Abstract
Natural autoreactive monoclonal IgMs have demonstrated potential as therapeutic agents for CNS disease. These antibodies bind surface antigens on specific CNS cells activating intracellular repair-promoting signals. IgMs that bind to surface antigens on oligodendrocytes enhanced remyelination in animal models of multiple sclerosis. IgMs that bind to neurons stimulate neurite outgrowth and prevent neuron apoptosis. The neuron-binding IgMs may have utility in CNS axon- or neuron-damaging diseases such as amyotrophic lateral sclerosis, stroke, spinal cord injury or secondary progressive multiple sclerosis. Recombinant remyelination-promoting IgMs have been generated for formal toxicology studies and, after FDA approval, a Phase I clinical trial. Natural autoreactive monoclonal antibodies directed against CNS cells represent novel therapeutic molecules to induce repair of the nervous system.
Natural autoreactive (NA) monoclonal IgM antibodies can promote central nervous system (CNS) protection and repair. These repair-promoting IgMs have characteristics of classic NA antibodies. For example, they are generally of the IgM isotype; encoded by germline genes with few somatic mutations; and polyreactive with low affinity with a range of structurally unrelated, self and non-self antigens, specifically cytoskeleton, nuclear proteins and DNA 1. In addition, NA monoclonal antibodies that promote CNS protection and repair bind specifically to surface plasma membrane antigens, which activate intracellular signals that promote neuron or glial cell survival 2, 3 and cross the blood-brain-barrier to accumulate within injured regions of the CNS4. Other independent investigators have also demonstrated that human NA monoclonal antibodies cross the blood-brain barrier and localize to normal and injured CNS tissues in vivo 5. The therapeutic human IgMs bind to membrane antigens, which are destroyed following treatments that disrupt cellular architecture including chemical/biochemical fixation, dehydration, solubilization, extraction or digestion and physical/mechanical crush forces or extreme temperatures 6, 7. The human IgMs demonstrate specific affinity only when membranes are maintained under live physiological conditions 6–8. Cell signals are activated through direct antibody-protein-glycolipid binding interactions. 2, 3, 9–11.
Identification of Oligodendrocyte Binding Antibodies That Promote CNS Repair
We employed a novel strategy to identify human monoclonal antibodies that promote remyelination 12. Monoclonal antibodies were isolated from the sera of patients with monoclonal gammopathy. Selection criteria included a serum monoclonal immunoglobulin concentration of greater than 3 g/dL and a lack of neurologic or antibody-associated pathologies. We screened antibodies for binding to myelin in live CNS tissue slices and to the surface of live oligodendrocytes in culture 12. Six of 52 serum-derived human IgMs (sHIgM) and zero of 50 serum-derived human IgGs (sHIgG) bound in these assays. Two IgMs (sHIgM22 and sHIgM46) promoted significant remyelination in vivo 12. A recombinant version of sHIgM22, rHIgM22, was engineered by cloning the antibody variable region DNA sequence into an expression vector 9, 13. rHIgM22 promoted myelin repair in the Theiler's virus infection-induced model of MS equal to the serum-derived form 9. Gram quantities of GMP-grade rHIgM22 have been purified for formal toxicology studies prior to Phase I clinical trials. Our development of rHIgM22 established an infrastructure for rapid translation of additional human antibodies from basic science to clinical therapies.
We successfully used the same strategy to identify additional human IgMs for testing in other models of neurologic injury and disease. Two neuron-binding antibodies (sHIgM12 and sHIgM42) stimulated neurite extension 10. A dendritic cell-binding antibody (B7DC XAb) mediated melanoma tumor clearance from lungs 14. Several beta-amyloid (Aβ1–40 and Aβ1–42)-binding human antibodies will be tested in animal models of Alzheimer’s disease. This strategy for identifying human Abs that directly signal cells has the potential to generate therapeutic antibodies for a broad range of human diseases.
Specific Antibody-glycolipid-protein Interactions Mediate rHIgM22 Binding to Both Myelin and Oligodendrocyte Membrane Surface
Several mouse IgMs, including A2B5, O1, O4, HNK-1, SCH79.08 and SCH94.03, bind oligodendrocytes and promote remyelination in mouse models of multiple sclerosis (Table) 7. A2B5, O1, and O4 bind to surface glycolipid antigens on less-differentiated oligodendrocytes 15–17. HNK-1, SCH79.08, SCH94.03 and rHIgM22 bind to antigens on the surface of relatively mature oligodendrocytes and myelin. We hypothesized that antibody-mediated remyelination required binding to oligodendrocyte membrane glycolipids.
