Abstract
Endotoxin tolerance reprograms cell responses to LPS by repressing expression of proinflammatory cytokines, while not inhibiting production of anti-inflammatory cytokines and antimicrobial effectors. Molecular mechanisms of induction and maintenance of endotoxin tolerance are incompletely understood, particularly with regard to the impact of endotoxin tolerization on signalosome assembly, activation of adaptor-kinase modules, and expression of negative regulators of TLR signaling in human cells. In this study, we examined LPS-mediated activation of MyD88-dependent and Toll-IL-1R-containing adaptor inducing IFN-β (TRIF)-dependent pathways emanating from TLR4 and expression of negative regulators of TLR signaling in control and endotoxin-tolerant human monocytes. Endotoxin tolerization suppressed LPS-inducible TLR4-TRIF and TRIF-TANK binding kinase (TBK)1 associations, induction of TBK1 kinase activity, activation of IFN regulatory factor (IRF)-3, and expression of RANTES and IFN-β. Tolerance-mediated dysregulation of the TLR4-TRIF-TBK1 signaling module was accompanied by increased levels of suppressor of IκB kinase-ε (SIKE) and sterile α and Armadillo motif-containing molecule (SARM). LPS-tolerant cells showed increased expression of negative regulators Toll-interacting protein (Tollip), suppressor of cytokine signaling (SOCS)-1, IL-1R-associated kinase-M, and SHIP-1, which correlated with reduced p38 phosphorylation, IκB-α degradation, and inhibited expression of TNF-α, IL-6, and IL-8. To examine functional consequences of increased expression of Tollip in LPS-tolerized cells, we overexpressed Tollip in 293/TLR4/MD-2 transfectants and observed blunted LPS-inducible activation of NF-κB and RANTES, while TNF-α responses were not affected. These data demonstrate dysregulation of TLR4-triggered MyD88- and TRIF-dependent signaling pathways and increased expression of negative regulators of TLR signaling in endotoxin-tolerant human monocytes.
Keywords: lipopolysaccharide, signal transduction, suppression/anergy, cell activation, monocytes/macrophages
Introduction
TLRs are principal sensors of bacterial, viral, and fungal pathogen-associated molecular patterns (PAMPs) and “danger” molecules released by host cells subjected to damage inflicted by infection, inflammation, or stress [1,2,3]. These signaling receptors express an ectodomain implicated in ligand recognition, a transmembrane domain, and a cytoplasmic portion that contains the Toll-IL-1R (TIR) signaling domain [1]. TLRs are expressed by many cell types, including epithelial and endothelial cells, monocytes, macrophages, dendritic cells, and T- and B-lymphocytes either on the cell surface (TLR2, TLR4, TLR5, TLR11) or in intracellular endosomes (TLR3, TLR7, TLR8, TLR9) [4]. They sense microbial PAMPs, including lipids (TLR2 and TLR4) [3, 5], proteins (TLR5 and TLR11) [6, 7], nucleic acids (TLR3, TLR7, TLR8, TLR9) [8,9,10], as well as endogenous “danger” molecules [11,12,13]. Ligand recognition induces TLRs dimerization that brings together their TIR domains, creating “docking” platforms for recruitment of adaptor proteins and kinases that activate signaling cascades culminating in expression of cytokines, chemokines, and type I IFNs [1, 14].
TLRs mediate cell activation via engagement of MyD88- and/or TIR domain-containing adaptor inducing IFN-β (TRIF)-dependent signaling pathways. MyD88 is an adaptor protein used by all TLRs except of TLR3 [15,16,17,18,19]. It interacts with TLRs via homotypic TIR-TIR domain interactions but requires a bridging adaptor, MyD88 adaptor like (MAL) (also called TIR domain-containing adaptor protein, TIRAP) to associate with TLR2 and TLR4 [20, 21]. MyD88 mediates TLR2/4-triggered activation of MAPKs, transcription factors, and production of proinflammatory cytokines via proximal kinase-adaptor modules involving IL-1R-associated kinases 1-4, TGF-β-activated kinase 1 and adaptor TNFR-associated factor (TRAF)-6 [16,17,18,19, 22,23,24]. In contrast, TLR4 engages TRIF via a bridging adaptor, TRIF-related adaptor molecule (TRAM), to signal IFN regulatory factor (IRF) 3 activation and expression of type I IFNs [25, 26]. TLR7-9 use only MyD88 for activation of type I IFNs via the MyD88-IRAK4-IRAK1-IRF7 axis [27,28,29] and proinflammatory cytokines via the MyD88- IRF5 complex [30]. TLR3 relies solely on TRIF to activate NF-κB, proinflammatory cytokines (via receptor-interacting protein-1 and TRAF-6) and type I IFNs (via TANK-binding kinase (TBK)1 and transcription factors IRF3 and IRF7) [1, 15].
