Abstract
Enterobacter sakazakii (ES) is an emerging pathogen that causes meningitis and necrotizing enterocolitis in infants. Dendritic cells (DCs) are professional phagocytic cells that play an essential role in host defense against invading pathogens, however, the interaction of ES with DCs is not known. Here, we demonstrate that ES targets DC-SIGN to survive in myeloid DCs for which outer membrane protein A (OmpA) expression in ES is critical, although it is not required for uptake. In addition, DC-SIGN expression was sufficient to cause a significant invasion by ES in HeLa cells and intestinal epithelial cells, which are normally not invaded by ES. OmpA+ ES prevented the maturation of DCs by triggering the production of high levels of IL-10 and TGF-β and by suppressing the activation of MAP kinases. Pretreatment of DCs with antibodies to IL-10 and TGF-β or of bacteria with anti-OmpA antibodies significantly enhanced the maturation markers on DCs. Furthermore, DCs pretreated with various inhibitors of MAP kinases prohibited the increased production of pro-inflammatory cytokines stimulated by LPS or OmpA− ES. LPS pretreatment followed by OmpA+ ES infection of DCs failed to induce maturation of DCs, indicating that OmpA+ ES renders the cells in immunosuppressive state to external stimuli. Similarly, OmpA+ ES infected DCs failed to present antigen to T cells as indicated by the inability of T cells to proliferate in mixed lymphocyte reaction. We conclude that ES interacts with DC-SIGN to subvert the host immune responses by disarming MAP kinase pathway in DCs.
Keywords: Dendritic cells, Bacteria, Cell differentiation
INTRODUCTION
Enterobacter sakazakii (ES) is a fairly ubiquitous organism, which can be found in milk powder, rice, vegetables, cheese, sausage meat, teas, and various spices (1–4). However, most of the attention of ES related contamination of food products has focused on powdered infant formulae (5, 6). The Food and Drug Administration published a warning regarding the presence of ES in baby formula in 2002 and later several times (7). ES may exhibit long-term persistence in dried infant formula and has been described as the only organism isolated after a 2.5-year period of storage (8). Infants requiring formula feeding are at high risk for developing life threatening ES infections, which are associated with significantly high morbidity and mortality rates ranging from 33% to 80% (9–11). More than half of the survivors suffer irreversible neurological sequelae, resulting in quadriplegia, developmental impedance, and impaired sight and hearing (12). Premature (< 28 days old) or low birth-weight (< 2500 g) infants are more susceptible to ES infections (13, 14). The risk also appears to be particularly high in children with impaired immune defenses. Clinical presentations include meningitis (complicated by ventriculitis, brain abscess, cerebral infarction and cyst formation), septicemia and necrotizing enterocolitis (NEC) in infants (9). To date, a very few studies have been focused on the pathogenic mechanisms involved in the development of meningitis or NEC. Our recent studies have demonstrated that infection of newborn rats or mice with ES induces meningitis within 72 h post-infection for which outer membrane protein A (OmpA) expression is critical (15). Similarly, newborn rats under hypoxia conditions also develop NEC by ES (16, 17). Nonetheless, the interaction of ES with professional phagocytes is not known. We speculate that ES might be interacting with resident macrophages and/or dendritic cells initially in intestinal wall and therefore requires strategies to evade the phagocytic mechanisms of these cells for initiation of the disease.
Dendritic cells (DCs) constitute a system of hematopoietic cells that are rare but ubiquitously distributed (18). Immature DCs are seeded throughout peripheral tissues to act as sentinels against invading pathogens (19). These antigen-presenting cells also play an important role in the modulation of specific immune responses. Upon pathogen capture, DCs are activated, process pathogen into antigenic peptides for presentation in association with either MHC II or non-classical MHC-like molecules such as CD1, and migrate to the secondary lymphoid organs where they activate naïve T cells to initiate adaptive immune response (20, 21). Activation of DCs is associated with the expression of costimulatory markers on their surface such as HLA-DR, CD40 and CD86. Phagocytosis of bacteria as well as contact with bacterial toxins or components of bacterial cell wall can activate resting DCs, resulting in the initiation of immune response and elimination of the pathogen (22–24). However, many pathogens have turned DCs into allies either by inactivating infected DCs and rendering them tolerogenic or by inducing the production of immunosuppressive factors such as IL-10 and TGF-β (25–27). To sense pathogens, DCs express pathogen recognition receptors like C-type lectins (28). DC-SIGN (dendritic cell-specific ICAM-grabbing non-integrin, where ICAM is intercellular adhesion molecule) is a calcium-dependent C-type lectin expressed by DCs, containing a carbohydrate recognition domain (CRD) at its extracellular COOH-terminal end that recognizes mannose-rich molecules (29). DC-SIGN was initially described as a receptor for ICAM-3 at the surface of T cells, triggering the formation of the immunological synapse between DCs and naive T lymphocytes (30). Interestingly, DC-SIGN binds to HIV and simian immunodeficiency viruses, and is involved in the trans-infection of CD4 T lymphocytes by HIV- or SIV-infected DCs (31–34). DC-SIGN has also been implicated in the phagocytosis of Candida albicans, Mycobacterium tuberculosis, a lgtB mutant of Neisseria gonorrhoeae, a non pathogenic E. coli strain and Yersinia pestis (35–39). Although it is not known whether DCs are primary targets for ES during the infectious process, the knowledge about the steps involved in the pathogenesis of meningitis is essential to combat ES infections. Here, we demonstrate for the first time that ES exploits DC-SIGN to enter DCs and interferes with the maturation of DCs by altering the MAP kinase pathway to render them tolerogenic.
MATERIALS AND METHODS
Bacterial strains, cells, and reagents
Enterobacter sakazakii (51329) and rat intestinal epithelial cells, IEC-6 (used between passages 20–26), were obtained from ATCC (Manassas, VA). IEC-6 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum, 1 U ml−1 insulin, 100 U ml−1 penicillin G, and 100 U/ml−1 streptomycin. All general chemicals, LPS isolated from E. coli K12, and mannan were purchased from Sigma (St. Louis, MO). Mermaid is a DC-SIGN-like molecule expressed by the marine nematode Laxus oneistus. Recombinant histidine tagged mermaid (His-Mermaid) was expressed and purified as described previously (40). DC-SIGN cDNA cloned into pcDNA3 (41) and anti-DC-SIGN antibodies (42) were obtained through NIH AIDS Research and Reference Reagent program. Antibodies to phosophorylated forms of p38, ERK1/2, JNK, anti-CD64 antibody, and human recombinant TNF-α, IL-1β and PGE-2 were obtained from BD Biosciences (San Jose, CA). MAP kinase inhibitors were purchased from Calbiochem (San Diego, CA) and Sigma. For studies using chloramphenicol, optimization of time kill curves was performed in our bacterial culture system. At doses of 3–10 µg ml−1, bacterial concentrations (over 105–107 colony forming units (cfu) ml−1) remained static up to 24 h, as determined by serial dilution, plating on blood agar, and enumerating the bacteria after overnight incubation. Bacterial cultures from overnight incubation were centrifuged, washed with saline, and kept in water bath 30 min to obtain heat-killed ES.
Generation of OmpA− ES and complementation with ompA gene
ES 51329 was grown in LB or tryptic soy broth medium without any antibiotics. ES was transformed with the plasmid pUC13 containing the gfp gene. Transformants were selected by ampicillin (100 µg/ml), and assessed for GFP expression by viewing under ultraviolet light. The ompA-deletion mutant of ES was constructed by replacing ompA with a kanamycin (Km) cassette. Briefly, a spontaneous rifampicin-resistant mutant was isolated and named ES51R. A 1.77-kb DNA containing ompA was amplified from ES with primers: 5’-GTGAGCTCCGGGCTAAAAATTCACTCAA (containing a SacI site), and 5’-CAGGTACCATCGTGCAGCTGATTGA (containing a KpnI site). The DNA was cloned into pEP185.2 at the same sites, and the internal 876-bp NruI-BglII fragment was replaced with a 1.2-kb Km cassette from pUC-4K (Pharmacia). The recombinant plasmid was transferred from E. coli to ES51R by conjugation, and double-crossover mutants were selected. The deletion of ompA in ES51R was verified by PCR with the above primers. To restore the OmpA expression in OmpA− ES, the plasmid pEP185.2 containing ES ompA gene was transferred into the mutant, selecting for chloroamphenicol resistance. Expression of OmpA in the complemented strain, pOmpA+ ES and OmpA− ES was confirmed by Western blot using rabbit antiserum developed against E. coli OmpA, which also recognizes the OmpA of ES (43).
Dendritic cell culture and activation
Dendritic cells were generated from human peripheral blood mononuclear cells (PBMCs) as described previously (44, 45). Briefly, monocytes were prepared from PBMCs by positive selection using CD14 immunomagnetic beads (Miltenyi Biotec, Auburn, CA). CD14+ isolated cells were then cultured in RPMI supplemented with 10% FCS, 2.4 mM L-glutamine (Invitrogen, Carlsbad, CA), 50 ng ml−1 human recombinant GM-CSF and 20 ng ml−1 human recombinant IL-4 (Peprotech, Rocky Hill, NJ). DCs were used after 7 days of culture and phenotype was determined by FACS caliber flow cytometer (BD Biosciences, San Jose, CA). Immature DCs were CD3-negative, CD14-low, CD19-negative, CD83-negative, CD25-negative and expressed low levels of HLA-DR, CD40, CD86 and CD1a. For stimulation experiments, DCs (5 × 104 ml−1) were cultured with live or killed ES at a multiplicity of infection of 10 (cell to bacteria ratio 1:10) for 24 and 48 h. DCs were also stimulated with lipopolysaccharide (LPS, Sigma, St. Louis, MO) at a concentration of 10 ng ml−1as well as with a maturation mixture (MM) of TNF-α (10 ng ml−1), IL-1α (10 ng ml−1), and prostaglandin E2 (PGE2) (1 µg ml−1) (46). In some experiments, DCs were pretreated for 20 min with anti-DC-SIGN antibody (5 µg ml−1), mannan, (500 µg ml−1) or dextran (500 µg ml−1) or ES was pretreated for 30 min with His-Mermaid (10 µg ml−1). The concentrations used were based on our preliminary data and were selected based on the fact that at these concentrations, there was no influence on the survival of bacteria or DCs. Prior to antibody staining, an aliquot of DC culture was stained with trypan blue as well as with Annexin V kit to assess the amount of cell death in co-culture.
Transfection of HeLa and IEC-6 cells
HeLa– and IEC-6-DC-SIGN cells were generated by transfecting the respective cells with an expression plasmid containing human DC-SIGN gene, followed by selection for stable surface DC-SIGN expression as originally described (47). For invasion assays, ES (107 CFU/ml) were added to confluent monolayers of HeLa or IEC-6 cells separately at bacteria to a cell ratio of 100:1 and incubated for 6 h. The monolayers were washed three times with RPMI 1640 medium followed by addition of gentamicin (100 µg ml−1) and further incubated for 1 h at 37 °C. The cells were then washed three times with RPMI 1640 and lysed with 0.5% of Triton X-100. The released bacteria were diluted with saline and enumerated by plating on blood agar. The total cell associated bacteria were determined as described for the invasion, except that the gentamicin step was omitted.
Determination of ES uptake by DCs and intracellular survival
DCs (5 × 104 cells ml−1) were washed three times in culture medium without antibiotics and then placed in 500 µl of culture medium in 12 × 75 mm polystyrene snap-cap tubes (BD Falcon, San Jose, CA). Varying concentrations of bacteria (in 10 µl) were added to the tubes. DCs and bacteria were then incubated for 1 h at 37°C. At different incubation periods, the co-cultures were centrifuged at a low-speed; aliquots from the supernatants were diluted, and plated on blood agar. The number of bacteria present in the supernatants was subtracted from the bacteria added to co-cultures to obtain the number of bacteria entered DCs. To assess intracellular bacteria at different times post-exposure, gentamicin was added to DC-bacteria co-culture tubes at a final concentration of 100 µg ml−1 and incubated for an additional 60 min at 37°C. The co-cultures were washed three times in RPMI containing no antibiotics and reconstituted with antibiotic-free culture medium. The cultures were then assessed immediately for intracellular bacteria (time period 0) or placed again at 37°C in culture medium containing 30 µg ml−1 gentamicin. DC-bacteria co-cultures were washed twice with RPMI, the cells were lysed with 100 µl of 0.5% Triton X-100, and the released intracellular bacteria were enumerated by plating the dilutions on blood agar. Results were expressed as percentage viable bacteria taken up by DCs at respective sampling time intervals. For inhibition studies, antibodies were incubated with either with DCs (anti-DC-SIGN and anti-CD64 antibodies) or OmpA+ ES (anti-OmpA antibodies) for one hour prior to adding to each other.
