Abstract
Objective
To provide a quantitative algorithm for classifying primary progressive aphasia (PPA) into agrammatic (PPA-G), semantic (PPA-S) and logopenic (PPA-L) variants, each of which is known to have a different probability of association with Alzheimer’s disease (AD) versus frontotemporal lobar degeneration (FTLD).
Design
Prospectively and consecutively enrolled 16 PPA patients tested with neuropsychological instruments and magnetic resonance imaging (MRI).
Setting
University medical center.
Participants
PPA patients recruited nationally in the USA as part of a longitudinal study.
Results
A two-dimensional template, reflecting performance on tests of syntax (Northwestern Anagram Test) and lexical semantics (Peabody Picture Vocabulary Test), classified all 16 patients in concordance with a clinical diagnosis that had been made prior to the administration of the quantitative tests. All three subtypes had distinctly asymmetrical atrophy of the left perisylvian language network. Each subtype also had distinctive peak atrophy sites. Only PPA-G had peak atrophy in the IFG (Broca’s area), only PPA-S had peak atrophy in the anterior temporal lobe, and only PPA-L had peak atrophy in area 37.
Conclusions
Once an accurate root diagnosis of PPA is made, subtyping can be quantitatively guided using a two-dimensional template based on orthogonal tasks of grammatical competence and word comprehension. Although the choice of tasks and precise cut-off levels may evolve in time, this set of 16 patients demonstrates the feasibility of using a simple algorithm for clinico-anatomical classification in PPA. Prospective studies will show whether this suptyping can improve the clinical prediction of underlying neuropathology.
INTRODUCTION
The classification of primary progressive aphasia (PPA) into subtypes has acquired new relevance in light of post-mortem series and in vivo amyloid imaging showing that individual variants have different likelihoods of being caused by Alzheimer’s disease (AD) versus frontotemporal lobar degeneration (FTLD). The most frequent associations have been reported between the agrammatic variant (PPA-G) and FTLD with tauopathy (FTLD-T), the semantic variant (PPA-S) and FTLD with ubiquitin/TDP-43 proteinopathy (FTLD-TDP), and the logopenic variant (PPA-L) and AD 1–3.
In the absence of definitive in vivo biomarkers for these diseases, the reliable classification of PPA assumes considerable relevance for increasing the accuracy with which the nature of the underlying pathology can be predicted. This is particularly important for early-onset dementias where the concordance between clinical predictions and post-mortem confirmation can be quite low. Although numerous studies have described clinical and neuropsychological characteristics of PPA subtypes, few have included an unselected prospective cohort investigated with a unified battery of easily administered tests specifically chosen to probe the defining features of the subtypes.
This study reports an empirically established two-dimensional quantitative template, derived from performance on tests of syntax and lexical semantics, that successfully classified 16 consecutively investigated PPA patients. The biological validity of the resultant classification was supported by the presence of distinctive anatomical patterns of peak cortical atrophy in each variant. Whether this classification also corresponds to differential neuropathological processes remains to be determined by prospective studies.
METHODS
Recruitment occurred in the context of an NIH-funded project that brought patients from throughout the USA to Northwestern University for a 3-day intensive research program. All patients who fulfilled criteria for PPA, who could complete the five key diagnostic tests, and who had an MRI scan suitable for quantitative morphometry were included. Only scans obtained within a few days of neuropsychological testing were used.
The root diagnosis of PPA was made on the basis of a progressive language disturbance (i.e., aphasia) that is initially the most salient feature of the clinical picture (i.e., primary) and that is caused by neurodegeneration (i.e., is progressive) 4–6. The presence of an aphasia was established by aphasia quotients (AQ) derived from administration of the Western Aphasia Battery (WAB)7. All patients were right handed as determined by the Edinburgh scale 8, 8 were male and 8 female (Table 1). Duration of disease at the time of testing varied from 2 to 7.5 years. The progressive nature of the deficits and the fact that the language disorder was the chief problem during the initial few years of the disease were documented by history taken from the patient, medical records, and from at least one additional informant who lived in the same household 9. All patients had received a descriptive diagnosis of PPA-G, PPA-L, or PPA-S based on an initial office evaluation (Table 2), and prior to the administration of the quantitative tests, by clinicians with extensive experience with this disease (MM and SW). Five language tests provided the basis for the quantitative classification.
Table 1.
Subject characteristics.