Alterations of the molecular mechanisms controlling TLR signaling result in allergic, autoimmune, and inflammatory diseases, systemic inflammatory response syndrome, sepsis, and septic shock [31, 32]. A subset of septic shock patients shows attenuated LPS-induced proinflammatory cytokine expression by macrophages, unchanged or increased production of anti-inflammatory cytokines, and increased incidence of secondary infections [33,34,35]. Such a phenotype resembles “reprogramming” of macrophage responses to TLR agonists observed in endotoxin tolerance, which is characterized by suppressed induction of proinflammatory cytokines and increased or unchanged expression of anti-inflammatory and antimicrobial mediators [32, 36, 37]. Therefore, endotoxin tolerance may have evolved as a mechanism curtailing excessive inflammatory responses and preventing septic shock. While LPS tolerance was initially reported to occur because of suppressed cell surface expression of TLR4/MD2 [38], other studies showed unaltered TLR4/MD2 expression, but suppressed TLR4 and MAL tyrosine phosphorylation [39, 40], MyD88 recruitment to TLR4 [41], IRAK-1-MyD88 interactions [42], and IRAK-1 activation [41,42,43] as molecular hallmarks of endotoxin tolerance. Alterations in subunit composition of NF-κB, p65 phosphorylation, and changes in chromatin remodeling have also been reported as downstream mechanisms responsible for macrophage reprogramming in LPS tolerance [37, 44, 45].
Little is known regarding how activation of proximal elements of TLR4 signaling involved in MyD88- and TRIF-dependent signaling is affected by induction of endotoxin tolerance and whether LPS tolerization is underlain by changes in expression levels of negative regulators of TLR signaling. In this study, we examined LPS-inducible TLR4-TRIF and TRIF-TBK1 signalosome assembly, activation of TBK1 and the transcription factor IRF3, and expression of MyD88- and TRIF-dependent cytokines in control and endotoxin-tolerant human monocytes. In addition, we analyzed whether endotoxin tolerization modulates mRNA and protein expression of negative regulators of TLR4 signaling pathways. Our results indicate severe down-regulation of LPS-inducible TLR4-TRIF and TRIF-TBK1 associations, TBK1 activation, and induction of IRF3 as proximal signaling hallmarks of endotoxin tolerance in human monocytes. Furthermore, we show up-regulated expression of Toll-interacting protein (Tollip), suppressor of cytokine signaling (SOCS)-1, SHIP-1; IRAK-M, suppressor of IκB-ε (SIKE) and sterile-α and Armadillo motif containing protein (SARM) as negative switches responsible for reprogramming of MyD88- and TRIF-dependent signaling cascades.
MATERIALS AND METHODS
Reagents and cell culture
Antibodies against IκB-α, IRF3, lamin B, β-actin, SOCS-1, donkey anti-goat IgG-Texas Red, and donkey anti-rabbit IgG-FITC were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-phospho (p)-p38 and anti-p38 were purchased from Promega (Madison, WI, USA), anti-p-IRF3, anti-TRIF, and anti-TBK1 Abs were obtained from Cell Signaling (Danvers, MA, USA), anti-HLA A, B, C Ab was obtained from BD Biosciences Pharmingen (San Diego, CA, USA) and anti-Tollip Ab was obtained from Alexis Biochemicals (San Diego, CA, USA). Ultrapure Escherichia coli 0111:B4 LPS repurified according to Hirschfeld et al. [46] and free of lipoproteins was obtained from Invivogen (San Diego, CA, USA), and Vectashield hardset mounting medium with DAPI was from Vector Laboratories (Burlingame, CA, USA). Human embryonic kidney (HEK) 293T cells were from American Type Culture Collection (Manassas, VA, USA), and maintained in DMEM supplemented with 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen, San Diego, CA, USA), and 10% FBS (HyClone, Logan, UT, USA) (complete DMEM). HEK293 cells stably expressing untagged TLR4 and Flag-MD-2 (293/TLR4/MD-2) were kindly provided by Dr. Douglas T. Golenbock (University of Massachusetts Medical School, Worchester, MA, USA). These cell lines were maintained in complete DMEM medium (Mediatech, Inc., Manassas, VA, USA) supplemented with 0.5 mg/ml neomycine (Sigma, St. Louis, MO, USA). Human monocytes were isolated from whole blood by counter flow centrifugal elutriation from PBMC that were obtained by leukapheresis of blood from healthy human volunteers at the Department of Transfusion Medicine, National Institutes of Health. Monocytes were cultured in RPMI 1640 medium (Mediatech, Inc.) supplemented with 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, (Invitrogen), 5 × 10−5 M β-mercaptoethanol (Sigma), and 5% FBS (HyClone). All studies were approved by the Institutional Review Board, University of Maryland, Baltimore.
Recombinant plasmids and transient transfection
pCDNA3-human (hu)TLR4, pCDNA3-huCD14, and pELAM-luciferase were obtained from Dr. Douglas T. Golenbock (University of Massachusetts Medical School, Worcester, MA). pEFBOS-His/Flag-huMD-2 was provided by Dr. Kensuke Miyake (Research Institute for Microbial Diseases, Osaka University, Osaka, Japan), pGL3-RANTES-luciferase reporter plasmid was from Dr. John Hiscott (McGill University, Montreal, Canada), pCDNA3-Flag-Tollip was published previously [47], and thymidine kinase-driven Renilla luciferase (pRL-TK) was from Promega. The luciferase reporter genes IFN-β (p125-luciferase) [40], upstream activation sequence (UAS)-luciferase, IRF3-Gal4, and DNA binding domain (DBD)-Gal4 [48] were published. pGEX-4T2 and pGEX-4T2-IRF3 were described elsewhere [48] and used for transformation of BL21 cells. Expression of GST and GST-IRF3 fusion proteins was induced after treatment of transformed bacteria for 4 h at 24°C with 0.5 mM isopropyl β-D-thio-galactoside (Sigma), and recombinant GST (a control substrate) and GST-IRF3 (aa 380-427, TBK1 substrate) were purified from bacterial lysates, according to standard protocols, using bulk GST purification and detection modules (GE HealthCare, Piscataway, NJ, USA). HEK293T or 293/TLR4/MD-2 cells were cultured overnight in 150 mm TC dishes (5×106 cells per dish) and cotransfected for 3 h with expression vectors as described in the figure legends (25 μg total plasmid DNA per dish) using Superfect transfection reagent (Qiagen, Valencia, CA, USA). After 48 h, cellular extracts were prepared as described [39, 40] and used for immunoprecipitation or subjected to immunoblotting.