Table 1.
Properties of CNS-Reactive Signaling Antibodies
| Antibody1 | CNS Surface Membrane Target | CNS Cell Signals 2 | Repair |
|---|---|---|---|
| A2B5 MIgM | c-series gangliosides (GT3, GQ1c and GP1c) | Ca2+ | Remyelination |
| HNK-1 MIgM | Sulfated Glucuronosyl Glycosphingolipids | Ca2+ | Remyelination |
| O1 MIgM | Galactosyl Cerebroside (GalC) | Ca2+ | Remyelination |
| O4 MIgM | Sulfatide (GalC-Sulfate) | Ca2+, P-MAPK, caspase-3I, P-Srcfk | Remyelination |
| SCH94.03 MIgM | Myelin | Ca2+ | Remyelination |
| 79.08 MIgM | Myelin basic protein (MBP) | Ca2+ | Remyelination |
| rHIgM22 | Myelin | Ca2+, P-MAPK, caspase-3I, P-Srcfk | Remyelination |
| sHIgM46 | Myelin | Ca2+ | Remyelination |
| rHIgM12 | Neuronal Membrane | Ca2+, caspase-3I | Neuroprotection, Neurite outgrowth |
| sHIgM42 | Neuronal Membrane | Ca2+, caspase-3I | Neuroprotection, Neurite outgrowth |
| sHIgM39 isotype control | None | No | None |
hybridoma (h), serum (s) recombinant (r), mouse (M), human (H), isotype control (control)
calcium influx (Ca2+), Inhibition of caspase-3 activation (I-caspase-3), MAPK phosphorylation (P-MAPK), Src family kinase phosphorylation (P-Src-fk)
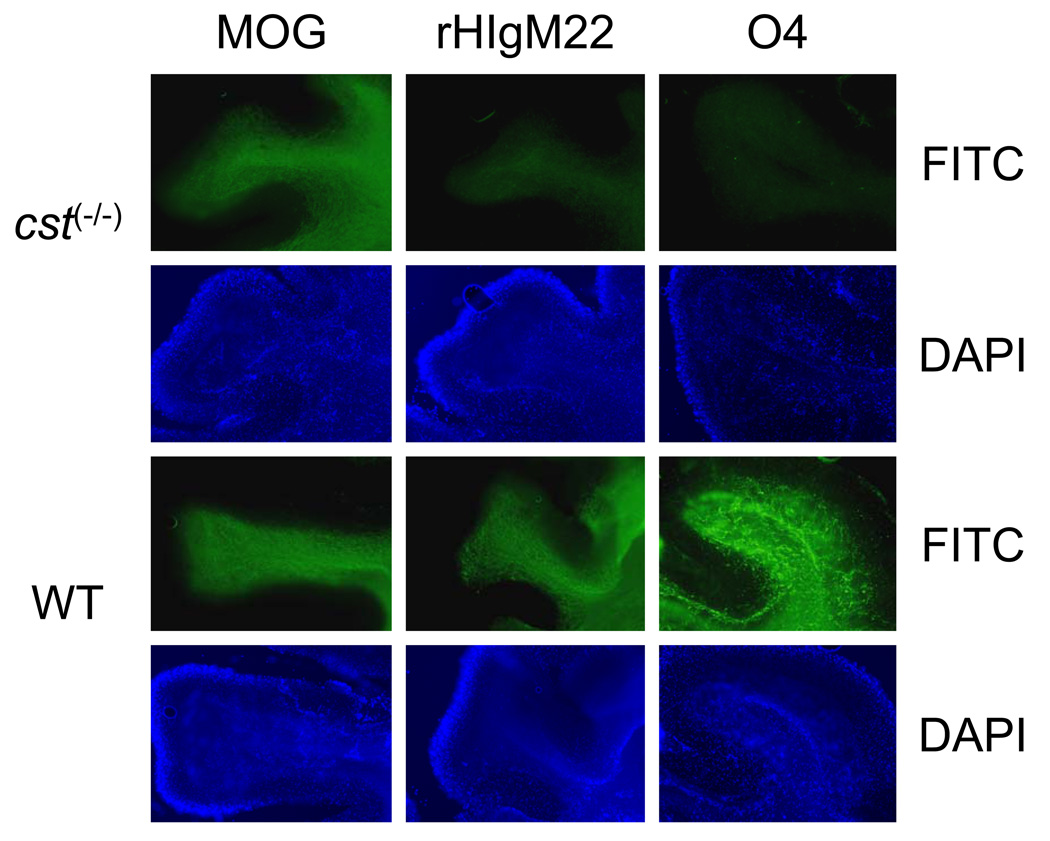
Binding IgMs to CNS tissue from glycolipid knock out mice demonstrates that the molecules bound by rHIgM22 in CNS myelin depend upon components of the glycolipid synthesis pathway. Binding of rHIgM22 to the plasma membrane requires the presence of a substrate of cerebroside sulfotransferase (CST). Normally, the cerebroside galactosyltransferase (CGT) enzyme converts ceramide to galactosylcerebroside (GalC) in oligodendenrocytes. Then the enzyme CST converts GalC to sulfatide. Immunofluorescence of live CNS tissue slices demonstrates the strong affinity of rHIgM22 for densely myelinated axons in wild type mice (Figure 1). rHIgM22 and antibodies against myelin oligodendrocyte glycoprotein (MOG), expressed on mature myelin, bound to densely myelinated fiber tracts and to individual myelinated axons in the cerebellum. In contrast, rHIgM22 affinity for white matter tracts was abolished in CNS tissue from mice lacking sulfatide (cst (−/−))18. Similarly O4 antibody which labels sulfatide was absent in cst (−/−) mice. In addition, rHIgM22 binding was not detected in other sulfatide-expressing tissues including peripheral nervous system myelin and Schwann