| Age at Testing Gender | Education in years | Handedness Edinburgh | Symptom Duration(years) | WAB AQ(100) | % Correct Word Assoc(16) | % Correct PPVT (36) | % Correct NAT (10) | % Correct BNT (60) | % BDAE Fluency(7 | WAB Repetitio(100) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 62/M | 20 | +80 | 5 | 82.3 | 100 | 100 | 50 | 98.3 | 42.9 | 80 |
| P2 | 59/M | 12 | +95 | 3 | 79.9 | 100 | 94.4 | 40 | 81.7 | 57.1 | 78 |
| P3 | 61/F | 18 | +100 | 5 | 75.3 | 100 | 100 | 50 | 88.3 | 42.9 | 88 |
| P4 | 56/F | 14 | +100 | 2 | 65.4 | 100 | 83.3 | 10 | 81.7 | 14.3 | 42 |
| P5 | 53/F | 16 | +100 | 3 | 65.9 | 81.3 | 22.2 | 60 | 6.7 | 100 | 74 |
| P6 | 63/M | 18 | +100 | 5.5 | 88.2 | 100 | 47.2 | 100 | 23.3 | 100 | 97 |
| P7 | 54/F | 12 | +100 | 3 | 83.2 | 93.8 | 27.8 | 100 | 10 | 100 | 91 |
| P8 | 64/F | 16 | +100 | 7.5 | 80.6 | 91.7 | 38.9 | 100 | 8.3 | 100 | 88 |
| P9 | 56/F | 18 | +100 | 2.5 | 75.5 | 75 | 38.9 | 100 | 5 | 100 | 85 |
| P10 | 69/M | 15 | +100 | 2.5 | 92 | 100 | 97.2 | 90 | 98.3 | 71.4 | 90 |
| P11 | 63/M | 18 | +100 | 2.5 | 97.1 | 100 | 100 | 10 | 96.7 | 100 | 89 |
| P12 | 58/M | 16 | +100 | 2 | 86.9 | 100 | 97.2 | 90 | 90 | 100 | 90 |
| P13 | 65/F | 13 | +100 | 5 | 78.6 | 100 | 83.3 | 70 | 83.3 | 71.4 | 88 |
| P14 | 75/F | 16 | +100 | 2.5 | 97.2 | 100 | 97.2 | 100 | 88.3 | 100 | 90 |
| P15 | 64/M | 18 | +90 | 2 | 93.2 | 100 | 100 | 70 | 98.3 | 85.7 | 89 |
| P16 | 48/M | 16 | +80 | 6 | 83.2 | 100 | 100 | 100 | 98.3 | 71.4 | 90 |
A “+” score in the Edinburgh scale indicates the degree of right handedness. Abbreviations: WAB AQ- Western Aphasia Battery Aphasia Quotient. PPVT- Peabody Picture Vocabulary test. NAT- Northwestern Anagram Test. BNT- Boston Naming Test. BDAE- Boston Diagnostic Aphasia Examination. Numbers in parentheses indicate highest possible raw scores.
Table 2.
Criteria for PPA and its subtypes
| Criteria for the Root Diagnosis of PPA |
|
| Criteria for PPA-G (agrammatic variant): |
| Descriptive: The central feature is an abnormality of syntax (word-order) or some other aspect of grammar in spoken or written language in the presence of relatively preserved single word comprehension. Fluency is usually impaired and speech is usually effortful and hesitant. |
| Quantitative: NAT <60%, PPVT ≥60%. |
| Criteria for PPA-S (semantic variant): |
| Descriptive:The central feature is an abnormality of single word comprehension in the presence of relatively preserved grammar and fluency. Output is circumlocutory, occasionally uninformative and frequently paraphasic. Naming is severely impaired. |
| Quantitative: PPVT <60%, NAT ≥60%. |
| Criteria for PPA-L (logopenic variant): |
| Descriptive: The central feature is intermittent word-finding hesitations and phonemic paraphasias. Naming is impaired but not as severely as in PPA-S and improves upon phonemic cueing. Repetition may be impaired. Fluent output in casual conversation can alternate with dysfluent speech, which emerges when the patient needs to convey precise information and cannot use circumlocution. Spelling can be impaired |
| Quantitative: PPVT and NAT are both ≥60%. |
| Criteria for PPA-M (mixed): |
| Descriptive: Combination of agrammatism with comprehension deficit, usually accompanied by poor fluency and frequent paraphasias. |
| Quantitative: PPVT and NAT are both < 60%. |
Single word comprehension
Word comprehension (lexical semantics) is commonly tested by asking the patient to match a word to a picture. The auditory word comprehension subtest of the WAB was too easy. We therefore opted to use the PPVT-IV 10, and selected a subset of 36 moderately difficult items (157–192). Each item requires the patient to match a word representing an object, action or attribute to one of 4 picture choices. Because performance on the PPVT could potentially be confounded by problems of picture recognition, its face validity as a measure of word comprehension was further established by comparing scores to a word-word association task in which patients decided which of two pairs contained semantically matching words (e.g., horse-saddle versus horse-slippers). Only the PPA-S patients with the lowest PPVT scores showed less than 100% performance on the word-word association task (Table 1). However, the impairment on this task was milder than on the PPVT. In the future, a more difficult form of the word-word association task could be substituted for the PPVT to eliminate potential interference from picture recognition deficits.
Syntax
Syntax, a major component of grammar, regulates the proper ordering of words into sentences. Its assessment is challenging. The WAB, for example, has no subtest for assessing syntax. In traditional aphasiology, fluency and phrase length have been used as surrogates for grammatical competence. However, it becomes difficult to decide if apparent agrammatism in a dysfluent patient represents an economy of expression, consequences of dysarthria, or a true insensitivity to rules of syntax. To circumvent these problems, we designed the Northwestern Anagram Test (NAT) 11. During the administration of the NAT, the patient is asked to order single words, each printed on a separate card, to be syntactically consistent with an action depicted in a target picture 11. Printed words and arrows label each actor and action in the picture to minimize the impact of single word comprehension deficits on performance. Correct performance therefore specifically reflects the ability to order words into a sentence that has a syntactic structure consistent with the depicted action. This test correlates with other tests of grammatical sentence production but not with tests of naming, single word comprehension or motor speech production. For the purpose of PPA subtyping, we chose a subset of 10 items from the NAT containing object-extracted wh- questions (Obj-Wh) and object-clefts constructions (OC). These items of intermediate difficulty could be performed even by PPA-S patients with prominent word comprehension deficits. The NAT inclusive of the 10-item subset can be downloaded at http://www.soc.northwestern.edu/NorthwesternAnagramTest/.