Luciferase reporter assays
For NF-κB reporter assays, HEK293T cells were plated in 24-well plates (105 cells per well), grown overnight and cotransfected using Superfect (Qiagen) with pCDNA3-TLR4 (50 ng per well), pCDNA3-CD14 (20 ng per well), pEFBOS-MD2 (2 ng per well), pELAM-luciferase (200 ng per well each), and pRL-TK (50 ng per well), and total plasmid DNA amount was adjusted to 1 μg/well with pCDNA3. RANTES reporter assays were carried out essentially as described for NF-κB assays except pELAM-luciferase was replaced with pRANTES-luciferase [40]. For IRF3 reporter assays, 293/TLR4/MD-2 stable transfectants (105 cells per well in 24-well plates) were cotransfected with Gal4-IRF3 (200 ng per well), a luciferase reporter gene containing the Gal4 upstream activation sequence (UAS-luciferase) (200 ng per well), and pRL-TK (50 ng per well) using Superfect (Qiagen) (in control cultures, IRF3-Gal4 was replaced with DBD-Gal4). Following transfections, cells were recovered for 24 h, treated as indicated in the figure legends, lysed in a passive lysis buffer (Promega), and firefly luciferase vs. Renilla luciferase activities were measured with dual luciferase reporter assay system (Promega) on a Berthold LB9507 luminometer (Berthold Technologies, Bad Wildbad, Germany).
Isolation of RNA and real-time PCR
Total RNA was isolated with RNeasy kits (Qiagen), followed by DNase digestion and repurification as recommended by the manufacturer. cDNA was prepared from 1 μg RNA using Reverse Transcription System (Promega), and subjected to quantitative real-time PCR analysis with gene-specific primers for human hypoxanthine-guanine phosphoribosyltransferase (HPRT), IL-8, IL-6, TNF-α, IFN-β, RANTES, SOCS-1, Tollip, SHIP-1, SARM-1, and SIKE on a MyIQ real-time PCR system (Bio-Rad, Hercules, CA, USA). The following primers were used: HPRT, forward: 5′-ACCAGTCAACAGGGGACATAAAAG-3′, reverse: 5′-GTCTGCATTGTTTTGCCAGTGTC-3′; TNF-α, forward: 5′-CCCAGGCAGTCAGATCATCTTC-3′, reverse: 5′-GCTTGAGGGTTTGCTACAACATG-3′; IL-6, forward: 5′-GAAAGCAGCAAAGAGGCACTG-3′, reverse: 5′-GAAGCATCCATCTT TTTCAGCC-3′; IL-8, forward: 5′-CACCGGAAGGAACCATCTCACT-3′, reverse: 5′-TGCACCTTCACACAGAGCTGC-3′; RANTES, forward: 5′-TTTGTCACCCGAAAGAACCG-3′, reverse: 5′-CAAGGACTCTCCATCCTAGCTCAT-3′; IRAK-M, forward: 5′-GCGGGCAAAGTTAAGACCAT-3′, reverse: 5′-TCCTCAGGCCTTCATCAGAA-3′; IFN-β, forward: 5′-ACTGCCTCAAGGACAGGATG-3′, reverse: 5′-AGCCAGGAGGTTCTCAACAA-3; SOCS-1, forward: 5′-TAGCACACAACCAGGTGGCA-3′, reverse: 5′-GCTCTGCTGCTGTGGAGACTG-3′; Tollip, forward: 5′-GACCACCGTCAGCACTCAG-3, reverse: 5′-GGTCATGCCGTAATTCTTGG-3; SHIP-1, forward: 5′-GCGTGCTGTATCGGAATTGC-3′, reverse, 5′-CACAG GGTATTGCAGATGGG-3′; SIKE, forward: 5′-TGGATGCTGAACCAGTCC TG-3′, reverse: 5′-GATGGCTTGGGAAGCAGTGT-3′; SARM-1, forward: 5′-TGCTCGAC TCTAACCGCTTG-3′, reverse: 5′-AGGCGTTTCAGGCTCTGGAT-3′. Data were processed using 2−ΔΔCT method, as described previously [49]. Melting curve analysis and sequencing of purified PCR products were employed to ensure the specificity of PCR amplification.
Immunoprecipitation and immunoblotting
Cell extracts were prepared using a lysis buffer containing 20 mM HEPES (pH 7.4), 0.5% Triton X-100, 150 mM NaCl, 12.5 mM β-glycerophosphate, 50 mM NaF, 1 mM DTT, 1 mM sodium orthovanadate, 2 mM EDTA, 1 mM PMSF, and protease inhibitor cocktail (Roche). Nuclear extracts were prepared using Nuclear Extract kit (Active Motif, Carlsbad CA, USA), according to the manufacturer’s recommendations. For immunoprecipitation (IP), cell extracts were preincubated for 2 h at 4°C with 20 μl of protein G-agarose (50% slurry), beads were removed by centrifugation, and precleared lysates were incubated with the respective Ab (1-4 μg per sample) overnight at 4°C. Thereafter, immune complexes were captured by incubation for 4 h at 4°C with protein G agarose (50 μl per sample), beads were extensively washed with ice-cold lysis buffer and resuspended in Laemmli buffer (Bio-Rad). Immunoprecipitates and cell and nuclear extracts were subjected to immunoblot analysis by separating proteins on 4-20% polyacrylamide minigels (Invitrogen), transferring to Immobilon-P membranes (Millipore), and probing with corresponding Abs as described previously [39, 40].
