the R176Q/+ (n=10) or WT (n=8) mice studied.
Fig. 5. Spontaneous ventricular tachycardias in neonatal R176Q/+ knock-in mice.
A, Lead II surface electrocardiograms in 10-day old WT (left) and R176Q/+ knock-in (right) neonatal mice. Letter ‘P’ indicates P wave; the letters ‘QRS’ mark the QRS complex. B, Episode of ventricular tachycardia evoked in a 9 day-old R176Q/+ knock-in mouse following injection of epinephrine and caffeine. Seconds after termination of this tachycardia, the mouse stopped breathing and the electrocardiogram showed low voltage cardiac activity consistent with sudden death.
Table 1. Measurement of surface ECG parameters in WT and R176Q/+ neonates.
PR-interval from beginning of P waves to the peak of R wave; RR- interval between two consecutive R wave peaks; QRS - duration of interval between beginning of Q wave to peak of S wave; QTc - duration of QT interval corrected for heart rate; HR- heart rate; NS, non-significant.
| WT (n=5) | R176Q/+ (n=4) | ||||
|---|---|---|---|---|---|
| Baseline | |||||
| Mean | SEM | Mean | SEM | P value | |
| HR (bpm) | 425.9 | 11.3 | 417.5 | 20.0 | 0.730 |
| PR (ms) | 42.6 | 1.2 | 41.1 | 2.5 | 0.603 |
| QRS (ms) | 8.4 | 0.6 | 6.5 | 0.1 | 0.027 |
| QT (ms) | 23.3 | 2.4 | 28.0 | 2.6 | 0.237 |
| QTc (ms) | 19.6 | 1.8 | 23.3 | 2.4 | 0.256 |
Subsequently, an arrhythmia challenge was conducted by injecting epinephrine (2 mg/kg i.p.) and caffeine (120 mg/kg i.p.) into the pups, a protocol known to evoke catecholamine-dependent arrhythmias in adult mice with RYR2 mutations 15. Whereas spontaneous (i.e., non-paced) ectopic beats and ventricular tachycardia occurred in only 7% of WT mice (1 out of 15), 41% (7 out of 17) R176Q/+ knock-in mice developed multiple ectopic beats and episodes of ventricular tachycardia (P=0.041) (Fig. 5B). Although most of these arrhythmias were self-terminating, we observed the sudden death of three R176Q/+ pups during or following an episode of ventricular arrhythmia. These data suggest that the increased incidence of SIDS in R176Q/+ might be caused by cardiac arrhythmias that are induced under conditions of catecholaminergic stress.
To exclude potential confounding effects of anesthesia on arrhythmogenicity, we recorded surface electrocardiograms in unanesthetized 1-day-old WT (n=7) and R176Q/+ (n=8) mice. There were no differences in HR, PR, QRS, or QTc intervals comparing R176Q/+ mice and WT littermates, although the averaged heart rates in awake mice (WT: 505±22 bpm; R176Q/+: 476±18 bpm; P=0.325.) were higher compared to anesthetized mice. Consistent with studies in 3-10 day-old mice, we did not observe episodes of bradycardia in any of the WT or R176Q/+ mice studied.
