Abstract
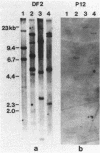
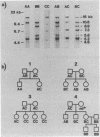
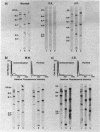
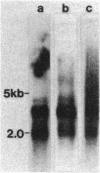
In paroxysmal nocturnal hemoglobinuria (PNH), an acquired hemolytic anemia, deficiency of decay accelerating factor (DAF) renders blood cells susceptible to increased deposition of autologous complement activation fragments (C3b) and complemented-mediated injury. To investigate the mechanism of the DAF defect, DNA and mRNA from normal and PNH leukocytes were compared in blot hybridization assays by using DAF cDNA and oligonucleotide probes. Southern analyses of DNA from normal cells revealed a single gene spanning approximately equal to 35 kilobases of DNA. Six HindIII banding patterns were distinguishable among normal individuals. In family studies, the patterns segregated as three homozygous and three heterozygous genotypes deriving from three haplotypes: A, B, and C with frequencies of 0.47, 0.36, and 0.17, respectively. Oligonucleotide mapping localized the polymorphic HindIII sites to two noncoding regions in the vicinity of exons encoding (i) the protein oligosaccharide-rich domain and (ii) the mRNA 3'-untranslated region. Analyses of DNA from DAF-negative leukocytes of eight PNH patients demonstrated restriction fragment profiles identical to those of normal individuals for all enzymes studied. Three patients had the BC (normals = 3/32), three patients had the AA (normals = 6/32), and two patients had the AC (normals = 8/32) HindIII genotype. Of the three PNH patients exhibiting the BC genotype, family studies of two demonstrated the expected inheritance patterns, and RNA gel blot analyses of two showed mRNA transcripts indistinguishable from those in normal cells. The absence of DAF gene or mRNA alterations in affected PNH cells that lack other glycolipid-anchored proteins as well as DAF argues that the lesion underlying PNH cells resides in the glycolipid-anchor pathway.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell R. D., Bentley D. R., Morley B. J. The factor B and C2 genes. Philos Trans R Soc Lond B Biol Sci. 1984 Sep 6;306(1129):367–378. doi: 10.1098/rstb.1984.0097. [DOI] [PubMed] [Google Scholar]
- Caras I. W., Davitz M. A., Rhee L., Weddell G., Martin D. W., Jr, Nussenzweig V. Cloning of decay-accelerating factor suggests novel use of splicing to generate two proteins. Nature. 1987 Feb 5;325(6104):545–549. doi: 10.1038/325545a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chow F. L., Hall S. E., Rosse W. F., Telen M. J. Separation of the acetylcholinesterase-deficient red cells in paroxysmal nocturnal hemoglobinuria. Blood. 1986 Apr;67(4):893–897. [PubMed] [Google Scholar]
- Cooper D. N., Schmidtke J. DNA restriction fragment length polymorphisms and heterozygosity in the human genome. Hum Genet. 1984;66(1):1–16. doi: 10.1007/BF00275182. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Eukaryotic protein modification and membrane attachment via phosphatidylinositol. Cell. 1987 Jan 30;48(2):179–181. doi: 10.1016/0092-8674(87)90419-3. [DOI] [PubMed] [Google Scholar]
- Davitz M. A., Low M. G., Nussenzweig V. Release of decay-accelerating factor (DAF) from the cell membrane by phosphatidylinositol-specific phospholipase C (PIPLC). Selective modification of a complement regulatory protein. J Exp Med. 1986 May 1;163(5):1150–1161. doi: 10.1084/jem.163.5.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hyman R. Cell-surface-antigen mutants of haematopoietic cells. Tools to study differentiation, biosynthesis and function. Biochem J. 1985 Jan 1;225(1):27–40. doi: 10.1042/bj2250027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänsch G. M., Schönermark S., Roelcke D. Paroxysmal nocturnal hemoglobinuria type III. Lack of an erythrocyte membrane protein restricting the lysis by C5b-9. J Clin Invest. 1987 Jul;80(1):7–12. doi: 10.1172/JCI113065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Medof M. E., Silber R., Nussenzweig V. Distribution of decay-accelerating factor in the peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria. J Exp Med. 1985 Jul 1;162(1):75–92. doi: 10.1084/jem.162.