Flow cytometry
Expression of CD40, CD86 and HLA-DR, associated with DC maturation and activation, was detected by staining with appropriate FITC-, phycoerythrin (PE)-, PE-CY5.5-, or allophycocyanin (APC)-coupled mouse monoclonal antibodies or mouse IgG isotype matched controls (eBiosciences, San Diego, CA). Cells were first pre-incubated for 20 minutes with IgG blocking buffer to mask non-specific binding sites and then further incubated with the indicated antibodies or an isotype control antibody for 30 min at 4°C. After incubation, the cells were washed three times with PBS containing 2% FBS and subsequently fixed with BD Cytofix (BD Biosciences, San Jose, CA). Cells were then analyzed by four-color flow cytometry using FACS calibur Cell Quest Pro software (BD Biosciences, San Jose, CA). DCs form a distinct population when separated by side and forward scatter parameters for which CD1a was used as a DC gating marker; this population formed the collection gate and at least 5000 events within this gate were collected for analysis. Binding of His-Mermaid to ES was evaluated by incubating 107 cfu ES with 500 mM His-Mermaid for 1 h at room temperature, washed, further incubated with FITC coupled secondary antibody and subjected to flow cytometry. BSA was used as a control protein. Activation of MAP kinase pathway was assessed using flow cytometry as described previously (48,49).
Confocal laser microscopy
DCs were infected with either OmpA+ or OmpA− ES for varying periods, washed with PBS and preincubated with IgG blocking buffer to prevent non-specific binding. Cells were then fixed with BD Cytofix (BD Biosciences), washed and incubated with 5 µg ml−1 anti-HLA-DR antibody (eBiosciences) followed by Alexa Fluor 488 goat anti-mouse IgG (Invitrogen, Carlsbad, CA). Cells were then allowed to adhere to poly L-lysine-coated slides for 10 min (Paul Marienfeld Gmbh and Co, Germany) and mounted in an antifade Vectashield solution containing 4', 6-diamidino-2-phenylindole (DAPI) (Vector laboratories, Burlingame, CA). The cells were viewed with a Leica (Wetzlar, Germany) DMRA microscope with Plan-apochromat oil immersion objective lenses. Images were acquired with a SkyVision-2/VDS digital charge-coupled device camera (12-bit; 1,280 by 1,024 pixels) in unbinned or 2 × 2 binned models into the EasyFISH software, saved as 16-bit monochrome images, and merged as 24-bit RGB TIFF images (Applied Spectral Imaging, Inc., Carlsbad, CA). The TIFF images were assembled using Adobe Photoshop 7.0.
Scanning and transmission electron microscopy
DCs were treated with ES and then fixed with 2% glutaraldehyde in 0.1M cacodylate buffer, pH 7.1. All samples were washed three times in 0.1 M cacodylate buffer for 15 minutes each. Then post-fixed for 20 minutes in 1% osmium tetroxide at 4°C followed by addition of EtOH (60%) Samples were dehydrated through 70, 80, 95, and 100% EtOH (two times, 15 min each) then into propylene oxide (two time, 15 min each) and into a 1:1, left overnight, capped, at room temperature. The 1:1 propylene oxide/Eponate mixture was decanted off and replaced with 100% Eponate mixture. The samples were polymerized at 70°C for 48 hours. Thin sections (~80 nm) were cut using a diamond knife, mounted on un-coated 300 mesh copper grids and stained with 5% uranyl acetate for 20 minutes. Observed and photographed in a transmission electron microscope (JEOL JEM 2100 LaB6) equipped with a Gatan Ultra Scan 1000 CCD camera. For SEM, DCs were grown on glass coverslips, fixed as described for TEM, and then transferred to the Critical Point Dryer holder, washed two times with buffer and dehydrated in 60, 70, 80, 95% EtOH (4 min each) and then into 100% EtOH (two times, 5 min each) before being dried to critical point in CO2 for 35 min. The coverslips were then mounted on aluminum SEM stubs with colloidal silver, and sputter coated with Pt/Pb, (80:20) for 20 seconds four times in argon gas then observed and photographed in a scanning electron microscope (JEOL JSM/6390LV) under high vacuum (University of Southern California, Los Angeles, CA).
Mixed lymphocyte reaction
The ability of infected DCs to activate naive T cells was assessed by allogeneic lymphoproliferation. DCs were harvested 24 h and 48 h after infection with ES, washed with PBS, and suspended in RPMI containing 10% serum without antibiotics or cytokines. Infected DCs were used to stimulate allogeneic naive T cells. Briefly in 96 well tissue culture plates, DCs and T cells were added in the ratio of 1: 300 and were co-cultured for 72 h, H3-thymidine was added and harvested for 18 h (50). Using liquid scintillation counter assessed the rate of incorporation of H3-thymidine and results were expressed in disintegration per minute. T cells were isolated from PBMCs using neuraminidase-treated sheep red blood cells as described previously (51).
Viability
The percentage of viable DCs was assessed by trypan blue as well as by Annexin V-FITC apoptosis kit (BD Biosciences, San Jose, CA). In all culture conditions, a proportion of cells (ranging from 5% to 15%) were trypan blue or Annexin V and/or propidium iodide positive. However, there was no significant difference observed in the proportion in cultures stimulated with medium, live, LPS, or killed ES.
Cytokine assays
Cytokine (TNF-α, IL-1β, IL-6, IL-12 p70, IL-10 and TGF-β) production in cell culture supernatants of DC-bacteria co-culture experiments collected after 24 and 48 h of incubation was carried out using Biosource ELISA kits (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions.
Statistical Analysis
Statistical significance was determined by paired, two-tailed Student’s t-test. P values <0.05 were considered to be statistically significant.
RESULTS
OmpA expression is necessary for the survival of ES in DCs
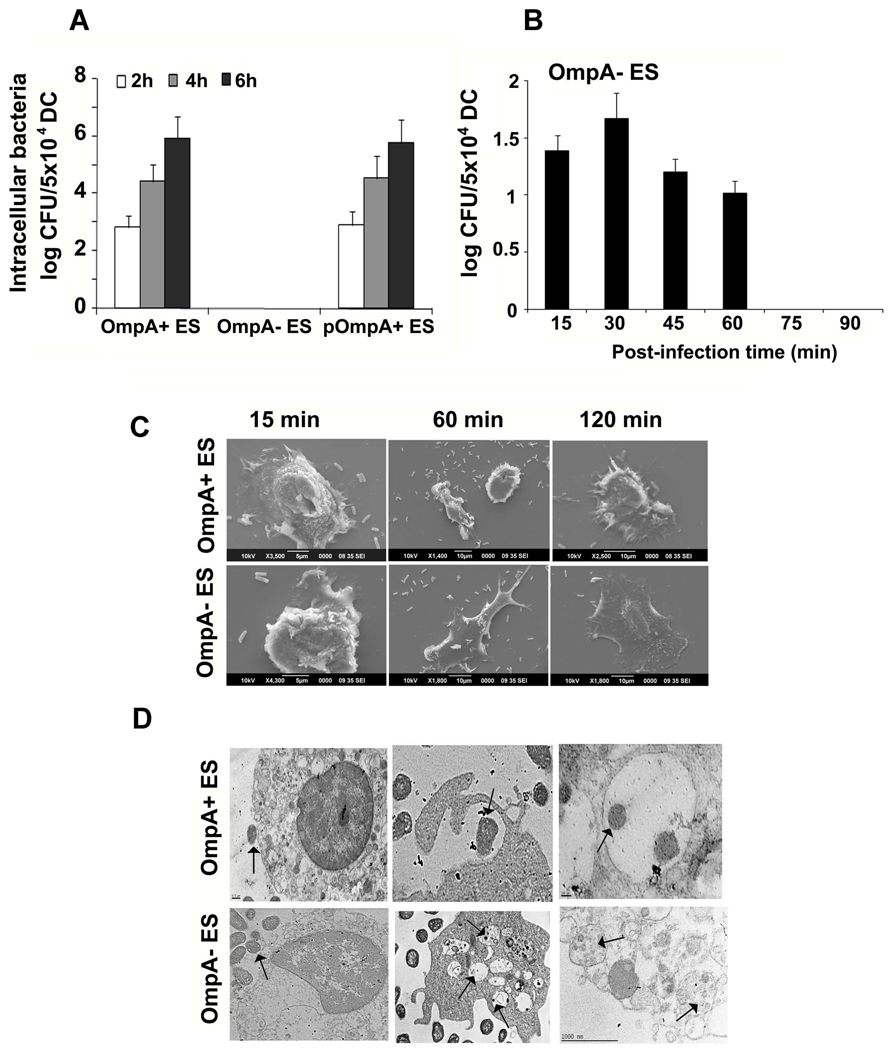
Our previous studies have demonstrated that OmpA expressing ES induces meningitis in newborn mice, whereas OmpA-ES did not, suggesting that OmpA expression may be important for survival in animals (15). However, its interaction with immune cells has not been studied to date. Therefore, to examine whether ES survives in DCs in vitro, myeloid DCs were infected with OmpA+ and OmpA− ES for varying periods. The results from gentamicin protection assays showed that OmpA+ ES survived inside DCs whereas OmpA− ES was killed within 2 h (Fig. 1A). To examine whether lack of OmpA− ES in DCs is not due to lack of entry into the cells, intracellular bacteria from 15 to 90 min post-infection was determined. OmpA− ES did enter the cells as early as 15 min and were killed by 75 min post-infection (Fig. 1B). To determine whether the observed survival of OmpA+ ES is due to the expression of OmpA, complementation of OmpA− ES with a plasmid containing the entire ompA gene was performed. The wild-type ES and the complemented strain, pOmpA+ ES expressed similar levels of OmpA as analyzed by Western blotting with anti-OmpA antibodies (data not shown). Phagocytosis assays with pOmpA+ ES restored the ability of OmpA−ES to persist in DCs, indicating that OmpA is involved in the survival of ES in DCs. Scanning electron microscopy of OmpA+ ES interaction with DCs revealed that ES was in the process of being engulfed by conventional phagocytosis by DCs at 15 min post-infection (Fig. 1C). The bacteria were completely engulfed by 60 min post-infection. DCs containing the bacteria showed rugged surface. OmpA− ES were also engulfed by 60 min post-infection, however, DCs revealed no rough morphology as that of OmpA+ ES infected cells. Transmission electron microscopy images demonstrated that OmpA+ ES was either attached to the cell surface or enclosed within the cytoplasmic vesicles with the characteristics of phagosomes (Fig. 1D). The fully phagocytosed ES was completely surrounded by intact membranes and sometimes two or more bacteria were seen in phagosomes, indicating that the bacteria might be multiplying inside DCs. In contrast, the OmpA− ES, although entered DCs were killed by the cells as observed by the presence of several vacuole like structures containing debris. These data suggest that both OmpA+ and OmpA− ES enter DCs with a similar frequency; however, OmpA+ ES survives and multiplies inside DCs, whereas OmpA− ES was killed within 2 h post-infection.
Figure 1. Intracellular survival of ES in DCs.
OmpA+ and OmpA− ES were incubated with DCs at an MOI of 10 for indicated time points. The number of intracellular bacteria (A and B) was determined using gentamicin protection assays as described in materials and methods. Data represent mean Log10 CFU per 5 × 104 DCs ± SD of triplicate samples from three independent experiments. DCs infected with the bacteria were processed from separate experiments for SEM (C) and TEM (D). Arrows indicate either intact or degraded bacteria.