In vitro kinase assay
In vitro kinase assays were performed as described previously [48, 50] using GST-IRF3 or GST as substrates. In brief, transfected or endogenous TBK1 proteins were immunoprecipitated with the α-TBK1Abs/protein G agarose, immune complexes were washed twice in a co-IP lysis buffer, three times in a kinase buffer containing 20 mM HEPES, pH 7.6, 50 mM NaCl, 10 mM MgCl2, 20 mM β-glycerophosphate, 1 mM sodium orthovanadate, 1 mM ATP, and then incubated for 25 min at 30°C in a kinase reaction with 1.0 μg of recombinant substrates (GST-IRF3 or GST), 1 mM ATP and 5 μCi [32P]ATP (Perkin Elmer) made up to a total volume of 20 μl with kinase buffer. Samples were resolved by SDS-PAGE, dried and subsequently exposed to an X-ray film.
Confocal microscopy
Monocytes (5×106 per ml in complete RPMI 1640) were incubated for 30 min with mouse α-MHC class I (A, B, C) Ab on ice, washed three times, stained with α-mouse IgG-Texas Red for 30 min on ice, and washed three times before fixation (to stain cell surface MHC class I molecules). Cells were subsequently fixed with 2% paraformaldehyde, permeablized with 100% ice-cold methanol for 10 min at −20°C and stained with rabbit α-IRF3 /α-rabbit IgG-FITC Abs (to detect intracellular IRF3); nuclei were costained with DAPI. Cells were seeded on glass slides by cytospin and mounted using Vectashield hardset mounting medium with DAPI. Confocal microscopy was performed using a LSM510 META Laser Scanning Microscope and a Plan-Apochromat 63×/1.4 oil objective, with the pinhole size for section thickness set at < 1 airy. Texas Red was excited with a 1.0 mW helium/neon laser emitting at 543 nm; for FITC, the 488 nm line of a 25 mW argon laser was used. Sequential scanning with separate laser excitation was used to avoid crosstalk between fluorophore signals, and images were processed with Zeiss LSM Image Browser Rel. 4.2 (Carl Zeiss).
Determination of cytokine production by ELISA
Levels of cytokines and chemokines in cell-free supernatants were determined by ELISA in the University of Maryland, Baltimore Cytokine Core Laboratory using commercially paired antibodies and recombinant standards obtained from R&D (TNF-α and RANTES), Biosource (IL-8; Camarillo, CA, USA), and Pierce Biotechnology (IL-6; Rockford, IL, USA). The lower detection limits for these assays were 3.9 pg/ml, 6.25 pg/ml, 3.9 pg/ml, and 31.25 pg/ml for TNF-α, IL-6, IL-8, and RANTES, respectively.
Statistical analysis
Statistical analysis was performed using the GraphPad Prism 5 program for Windows (Graphpad Software, San Diego, CA, USA). Statistical differences among experimental groups were evaluated by the Student’s t test with the level of significance set at P < 0.05. Values from summary of several experiments are expressed as mean ± sd, and results from one representative experiment are expressed as mean ± se.
RESULTS
Inhibited LPS-induced TLR4-TRIF and TRIF-TBK1 associations in endotoxin-tolerant human monocytes
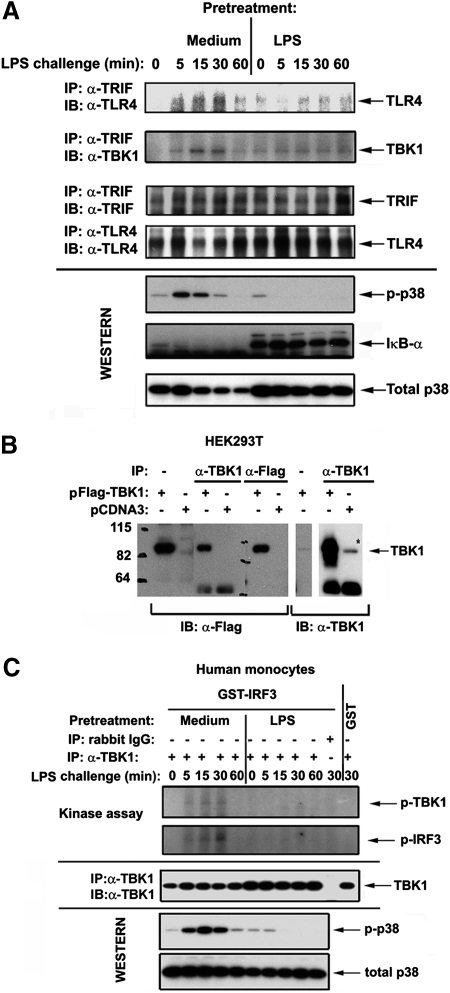
We sought to examine whether induction of endotoxin tolerance in human monocytes changes activation of TLR4-triggered TRIF-dependent pathway that employs the signaling axis consisting of the signaling adaptor TRIF, the kinase TBK1, and the transcription factor IRF3 [25, 26, 51]. Because recruitment of adaptor proteins to receptor complexes is an essential step for the activation of downstream signaling cascades [1, 15], we analyzed LPS-inducible recruitment of TRIF to TLR4. Coimmunoprecipitation demonstrated prominent LPS-initiated recruitment of TRIF to TLR4 in medium-pretreated monocytes within 5-15 min, followed by TRIF dissociation by 60 min (Fig. 1A, top panel, left half). In contrast, endotoxin-tolerant monocytes exhibited delayed recruitment of TRIF to TLR4 in response to LPS, and the amount of recruited TRIF was significantly lower compared with that detected in control cells (Fig. 1A, top panel, right half).