cells. These data support the hypothesis that rHIgM22 binding depends upon one or more CST-sulfated antigens present exclusively on the surface myelin of oligodendrocytes. rHIgM22 may target sulfatide or a number of sulfated antigens within the CNS including glucosylcerebroside sulfate, lactosylceramind-3-sulfate, seminolipid, bis-sulphoganglio tetraosylceramide, bis-sulphoganglio triaosylceramide. The data support the hypothesis that rHIgM22 recognizes a complex on oligodendrocytes dependent upon one or more sulfated antigens at the myelin plasma membrane.
Figure 1. rHIgM22-binding interactions are dependent upon a CST substrate.
Images (100x) of O4 (sulfatide; A,G), rHIgM22 (B,H) or MOG (C, I) reactivity shown using live cerebellar sections (200µm) subsequently fixed, FITC immunostained and DAPI counterstained (D–F, J–L). Wild-type showed reactivity to all in contrast cst (−/−) showed MOG but lacked O4 and rHIgM22 reactivity.
Oligodendrocytes express a repertoire of integrins during specific stages of development. Oligodendrocyte myelination correlates with the expression of laminin receptor α6β1 and vitronectin/fibronectin receptors αvβ1, αvβ3, αvβ5 and αvβ8 19. Recent studies in collaboration with Drs Jens Watzlawik and Richard Pagano at Mayo Clinic demonstrate a co-localization of rHIgM22 with β integrins on the plasma membrane of mature oligodendrocytes.
We propose that sulfated molecules and β integrin facilitate specific rHIgM22 binding to myelin and oligodendrocytes. In addition, the pentameric structure of the IgM molecule is necessary for remyelination and may be critical to cross-linking these antigens on the oligodendrocyte surface and inducing intracellular repair signals.
Identification of Neuron-Binding Antibodies for CNS Protection and Repair
Two neuron-binding human IgMs, sHIgM12 and sHIgM42, were identified using the strategy described earlier.10 These antibodies supported in vitro CNS neurite extension equal to the potent neurite stimulatory molecule laminin. Both IgMs bound to the surface of many types of cultured neurons but not to the surface of mature oligodendrocytes. Both IgMs stimulated neurite extension in the presence of CNS myelin, which normally inhibits outgrowth. These two IgMs are novel agents to promote neurite extension and are being tested in models of CNS disease where destructive of axons and neurons.