OmpA+ ES prevents the maturation and antigen presentation of DCs
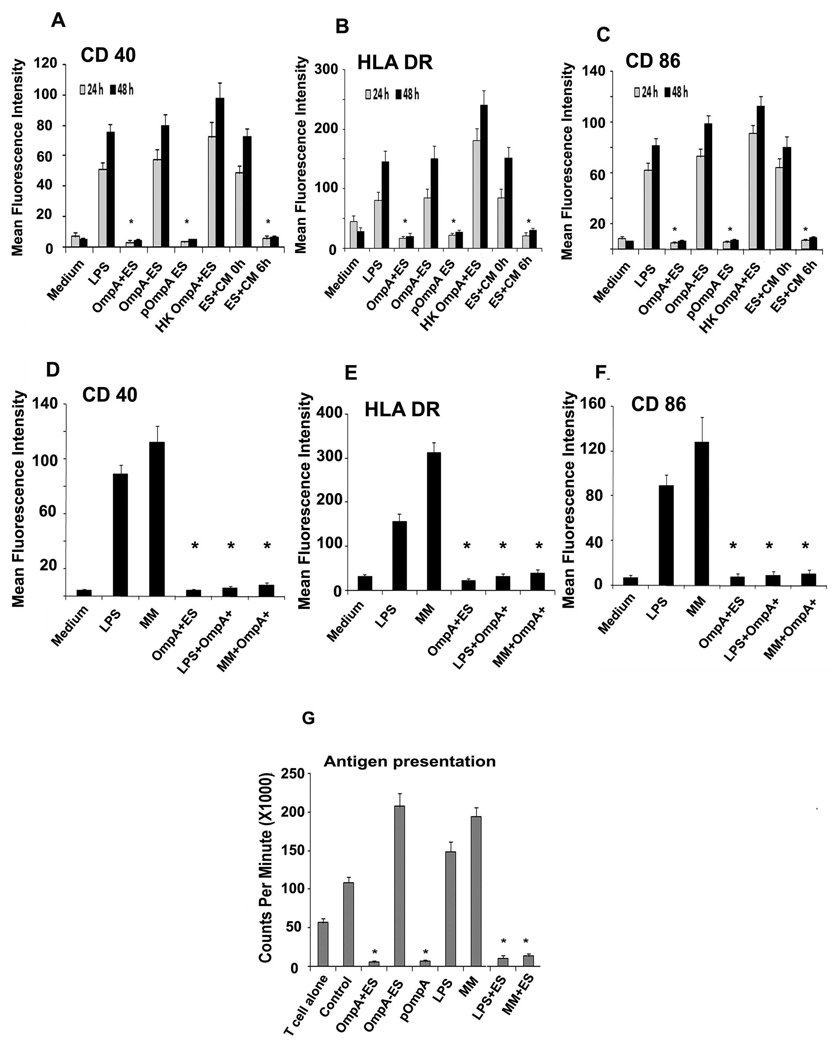
To test whether the survival of OmpA+ ES could also lead to a difference in maturation of DCs, we investigated the cell surface expression of CD40, CD86, and HLA-DR after 24 h and 48 h stimulation by flow cytometry. No increase in the expression of these markers on the surface of DCs was observed even after 48 h in DCs infected with OmpA+ ES (Fig. 2A to C). In contrast, OmpA− ES infected DCs showed significant expression of maturation markers. Consistent with the survival data, OmpA− ES transformed with ompA containing plasmid restored the capacity to prevent the maturation process. To determine whether the viability of bacteria has any effect on this distinct DC phenotype, DCs were infected with heat-killed ES. Interestingly, co-culturing of DCs with killed ES led to a significant increase in the expression of co-stimulatory markers. In addition, the requirement of bacterial protein synthesis in ES to prevent the maturation of DCs was examined by incubating DCs with ES treated with bacteriostatic doses of chloramphenicol (Fig. 2A–C, ES+CM 0 h). Similar to that observed by co-culturing with killed ES, the expression of co-stimulatory markers increased. However, a 6 h-delay in addition of chloroamphenicol (Fig. 2C, ES+CM 6 h) allowed sufficient protein synthesis to down-regulate co-stimulatory surface markers of DCs (Fig. 2A–C). The interference with DC maturation by ES raised the question, whether the bacteria would inhibit maturation induced by the LPS or maturation cocktail of TNF-α, IL-1β, and PGE-2, which are shown to be present at the site of infection in several other studies (52). Despite pretreatment with these inducers, DCs subsequently infected with OmpA+ ES showed down-regulation of maturation markers CD40, CD86, and HLA-DR (Fig. 2D–F). Taken together these data demonstrate that OmpA+ ES inhibited phenotypic maturation of DCs induced by LPS or proinflammatory stimuli, for which bacterial protein synthesis is required. In addition, infection of DCs with OmpA+ ES renders them into an immunosuppressive state.
Figure 2. Expression of maturation markers on the surface of DCs infected with ES.
OmpA+, OmpA−, pOmpA+ or heat-killed ES (HK OmpA+ ES) or LPS were incubated with DCs for 24 h and 48h. In some experiments, ES were pretreated with 20 µg ml−1 chloramphenicol for 1 h at 37°C, washed and adjusted the OD600 to obtain 107 cfu ml−1 , and then added at an MOI of 10 to DCs (ES+CM 0 h). In addition, chloramphenicol was added to bacteria-DC co-culture 6 h post-infection (ES+CM 6 h). DCs were then washed, stained with antibodies to CD40 (A), HLA-DR (B) and CD86 (C), fixed and analyzed by flow cytometry. In separate experiments, DCs were first stimulated with LPS or maturation medium (MM) for 24 h and then infected with OmpA+ ES for an additional 24 h. DCs were then washed and the expression of CD40 (D), HLA-DR (E), and CD86 (F) was analyzed by flow cytometry. The data represent geometric mean fluorescence intensity (MFI) of logarithmic data subtracted from isotype-matched controls. DCs infected with ES, LPS or MM were added to naïve T cells to examine the antigen presentation capacity of DCs as described in materials and methods (G). The error bars represent standard deviations from the means of triplicate samples. The results are representative of three independent experiments. The inhibition of cell surface marker expression or antigen presentation was significantly reduced in comparison to LPS infected DCs, *p<0.001 by two tailed t test.
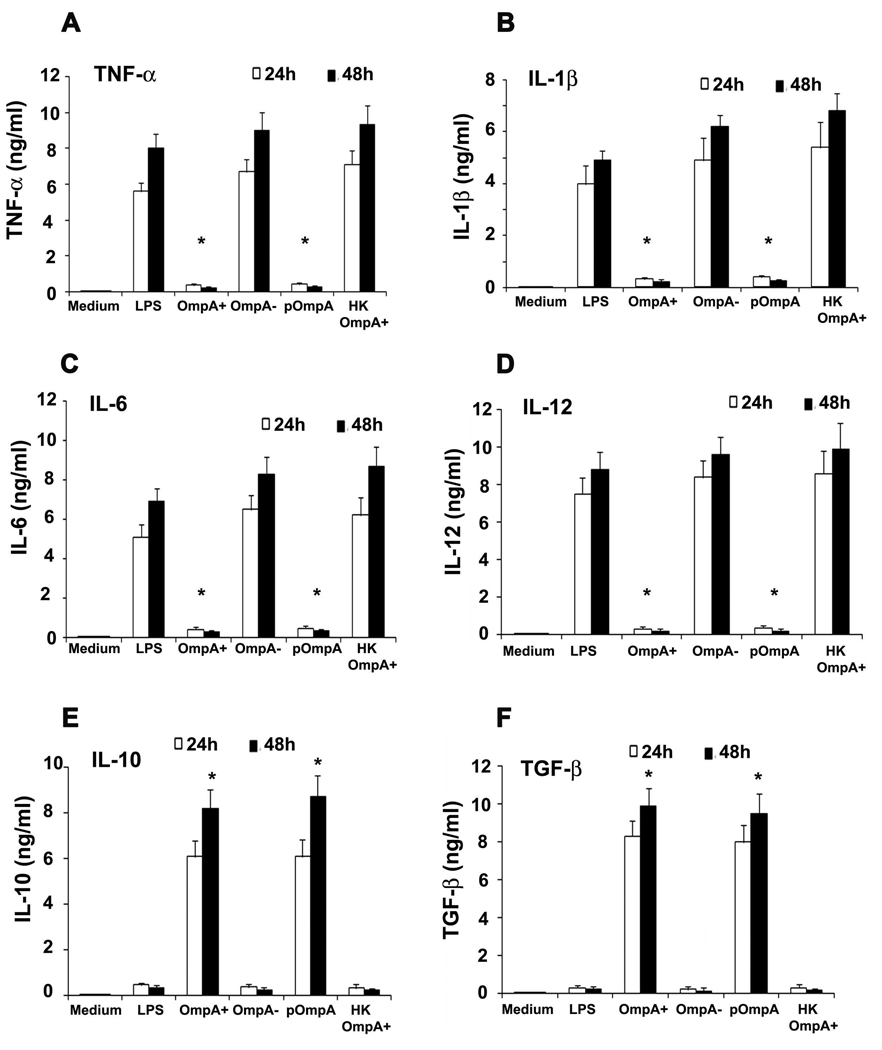
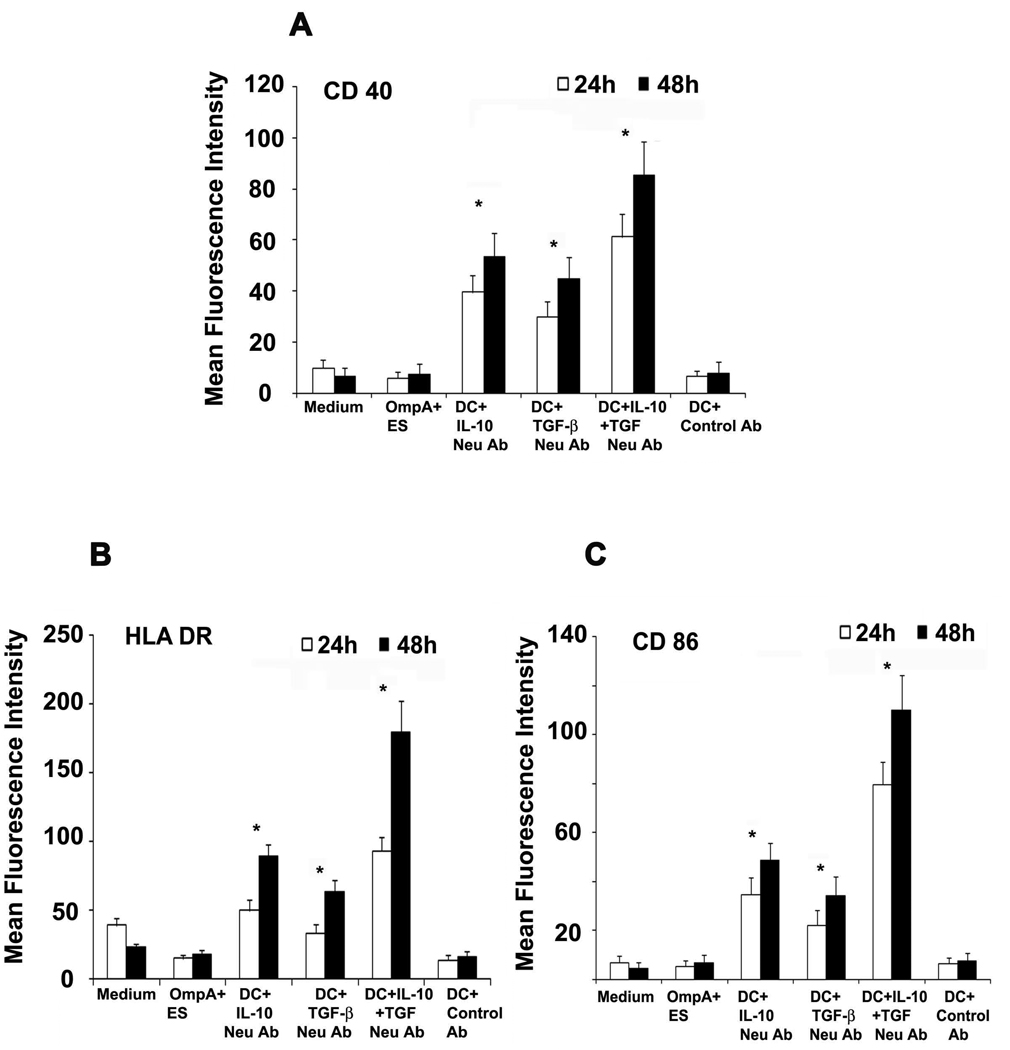
To further determine whether suppression of phenotypic maturation by OmpA+ ES correlates with prevention of functional maturation, DCs were tested for their ability to stimulate allogeneic purified naïve CD4+ T cells. Mature DCs normally induce significant allogeneic lymphoproliferation at DC and naïve T cell ratios of 1:300 (50). OmpA- ES activated DCs were effective in stimulating allogeneic CD4+ T cells, whereas OmpA+ ES treated DCs induce very weak or no T-cell proliferation (Fig. 2G). As observed with maturation markers, LPS treatment also induced the stimulation of CD4+ T cells, which was significantly decreased by OmpA+ ES infection. Similarly, maturation mixture induced activation of the cells was also prevented by infection of DCs with OmpA+ ES. Activation of DCs following exposure to bacteria is associated with secretion of chemokines and cytokines, which play a crucial role in deciding the ultimate outcome of an infection by switching on Th1 or Th2 immune response (53, 54). Therefore, we analyzed the cytokine profile of DCs infected with ES. We observed that OmpA+ ES, which persists and survives inside DCs triggered high levels of IL-10 and TGF-β and very low levels of proinflammatory cytokines (Fig. 3A to F). In contrast OmpA− ES, which could not survive inside DCs, led to higher production of proinflammatory cytokines and very low levels of anti-inflammatory cytokines indicating activation and maturation of DCs. To determine whether IL-10 and TGF-β production caused the poor responsiveness of DCs by OmpA+ ES, DCs were pretreated with neutralizing antibodies to IL-10 and TGF-β and infected with OmpA+ ES. As shown in Fig. 4, moderate expression of maturation markers in DCs pretreated with either IL-10 or TGF-β antibodies was observed, however a dramatic increase in the expression of CD40, HLA-DR and CD86 was observed when both the antibodies were incubated with DCs prior to infection. In contrast, DCs pretreated with isotype-matched control antibody and subsequently infected with OmpA+ ES failed to show activation of DCs. These results indicate that production of both IL-10 and TGF-β by DCs infected with OmpA+ ES plays an important role in preventing the maturation of these cells.