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Gottlieb A., Kinoshita T., Hall S., Silber R., Nussenzweig V., Rosse W. F. Relationship between decay accelerating factor deficiency, diminished acetylcholinesterase activity, and defective terminal complement pathway restriction in paroxysmal nocturnal hemoglobinuria erythrocytes. J Clin Invest. 1987 Jul;80(1):165–174. doi: 10.1172/JCI113043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Kinoshita T., Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984 Nov 1;160(5):1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Kinoshita T., Silber R., Nussenzweig V. Amelioration of lytic abnormalities of paroxysmal nocturnal hemoglobinuria with decay-accelerating factor. Proc Natl Acad Sci U S A. 1985 May;82(9):2980–2984. doi: 10.1073/pnas.82.9.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Lublin D. M., Holers V. M., Ayers D. J., Getty R. R., Leykam J. F., Atkinson J. P., Tykocinski M. L. Cloning and characterization of cDNAs encoding the complete sequence of decay-accelerating factor of human complement. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2007–2011. doi: 10.1073/pnas.84.7.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Walter E. I., Roberts W. L., Haas R., Rosenberry T. L. Decay accelerating factor of complement is anchored to cells by a C-terminal glycolipid. Biochemistry. 1986 Nov 4;25(22):6740–6747. doi: 10.1021/bi00370a003. [DOI] [PubMed] [Google Scholar]
- Medof M. E., Walter E. I., Rutgers J. L., Knowles D. M., Nussenzweig V. Identification of the complement decay-accelerating factor (DAF) on epithelium and glandular cells and in body fluids. J Exp Med. 1987 Mar 1;165(3):848–864. doi: 10.1084/jem.165.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson-Weller A., Burge J., Fearon D. T., Weller P. F., Austen K. F. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol. 1982 Jul;129(1):184–189. [PubMed] [Google Scholar]
- Nicholson-Weller A., March J. P., Rosen C. E., Spicer D. B., Austen K. F. Surface membrane expression by human blood leukocytes and platelets of decay-accelerating factor, a regulatory protein of the complement system. Blood. 1985 May;65(5):1237–1244. [PubMed] [Google Scholar]
- Nicholson-Weller A., March J. P., Rosenfeld S. I., Austen K. F. Affected erythrocytes of patients with paroxysmal nocturnal hemoglobinuria are deficient in the complement regulatory protein, decay accelerating factor. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5066–5070. doi: 10.1073/pnas.80.16.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Deficiency of an erythrocyte membrane protein with complement regulatory activity in paroxysmal nocturnal hemoglobinuria. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5430–5434. doi: 10.1073/pnas.80.17.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plunkett M. L., Sanders M. E., Selvaraj P., Dustin M. L., Springer T. A. Rosetting of activated human T lymphocytes with autologous erythrocytes. Definition of the receptor and ligand molecules as CD2 and lymphocyte function-associated antigen 3 (LFA-3). J Exp Med. 1987 Mar 1;165(3):664–676. doi: 10.1084/jem.165.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey-Campos J., Rubinstein P., Rodriguez de Cordoba S. Decay-accelerating factor. Genetic polymorphism and linkage to the RCA (regulator of complement activation) gene cluster in humans. J Exp Med. 1987 Jul 1;166(1):246–252. doi: 10.1084/jem.166.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse W. F., Dacie J. V. Immune lysis of normal human and paroxysmal nocturnal hemoglobinuria (PNH) red blood cells. I. The sensitivity of PNH red cells to lysis by complement and specific antibody. J Clin Invest. 1966 May;45(5):736–748. doi: 10.1172/JCI105388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse W. F., Parker C. J. Paroxysmal nocturnal haemoglobinuria. Clin Haematol. 1985 Feb;14(1):105–125. [PubMed] [Google Scholar]
- Zalman L. S., Wood L. M., Frank M. M., Müller-Eberhard H. J. Deficiency of the homologous restriction factor in paroxysmal nocturnal hemoglobinuria. J Exp Med. 1987 Feb 1;165(2):572–577. doi: 10.1084/jem.165.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]