Antibody-mediated Membrane Rearrangement Initiates Signaling
We propose that NA antibodies activate endogenous cellular mechanisms to protect CNS neurons and oligodendrocytes. Remyelination-promoting IgM antibodies bind to the surface of oligodendrocytes. However, not all IgMs that bind to oligodendrocytes promote remyelination. Oligodendrocyte binding did not perfectly predict the remyelinating potential of an IgM in vivo. Therefore, IgM-mediated repair requires other mechanisms besides strict binding. Specific signaling events are also required. Antibody-mediated signaling in oligodendrocytes is mediated through membrane rearrangement and microdomain signaling 2. Recent studies have demonstrated that mouse IgM antibody O4 and rHIgM22 bound to the surface of unfixed primary oligodendrocytes diffusely at 4°C. However, allowing membrane rearrangement at 15°C resulted in small punctate structures indicative of signaling microdomain clustering. sHIgM42 under the same conditions exhibited a similar punctate membrane pattern on neurons (Figure 2). Coincident with IgM-mediated membrane clustering on oligodendrocytes was the rapid activation of intracellular MAP kinases. The phosphorylation of several signaling proteins increased within 1 minute and persisted for 15 minutes in primary oligodendrocytes treated with rHIgM22.
Figure 2. Neuron-Binding Antibodies Demonstrate Temperature-Dependent, Surface Membrane Rearrangement.
sHIgM42 reactivity shown using live cerebellar granule neurons subsequently fixed, FITC immunostained and DAPI counterstained. sHIgM42 demonstrated binding interactions at 0°C (A) and membrane rearrangements at 15°C (B). Note formation of small punctuate domains along neurites at 15°C but not at 0°C.
Reparative IgMs Induce Rapid Calcium Influx and Inhibit Apoptosis
The in vivo remyelination-promoting ability of an antibody correlated with its ability to induce transient Ca2+ influx in oligodendrocytes in culture 3, 9. rHIgM22 binding to the oligodendrocyte plasma membrane induced signaling cascades that down regulated caspase-3 activation to prevent apoptosis. In addition, rHIgM22 altered gene expression upon calcium influx through CNQX-sensitive AMPA channels in oligodendrocytes. The neuron-binding antibodies sHIgM12 and sHIgM42 demonstrated robust rescue of cultured neurons from H2O2-induced apoptosis.
Antibody-mediated Activation of CNS Protection and Repair
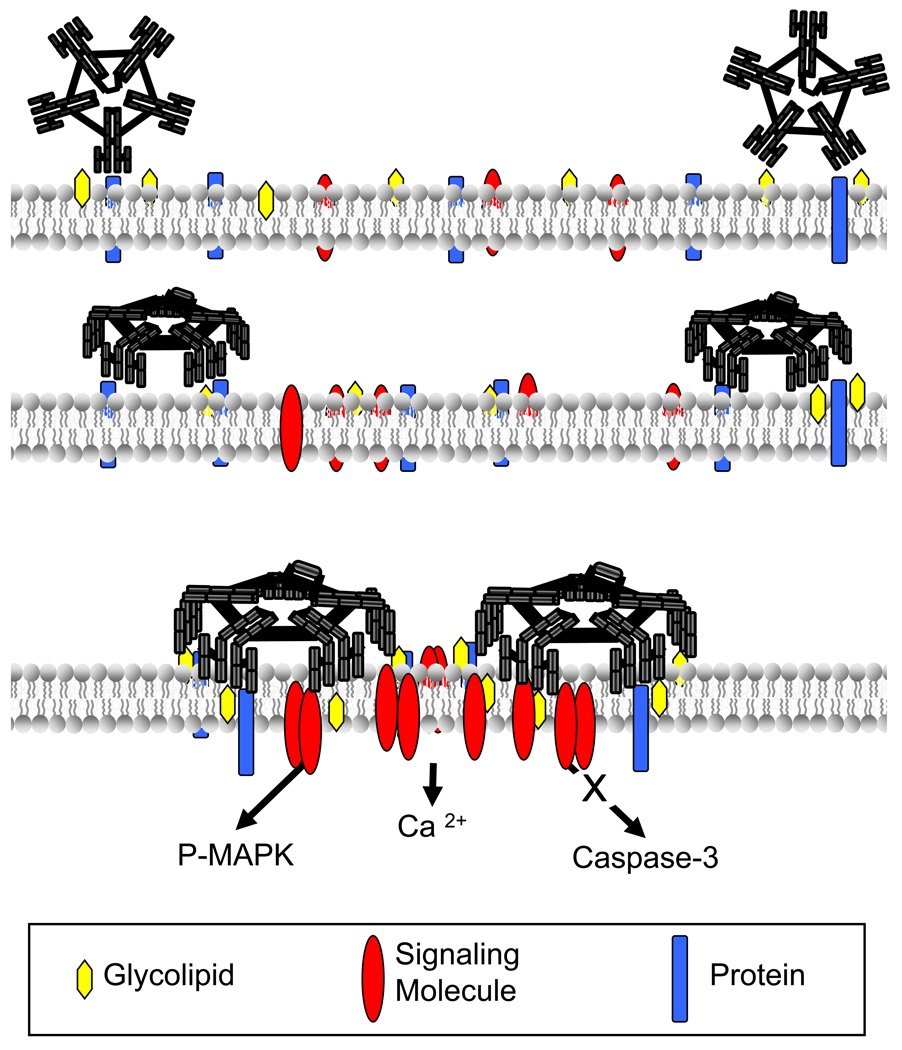
Strong parallels in character exist between remyelination promoting IgMs, rHIgM22 and O4, and the neurite outgrowth-promoting IgMs, sHIgM42 and sHIgM12. This suggests a common membrane-rearrangement mechanism that recruits signaling molecules into clustered microdomains and ultimately leads to specific cell responses in vitro and in vivo (Figure 3).