Figure 3. Cytokine production by DCs infected with ES.
DCs were exposed to medium alone, treated with various ES strains, LPS or heat killed (HK) ES for 24 and 48 h. Culture supernatants were collected and the levels of TNF-α (A), IL-1β (B), IL-6 (C), IL-10 (D), IL-12p70 (E), and TGF-β (F) were assessed by ELISA. The error bars represent standard deviations from the means of triplicate samples from four individual experiments. The suppression of cytokine production by OmpA+ or pOmpA+ ES was significant in comparison to OmpA− ES, LPS or HK ES induced levels, *p<0.001 by two tailed t test.
Figure 4. Effects of IL-10 and TGF-β neutralizing antibodies on DC activation.
DCs were pretreated with IL-10 or TGF B blocking antibodies or isotype matched antibody (Control Ab) and then infected with OmpA+ ES as described in materials and methods. The expression of CD40 (D), HLA-DR (E) and CD86 (F) was analyzed by flow cytometry. The error bars represent standard deviations from the means of triplicate samples. The results are representative of five independent experiments. The increase in the expression of surface markers in DCs treated with IL-10 and/or TGF-β is significantly greater compared to OmpA+ ES or control antibody treated DCs at both time points, *p<0.001 by two tailed t test.
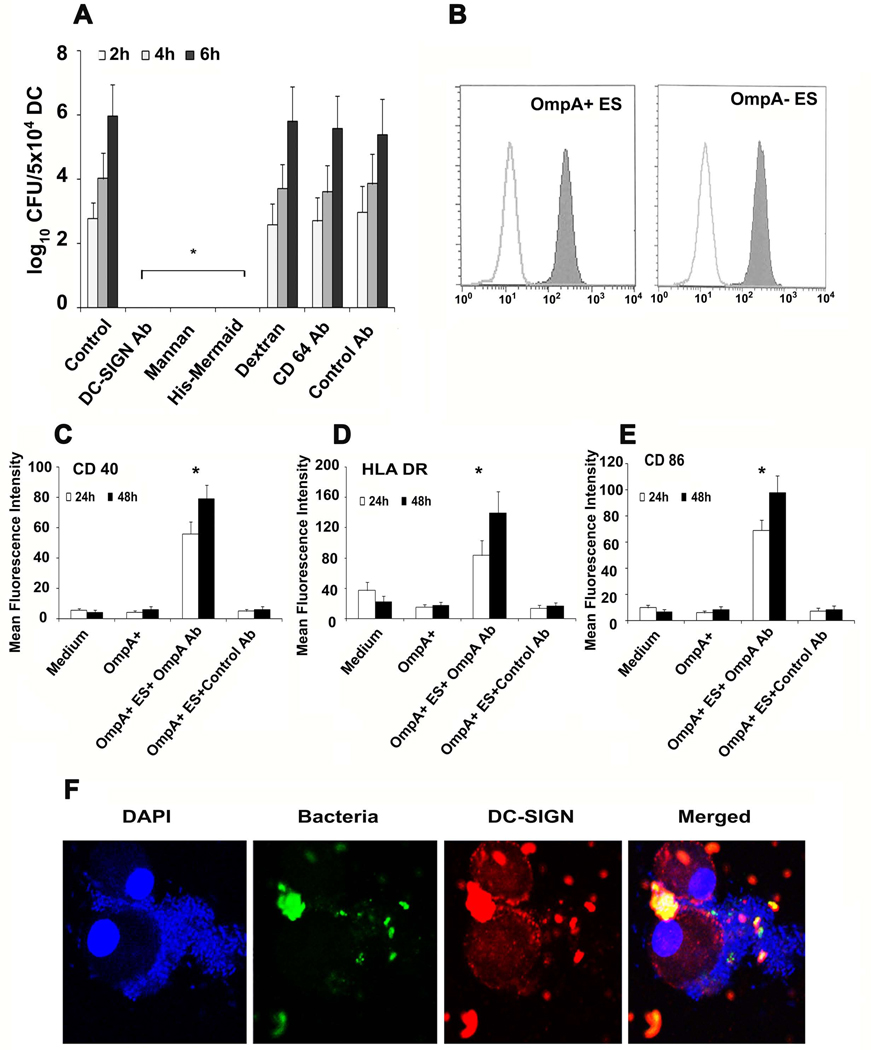
DC-SIGN is involved in internalization of ES by DCs
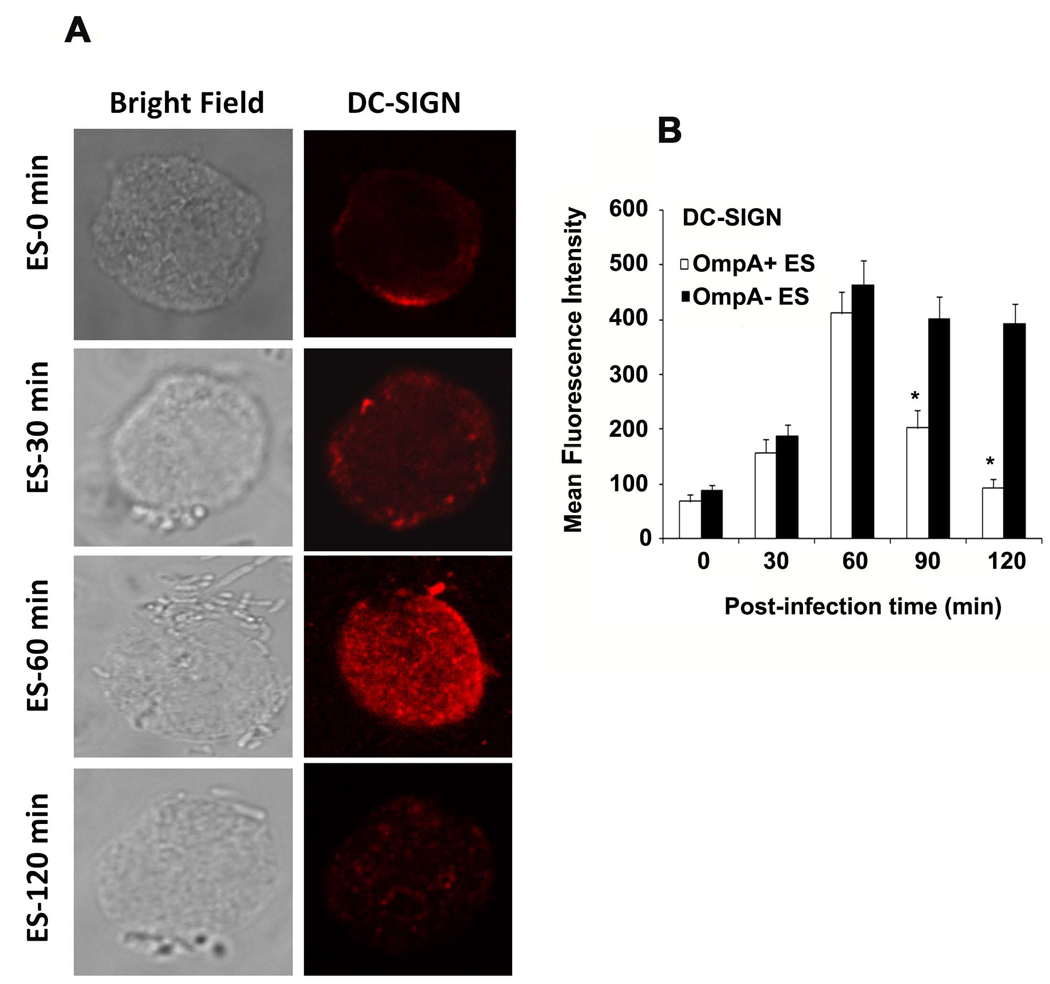
Several microorganisms such as HIV and Mycobacterium tuberculosis utilize the immunoreceptor DC-SIGN to escape immune surveillance by interfering with DC maturation (31–35). Therefore, we speculated that ES might be exploiting at similar strategy. To test whether ES interacts with DC-SIGN, DCs were pretreated with DC-SIGN blocking antibody for 20 minutes followed by addition of bacteria. As shown in Fig. 5A, OmpA+ ES failed to survive in DCs pretreated with DC-SIGN blocking antibody. To further confirm these findings, DCs were pretreated with mannan, which specifically binds mannose related receptors, including DC-SIGN and ES pretreated with His-Mermaid, which is a newly identified C-type lectin (40), which has been shown to compete with DC-SIGN for the binding to E. coli CS180, Yersinia pestis, and HIV-1 (31–38). Anti-CD64 antibody was also used to examine the role of other receptors in ES internalization. Pretreatment of DCs with these compounds completely inhibited the survival of OmpA+ ES in DCs, however pretreatment with dextran (used as control for mannan), anti-CD64 antibodies or isotype matched control antibodies had no effect. Of note, the concentrations of anti-DC-SIGN antibody, mannan and His-Mermaid, used in this study were found to have no effect on viability of either bacteria or DCs (data not shown). Lack of survival of OmpA+ ES in DCs pre-treated with anti-DC-SIGN antibodies was due to absence of entry of the bacteria into DCs (data not shown). OmpA− ES also could not enter DCs, indicating that both these strains are utilizing DC-SIGN to enter DCs, however, OmpA expression is important for the survival of the bacteria inside DCs. The inhibitory effect of His-Mermaid on ES entry into DC is due to the binding of His-Mermaid to both OmpA+ and OmpA− ES as evaluated by flow cytometry (Fig. 5B). To confirm whether the interaction of OmpA with DCs is required for distinct DC phenotype, OmpA+ ES was pretreated with anti-OmpA antibodies and cultured with DCs. Blocking of OmpA binding to DCs led to activation as shown by upregulation of CD40, HLA-DR and CD86 (Fig. 5C–E), indicating that OmpA might interact with DC-SIGN to suppress the maturation of DCs infected with OmpA+ ES. In addition, the spatial relationship of ES binding with DC-SIGN was examined by immunocytochemistry. DCs were stained using PE conjugated anti-DC-SIGN antibody and ES were visualized for the presence of GFP. As shown in the Fig. 5F and supplementary video 1, DC-SIGN was co-localized at the site of entry of bacteria. In addition, no bacteria were observed inside DCs following treatment of DCs with DC-SIGN blocking antibody, mannan or His–mermaid indicating that DC-SIGN is a receptor for ES (data not shown). Of note, the entry of OmpA+ ES into DCs increased the expression of DC-SIGN up to 60 min post-infection, which was reduced to basal levels by 120 min post-infection as shown by both immunocytochemistry and flow cytometry (Fig. 6A and B). DC-SIGN expression was also observed with OmpA− ES infection of DCs by 60 min post-infection, however, stayed at the similar level even after 120 min post-infection. These data suggest that the presence of OmpA+ ES in DCs suppresses the maturation and DC-SIGN expression on the surface of the cells.