Naming
The BNT was used to assess the confrontation naming of objects 12. It is a 60-item standardized test in which items are administered in order of decreasing frequency of occurrence in the language.
Fluency
There are several measures of fluency and the one selected for this study was “phrase length,” defined as the longest string of words produced without pause in a speech sample. Recorded samples while describing the “picnic” picture from the WAB were transcribed and rated by two raters on a 7-point scale for phrase length taken from the Boston Diagnostic Aphasia Examination (BDAE) 13. Although the BDAE also has a picture description task, the WAB picture has more actors and actions and provides more varied opportunities for speech production.
Repetition
Repetition was measured with the corresponding subtest from the WAB, which samples repetition of single words, phrases, and sentences.
Imaging
Scans were acquired and reconstructed with the FreeSurfer image analysis suite (version 4.1.0) as previously described 9. Thickness maps of the PPA group were statistically contrasted against 17 right-handed healthy volunteers (9 male, 8 female, mean age = 64.4 mean education = 16.29). There were no statistically significant differences in age or education between groups. Differences in thickness between the groups were calculated by conducting a general linear model on every vertex along the cortical surface. False Discovery Rate (FDR) was applied to adjust for multiple comparisons 14. A significance threshold of p < 0.01 was used to detect areas of peak cortical thinning (i.e., atrophy) in PPA compared to controls. Because of the small sample size, direct comparisons of subgroups was not performed.
RESULTS
Performances on the five language tests described above were first expressed as a percentage of the highest possible scores for that test (Figures 1–3), and then placed on a two dimensional map where the x and y axes reflected the percentage scores on tests of syntax (measured by the NAT) and comprehension (measured by the PPVT) (Figure 4). The 60% range of performance on each axis, chosen empirically to fit the diagnoses we had given during the initial office examination, divided the map into 4 quadrants (Figure 4).According to the resultant map:
Figures 1–3.
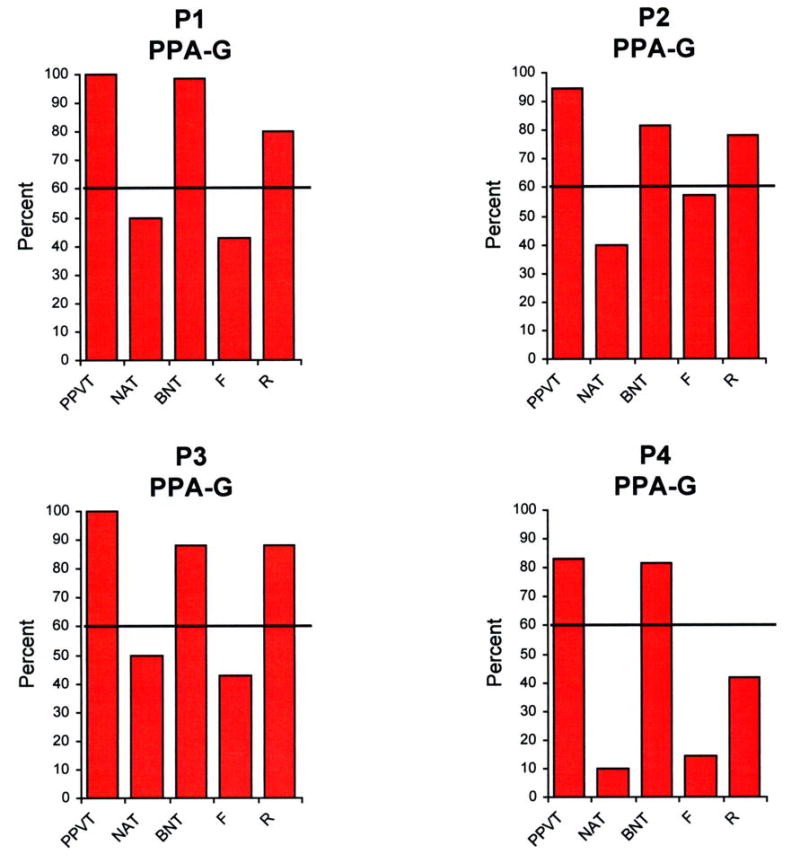
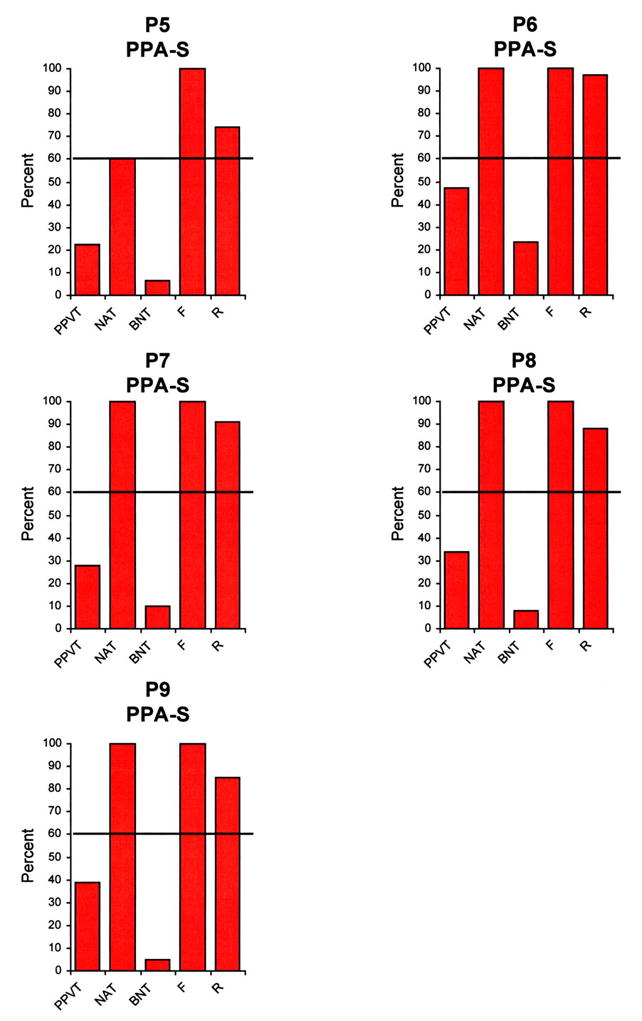
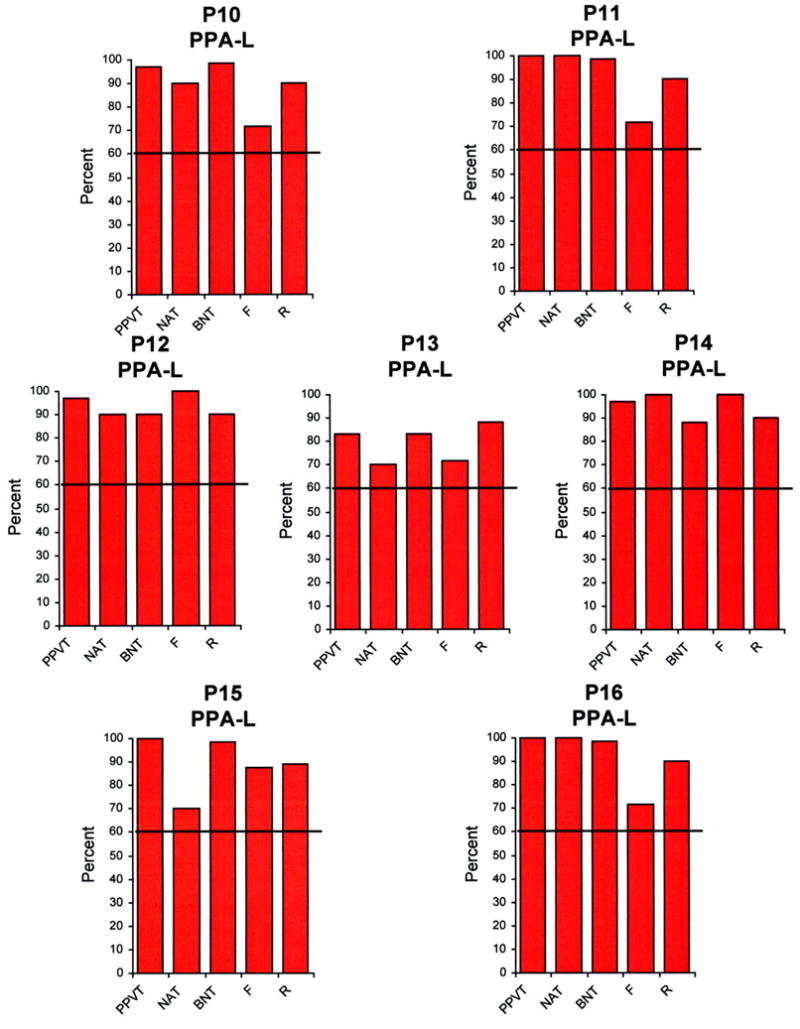
Performance of the 16 patients in the 5 language tests. The height of the bars represents performance as a percentage, where 100% reflects a perfect score for that task. PPVT- Peabody Picture Vocabulary Test. NAT- Northwestern Anagram Test. BNT- Boston Naming Test. F- Fluency. R- Repetition.
Figure 4.