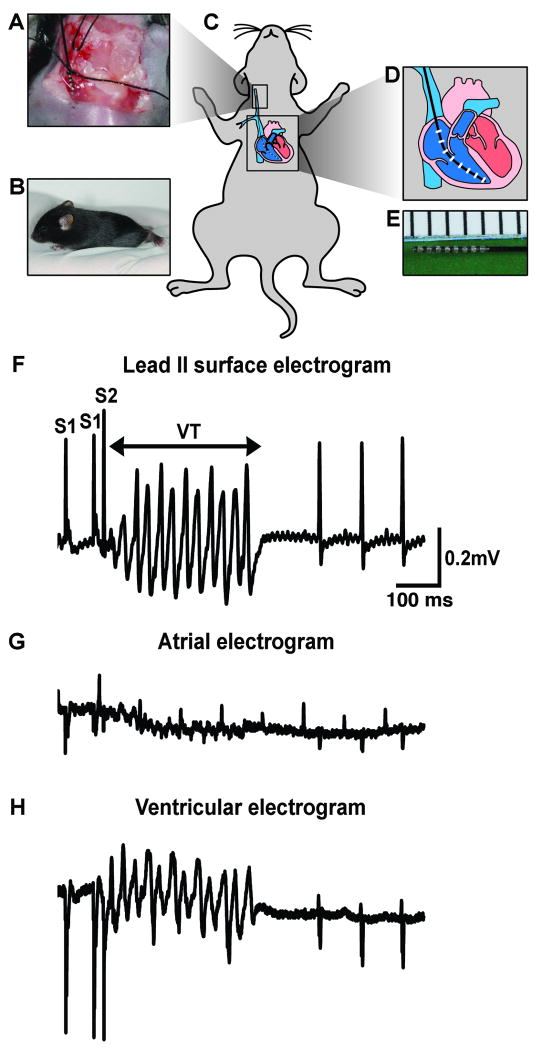
Enhanced arrhythmogenic susceptibility of R176Q/+ hearts
To confirm the presence of an arrhythmogenic substrate in the hearts of young R176Q/+ mice, we performed intracardiac programmed electrical stimulation in neonatal mice (Fig. 6A-E). After preparation of the right jugular vein (panel A) in neonatal mice (panels B-C), a 1.1F octapolar catheter (panels D, E) was advanced into the right ventricle of (panel C) under electrogram guidance. Surface ECG and intracardiac electrograms were recorded in mice 12-18 days of age, weighing 6.7 ± 0.2 grams, because the catheter could not be inserted into the jugular veins of younger mice. The basal heart rates in WT mice were 426±11 ms and in R176Q/+ mice 417±20 ms (P=0.730). Under basal conditions, overdrive pacing or a pacing protocol with one extra stimulus resulted in non-sustained ventricular tachycardia (NSVT) and episodes of premature ventricular contractions (PVC) in 17% (2 of 12) of R176Q/+ mice compared with 0% (0 of 12) of WT mice (P=0.478).
Fig. 6. Presence of an arrhythmogenic substate in young R176Q/+ knock-in mice.
(A) The right internal jugular vein was cannulated in 12-18 day-old neonatal mice (B). (C-E,) An 1.1 F octapolar catheter was advanced into the right ventricle allowing for simultaneous recording of surface ECG (F), atrial (G) and ventricular electrograms (H). (F-G) Example of ventricular tachycardia (VT) in R176Q/+ neonate induced with a single premature beat following isoproterenol injection.
Because arrhythmias in mice and patients with genetic mutations in RYR2 are almost always triggered by stress or catecholamines, isoproterenol (0.5 mg/kg) was administered to the neonatal mice intraperitoneally. ECG analysis revealed that isoproterenol elicited equal effects on heart rates (i.e., an ∼20% increase) in WT and R176Q/+ knock-in mice (data not shown). Following isoproterenol administration, ventricular tachycardia was inducible in 50% (6 of 12) of R176Q/+ pups (Fig. 6F-H), compared with 8% (1 of 12) of WT pups (P=0.069). Episodes of both monomorphic and polymorphic ventricular tachycardia were observed in R176Q/+ mice following isoproterenol. Isoproterenol did not induce conduction abnormalities or significant differences in QTc duration comparing R176Q/+ and WT mice. These findings suggest that the R176Q mutation in RYR2 enhances the susceptibility to arrhythmias in young mice, and that the increased arrhythmogenic substrate in these mice might lead to the enhanced incidence of SIDS.
Discussion
This study provides the first in vivo experimental evidence in a genetic mouse model for a possible pathogenic link between an ion channel mutation and sudden infant death syndrome (SIDS) and arrhythmias. Our findings demonstrate that an inherited gain-of-function Ryr2 mutation may represent a latent pathogenic substrate for sudden death during an early stage of life. In the presence of a suitable ‘trigger’ such as catecholamines, the pathogenic substrate may lead to triggered activity in the heart of young mice, associated with enhanced arrhythmogenesis and an increased probability of sudden death. These studies extend retrospective population-based linkage studies 22, 23 and biophysical studies of mutant ion channels 23, 24, and provide evidence for a pathogenic mechanism underlying ventricular arrhythmias and sudden death.