Figure 5. Binding of ES to DC-SIGN.
DCs were incubated with DC-SIGN blocking antibody, mannan, His-Mermaid, dextran, anti-CD64 antibody or control antibody for 1 h prior to incubating with ES. The number of intracellular survival ES was determined as described in materials and methods (A). In separate experiments, ES were treated with 10 µg of His-Mermaid and incubated for 1h, washed and the bound Mermaid was identified by probing with anti-His antibody followed by flow cytometry (B). Similarly, OmpA+ ES were incubated with either anti-OmpA antibodies (20 µl of antiserum with 106 cfu of bacteria) or isotype matched control antibody for 1 h on ice prior to adding to DCs. The expression of CD40, HLA-DR or CD86 was examined by flow cytometry (C–E). Panel F shows confocal microscopy of DCs infected with ES. DCs were co-cultured with GFP OmpA+ or OmpA− ES at an MOI of 10 for 15, 30, 60 and 120 min. The cells were washed, DCs were allowed to adhere to poly L-lysine slides, and then stained with anti-DC-SIGN antibody followed by Cy3 coupled secondary antibody. The slides were counter-stained with DAPI and visualized by confocal laser microscopy. Only data with OmpA+ ES at 60 min postinfection are shown. The error bars represent standard deviations from means of four individual experiments performed in triplicate. The decrease in the number of intracellular ES or increase in the expression of maturation markers was significantly greater in comparison to control, *p<0.001 calculated by Student’s t test.
Figure 6. OmpA+ ES suppresses the expression of DC-SIGN upon infection of DCs.
The expression of DC-SIGN following infection with ES was assessed at different time points by immunocytochemistry (A) and flow cytometry (B). The error bars represent standard deviations from the means of triplicate samples. The results are representative of three independent experiments. The decrease in the expression of DC-SIGN in DCs infected with OmpA+ ES was significantly lower compared to OmpA− ES infected DCs at 90 and 120 min postinfection, *p<0.001 by two tailed t test.
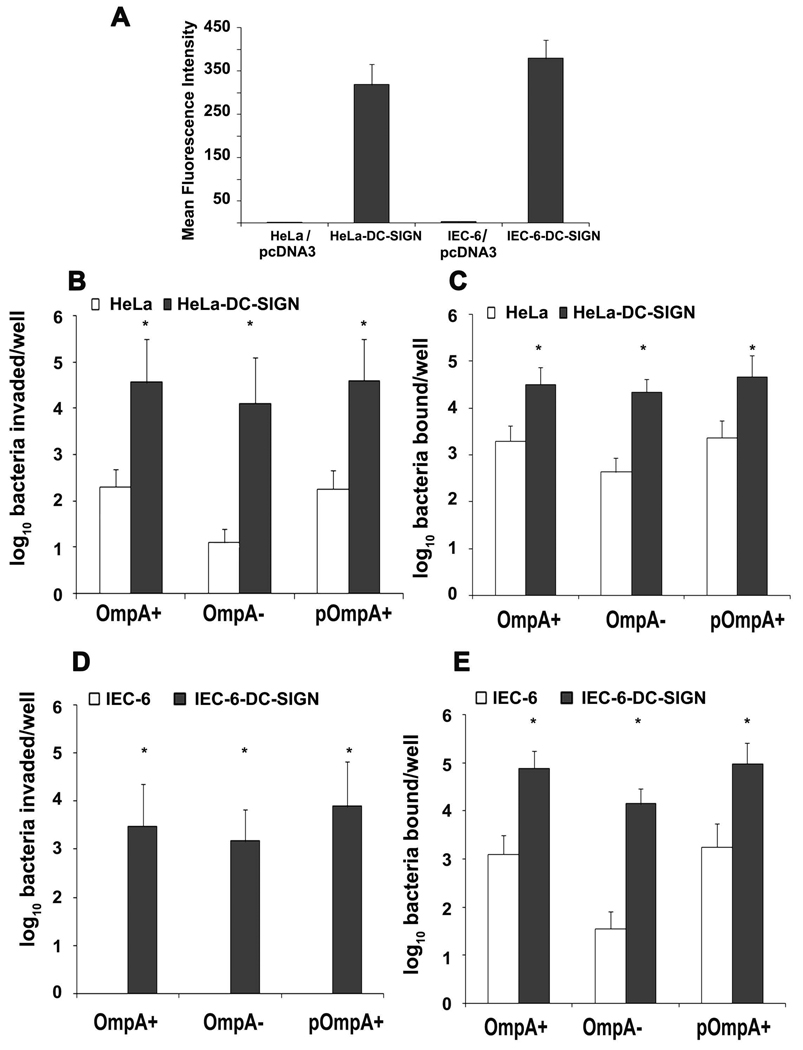
DC-SIGN expression is sufficient to increase ES uptake by HeLa cells and intestinal epithelial cells
To determine whether the expression of DC-SIGN is sufficient to allow the invasion of ES, a mammalian expression plasmid containing DC-SIGN cDNA was introduced into HeLa cells. The transfected cells were examined for the expression of DC-SIGN by flow cytometry using anti-DC-SIGN antibodies. As shown in Fig. 7A, HeLa cells transfected with DC-SIGN plasmid expressed significantly greater amounts of DC-SIGN in comparison to HeLa-pcDNA cells. The HeLa-DC-SIGN cells when subjected to invasion assays showed a 50-fold increase in invasion of both OmpA+ and OmpA− ES in comparison to plasmid alone transfected HeLa cells (Fig. 7B). Of note, the binding of both strains was also increased in comparison to HeLa-pcDNA cells (Fig. 7C). Earlier studies from our laboratory have shown that although 4 to 6% of OmpA+ ES bound to primary intestinal epithelial cells (IEC-6 cells), they failed to invade these cells. Therefore, IEC-6 cells were also transfected with DC-SIGN expressing plasmid construct and the resulting cells were examined for ES binding to and invasion (Fig. 7D and E). Significant increase in binding and invasion of both OmpA+ and OmpA− ES to IEC-6-DC-SIGN cells was observed indicating that ES directly interacts with DC-SIGN receptor. However, since OmpA−ES also invades DC-SIGN transfected cells, we conclude that OmpA does not play a significant role in the invasion of DCs, however, it is necessary for the survival inside DCs.
Figure 7. ES binds and invades HeLa and IEC-6 cells expressing DC-SIGN.
A mammalian expression plasmid containing DC-SIGN cDNA was introduced into HeLa and IEC-6 cells. The transfected cells were examined for the expression of DC-SIGN by flow cytometry using anti-DC-SIGN antibodies (A). In addition, the ability of OmpA+ and OmpA− ES to bind to and invade DC-SIGN expressing HeLa and IEC-6 cells were determined and compared with non-transfected cells (B–E). The error bars represent standard deviations of triplicate samples. The results are representative of three independent experiments. The increase in the binding or invasion of ES was significantly greater compared to control cells, *p<0.001 calculated by Student’s t test.
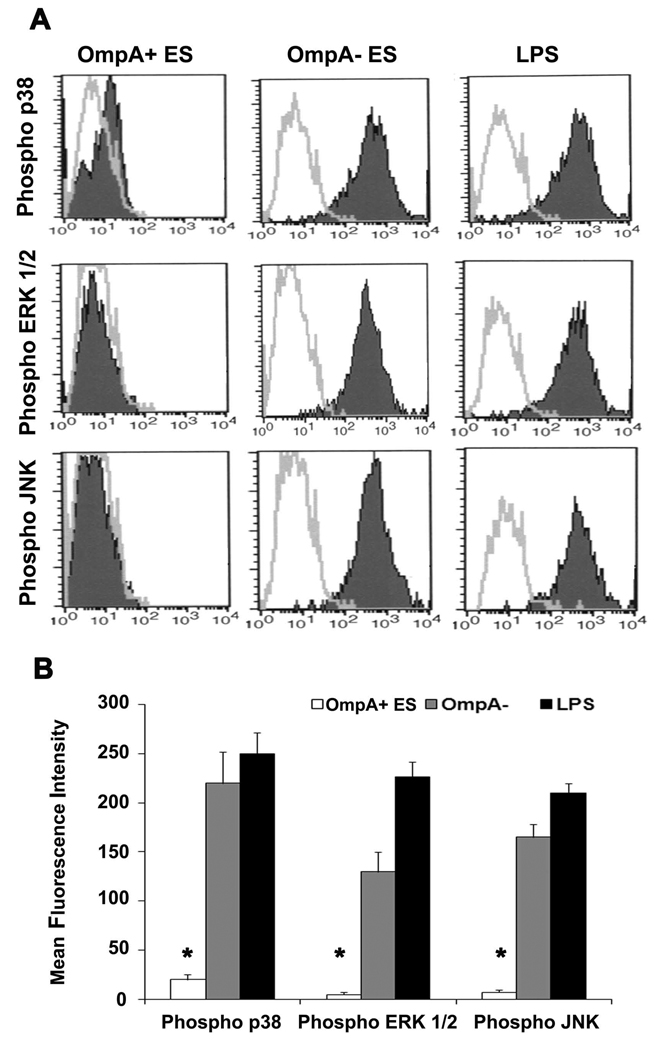
OmpA+ ES suppresses phosphorylation of MAP kinases involved in maturation of DCs
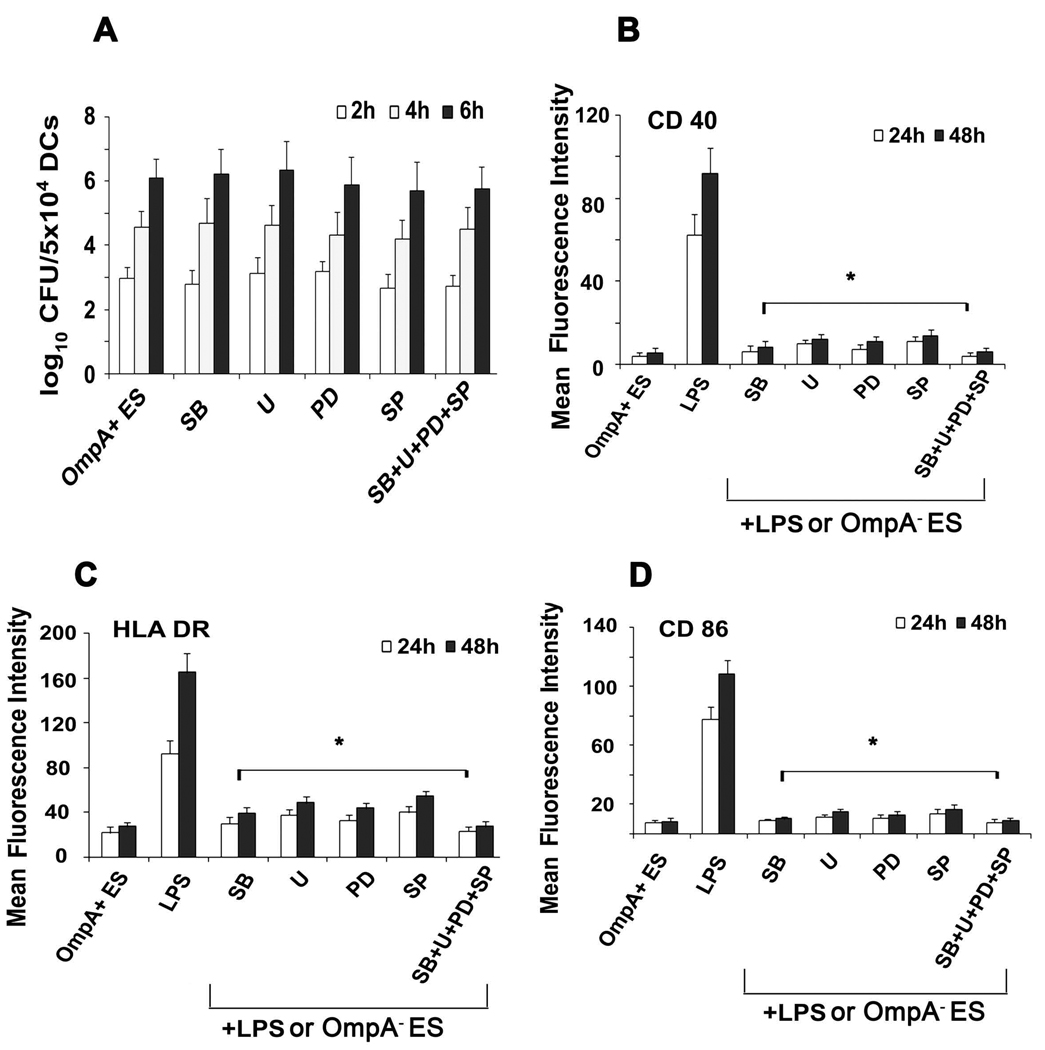
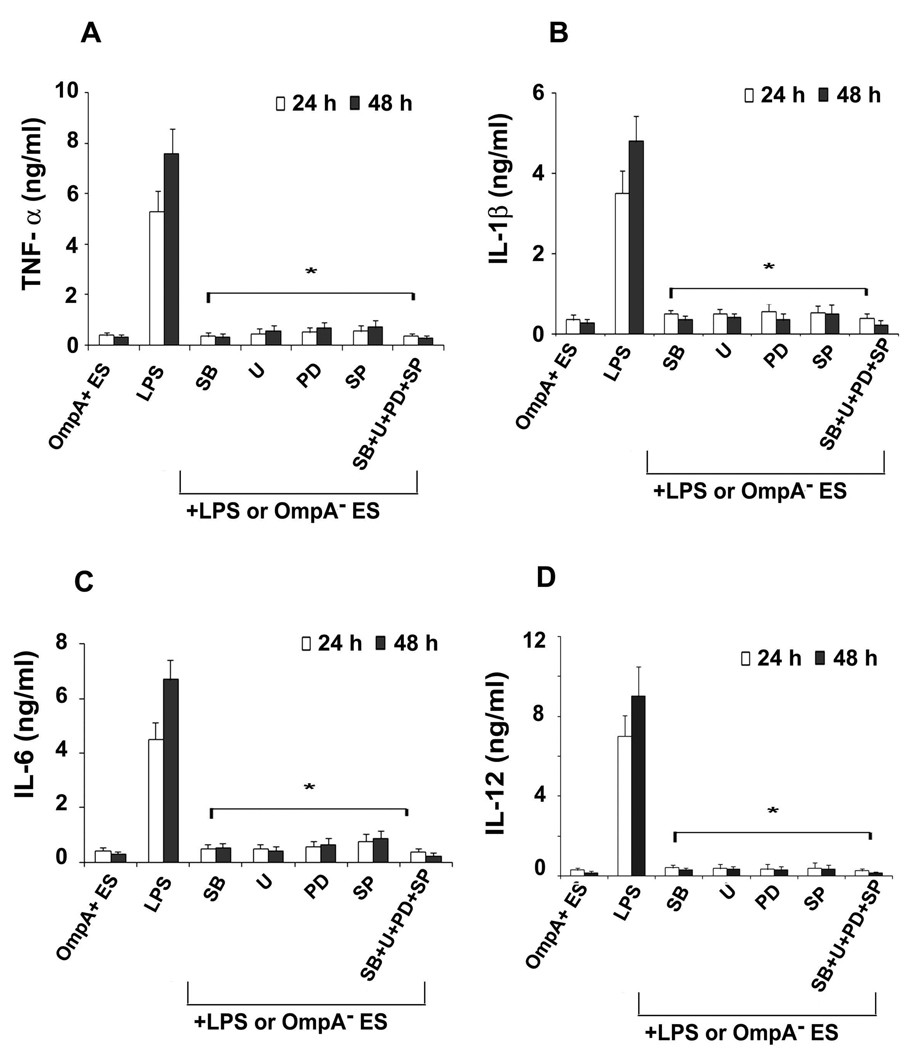
The MAP kinases have been shown to be involved in all aspects of the immune response, including the activation and maturation of DCs (55–58). Therefore, the influence of ES on various MAP kinases in DCs was determined. DCs infected with OmpA+ or OmpA− ES or LPS were stained with antibodies to phospho-p38, -ERK1/2, or -JNK and then subjected to flow cytometry. As shown in Fig. 8, DCs infected with OmpA+ ES showed basal level phosphorylation of p38, ERK1/2 and JNK in comparison to OmpA− ES in which all these molecules were phosphorylated. LPS also showed similar increase in phosphorylation of MAP kinases, indicating that OmpA+ ES suppresses the activation of MAP kinase pathway. The expression of non-phosphorylated MAP kinases was similar in all three treatments (data not shown). To determine whether the activation of MAP kinases is necessary for the entry of ES into DCs, the cells were pretreated with MAP Kinase inhibitors SB203580 (p38 kinase inhibitor), PD 98059 (MEK1 inhibitor), U0126 (inhibitor of MEK1 and MEK2, which are upstream of JNK) or with a JNK inhibitor SP600125 or with a combination of these inhibitors. The intracellular survival of ES was not affected by pre-treating the cells with MAP kinase inhibitors (Fig. 9A). In contrast, no upregulation of maturation markers was observed in DCs pre-treated with MAP kinase inhibitors followed by LPS treatment or OmpA− ES infection similar to that of OmpA+ ES induced levels (Fig. 9B to D). Maximum inhibitory effect was observed when DCs were pretreated with all the three inhibitors. Similarly, the production of pro-inflammatory cytokines was also significantly reduced in DCs pre-treated with MAP kinase inhibitors followed by LPS stimulation in comparison to LPS treated DCs (Fig. 10A to D). These data demonstrate that ES prevents the maturation of DCs by interfering with MAP kinase pathway, which is distinct from entry mechanisms.