Figure 1.
Endotoxin tolerization dysregulates LPS-mediated TLR4-TRIF, TRIF-TBK1 associations and inhibits TBK-1 activation in human monocytes. After prior exposure to medium or 10 ng/ml LPS for 20 h, human monocytes were washed and treated with medium or challenged with 100 ng/ml LPS. (A) Whole cell lysates were prepared, immunoprecipitated with α-TLR4 (H80) or α-TRIF Abs, and immune complexes were analyzed by immunoblotting with the indicated antibodies. Shown are data of a representative (n=3) experiments. (B) HEK293T cells were transfected with either empty vector (pCDNA3) or pCDNA3-Flag-TBK1 and recovered for 24 h; whole lysates were prepared and immunoprecipitated with α-Flag or α-TBK1 Abs. Samples of whole cell lysates and Flag- or TBK1-containing immune complexes were fractionated by SDS-PAGE; proteins were transferred onto PVDF membrane and immunoblotted with α-Flag or α-TBK1 Abs. Numbers on the left indicate molecular mass standards (kDa). The results of a representative (n=2) experiments are shown. (C) Cells were lysed and whole cell lysates were immunoprecipitated with α-TBK1 Ab or isotype control IgG. Immunoprecipitates were subjected to in vitro kinase assay, using GST-IRF3 and GST recombinant proteins as substrates (top two panels) or to Western blot analyses with α-TBK1 Ab to control total expression of endogenous TBK1 proteins. (A and C): Expression levels of p-p38, IκB-α and total p38 were analyzed in whole cell lysates by immunoblotting with the respective Abs to control for LPS inducibility/endotoxin tolerization. Shown are data of a representative experiment (n=3).
Next, we analyzed LPS-mediated associations of TBK1 with TRIF. To ensure that endogenous TBK1 proteins are specifically immunoprecipitated with commercial Ab against TBK1, Flag-TBK1 was expressed in HEK293T cells to compare Flag-reactive bands with those detectable by Abs against endogenous TBK1. Overexpressed Flag-TBK1 was immunoprecipitated with α-Flag Ab, followed by immunoblot detection with α-Flag vs. α-TBK1 Abs. Reciprocally, overexpressed Flag-TBK1 and endogenous TBK1 proteins were immunoprecipitated with α-TBK1 and subjected to Western blot analyses with α-Flag or α-TBK1 Abs to confirm specificity of detection. The α-Flag-reactive band corresponding to TBK1 (∼84 kDa) was detected in lysates from cells expressing Flag-TBK1 but not from pCDNA3-transfected cells (Fig. 1B). Bands with the same electrophoretic mobility were immunoreactive with Ab against endogenous TBK1 in whole cell lysates and in α-TBK1 immune complexes obtained from HEK293T cells overexpressing Flag-TBK1 (transfected TBK1) or transfected with pCDNA3 only (endogenous TBK1) (Fig. 1B). Figure 1B also shows that α-Flag Ab detected TBK1 captured by immunoprecipitation of overexpressed Flag-TBK1 with α-TBK1 Ab. Having demonstrated specific detection of TBK1 proteins with commercial α-TBK1 Ab, we then examined how endotoxin tolerization affects TLR4-initiated TRIF-TBK1 interactions. LPS stimulation of control monocytes led to an association of TRIF with TBK1 that was evident as early as 5 min, reached the plateau level within 15–30 min, and then rapidly declined, while LPS failed to trigger TRIF-TBK1 association in endotoxin-tolerant cells throughout the time course of stimulation (Fig. 1A, 2nd panel from the top). Altered TLR4-TRIF and TRIF-TBK1 associations were not due to lower expression of interacting components in LPS-tolerant cells, as comparable levels of total TLR4 and TRIF were detected in control and endotoxin-tolerant monocytes (Fig. 1A, 3rd and 4th panels from the top), and total TBK1 and p38 levels were even higher in LPS-tolerant cells (Fig. 1A, C and data not shown). These data show suppressed LPS-mediated TLR4-TRIF-TBK1 interactions in LPS-tolerant human monocytes.
Endotoxin tolerance inhibits LPS-inducible TBK-1 kinase activity and IRF3 activation
Because signalosome assembly within the TLR4 receptor complex is important for kinase activation [1, 15], we studied LPS-mediated induction of TBK1 kinase activity in control and endotoxin-tolerant human monocytes. In vitro kinase assay of immunoprecipitated endogenous TBK1 proteins revealed marked LPS-inducible TBK1 autophosphorylation in medium-pretreated human monocytes, whereas no response was observed in endotoxin-tolerant cells (Fig. 1C, top panel). Consistent with importance of TBK1 phosphorylation for the induction of its kinase activity [52], LPS stimulation of control monocytes induced TBK1-mediated phosphorylation of IRF3, a specific substrate for TBK1 [48], as early as 5 min that reached maximal response by 30 min (Fig. 1C, 2nd panel from the top). No phosphorylation of a control GST protein was detected, and immunoprecipitation of cell lysates of LPS-stimulated monocytes with an isotype control IgG did not show any kinase activity and did not pull down proteins reactive with α-TBK1 Ab, showing the specificity of our in vitro kinase assay for TBK1 (Fig. 1C, band 11). In contrast, prior exposure of monocytes to endotoxin completely abolished their ability to respond to LPS stimulation by the induction of TBK1 kinase activity, as judged by phosphorylation of IRF3 (Fig. 1C, 2nd panel from the top), in line with their blunted LPS-induced p38 phosphorylation (Fig. 1C, bottom panels).