Figure 3. Mechanism of NA Monoclonal Antibody-mediated CNS Repair.
Pentameric interactions of antibody binding to plasma membrane glycolipids (A) and proteins (B) on live cells induce membrane rearrangements. Signaling molecule (C) activation of pathways including calcium flux, MAP kinase activation and blocking caspase-3 activation that converge on transcriptional regulation of genes promoting CNS protection and repair.
We propose this class of autoreactive antibodies activates the cellular process of CNS protection and repair through direct signaling cascades. Although each antibody reacts to unique cell-specific antigens, binding to the appropriate cells activates the target cell in a conserved manner. Defining the common signaling components regulating changes in specific cells may lead to an understanding of the underlying mechanism of antibody-induced repair and result in the design of better strategies to promote remyelination and protect axons.
Acknowledgment
Work was supported by grants from National Institutes of Health(R01 NS 24180, R01 NS 32129, P01 NS 38468, R01 CA 104996, R01 CA096859), National Multiple Sclerosis Society (RG 317 2-B-8, CA 1011 A8-3), Multiple Sclerosis Society of Canada, Applebaum Foundation, Hilton Foundation, Peterson Foundation and Acorda Therapeutics, Inc. (Hawthorne, NY). Patents for promotion of remyelination are issued and owned by Mayo Clinic. Therefore, the authors have a potential financial conflict of interest. Drs. R. Pagano and J. Watzlawik have given the authors of this manuscript permission to cite their unpublished work with β integrins.
Contributor Information
Brent R. Wright, Email: rodriguez.moses@mayo.edu.
Arthur E. Warrington, Email: wright.brent@mayo.edu.
Dale E. Edberg, Email: edberg.dale@mayo.edu.
Moses Rodriguez, Email: warrington.arthur@mayo.edu.
References
- 1.Coutinho A, Kazatchkine MD, Avrameas S. Natural autoantibodies. Curr Opin Immunol. 1995 Dec;7(6):812–818. doi: 10.1016/0952-7915(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 2.Howe CL, Bieber AJ, Warrington AE, Pease LR, Rodriguez M. Antiapoptotic signaling by a remyelination-promoting human antimyelin antibody. Neurobiol Dis. 2004 Feb;15(1):120–131. doi: 10.1016/j.nbd.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Paz Soldan MM, Warrington AE, Bieber AJ, et al. Remyelination-promoting antibodies activate distinct Ca2+ influx pathways in astrocytes and oligodendrocytes: relationship to the mechanism of myelin repair. Mol Cell Neurosci. 2003 Feb;22(1):14–24. doi: 10.1016/s1044-7431(02)00018-0. [DOI] [PubMed] [Google Scholar]
- 4.Pirko I, Ciric B, Gamez J, et al. A human antibody that promotes remyelination enters the CNS and decreases lesion load as detected by T2-weighted spinal cord MRI in a virus-induced murine model of MS. Faseb J. 2004 Nov;18(13):1577–1579. doi: 10.1096/fj.04-2026fje. [DOI] [PubMed] [Google Scholar]
- 5.Banks WA, Farr SA, Morley JE, Wolf KM, Geylis V, Steinitz M. Anti-amyloid beta protein antibody passage across the blood-brain barrier in the SAMP8 mouse model of Alzheimer's disease: an age-related selective uptake with reversal of learning impairment. Exp Neurol. 2007 Aug;206(2):248–256. doi: 10.1016/j.expneurol.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller DJ, Rodriguez M. A monoclonal autoantibody that promotes central nervous system remyelination in a model of multiple sclerosis is a natural autoantibody encoded by germline immunoglobulin genes. J Immunol. 1995 Mar 1;154(5):2460–2469. [PubMed] [Google Scholar]