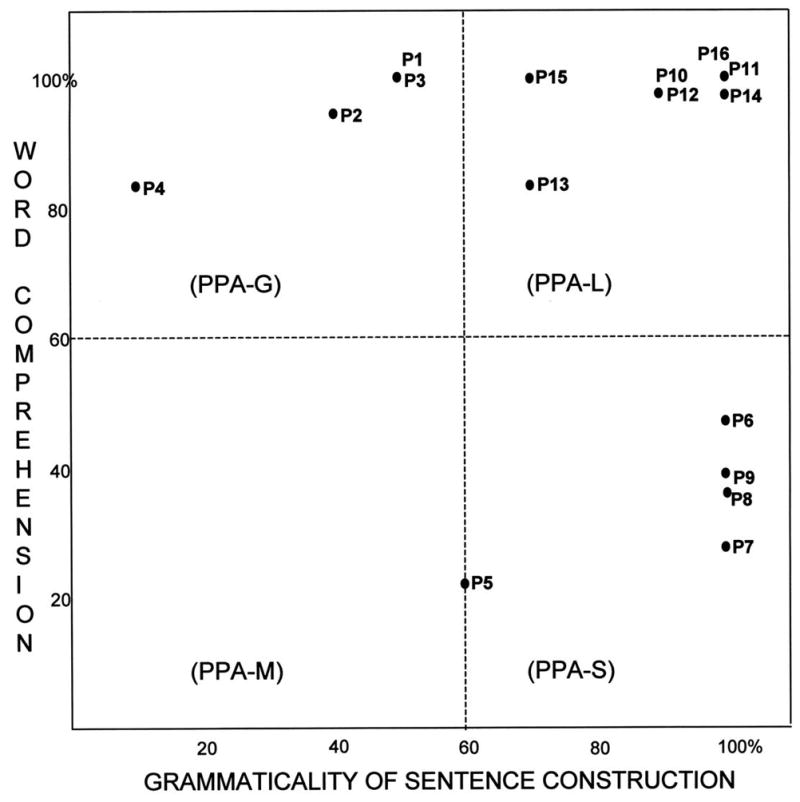
A 2-dimensional template based on single word comprehension and grammatical structure of sentences. The 60% performance level divides the template into four quadrants, one for each subtype. In this group of cases, the PPVT was used to assess word comprehension, and the NAT to assess grammar. The values in the x and y axes reflect the performance percentages shown in Figures 1–3.
The subtype is PPA-S if the PPVT is <60% and the NAT is ≥60%.
The subtype is PPA-G if the NAT is <60% but the PPVT is ≥60%.
The subtype is PPA-L if PPVT and NAT are both ≥60%.
The subtype is PPA-M if PPVT and NAT are both < 60%.
Of the 16 patients, 4 were included in the PPA-G group (P1-P4), 5 in the PPA-S group (P5-P9) and 7 in the PPA-L group (P10-P16). All subtypes displayed asymmetrically greater atrophy in the left hemisphere (Fig 5). Peak atrophy in PPA-G included the inferior frontal gyrus (IFG, Broca’s area) and the temporoparietal junction (TPJ). Additional atrophy was seen in premotor (PM) and dorsal prefrontal cortex (DF). The PPA-S group showed atrophy mostly in the anterior temporal lobe, including the superior (STG) middle (MTG), inferior (IFG) and fusiform gyri. The PPA-L group had peak atrophy in the TPJ and posterior parts of the ITG (Brodmann area 37). The IFG atrophy was prominent only in PPA-G, area 37 atrophy only in PPA-L, and anterior temporal atrophy only in PPA-S. The leftward asymmetry was most prominent in PPA-L.
Figure 5.
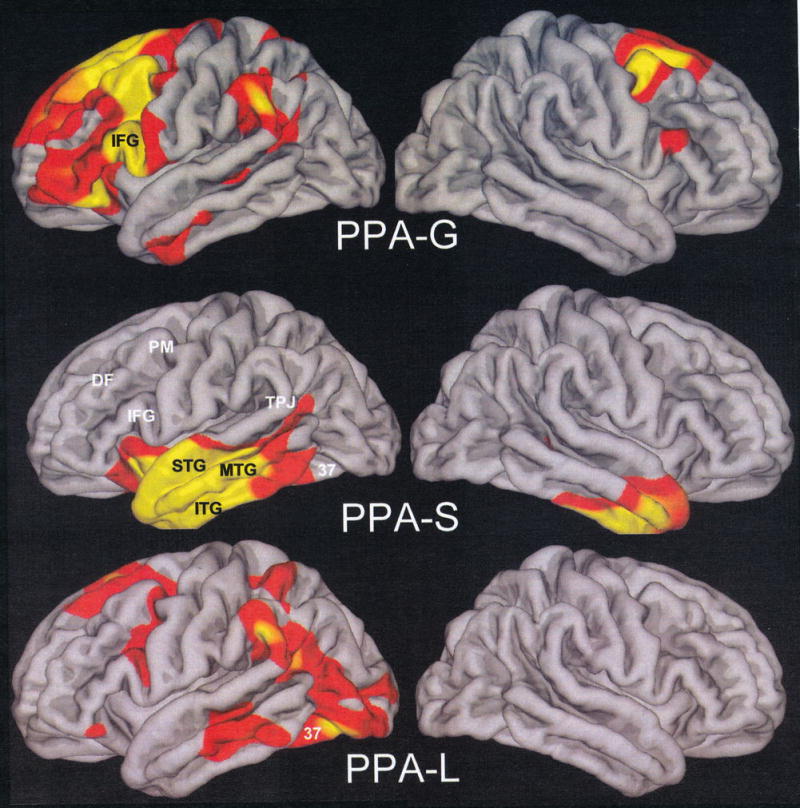
Distribution of cortical thinning. Red shading indicates a significance level of p<0.01, and the yellow p<0.001. Abbreviations: DF, Dorsolateral prefrontal cortex; IFG, Inferior frontal gyrus; ITG, Inferior temporal gyrus; MTG, Middle temporal gyrus; PM, Premotor cortex; STG, Superior temporal gyrus; TPJ, Temporoparietal junction; 37, Area 37 of Broadmann;
DISCUSSION
There is no one-to-one correspondence between anatomical components of the left perisylvian language network and specific language functions. In general, however, the frontal components are more closely related to fluency and grammar whereas the posterior and temporal components are more closely related to lexical semantics and object naming 15–17. Damage to different sectors of the language network can differentially hinder speech fluency, grammatical competence, word comprehension, word-finding, spelling, reading and object naming. Classical aphasiology, based predominantly on the investigation of patients with focal cerebrovascular disease, delineated Broca, Wernicke, conduction and transcortical aphasias as prototypical manifestations of damage to different parts of the network 18.