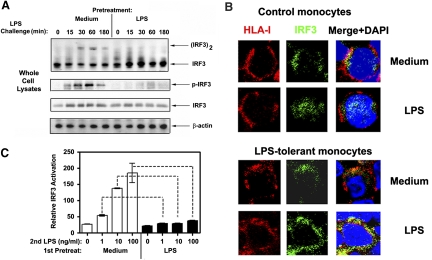
IRF3 is phosphorylated by TBK1, dimerizes and translocates to the nucleus to activate expression of TRIF-dependent genes [1, 48, 53]. Therefore, we used these parameters to examine the effect of endotoxin tolerization on LPS-inducible activation of IRF3. LPS stimulation of medium-pretreated monocytes led to dimerization of IRF3, as evidenced by the appearance of the slower migrating IRF3 dimer species on a native gel, and its phosphorylation, with both responses reaching plateau levels within 30-60 min after LPS challenge (Fig. 2A, top two panels). Immunoblot and confocal analyses also revealed IRF3 accumulation in nuclear extracts following LPS exposure of control human monocytes for 60-180 min (Fig. 2B, top two panels and data not shown). In contrast, endotoxin-tolerant monocytes showed severely impaired activation of IRF3 upon restimulation with LPS, as evidenced by the lack of IRF3 dimerization, phosphorylation, and nuclear translocation (Fig. 2A and B). Comparable levels of total IRF3 and β-actin proteins were observed in whole cell extracts (Fig. 2A), indicating that the differential activation of IRF3 observed in control and LPS-tolerant monocytes cannot be attributed to different protein loading.
Figure 2.
Endotoxin tolerance inhibits LPS-mediated activation of IRF3. Human monocytes were pretreated for 20 h with medium or 10 ng/ml LPS, washed and challenged with 100 ng/ml LPS for the indicated time points (A) or for 60 min (B). (A) Whole cell lysates were fractionated by native gel electrophoresis (top panel) or SDS-PAGE (other panels), transferred onto PVDF membranes and immunoblotted with the indicated Abs. β-actin immunoblots were used to control for protein loading. Data of a representative experiment (n=4) are presented. (B) Confocal analysis of IRF3 nuclear translocation. Monocytes were stained with mouse α-HLA/α-mouse IgG-Texas Red Abs before fixation (to stain cell surface HLA molecules), cells were fixed with 2% paraformaldehyde, permeablized with 100% ice-cold methanol, and stained with rabbit α-IRF3 /α-rabbit IgG-FITC Abs (to detect intracellular IRF3); nuclei were costained with DAPI. Cells were seeded on glass slides and analyzed by confocal microscopy, using a LSM510 META laser scanning microscope. Shown are results of a representative (n=3) experiment. (C) 293/TLR4/MD-2 cells were pretreated for 20 h with medium or 10 ng/ml LPS, washed, and transfected with expression plasmids encoding Gal4-IRF3 (DBD-IRF3 in control cultures), UAS-luciferase and Renilla luciferase. Following LPS stimulation for 5 h, firefly and Renilla luciferase activities were measured in cell lysates. Data were calculated as firefly luciferase/Renilla luciferase activities in Gal4-IRF3-expressing cells divided by those values obtained in Gal4-DBD-expressing cultures. Results from a representative experiment (n=3) are depicted.
To extend these results, we next analyzed the transactivation potential of IRF3 based on IRF3-driven activation of a reporter luciferase gene in response to LPS stimulation, as reported previously [48, 54]. We employed an assay for IRF-3 activation, which utilizes a hybrid protein consisting of the yeast Gal4 DNA-binding domain (DBD) fused to IRF-3 lacking its own DBD. Reporter gene expression from the Gal4 upstream activation sequence in this assay requires IRF-3 activation [48, 54]. 293/TLR4/MD-2 cells were pretreated with medium or tolerized with LPS, washed, transfected with expression vectors encoding Gal4-IRF3 and UAS-luciferase, and restimulated with LPS. Figure 2C shows that LPS activated IRF3-dependent expression of luciferase reporter in a dose-dependent manner in medium-pretreated 293/TLR4/MD-2 cells, reaching ∼15-fold stimulation, while this response was markedly inhibited in cells rendered endotoxin tolerant (maximal response was in the magnitude of threefold induction). Overall, our results demonstrate that endotoxin tolerance induction results in defective induction of TBK1 kinase activity and impaired activation of IRF3.