Figure 8. ES impairs phosphorylation of p38, ERK1/2 and JNK in DCs.
DCs were cultured in the presence of OmpA+ or OmpA− for 6 h, washed, and stimulated with LPS for 30 min. The cells were washed, fixed and then phosphorylation of p38, ERK1/2 and JNK was determined by flow cytometry after staining with respective phospho-specific antibodies (A). For quantitative measurements, the geometric mean fluorescence intensity was plotted for each MAP kinase (B). The results are representative of five independent experiments. The phosphorylation of MAP kinases in OmpA+ ES infected DCs was significantly lower compared to OmpA− ES infected or LPS treated cells, *p<0.001 calculated by Student’s t test.
Figure 9. Effect of MAP kinase inhibitors on phagocytosis and maturation of DCs.
DCs were treated with the p38 inhibitor SB203580 (SB), the ERK1/2 inhibitors UO126 (U), PD98059 (PD), the JNK inhibitor SP600125 (SP) or with a combination (SB+U+PD+SP) for 1 h and then incubated with bacteria. Percentage intracellular bacteria were assessed using gentamicin protection assay as described in material and methods section (A). Furthermore, bacteria treated with these MAP kinase inhibitors were stimulated with LPS for 24 and 48 h and expression of maturation markers CD40 (B), HLA-DR (C) and CD86 (D) was assessed using flow cytometry. The data is presented as geometric mean fluorescence intensity (MFI) of logarithmic data subtracted from isotype matched controls. The error bars represent standard deviations of triplicate samples from four individual experiments. The decrease in the expression of maturation markers in DCs pretreated with various inhibitors was significantly lower compared to LPS pretreated cells, *p<0.001 calculated by Student’s t test.
Figure 10. Inhibition of proinflammatory cytokines by MAP kinase inhibitors.
DCs were cultured for 1 h with the p38 inhibitor SB203580 (SB), the ERK1/2 inhibitors UO126 (U), PD98059 (PD), the JNK inhibitor SP600125 (SP) or with a combination (SB+U+PD+SP). After incubation, the cells were stimulated for 24 and 48 h with LPS or OmpA− ES and cytokine secretion was analyzed by ELISA. The error bars represent standard deviations of triplicate samples from six individual experiments. The production of cytokines in DCs pretreated with MAP kinase inhibitors was significantly lower in comparison to LPS treated cells, *p<0.001 calculated by Student’s t test.
DISCUSSION
Dendritic cells (DCs) play a crucial role in the initiation and modulation of pathogen-specific immune responses (18). Immature DCs in the periphery and submucosa sense the external environment and constantly monitor for pathogens (19). Once DC recognizes and captures a pathogen, it undergoes considerable changes, resulting in DC maturation (20–23). However, some of the pathogens interfere with the maturation of DCs and exploit them as replication permissive niche. Here, we show that exposure to ES that expresses OmpA can impair the maturation of myeloid DCs, triggering the production of IL-10 and TGF-β, the cytokines generally associated with immunosuppressive response (59–61). Remarkably, ES that lack OmpA induced the production of pro-inflammatory cytokines in infected DCs. Although both OmpA+ and OmpA− ES were efficiently taken up by DCs, the cells immediately killed OmpA− ES. In contrast, OmpA+ ES resists killing and multiplied in these cells, suggesting that OmpA expression is critical for the survival of ES in DCs. Complementation with OmpA gene restored the ability of OmpA− ES to persist in DCs highlighting the crucial role of OmpA for survival in DCs. Phagocytosis and the subsequent intracellular events control the generation of immune response and the fate of the pathogen. Phagocytosis of infectious organisms begins with binding of the organism to the cell. SEM and TEM studies revealed that ES was taken up by DCs in a conventional phagocytosis mechanism enclosed inside membrane-bound compartments of DCs. Two or more bacteria were also observed in a single phagosome like organelles.
The idea that DCs use DC-SIGN to capture microbial pathogens for delivery to lymphocytes emerged with the discovery of DC-SIGN as a receptor for gp120 antigen of HIV. Several studies have established that DCs serve as the carrier for HIV-1, with DC-SIGN as the receptor for viral particles and delivering them to target cells such as CD4 lymphocytes (31–33). A similar concept also applies to ES, as it binds DC-SIGN to enter DCs. Anti-DC-SIGN antibodies and mannan, which affect DC-SIGN binding ability, and His-Mermaid, which can compete with DC-SIGN for ES binding, all significantly prevented the entry of ES into DCs, indicating that ES interacts with DC-SIGN. Although several mannose C-type lectin receptors are present on DCs, including DC-SIGN, langerin, and the mannose receptor, ES binding and entry was completely prevented with anti-DC-SIGN antibody. Therefore, it is possible that ES interacts specifically with DC-SIGN to bind to and enter DCs. However, the absence of OmpA did not affect the ability of ES to enter DCs or to bind His-Mermaid, suggesting that ES might be entering DCs via the interaction of mannose residues present on LPS with DC-SIGN. DC-SIGN binds several mannose-containing glycoconjugates as well as fucose-containing Lewis blood group antigens (Lex, Ley, Lea and Leb). However, the core LPS of E. coli K12, H. ducreyi, N. gonorrhoeae, S. typhimurium do not contain either mannose or fucose, but GlcNAc is part of the core region (34). Zhang et al have shown that when the GlcNAc epitopes of the core region have been removed, the ability of LPS from these bacteria to bind DC-SIGN is decreased or lost (34).