The left perisylvian language network can also become the preferential target of degenerative disease. The resultant syndrome, a progressive and initially isolated language impairment, is known as PPA. As in the case of aphasias caused by cerebrovascular accidents, the aphasia in PPA can display numerous patterns. However, the clinico-anatomical correlations established in acute cerebrovascular lesions are not necessarily generalizable to those encountered in PPA 19. The differences probably reflect the slow destruction of tissue by neurodegenerative disease, residual survival of neurons even in the most atrophic areas, and compensatory reorganizations of synaptic circuitry.
Recent developments showing that individual aphasic patterns are differentially associated with the neuropathology of AD, FTLD-TDP and FTLD-T have rekindled the need to establish a reliable subtyping of PPA. A widespread practice has been to use the progressive nonfluent aphasia (PNFA) and semantic dementia (SD) syndromes of Neary et al.20 as the two major variants of progressive aphasia. However, the PNFA designation, based on the core feature of “nonfluent spontaneous speech with at least one of the following: agrammatism, phonemic paraphasias, anomia” appears, in retrospect, to have been too broad. The introduction of a logopenic PPA variant by Gorno-Tempini 21 has led to the division of PNFA into agrammatic (PPA-G) and logopenic (PPA-L) subtypes. The logopenic patients may be dysfluent because of word-finding hesitations but do not show major impairments of grammar. The logopenic variant also subsumes PPA patients with a “fluent,” circumlocutory aphasia who have normal comprehension and therefore do not comfortably fit the SD designation. The heuristic value of this subdivision of PNFA was demonstrated by post-mortem investigations showing that PPA-L has a high association (60% in our post-mortem series) with AD pathology whereas the PPA-G variant has a high (80% in our series) association with FTLD-T 2.
The use of the SD nomenclature raises analogous concerns of heterogeneity. Its two core clinical features, which must both be present, include “loss of word meaning” as well as “perceptual disorder” characterized by prosopagnosia and/or associative agnosia 20. The SD designation could therefore subsume patients who are equally aphasic and agnosic and who would therefore not fulfill the PPA criteria in Table 2. Moreover, PPA patients with poor comprehension may not qualify for the diagnosis of SD in the absence of at least some perceptual disorder. We addressed this question in a recent study where we characterized the semantic variant of PPA (PPA-S) as a syndrome where word comprehension deficit is the only obligatory core feature and the major cause of disability 9. The reliable and reproducible diagnosis of PPA-S is of considerable practical import since this subtype has a high likelihood of being associated with the neuropathology of FTLD-TDP 3, 6.
Previous subtyping approaches, such as the one by Neary et al 20, have generally relied on lists of features, but have rarely specified quantitative boundaries or specific instruments. One of the several challenges has been the implicit use of the term “fluency”, which can be impaired by damage outside the language network, as a surrogate for grammatical competence, which is a core function of the language network. This is one reason why so many patients with effortful speech have been described as having PNFA, sometimes without full documentation of a language impairment. In the current study, we used a newly developed and easily administered instrument, the Northwestern Anagram Test (NAT), to directly assess the production of syntactically correct sentences. The testing method minimizes the influence of poor single word comprehension and working memory deficits on performance and dissociates low fluency from grammatical competence.
We chose the PPVT for single word comprehension. Scores on the PPVT had no significant correlation with NAT scores and therefore assessed an orthogonal aspect of language function. The face validity of the PPVT as a test of word comprehension was shown by its high concordance with the purely verbal paired word association test we administered the same patients. We selected a subset of items with difficulty levels that are likely to avoid floor or ceiling effects. However, the cut-off level we chose for this group of patients may need to be altered for populations with different educational levels.
The two-dimensional mapping, based on the PPVT and the NAT, with cutoff levels at 60%, allowed us to subtype all patients in a manner that fit the descriptive clinical diagnosis made prior to the availability of the PPVT and NAT scores. The other language tests shown in Figures 1–3 provided supplementary but less specific information. Severe impairments of naming on the BNT were only seen in PPA-S. However, a low BNT is unlikely to be specific to PPA-S since low scores could also reflect impairments of lexical retrieval even when comprehension is intact. Fluency was the lowest in PPA-G but there were 3 PPA-L patients who also had distinctly abnormal fluency scores (P10, P13, P16) even in the absence of motor or apraxic speech impairments. Repetition abnormalities have been reported to constitute a distinguishing feature of PPA-L 22. This was not the case in our patients, probably because the WAB repetition subtest is too easy for patients with relatively mild impairment. Naming was preserved in some PPA-L patients and only mildly impaired in others, leaving word-finding hesitations as the major area of impairment in the spoken language of these patients. In clinical practice, we do see PPA-L patients with prominent retrieval-based object naming deficits, although such patients were not represented in the current sample.