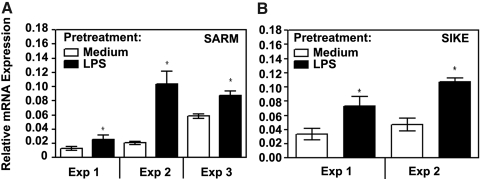
Endotoxin-tolerant monocytes show increased expression of SARM, SIKE, and decreased activation of TRIF-dependent cytokines
TLR signaling is negatively regulated at multiple levels by intermediates affecting TLR signaling cascades [4, 55]. SARM and SIKE were identified as negative regulators of TRIF and TBK1 [56, 57] that influence preferentially the TRIF-dependent signaling cascades. Therefore, it was of interest to examine whether induction of endotoxin tolerance is associated with changes in expression levels of SARM and SIKE. Real-time PCR analyses revealed that prior exposure to LPS led to marked up-regulation of SARM and SIKE mRNA expression compared with their levels observed in control, medium-pretreated monocytes (Fig. 3A, B). Stimulation of control, medium-pretreated monocytes with LPS for 3 and 6 h resulted in ∼2.5-3-fold increases of SARM mRNA levels, which was significantly lower compared with elevated SARM expression exhibited by endotoxin-tolerized monocytes treated with medium or stimulated with LPS at all time points studied (Supplemental Fig. 1A). In contrast to SARM, LPS stimulation within 6 h did not affect SIKE mRNA levels in control, medium-petreated monocytes. Endotoxin tolerized cells showed ∼5-times higher basal expression of SIKE mRNA than control monocytes, which was slightly (∼1.6-fold) increased in response to LPS challenge (Supplemental Fig. 1B). These data indicate that endotoxin tolerization leads to increased expression of SARM and SIKE mRNA.
Figure 3.
Increased expression of SARM and SIKE mRNA in endotoxin-tolerant human monocytes. Human monocytes were pretreated for 20 h with medium or 10 ng/ml LPS, as shown. RNA was isolated, reverse-transcribed, and analyzed by real-time PCR to determine expression levels of SARM (A) and SIKE (B) mRNA. Results of three (SARM) and two (SIKE) independent experiments (mean ± se) are depicted.
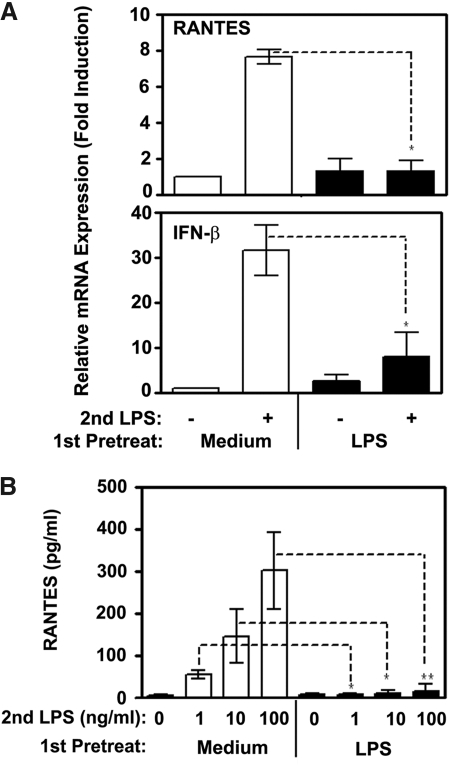
To link dysregulated signalosome assembly and TBK1/IRF3 activation within the TLR4-TRIF-TBK1-IRF3 signaling axis with functional endpoints, we analyzed LPS-inducible gene expression and secretion of the TRIF-dependent cytokines, RANTES, and IFN-β [37, 54]. Whereas LPS mediated 7- and 31-fold increases in expression of RANTES and IFN-β mRNA in medium-pretreated cells, endotoxin-tolerant cells restimulated with LPS exhibited only 1.2- and 7.1-fold increases in gene expression of these cytokines (Fig. 4A). Furthermore, deficient LPS-induced RANTES secretion was seen in endotoxin-tolerized monocytes, in contrast to marked LPS responses of control cells (Fig. 4B). These data indicate that LPS-inducible expression of TRIF-dependent cytokines is significantly inhibited in endotoxin-tolerant human monocytes.
Figure 4.
Inhibited LPS-inducible expression of RANTES and IFN-β in endotoxin-tolerant human monocytes. After prior exposure to medium or 10 ng/ml LPS for 20 h, human monocytes were washed and treated with medium or challenged with 100 ng/ml LPS for 1h (A) or with the indicated concentrations of LPS for 24 h (B). (A): Following stimulation, RNA was isolated, reverse-transcribed and analyzed by real-time PCR with gene-specific primers for RANTES, IFN-β and HPRT. Results of a representative experiment (n=5) are depicted. (B) RANTES secretion levels were assayed in cell-free culture supernatants by ELISA. Shown are data (mean ± sd) of 5 independent experiments. *, P < 0.05; **, P < 0.01.