Approximately 3,000 apparently healthy infants that die each year are classified as SIDS 25. A number of risk factors have been identified so far which include: young maternal age with low educational levels and socioeconomic status, poor prenatal care, exposure to cigarette smoke during and after pregnancy, prematurity, low birth weight, male sex, African American race, overheating, prone sleeping position, and sleeping on a soft surface 3. In addition, inborn errors of metabolism, respiratory dysfunction, cardio-respiratory instability, and cardiac arrhythmias have been proposed as mechanisms underlying SIDS 26.
Cardiac arrhythmias due to mutations in ion channels have been proposed as mechanisms for SIDS more than three decades ago 25, and were subsequently demonstrated in a case of “near-miss” SIDS for long QT syndrome 6. Consequently, long QT syndrome 6, 22, 27, short QT syndrome 28, and Brugada syndrome 29 have all been implicated as causes of SIDS. Based on postmortem studies of SIDS victims, it has been estimated that up to 10-15% of SIDS cases might result from cardiac channelopathies 30. Recently, cardiac ryanodine receptors have also been added to the list of potassium and sodium channels as possible causes of SIDS 9, as two new mutations were identified in a cohort of SIDS victims 9.
Inherited mutations in the RYR2 gene have also been associated with catecholaminergic polymorphic ventricular tachycardia (CPVT) and possibly arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) in older children and adults 12, 31. Moreover, several CPVT-associated RYR2 mutation were also identified in cohorts of young victims (mean age 12-14 years) of sudden unexplained death 32, 33. These clinical findings suggest that SIDS and childhood sudden unexplained death may, in fact, be early and delayed manifestations of the same arrhythmia syndrome, namely CPVT. Experimental studies support this hypothesis, as the gain-of-function biophysical defects in RyR2 Ca2+ channels caused by CPVT-linked mutations are similar to those caused by SIDS-associated RyR2 mutations (R2267H and S4564R) 9, 10, 34. While it is a limitation of our study that the R176Q mutation studied in this paper was identified in a 15-year-old proband 35 and not in a neonatal victim of SIDS, we do believe that the R176Q/+ knock-in mouse represents a suitable model to study the physiological effects of a gain-of-function defect in RyR2 channels. In future studies, we plan to generate knock-in mice carrying RYR2 mutations identified in younger victims of SIDS.
Tiso et al. 12 have suggested that mutations in RyR2 including R176Q cause ARVC/D. This study has been controversial in recent years as the association between RyR2 mutations and ARVC/D has not been confirmed by other groups 11. Previous studies in adult R176Q/+ mice demonstrated the absence of right ventricular (RV) dysplasia and interstitial fibrosis, although subtle RV diastolic dysfunction was detected in these mice 15. Histological studies in neonatal R176Q/+ mice that either died unexpectedly or were electively sacrificed on postnatal day 1 also revealed an absence of RV dysplasia or interstitial fibrosis. Thus, it is likely that the R176Q/+ mutation in RyR2 causes an arrhythmogenic phenotype in the absence of structural heart disease.
Our arrhythmia findings in young R176Q/+ mice are similar to those made previously in adult R176Q/+ mice that develop CPVT 15. Unfortunately, it is not possible to perform telemetric studies in neonatal R176Q/+ mice as the radiotransmitter (4 gram) is about the same size as the neonatal mice (2-7 grams). Nevertheless, our data in 2-10 days old mice revealed an increased incidence of ventricular arrhythmias strongly suggesting that these are the cause of sudden death in R176Q/+ mice. Postma et al. 36 demonstrated that some carriers of RyR2 mutations exhibit bradycardia. Our data show that R176Q/+ mutant mice exhibit slightly lower heart rates, although these differences were not significant. Based on our experimental findings, ventricular tachycardias are the most likely cause of sudden unexplained death in neonatal R176Q/+ mice, although bradycardia and atrioventricular block could not be excluded as a possible cause of death.