All three subtypes had asymmetrical left hemisphere atrophy that involved the perisylvian and additional temporal components of the language network. Each group also had unique anatomical signatures of peak atrophy sites within the language network. These anatomical patterns agree with those described by Gorno-Tempini and colleagues 21 and therefore confirm the biological validity of the subtyping method described here.
The distinctive atrophy patterns were concordant with the clinical profiles. The areas of peak atrophy in PPA-S, the subtype characterized by word comprehension deficits, overlapped parts of the language network known to mediate word comprehension 23, 24. The IFG was severely atrophied only in PPA-G, a relationship that is consistent with the role of this area in syntax, fluency and other aspects of grammatical competence 25. The atrophy in PPA-G also extended into other areas of premotor and dorsolateral frontal cortex, a distribution that may reflect the close relationship of this variant to corticobasal degeneration 26.
In PPA-L, the major atrophy was in the posterior parts of the language network, including TPJ and area 37. In light of new functional imaging data, it seems as if the TPJ, partially overlapping Wernicke’s area, may not be all that critical for decoding word meaning and that this aspect of language may be more closely dependent on more anterior parts of the lateral temporal lobe 23. The TPJ, especially the posterior part of the superior temporal gyrus, may play a particularly important role in phonological encoding 27 and its atrophy in PPA-L may underlie the frequent phonemic paraphasias described in this variant 22. Area 37 has been linked to modality-independent lexical access 16, an affiliation that is consistent with the word-finding impairment characteristic of PPA-L.
The delineation of PPA-L based on the preservation of grammar and semantics may raise the concern that it may merely reflect a less severe form of PPA rather than a separate variant. However, it should be pointed out that the impaired word-finding in PPA-L, often accompanied by additional errors of spelling and calculation, can cause as much functional disability as arises in the other PPA variants. As the disease progresses, the PPA-L patients may become more and more nonfluent because of frequent word-finding hesitations. In our experience, however, such progression rarely, if ever, leads to the emergence of the prominent impairments of syntax or semantics characteristic of PPA-G and PPA-S. The PPA-L variant therefore has a trajectory of progression that continues to distinguish it from the other PPA subtypes.
The most critical step in the process of subtyping is the accurate root diagnosis of PPA and its delineation from patients whose main problem lies in the areas of visual agnosia, motor speech impairment or amotivational states. Equally important is the need to eliminate those patients whose progressive aphasia emerges on a background of equally severe amnesia, agnosia or apathy. Once the root diagnosis of PPA has been made, the subsequent clinico-anatomical subtyping can be achieved on the basis of two easily administered tests of syntax and semantics (Table 2). The literature indicates that PPA-G, PPA-S and PPA-L have different probabilities of being linked to AD, FTLD-T and FTLD-TDP 2, 3. Future post-mortem studies will show if the subtyping algorithm described here, and validated on a relatively small sample of 16 patients, will lead to similar relationships in additional samples and reliably improve the prediction of the underlying neuropathology.
All PPA subtypes share the common denominator of selective atrophy within the language network. Our subtyping approach is based on the nature of the most impaired language function at the early to middle stages of disease severity. This does not mean that other language functions within a subtype are intact. For example, patients with PPA-S may have a substantial proportion of their naming errors caused by lexical retrieval rather than word comprehension impairments, and many PPA-L and PPA-G patients may show impairments of semantic priming 9, 28, 29. It is therefore important to keep in mind that, while subtypes are defined by the nature of the most severe impairment, inter-subtype boundaries become fuzzy when components of language function other than those of peak impairment are considered. As the disease progresses, furthermore, testing may become increasingly more difficult and subtypes may no longer be identifiable. We should also point out that “grammar” and “word comprehension” are exceedingly complex constructs and that the NAT and PPVT capture only a fragment of the corresponding processes. Nonetheless, our goal was to provide a conceptual framework for mapping subtypes according to performance along these two orthogonal subdomains of language, with the two tests serving as reliable (albeit partial) markers of impairment.
Methods of classification tend to evolve and the current approach will almost certainly be improved in the future. Other tests of grammar and semantics may prove more useful and the cut-off level of performance may need to be adjusted to accommodate different linguistic and educational backgrounds. The goal of the current report is to demonstrate the feasibility of a simple two-dimensional template for mapping the major subtypes of PPA. Eventually biomarkers will emerge and clinical subtyping will no longer serve the purpose of predicting the underlying neuropathology. Even then, however, subtyping will help to explore the molecular mechanisms that make individual sectors of the language network the selective targets of different neuropathological diseases.
Acknowledgments
Supported by DC008552 from the National Institute on Deafness and Communication Disorders and AG13854 (Alzheimer Disease Center) from the National Institute on Aging.