To further confirm that ES interacts with DC-sign, binding of ES to His-Mermaid, a DC-SIGN like molecule was determined by flow cytometry. The carbohydrate recognition domain of Mermaid shares both structural and functional similarity with that of DC-SIGN (40). Strong binding of ES to His-Mermaid was observed indicating that there is a specific interaction between DC-SIGN and ES. Further ES was also observed to invade HeLa cells expressing DC-SIGN with a fifty-fold greater efficiency in comparison to plasmid-alone transfected cells. In addition, ES was able to invade DC-SIGN expressing IEC-6 cells, which are non-invasive by this pathogen, substantiating the evidence that engaging DC-SIGN is sufficient for the invasion. Our results clearly support the notion that ES utilizes the DC-SIGN receptor to invade and replicate inside DCs. Interestingly, the survival of OmpA+ ES in DCs requires bacterial protein synthesis as chloroamphenicol treated ES could not survive in the DCs although entered normally, indicating that the expression of OmpA+ ES might interact with certain cellular components to induce the secretion of bacterial proteins into phagosome. Of note, blocking of OmpA interaction with DCs by anti-OmpA antibody prevented the suppressive effects of the bacterium in DCs, indicating that OmpA might be interacting with DC-SIGN, which triggers the secretion of proteins into DCs and thereby suppresses DC function. Additional studies are needed to identify these proteins produced by ES in DCs.
DCs are the most potent antigen-presenting cells capable of activating naive T lymphocytes, and hence play a central role in the induction of adaptive immunity (18–20). Immature DCs sample and process antigens, and efficiently sense a large variety of signals from the surrounding environment. ES infected DCs fail to present antigen to T cells as indicated by the inability of T cells to proliferate in mixed lymphocyte reaction. Strong T cell immune responses are instrumental in controlling microbial infections. Our studies support the notion that interference with DC function is a mechanism of pathogenicity employed by ES to evade T-cell recognition. The inability of DCs to present antigen to T cells can have serious consequences like chronicity and recurrence of infection. The suppression of T cell immune responses also could be due to the production of anti-inflammatory cytokine production in microbial infections. The nature of microbial stimulus exerts a potent influence on the ability of DCs to produce distinct cytokines and to induce TH1 versus TH2 responses (53, 54). The cytokine profile of DCs infected with OmpA+ ES showed higher production of IL-10 and TGF-β and very low levels of proinflammatory cytokines, a phenotype associated with tolerogenic DCs (59–61). In contrast, infection of DCs with OmpA− ES led to higher production of proinflammatory cytokines indicating activation and maturation of DCs. Thus, ES could exploit IL-10 and TGF-β producing tolerogenic DCs to escape potent host immune defense mechanisms.
Signals emanating from many cell-surface receptors and environmental cues converge on mitogen-activated protein (MAP) kinases, which in turn phosphorylate and activate various transcription factors and other molecular effectors (62–64). MAP kinases comprise three major groups: the extracellular signal-regulated protein kinases (ERK1 and ERK2), the c-Jun amino terminal kinases (JNK) and the p38 MAP kinases (65–67). A beneficial strategy used by many pathogens is to interfere with the phosphorylation cascades in the intracellular-signaling pathways of the host cell. Our studies revealed that ES severely impairs the phosphorylation of p38, ERK1/2 and c-JNK. On par with these findings, pretreatment of DCs with inhibitors of MAP kinase pathway prevented the activation and maturation of DCs stimulated by LPS or OmpA− ES as observed with OmpA+ ES. Thus, ES prevents the activation and maturation of DCs by compromising the MAP kinase pathway. Nevertheless, MAP kinase inhibitor pretreatment did not affect the entry of ES in DCs.
The present study highlights the fundamental role of two molecules in ES pathogenesis, the DC-specific immunoreceptor DC-SIGN and the ES outer membrane protein OmpA. Host DC-SIGN mediates ES access to DCs and exert profound immunosuppressive effects thereupon by targeting MAP kinase activity. On the bacterial side, the suppression of MAP kinase activation and subsequent prevention of the expression of maturation markers require the expression of OmpA in ES. The absence of presentation of bacterial antigens to T cells ensures ES survival inside DCs and likely provides ES with a niche to multiply. This may help in reaching a high degree of bacteremia required to cross the blood brain barrier and subsequently cause meningitis in neonates.
Supplementary Material
ACKNOWLEDGMENTS
The following reagents were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: pcDNA3-DC-SIGN (Cat# 5444) from Drs. S. Pohlmann, F. Baribaud, F. Kirchhoff and R. W. Doms and DC-SIGN antibody (Clone 120526, Cat# 6886). We also thank Ernesto Barron and Douglas Hauser for their help in scanning and transmission electron microscopy at University of Southern California School of Medicine, Los Angeles.
This work was supported by NIH grant AI40567 (N.V.P) and by the Austrian Science Fund (FWF) Grant P17710-B12 (S.B.).
Abbreviations used
- ES
Enterobacter sakazakii
- OmpA
Outer membrane protein A
- DCs
Dendritic cells
- DC-SIGN
DC-specific ICAM non-integrin
- LPS
Lipopolysaccharide
- MAP kinases
mitogen activated protein kinases
- MM
Maturation mixture
Footnotes
DISCLOSURES
The authors have no financial conflict of interest.
REFERENCES
- 1.Friedemann M. Enterobacter sakazakii in food and beverages (other than infant formula and milk powder) Int. J. Food. Microbiol. 2007;116:1–10. doi: 10.1016/j.ijfoodmicro.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 2.Kim HY, Lee Y, Beuchat LR, Yoon BJ, Ryu JH. Microbiological examination of vegetable seed sprouts in Korea. J. Food Prot. 2009;72:856–859. doi: 10.4315/0362-028x-72.4.856. [DOI] [PubMed] [Google Scholar]
- 3.Baumgartner A, Grand M, Liniger M, Iversen C. Detection and frequency of Cronobacter spp. (Enterobacter sakazakii) in different categories of ready-to-eat foods other than infant formula. Int. J. Food Microbiol. 2009 doi: 10.1016/j.ijfoodmicro.2009.04.009. In Press. [DOI] [PubMed] [Google Scholar]
- 4.Kandhai MC, Reij MW, Gorris LG, Guillaume-Gentil O, van Schothorst M. Occurrence of Enterobacter sakazakii in food production environments and households. Lancet. 2004;363:39–40. doi: 10.1016/S0140-6736(03)15169-0. [DOI] [PubMed] [Google Scholar]
- 5.Drudy D, Mullane NR, Quinn T, Wall PG, Fanning S. Enterobacter sakazakii: an emerging pathogen in powdered infant formula. Clin. Infect. Dis. 2006;42:996–1002. doi: 10.1086/501019. [DOI] [PubMed] [Google Scholar]
- 6.Giovannini M, Verduci E, Ghisleni D, Salvatici E, Riva E, Agostoni C. Enterobacter sakazakii: an emerging problem in paediatric nutrition. J. Int. Med. Res. 2008;36:394–399. doi: 10.1177/147323000803600303. [DOI] [PubMed] [Google Scholar]
- 7.Anonymous: International Commission on Microbiological Specifications for Foods (ICMSF) Microbiological Testing in Food Safety Management. New York: Kluwer Academic/Plenum Publishers; 2002. Microorganisms in Food 7. [Google Scholar]
- 8.Riedel K, Lehner A. Identification of proteins involved in osmotic stress response in Enterobacter sakazakii by proteomics. Proteomics. 2007;7:1217–1231. doi: 10.1002/pmic.200600536. [DOI] [PubMed] [Google Scholar]
- 9.Hunter CJ, Petrosyan M, Ford HR, Prasadarao NV. Enterobacter sakazakii: an emerging pathogen in infants and neonates. Surg. Infect. (Larchmt) 2008;9:533–539. doi: 10.1089/sur.2008.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skovgaard N. New trends in emerging pathogens. Int. J. Food Microbiol. 2007;120:217–224. doi: 10.1016/j.ijfoodmicro.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 11.Mullane NR, Iversen C, Healy B, Walsh C, Whyte P, Wall PG, Quinn T, Fanning S. Enterobacter sakazakii an emerging bacterial pathogen with implications for infant health. Minerva. Pediatr. 2007;59:137–148. [PubMed] [Google Scholar]
- 12.Bowen AB, Braden CR. Invasive Enterobacter sakazakii disease in infants. Emerg. Infect. Dis. 2006;12:1185–1189. doi: 10.3201/eid1208.051509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willis J, Robinson JE. Enterobacter sakazakii meningitis in neonates. Pediatr. Infect. Dis. J. 1988;7:196–199. doi: 10.1097/00006454-198803000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Nazarowec-White M, Farber JM. Enterobacter sakazakii: a review. Int. J. Food Microbiol. 1997;34:103–113. doi: 10.1016/s0168-1605(96)01172-5. [DOI] [PubMed] [Google Scholar]
- 15.Mittal R, Wang Y, Hunter CJ, Gonzalez-Gomez I, Prasadarao NV. Brain damage in newborn rat model of meningitis by Enterobacter sakazakii: a role for outer membrane protein A. Lab. Invest. 2009;89:263–277. doi: 10.1038/labinvest.2008.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunter CJ, Williams M, Petrosyan M, Guner Y, Mittal R, Mock D, Upperman JS, Ford HR, Prasadarao NV. Lactobacillus bulgaricus prevents intestinal epithelial cell injury caused by Enterobacter sakazakii-induced nitric oxide both in vitro and in the newborn rat model of necrotizing enterocolitis. Infect. Immun. 2009;77:1031–1043. doi: 10.1128/IAI.01192-08. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Hunter CJ, Singamsetty VK, Chokshi NK, Boyle P, Camerini V, Grishin AV, Upperman JS, Ford HR, Prasadarao NV. Enterobacter sakazakii enhances epithelial cell injury by inducing apoptosis in a rat model of necrotizing enterocolitis. J. Infect. Dis. 2008;198:586–593. doi: 10.1086/590186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinman RM. Dendritic cells: understanding immunogenicity. Eur. J. Immunol. 2007;37 Suppl 1:S53–S60. doi: 10.1002/eji.200737400. [DOI] [PubMed] [Google Scholar]
- 19.Steinman RM, Bonifaz L, Fujii S, Liu K, Bonnyay D, Yamazaki S, Pack M, Hawiger D, Iyoda T, Inaba K, Nussenzweig MC. The innate functions of dendritic cells in peripheral lymphoid tissues. Adv. Exp. Med. Biol. 2005;560:83–97. doi: 10.1007/0-387-24180-9_12. [DOI] [PubMed] [Google Scholar]
- 20.Rossi M, Young JW. Human dendritic cells: potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J. Immunol. 2005;175:1373–1381. doi: 10.4049/jimmunol.175.3.1373. [DOI] [PubMed] [Google Scholar]
- 21.Bousso P. T-cell activation by dendritic cells in the lymph node: lessons from the movies. Nat. Rev. Immunol. 2008;8:675–684. doi: 10.1038/nri2379. [DOI] [PubMed] [Google Scholar]
- 22.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 23.Steinman RM. Some interfaces of dendritic cell biology. APMIS. 2003;111:675–697. doi: 10.1034/j.1600-0463.2003.11107802.x. [DOI] [PubMed] [Google Scholar]