In vitro studies, using single channel recordings of RyR2 with the two SIDS mutations have demonstrated an increased open probability of the mutant channels, which was enhanced by β-adrenergic stimulation 9. In addition, the electrophysiological studies in neonatal R176Q/+ mice suggest that ectopic activity and ventricular tachycardias occur almost exclusively in the presence of a triggering event (i.e., β-adrenergic stimulation or cardiac pacing). These findings are consistent with clinical observations that ventricular tachycardias are almost exclusively seen in CPVT patients after isoproterenol 31. We observed both monomorphic and polymorphic ventricular arrhythmias in young R176Q/+ mice, whereas patients with CPVT tend to exhibit bidirectional or polymorphic VTs 31. At present, it remains unclear if the occurrence of monomorphic VTs in R176Q/+ mice is due to species differences or due to the young age at which the mice are studied. Nevertheless, these electrophysiological data suggest that catecholamine-induced cardiac arrhythmias are a likely mechanism of increased sudden cardiac death incidence observed in the young R176Q/+ mice.
The triple risk model of SIDS proposes the simultaneous occurrence of an intrinsic susceptibility, an exogenous stressor, and a critical development period 37. Our data are consistent with this model as the R176Q/+ mice have a genetic susceptibility due to the RYR2 mutation, develop arrhythmias only following catecholaminergic stimulation (stressor), and are most susceptible during first few neonatal days, which is possibly associated with developmental changes that increase the likelihood of arrhythmias. Somewhat similar evidence for sudden death during infancy was obtained in transgenic rabbit overexpressing the HERG-G628S mutation in the heart 38. Moreover, Nuyens et al. 39 reported that knock-in mice with a deletion of amino-acids 1505-1507 (KPQ) in the cardiac SCN5A Na+ channel develop embryonic death, whereas heterozygous mice are susceptible to ventricular arrhythmias. These and other animal models may enhance our understanding the molecular and cellular pathways involved in neonatal arrhythmogenesis, which might help to identify children at risk for SIDS and to develop new preventive treatments for high-risk individuals. Future studies of R176Q/+ mutant mice in a different inbred background may identify modifiers of the phenotype that might translate in patient populations susceptible to SIDS. In conclusion, our findings demonstrate that a gain-of-function mutation in RyR2 confers an increased risk of cardiac arrhythmias and sudden death in young mice, and suggest that young R176Q/+ mice may be used as a model for elucidating the complex interplay between genetic and environmental risk factors associated with SIDS.
Supplementary Material
Acknowledgments
We thank Bhavini Patel for her excellent technical assistance. The R176Q knock-in mice were generously provided by Dr. Susan Hamilton.
Funding Sources: These studies were supported by a grant of the March of Dimes foundation (MOD24172). X.H.T.W. is a W.M. Keck Foundation Distinguished Young Scholar in Medical Research, and is also supported by grants of the NIH/NHLBI (R01-HL089598), and American Heart Association (0535310N). N.M. is supported by NIH grant T32-HL007706, and S. Sarma by T32-HL007706. R.J.v.O. is a recipient of the 2008-2010 American Physiological Society Postdoctoral Fellowship in Physiological Genomics. N.L. is the recipient of Michel Mirowski International Fellowship in Cardiac Pacing and Electrophysiology from the Heart Rhythm Society. M.V. is funded by grants from the NIH/NHLBI R21-HL085215 and the Houston Texans.
Footnotes
Sudden infant death syndrome (SIDS) is the leading cause of death in infants less than 1 year of age. Most SIDS deaths occur in infants that are seemingly healthy and are not preceded by warning signs. The exact etiology of SIDS occurs remains elusive, but many experts believe that a combination of several factors are involved. The triple risk model of SIDS proposes simultaneous occurrence of a biological susceptibility, an outside stressor, and a critical development period. It has been suggested that inherited mutations in ion channels might lead to a vulnerability to lethal cardiac arrhythmias in infants. Interestingly, mutations in the ryanodine receptor (RyR2) calcium channel gene were identified in several victims of SIDS. We used a knock-in mouse model of mutation R176Q in RyR2 to determine whether defective RyR2 calcium channel function would result in sudden infant death due to cardiac arrhythmias. Our results revealed an increased incidence of sudden unexpected death in R176Q knock-in mice during the first weeks of life. Surface and intracardiac ECG recordings demonstrated the presence of an arrhythmogenic substrate and ventricular arrhythmias in infant R176Q mice. Using optical mapping experiments, we also found an increased incidence of spontaneous calcium release events via defective RyR2 in R176Q mouse hearts. These studies suggest that a genetic mutation in RyR2 in infant mice may predispose them to ventricular arrhythmias and sudden death.