References
- 1.Rabinovici GD, Jagust WJ, Furst AJ, et al. Aβ amyloid and glucose metabolism in three variants of primary progressive aphasia. Annals of Neurology. 2008;64:388–401. doi: 10.1002/ana.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mesulam M, Wicklund A, Johnson N, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Annals of Neurology. 2008;63:709–719. doi: 10.1002/ana.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knibb JA, Xuereb JH, Patterson K, Hodges JR. Clinical and pathological characterization of progressive aphasia. Annals of Neurology. 2006;59:156–165. doi: 10.1002/ana.20700. [DOI] [PubMed] [Google Scholar]
- 4.Mesulam M–M, Weintraub S. Primary progressive aphasia and kindred disorders. In: Duyckaerts C, Litvan I, editors. Handbook of Clinical Neurology. New York: Elsevier; 2008. pp. 573–587. [DOI] [PubMed] [Google Scholar]
- 5.Mesulam M-M. Primary progressive aphasia: A language-based dementia. New England Journal of Medicine. 2003;348:1535–1542. doi: 10.1056/NEJMra022435. [DOI] [PubMed] [Google Scholar]
- 6.Rogalski EJ, Mesulam M–M. Clinical trajectories and biological features of primary progressive aphasia (PPA) Current Alzheimer Research. 2009:6. doi: 10.2174/156720509788929264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kertesz A. Western Aphasia Battery. San Antonio, Texas: The Psychological Corporation; 1982. [Google Scholar]
- 8.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;87:256–259. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 9.Mesulam M–M, Rogalski E, Wieneke C, et al. Neurology of anomia in the semantic subtype of primary progressive aphasia. Brain. 2009 doi: 10.1093/brain/awp138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn LA, Dunn LM. Peabody Picture Vocabulary Test-4: Pearson. 2006 [Google Scholar]
- 11.Weintraub S, Mesulam M–M, Wieneke C, Rademaker A, Rogalski EJ, Thompson CK. The Northwestern Anagram Test: measuring sentence production in primary progressive aphasia. American Journal of Alzheimer’s Disease and Other Dementias. doi: 10.1177/1533317509343104. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 13.Goodglass H, Kaplan E, Barresi B. Boston Diagnostic Aphasia Examination. 3. Austin: Pro-Ed; 2001. [Google Scholar]
- 14.Genovese CR, Lazar NA, Nichols TE. Thresholding of statistical maps in functional imaging using the false discovery rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 15.Price CJ. The anatomy of language: contributions from functional neuroimaging. Journal of Anatomy. 2000;197:335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeLeon J, Gottesman RF, Kleinman JT, et al. Neural regions assential for distinct cognitive processes underlying picture naming. Brain. 2007;130:1408–1422. doi: 10.1093/brain/awm011. [DOI] [PubMed] [Google Scholar]
- 17.Mesulam M-M. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Annals of Neurology. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- 18.Benson F. Aphasia and related disorders: a clinical approach. In: Mesulam M–M, editor. Principles of Behavioral Neurology. Philadelphia: F. A. Davis; 1985. pp. 193–238. [Google Scholar]
- 19.Mesulam M–M, Weintraub S. Spectrum of primary progressive aphasia. In: Rossor MN, editor. Unusual Dementias. London: Baillière Tindall; 1992. pp. 583–609. [PubMed] [Google Scholar]
- 20.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration. A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 21.Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology. 2004;55:335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorno-Tempini ML, Brambati SM, Ginex V, et al. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71:1227–1234. doi: 10.1212/01.wnl.0000320506.79811.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gitelman DR, Nobre AC, Sonty S, Parrish TB, Mesulam M-M. Language network specializations: An analysis with parallel task design and functional magnetic resonance imaging. NeuroImage. 2005;26:975–985. doi: 10.1016/j.neuroimage.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 24.Spitsyna G, Warren JE, Scott SK, Turkheimer FE, Wise RJS. Converging language streams in the human temporal lobe. Journal of Neuroscience. 2006;26:7328–7336. doi: 10.1523/JNEUROSCI.0559-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Indefrey P, Hellwig F, Herzog H, Seitz RJ, Hagoort P. Neural responses to the production and comprehension of syntax in identical utterances. Brain and Language. 2004;89:312–319. doi: 10.1016/S0093-934X(03)00352-3. [DOI] [PubMed] [Google Scholar]
- 26.Kertesz A, Martinez-Lage P, Davidson W, Munoz DG. The corticobasal degeneration syndrome overlaps progressive aphasia and frontotemporal dementia. Neurology. 2000;55:1368–1375. doi: 10.1212/wnl.55.9.1368. [DOI] [PubMed] [Google Scholar]
- 27.Graves WW, Grabowski TJ, Mehta S, Gupta P. The left posterior superior temporal gyrus participates specifically in accessing lexical phonology. Journal of Cognitive Neuroscience. 2008;20:1698–1710. doi: 10.1162/jocn.2008.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogalski E, Rademaker A, Mesulam M, Weintraub S. Covert processing of words and pictures in nonsemantic variants of primary progressive aphasia. Alzheimer’s Disease and Associated Disorders. 2008 doi: 10.1097/WAD.0b013e31816c92f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vandenberghe RR, Vandenbulcke M, Weintraub S, et al. Paradoxical features of word finding difficulty in primary progressive aphasia. Annals of Neurology. 2005;57:204–209. doi: 10.1002/ana.20362. [DOI] [PubMed] [Google Scholar]