- 7.Asakura K, Miller DJ, Pease LR, Rodriguez M. Targeting of IgMkappa antibodies to oligodendrocytes promotes CNS remyelination. J Neurosci. 1998 Nov 1;18(19):7700–7708. doi: 10.1523/JNEUROSCI.18-19-07700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller DJ, Njenga MK, Parisi JE, Rodriguez M. Multi-organ reactivity of a monoclonal natural autoantibody that promotes remyelination in a mouse model of multiple sclerosis. J Histochem Cytochem. 1996 Sep;44(9):1005–1011. doi: 10.1177/44.9.8773566. [DOI] [PubMed] [Google Scholar]
- 9.Mitsunaga Y, Ciric B, Van Keulen V, et al. Direct evidence that a human antibody derived from patient serum can promote myelin repair in a mouse model of chronic-progressive demyelinating disease. Faseb J. 2002 Sep;16(10):1325–1327. doi: 10.1096/fj.01-0994fje. [DOI] [PubMed] [Google Scholar]
- 10.Warrington AE, Bieber AJ, Van Keulen V, Ciric B, Pease LR, Rodriguez M. Neuron-binding human monoclonal antibodies support central nervous system neurite extension. J Neuropathol Exp Neurol. 2004 Jun;63(5):461–473. doi: 10.1093/jnen/63.5.461. [DOI] [PubMed] [Google Scholar]
- 11.Howe CL, Mayoral S, Rodriguez M. Activated microglia stimulate transcriptional changes in primary oligodendrocytes via IL-1beta. Neurobiol Dis. 2006 Sep;23(3):731–739. doi: 10.1016/j.nbd.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Warrington AE, Asakura K, Bieber AJ, et al. Human monoclonal antibodies reactive to oligodendrocytes promote remyelination in a model of multiple sclerosis. Proc Natl Acad Sci U S A. 2000 Jun 6;97(12):6820–6825. doi: 10.1073/pnas.97.12.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warrington AE, Bieber AJ, Ciric B, Pease LR, Van Keulen V, Rodriguez M. A recombinant human IgM promotes myelin repair after a single, very low dose. J Neurosci Res. 2007 Apr;85(5):967–976. doi: 10.1002/jnr.21217. [DOI] [PubMed] [Google Scholar]
- 14.Radhakrishnan S, Nguyen LT, Ciric B, et al. Immunotherapeutic potential of B7-DC (PD-L2) cross-linking antibody in conferring antitumor immunity. Cancer Res. 2004 Jul 15;64(14):4965–4972. doi: 10.1158/0008-5472.CAN-03-3025. [DOI] [PubMed] [Google Scholar]
- 15.Fredman P, Magnani JL, Nirenberg M, Ginsburg V. Monoclonal antibody A2B5 reacts with many gangliosides in neuronal tissue. Arch Biochem Biophys. 1984 Sep;233(2):661–666. doi: 10.1016/0003-9861(84)90492-2. [DOI] [PubMed] [Google Scholar]
- 16.Sommer I, Schachner M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol. 1981 May 30;83(2):311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]
- 17.Chou DK, Ilyas AA, Evans JE, Costello C, Quarles RH, Jungalwala FB. Structure of sulfated glucuronyl glycolipids in the nervous system reacting with HNK-1 antibody and some IgM paraproteins in neuropathy. J Biol Chem. 1986 Sep 5;261(25):11717–11725. [PubMed] [Google Scholar]
- 18.Hirahara Y, Bansal R, Honke K, Ikenaka K, Wada Y. Sulfatide is a negative regulator of oligodendrocyte differentiation: development in sulfatide-null mice. Glia. 2004 Feb;45(3):269–277. doi: 10.1002/glia.10327. [DOI] [PubMed] [Google Scholar]
- 19.Milner R, Frost E, Nishimura S, et al. Expression of alpha vbeta3 and alpha vbeta8 integrins during oligodendrocyte precursor differentiation in the presence and absence of axons. Glia. 1997 Dec;21(4):350–360. [PubMed] [Google Scholar]