Conflict of Interest Disclosures: None.
References
- 1.Krous HF, Byard RW, Rognum TO. Pathology research into sudden infant death syndrome: where do we go from here? Pediatrics. 2004;114:492–494. doi: 10.1542/peds.114.2.492-a. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell EA. Recommendations for sudden infant death syndrome prevention: a discussion document. Arch Dis Child. 2007;92:155–159. doi: 10.1136/adc.2005.076752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moon RY, Horne RS, Hauck FR. Sudden infant death syndrome. Lancet. 2007;370:1578–1587. doi: 10.1016/S0140-6736(07)61662-6. [DOI] [PubMed] [Google Scholar]
- 4.Maron BJ, Clark CE, Goldstein RE, Epstein SE. Potential role of QT interval prolongation in sudden infant death syndrome. Circulation. 1976;54:423–430. doi: 10.1161/01.cir.54.3.423. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz PJ, Segantini A. Cardiac innervation, neonatal electrocardiography, and SIDS. A key for a novel preventive strategy? Ann N Y Acad Sci. 1988;533:210–220. doi: 10.1111/j.1749-6632.1988.tb37250.x. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz PJ, Priori SG, Dumaine R, Napolitano C, Antzelevitch C, Stramba-Badiale M, Richard TA, Berti MR, Bloise R. A molecular link between the sudden infant death syndrome and the long-QT syndrome. N Engl J Med. 2000;343:262–267. doi: 10.1056/NEJM200007273430405. [DOI] [PubMed] [Google Scholar]
- 7.Tester DJ, Ackerman MJ. Postmortem long QT syndrome genetic testing for sudden unexplained death in the young. J Am Coll Cardiol. 2007;49:240–246. doi: 10.1016/j.jacc.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Van Norstrand DW, Tester DJ, Ackerman MJ. Overrepresentation of the proarrhythmic, sudden death predisposing sodium channel polymorphism S1103Y in a population-based cohort of African-American sudden infant death syndrome. Heart Rhythm. 2008;5:712–715. doi: 10.1016/j.hrthm.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tester DJ, Dura M, Carturan E, Reiken S, Wronska A, Marks AR, Ackerman MJ. A mechanism for sudden infant death syndrome (SIDS): stress-induced leak via ryanodine receptors. Heart Rhythm. 2007;4:733–739. doi: 10.1016/j.hrthm.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, Sun J, Guatimosim S, Song LS, Rosemblit N, D'Armiento JM, Napolitano C, Memmi M, Priori SG, Lederer WJ, Marks AR. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 11.Wehrens XH. The molecular basis of catecholaminergic polymorphic ventricular tachycardia: What are the different hypotheses regarding mechanisms? Heart Rhythm. 2007;4:794–797. doi: 10.1016/j.hrthm.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tiso N, Stephan DA, Nava A, Bagattin A, Devaney JM, Stanchi F, Larderet G, Brahmbhatt B, Brown K, Bauce B, Muriago M, Basso C, Thiene G, Danieli GA, Rampazzo A. Identification of mutations in the cardiac ryanodine receptor gene in families affected with arrhythmogenic right ventricular cardiomyopathy type 2 (ARVD2) Hum Mol Genet. 2001;10:189–194. doi: 10.1093/hmg/10.3.189. [DOI] [PubMed] [Google Scholar]
- 13.Priori SG, Napolitano C. Intracellular calcium handling dysfunction and arrhythmogenesis: a new challenge for the electrophysiologist. Circ Res. 2005;97:1077–1079. doi: 10.1161/01.RES.0000194556.41865.e2. [DOI] [PubMed] [Google Scholar]
- 14.Tester DJ, Kopplin LJ, Creighton W, Burke AP, Ackerman MJ. Pathogenesis of unexplained drowning: new insights from a molecular autopsy. Mayo Clin Proc. 2005;80:596–600. doi: 10.4065/80.5.596. [DOI] [PubMed] [Google Scholar]