- 24.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 25.Moutaftsi M, Mehl AM, Borysiewicz LK, Tabi Z. Human cytomegalovirus inhibits maturation and impairs function of monocyte-derived dendritic cells. Blood. 2002;99:2913–2921. doi: 10.1182/blood.v99.8.2913. [DOI] [PubMed] [Google Scholar]
- 26.Gelderblom HC, Nijhuis LE, de Jong EC, te Velde AA, Pajkrt D, Reesink HW, Beld MG, van Deventer SJ, Jansen PL. Monocyte-derived dendritic cells from chronic HCV patients are not infected but show an immature phenotype and aberrant cytokine profile. Liver Int. 2007;27:944–953. doi: 10.1111/j.1478-3231.2007.01507.x. [DOI] [PubMed] [Google Scholar]
- 27.Bueno SM, Tobar JA, Iruretagoyena MI, Kalergis AM. Molecular interactions between dendritic cells and Salmonella: escape from adaptive immunity and implications on pathogenesis. Crit. Rev. Immunol. 2005;25:389–403. doi: 10.1615/critrevimmunol.v25.i5.40. [DOI] [PubMed] [Google Scholar]
- 28.van Kooyk Y. C-type lectins on dendritic cells: key modulators for the induction of immune responses. Biochem. Soc. Trans. 2008;36:1478–1481. doi: 10.1042/BST0361478. [DOI] [PubMed] [Google Scholar]
- 29.den Dunnen J, Gringhuis SI, Geijtenbeek TB. Innate signaling by the C-type lectin DC-SIGN dictates immune responses. Cancer Immunol. Immunother. 2009;58:1149–1157. doi: 10.1007/s00262-008-0615-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou T, Chen Y, Hao L, Zhang Y. DC-SIGN and immunoregulation. Cell. Mol. Immunol. 2006;3:279–283. [PubMed] [Google Scholar]
- 31.Geijtenbeek TB, van Kooyk Y. DC-SIGN: a novel HIV receptor on DCs that mediates HIV-1 transmission. Curr. Top. Microbiol. Immunol. 2003;276:31–54. doi: 10.1007/978-3-662-06508-2_2. [DOI] [PubMed] [Google Scholar]
- 32.Wu L, KewalRamani VN. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat. Rev. Immunol. 2006;6:859–868. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu Kimata MT, Cella M, Biggins JE, Rorex C, White R, Hicks S, Wilson JM, Patel PG, Allan JS, Colonna M, Kimata JT. Capture and transfer of simian immunodeficiency virus by macaque dendritic cells is enhanced by DC-SIGN. J. Virol. 2002;76:11827–11836. doi: 10.1128/JVI.76.23.11827-11836.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang P, Snyder S, Feng P, Azadi P, Zhang S, Bulgheresi S, Sanderson KE, He J, Klena J, Chen T. Role of N-acetylglucosamine within core lipopolysaccharide of several species of gram-negative bacteria in targeting the DC-SIGN (CD209) J. Immunol. 2006;177:4002–4011. doi: 10.4049/jimmunol.177.6.4002. [DOI] [PubMed] [Google Scholar]
- 35.van Kooyk Y, Appelmelk B, Geijtenbeek TB. A fatal attraction: Mycobacterium tuberculosis and HIV-1 target DC-SIGN to escape immune surveillance. Trends Mol. Med. 2003;9:153–159. doi: 10.1016/s1471-4914(03)00027-3. [DOI] [PubMed] [Google Scholar]
- 36.Tailleux L, Schwartz O, Herrmann JL, Pivert E, Jackson M, Amara A, Legres L, Dreher D, Nicod LP, Gluckman JC, Lagrange PH, Gicquel B, Neyrolles O. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 2003;197:121–127. doi: 10.1084/jem.20021468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cambi A, Gijzen K, de Vries JM, Torensma R, Joosten B, Adema GJ, Netea MG, Kullberg BJ, Romani L, Figdor CG. Candida The C-type lectin DC-SIGN (CD209) is an antigen-uptake receptor for Candida albicans on dendritic cells. Eur. J. Immunol. 2003;33:532–538. doi: 10.1002/immu.200310029. [DOI] [PubMed] [Google Scholar]
- 38.Zhang P, Skurnik M, Zhang SS, Schwartz O, Kalyanasundaram R, Bulgheresi S, He JJ, Klena JD, Hinnebusch BJ, Chen T. Human dendritic cell-specific intercellular adhesion molecule-grabbing nonintegrin (CD209) is a receptor for Yersinia pestis that promotes phagocytosis by dendritic cells. Infect. Immun. 2008;76:2070–2079. doi: 10.1128/IAI.01246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang P, Schwartz O, Pantelic M, Li G, Knazze Q, Nobile C, Radovich M, He J, Hong SC, Klena J, Chen T. DC-SIGN (CD209) recognition of Neisseria gonorrhoeae is circumvented by lipooligosaccharide variation. J. Leukocyte Biol. 2006;79:731–738. doi: 10.1189/jlb.0405184. [DOI] [PubMed] [Google Scholar]
- 40.Bulgheresi S, Schabussova I, Chen T, Mullin NP, Maizels RM, Ott JA. A new C-type lectin similar to the human immunoreceptor DC-SIGN mediates symbiont acquisition by a marine nematode. Appl. Environ. Microbiol. 2006;72:2950–2956. doi: 10.1128/AEM.72.4.2950-2956.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pöhlmann S, Baribaud F, Lee B, Leslie GJ, Sanchez MD, Hiebenthal-Millow K, Münch J, Kirchhoff F, Doms RW. DC-SIGN interactions with human immunodeficiency virus type 1, type 2 and simian immunodeficiency virus. J. Virol. 2001;75:4664–4672. doi: 10.1128/JVI.75.10.4664-4672.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jameson B, Baribaud F, Pöhlmann S, Ghavimi D, Mortari F, Doms RW, Iwasaki A. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J. Virol. 2002;76:1866–1875. doi: 10.1128/JVI.76.4.1866-1875.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singamsetty VK, Wang Y, Shimada H, Prasadarao NV. Outer membrane protein A expression in Enterobacter sakazakii is required to induce microtubule condensation in human brain microvascular endothelial cells for invasion. Microb. Pathog. 2008;45:181–191. doi: 10.1016/j.micpath.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dixon GL, Newton PJ, Chain BM, Katz D, Andersen SR, Wong S, van der Ley P, Klein N, Callard RE. Dendritic cell activation and cytokine production induced by group B Neisseria meningitidis: interleukin-12 production depends on lipopolysaccharide expression in intact bacteria. Infect. Immun. 2001;69:4351–4357. doi: 10.1128/IAI.69.7.4351-4357.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones HE, Uronen-Hansson H, Callard RE, Klein N, Dixon GLJ. The differential response of human dendritic cells to live and killed Neisseria meningitides. Cell. Microbiol. 2007;9:2856–2869. doi: 10.1111/j.1462-5822.2007.01001.x. [DOI] [PubMed] [Google Scholar]
- 46.Banks KE, Humphreys TL, Li W, Katz BP, Wilkes DS, Spinola SM. Haemophilus ducreyi partially activates human myeloid dendritic cells. Infect. Immun. 2007;75:5678–5685. doi: 10.1128/IAI.00702-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.SolFoulon N, Moris A, Nobile C, Boccaccio C, Engering A, Abastado JP, Heard JM, van Kooyk Y, Schwartz O. HIV-1 Nef-induced upregulation of DC-SIGN in dendritic cells promotes lymphocyte clustering and viral spread. Immunity. 2002;16:145–155. doi: 10.1016/s1074-7613(02)00260-1. [DOI] [PubMed] [Google Scholar]
- 48.Schulz KR, Danna EA, Krutzik PO, Nolan GP. Single-cell phospho-protein analysis by flow cytometry. Curr. Protoc. Immunol. 2007 doi: 10.1002/0471142735.im0817s78. Chapter 8:Unit 8.17. [DOI] [PubMed] [Google Scholar]
- 49.Krutzik PO, Nolan GP. Intracellular phospho-protein staining techniques for flow cytometry: monitoring single cell signaling events. Cytometry A. 2003;55:61–70. doi: 10.1002/cyto.a.10072. [DOI] [PubMed] [Google Scholar]
- 50.Hanekom WA, Mendillo M, Manca C, Haslett PA, Siddiqui MR, Barry C, 3rd, Kaplan G. Mycobacterium tuberculosis inhibits maturation of human monocyte-derived dendritic cells in vitro. J. Infect. Dis. 2003;188:257–266. doi: 10.1086/376451. [DOI] [PubMed] [Google Scholar]
- 51.Kanof ME. Isolation of T cells using rosetting procedures. Curr. Protoc. Immunol. 2001 doi: 10.1002/0471142735.im0702s19. Chapter 7:Unit 7.2. [DOI] [PubMed] [Google Scholar]
- 52.Sojka DK, Lazarski CA, Huang YH, Bromberg I, Hughson A, Fowell DJ. Regulation of immunity at tissue sites of inflammation. Immunol. Res. 2009 doi: 10.1007/s12026-009-8105-x. in press. [DOI] [PubMed] [Google Scholar]
- 53.Zou GM, Tam YK. Cytokines in the generation and maturation of dendritic cells: recent advances. Eur. Cytokine Netw. 2002;13:186–199. [PubMed] [Google Scholar]
- 54.Lukacs-Kornek V, Engel D, Tacke F, Kurts C. The role of chemokines and their receptors in dendritic cell biology. Front. Biosci. 2008;13:2238–2252. doi: 10.2741/2838. [DOI] [PubMed] [Google Scholar]
- 55.Nakahara T, Uchi H, Urabe K, Chen Q, Furue M, Moroi Y. Role of c-Jun N-terminal kinase on lipopolysaccharide induced maturation of human monocyte-derived dendritic cells. Int. Immunol. 2004;16:1701–1709. doi: 10.1093/intimm/dxh171. [DOI] [PubMed] [Google Scholar]
- 56.Arrighi JF, Rebsamen M, Rousset F, Kindler V, Hauser C. A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-alpha, and contact sensitizers. J. Immunol. 2001;166:3837–3845. doi: 10.4049/jimmunol.166.6.3837. [DOI] [PubMed] [Google Scholar]
- 57.Miyazawa M, Ito Y, Kosaka N, Nukada Y, Sakaguchi H, Suzuki H, Nishiyama N. Role of MAPK signaling pathway in the activation of dendritic type cell line, THP-1, induced by DNCB and NiSO4. J. Toxicol. Sci. 2008;33:51–59. doi: 10.2131/jts.33.51. [DOI] [PubMed] [Google Scholar]
- 58.Aicher A, Shu G, Magaletti D, Mulvania T, Pezzutto A, Craxton A, Clark EA. Differential role for p38 mitogen-activated protein kinase in regulating CD40-induced gene expression in dendritic cells and B cells. J. Immunol. 1999;163:5786. [PubMed] [Google Scholar]
- 59.Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Ka¨mpgen E, Romani N, Schuler G. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J. Exp. Med. 1996;184:741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat. Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 61.Ding L, Shevach EM. IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function. J. Immunol. 1992;148:3133–3139. [PubMed] [Google Scholar]
- 62.Park SM, Kim HS, Choe J, Lee TH. Differential induction of cytokines genes and activation of mitogen-activated protein kinase family by soluble CD40 ligand and TNF in a human follicular dendritic cell line. J. Immunol. 1999;163:631. [PubMed] [Google Scholar]
- 63.Mäkelä SM, Strengell M, Pietilä TE, Osterlund P, Julkunen I. Multiple signaling pathways contribute to synergistic TLR ligand-dependent cytokine gene expression in human monocyte-derived macrophages and dendritic cells. J. Leukoc. Biol. 2009;85:664–672. doi: 10.1189/jlb.0808503. [DOI] [PubMed] [Google Scholar]
- 64.Rincón M, Flavell RA, Davis RA. The JNK and P38 MAP kinase signaling pathways in T cell-mediated immune responses. Free Radic. Biol. Med. 2000;28:1328–1337. doi: 10.1016/s0891-5849(00)00219-7. [DOI] [PubMed] [Google Scholar]
- 65.Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta. 2007;1773:1358–1375. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 66.Zhang YL, Dong C. MAP kinases in immune responses. Cell. Mol. Immunol. 2005;2:20–27. [PubMed] [Google Scholar]
- 67.Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu. Rev. Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.