- 15.Kannankeril PJ, Mitchell BM, Goonasekera SA, Chelu MG, Zhang W, Sood S, Kearney DL, Danila CI, De Biasi M, Wehrens XH, Pautler RG, Roden DM, Taffet GE, Dirksen RT, Anderson ME, Hamilton SL. Mice with the R176Q cardiac ryanodine receptor mutation exhibit catecholamine-induced ventricular tachycardia and cardiomyopathy. Proc Natl Acad Sci U S A. 2006;103:12179–12184. doi: 10.1073/pnas.0600268103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kingsley-Kallesen M, Mukhopadhyay SS, Wyszomierski SL, Schanler S, Schutz G, Rosen JM. The mineralocorticoid receptor may compensate for the loss of the glucocorticoid receptor at specific stages of mammary gland development. Mol Endocrinol. 2002;16:2008–2018. doi: 10.1210/me.2002-0103. [DOI] [PubMed] [Google Scholar]
- 17.de Diego C, Chen F, Xie LH, Dave AS, Thu M, Rongey C, Weiss JN, Valderrabano M. Cardiac alternans in embryonic mouse ventricles. Am J Physiol Heart Circ Physiol. 2008;294:H433–440. doi: 10.1152/ajpheart.01165.2007. [DOI] [PubMed] [Google Scholar]
- 18.Sood S, Chelu MG, van Oort RJ, Skapura D, Santonastasi M, Dobrev D, Wehrens XH. Intracellular calcium leak due to FKBP12.6 deficiency in mice facilitates the inducibility of atrial fibrillation. Heart Rhythm. 2008;5:1047–1054. doi: 10.1016/j.hrthm.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell GF, Jeron A, Koren G. Measurement of heart rate and Q-T interval in the conscious mouse. Am J Physiol. 1998;274:H747–751. doi: 10.1152/ajpheart.1998.274.3.H747. [DOI] [PubMed] [Google Scholar]
- 20.Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, Coromilas J, Landry DW, Marks AR. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004;304:292–296. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- 21.Chelu MG, Sarma S, Sood S, Wang S, Van Oort RJ, Skapura DS, Li N, Santonastasi M, Muller FU, Schmitz W, Schotten U, Anderson ME, Valderrabano M, Dobrev D, Wehrens XH. Calmodulin kinase II–mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–1951. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ackerman MJ, Siu BL, Sturner WQ, Tester DJ, Valdivia CR, Makielski JC, Towbin JA. Postmortem molecular analysis of SCN5A defects in sudden infant death syndrome. Jama. 2001;286:2264–2269. doi: 10.1001/jama.286.18.2264. [DOI] [PubMed] [Google Scholar]
- 23.Plant LD, Bowers PN, Liu Q, Morgan T, Zhang T, State MW, Chen W, Kittles RA, Goldstein SA. A common cardiac sodium channel variant associated with sudden infant death in African Americans, SCN5A S1103Y. J Clin Invest. 2006;116:430–435. doi: 10.1172/JCI25618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhodes TE, Abraham RL, Welch RC, Vanoye CG, Crotti L, Arnestad M, Insolia R, Pedrazzini M, Ferrandi C, Vege A, Rognum T, Roden DM, Schwartz PJ, George AL., Jr Cardiac potassium channel dysfunction in sudden infant death syndrome. J Mol Cell Cardiol. 2008;44:571–581. doi: 10.1016/j.yjmcc.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin JA, Kung HC, Mathews TJ, Hoyert DL, Strobino DM, Guyer B, Sutton SR. Annual summary of vital statistics: 2006. Pediatrics. 2008;121:788–801. doi: 10.1542/peds.2007-3753. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz PJ, Stramba-Badiale M, Segantini A, Austoni P, Bosi G, Giorgetti R, Grancini F, Marni ED, Perticone F, Rosti D, Salice P. Prolongation of the QT interval and the sudden infant death syndrome. N Engl J Med. 1998;338:1709–1714. doi: 10.1056/NEJM199806113382401. [DOI] [PubMed] [Google Scholar]
- 27.Arnestad M, Crotti L, Rognum TO, Insolia R, Pedrazzini M, Ferrandi C, Vege A, Wang DW, Rhodes TE, George AL, Jr, Schwartz PJ. Prevalence of long-QT syndrome gene variants in sudden infant death syndrome. Circulation. 2007;115:361–367. doi: 10.1161/CIRCULATIONAHA.106.658021. [DOI] [PubMed] [Google Scholar]
- 28.Giustetto C, Di Monte F, Wolpert C, Borggrefe M, Schimpf R, Sbragia P, Leone G, Maury P, Anttonen O, Haissaguerre M, Gaita F. Short QT syndrome: clinical findings and diagnostic-therapeutic implications. Eur Heart J. 2006;27:2440–2447. doi: 10.1093/eurheartj/ehl185. [DOI] [PubMed] [Google Scholar]
- 29.Skinner JR, Chung SK, Montgomery D, McCulley CH, Crawford J, French J, Rees MI. Near-miss SIDS due to Brugada syndrome. Arch Dis Child. 2005;90(5):528–529. doi: 10.1136/adc.2004.058115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wehrens XH, Marks AR. Sudden unexplained death caused by cardiac ryanodine receptor (RyR2) mutations. Mayo Clin Proc. 2004;79:1367–1371. doi: 10.4065/79.11.1367. [DOI] [PubMed] [Google Scholar]
- 31.Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, Sorrentino V, Danieli GA. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 32.Tester DJ, Spoon DB, Valdivia HH, Makielski JC, Ackerman MJ. Targeted mutational analysis of the RyR2-encoded cardiac ryanodine receptor in sudden unexplained death: a molecular autopsy of 49 medical examiner/coroner's cases. Mayo Clin Proc. 2004;79:1380–1384. doi: 10.4065/79.11.1380. [DOI] [PubMed] [Google Scholar]
- 33.Creighton W, Virmani R, Kutys R, Burke A. Identification of novel missense mutations of cardiac ryanodine receptor gene in exercise-induced sudden death at autopsy. J Mol Diagn. 2006;8:62–67. doi: 10.2353/jmoldx.2006.050081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehnart SE, Wehrens XH, Laitinen PJ, Reiken SR, Deng SX, Cheng Z, Landry DW, Kontula K, Swan H, Marks AR. Sudden death in familial polymorphic ventricular tachycardia associated with calcium release channel (ryanodine receptor) leak. Circulation. 2004;109:3208–3214. doi: 10.1161/01.CIR.0000132472.98675.EC. [DOI] [PubMed] [Google Scholar]
- 35.Bauce B, Nava A, Rampazzo A, Daliento L, Muriago M, Basso C, Thiene G, Danieli GA. Familial effort polymorphic ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy map to chromosome 1q42-43. Am J Cardiol. 2000;85:573–579. doi: 10.1016/s0002-9149(99)00814-0. [DOI] [PubMed] [Google Scholar]
- 36.Postma AV, Denjoy I, Kamblock J, Alders M, Lupoglazoff JM, Vaksmann G, Dubosq-Bidot L, Sebillon P, Mannens MM, Guicheney P, Wilde AA. Catecholaminergic polymorphic ventricular tachycardia: RYR2 mutations, bradycardia, and follow up of the patients. J Med Genet. 2005;42:863–870. doi: 10.1136/jmg.2004.028993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate. 1994:194–197. doi: 10.1159/000244052. [DOI] [PubMed] [Google Scholar]
- 38.Brunner M, Peng X, Liu GX, Ren XQ, Ziv O, Choi BR, Mathur R, Hajjiri M, Odening KE, Steinberg E, Folco EJ, Pringa E, Centracchio J, Macharzina RR, Donahay T, Schofield L, Rana N, Kirk M, Mitchell GF, Poppas A, Zehender M, Koren G. Mechanisms of cardiac arrhythmias and sudden death in transgenic rabbits with long QT syndrome. J Clin Invest. 2008;118:2246–2259. doi: 10.1172/JCI33578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nuyens D, Stengl M, Dugarmaa S, Rossenbacker T, Compernolle V, Rudy Y, Smits JF, Flameng W, Clancy CE, Moons L, Vos MA, Dewerchin M, Benndorf K, Collen D, Carmeliet E, Carmeliet P. Abrupt rate accelerations or premature beats cause life-threatening arrhythmias in mice with long-QT3 syndrome. Nat Med. 2001;7:1021–1027. doi: 10.1038/nm0901-1021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.