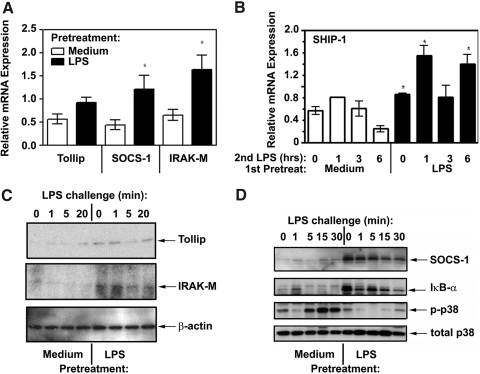
LPS tolerization up-regulates Tollip, SOCS-1, IRAK-M, and SHIP-1 expression and suppresses TLR4-inducible IκB-α degradation, p38 phosphorylation, and expression of proinflammatory cytokines and chemokines
Previous studies by us and others have demonstrated inhibited recruitment of MyD88 and suppressed IRAK1 activation as molecular hallmarks of endotoxin tolerance [39,40,41,42,43]. Because Tollip, SOCS-1, IRAK-M, and SHIP-1 are negative regulators of TLR signaling implicated in interference with recruitment of MyD88 and IRAK kinases to TLR4 and IRAK-1 activation [47, 58,59,60,61], we examined whether endotoxin tolerization affects expression levels of these molecules. Real-time PCR of endotoxin-tolerized monocytes cultured with medium showed increased levels of Tollip, SOCS-1, IRAK-M, and SHIP-1 mRNA compared with those observed control, medium-pretreated cells not exposed to LPS (Fig. 5A, B). LPS restimulation of either control or endotoxin-tolerized monocytes within 6 h did not appreciably modulate expression levels of Tollip and IRAK-M mRNA compared with their basal expression, but endotoxin-tolerized cells preserved patterns of significantly higher Tollip and IRAK-M mRNA levels at all time points (Supplemental Fig. 2, top and middle panels). In contrast to Tollip and IRAK-M, SOCS-1 mRNA expression was up-regulated by LPS within 3-6 h postchallenge in control monocytes and remained elevated in endotoxin-tolerant cells throughout the challenge procedures (Supplemental Fig. 2, bottom panel). Because patterns of mRNA expression do not always correspond to protein expression levels, we next carried out immunoblot analyses of control vs. endotoxin-tolerant monocytes that demonstrated markedly up-regulated expression of Tollip, SOCS-1, and IRAK-M proteins (Fig. 5C, D) in tolerized cells. These negative regulators preferentially affect the MyD88-dependent pathway, targeting IRAK-1 activation (SHIP-1 and Tollip) [58], dissociation of IRAK-4/IRAK-1 from MyD88 (IRAK-M) [60], and MAL ubiquitination and proteolytic degradation (SOCS-1) [59]. In line with up-regulated expression of negative regulators of MyD88-dependent signaling, endotoxin-tolerant cells showed impaired LPS-mediated degradation of IκB-α and phosphorylation of p38 (Fig. 5D). Different activation of signaling intermediates was not due to variations in protein loading, as evidenced by equal expression levels of total p38 and β-actin seen in the samples analyzed (Fig. 5C, D, bottom).
Figure 5.
Effect of endotoxin tolerance on expression of Tollip, SOCS-1, IRAK-M, and SHIP-1. Human monocytes were pretreated for 20 h with medium or 10 ng/ml LPS, washed and treated with medium, or restimulated with 100 ng/ml LPS as shown. RNA was isolated, reverse-transcribed, and analyzed by real-time PCR to determine expression levels of Tollip, SOCS-1, IRAK-M (A), and SHIP-1 (B). The results of five independent experiments (mean ± sd) are shown. (C) and (D) Whole cell lysates were examined by Western blot analyses to determine protein expression of Tollip, IRAK-M (C) and SOCS-1 (D), using the corresponding Abs. Total p38 and β-actin immunoblots were run to control for protein loading, and IκB-α and p-p38 proteins were immunoblotted to monitor for LPS inducibility/tolerization. Results of a representative experiment (n=3) are shown.
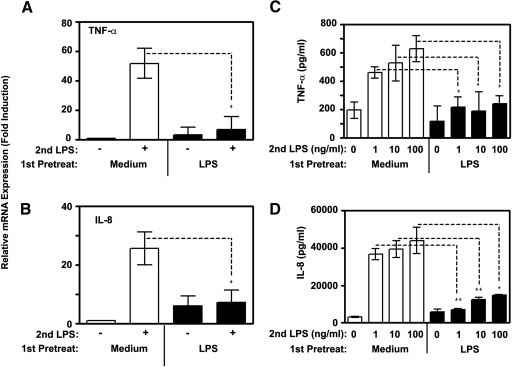
As NF-κB and MAPKs are principal for activation of cytokines and chemokines [62, 63], we next examined LPS-induced gene expression and secretion of proinflammatory cytokines TNF-α, IL-6, and chemokine IL-8 in control and endotoxin-tolerized monocytes. LPS challenge of medium-pretreated monocytes increased expression of TNF-α and IL-8 mRNA by 51.8- and 27-fold, respectively, while exerting only 3.4- and 2.1-fold increases in endotoxin-tolerant cells (Fig. 6A, B). Likewise, endotoxin tolerance induction strongly impaired LPS-inducible secretion of proinflammatory cytokines TNF-α, IL-6, and chemokine IL-8, whereas robust secretion of these cytokines was observed in LPS-stimulated control monocytes (Fig. 6C, D, Supplemental Fig. 3). Taken together, these results indicate increased expression levels of Tollip, SOCS-1, IRAK-M, and SHIP-1 in endotoxin-tolerant human monocytes that correlate with impaired LPS-inducible degradation of IκB-α, phosphorylation of p38 and inhibited expression of proinflammatory cytokines and chemokines.
Figure 6.
Deficient expression of proinflammatory cytokines and chemokines in endotoxin-tolerant human monocytes. Human monocytes were pretreated for 20 h with medium or 10 ng/ml LPS, washed three times, and treated with medium or restimulated with 100 ng/ml LPS for 3h (A and B), or for the indicated time points (C and D). (A and B) Total RNA was isolated, reverse-transcribed and subjected to real-time PCR analyses for the indicated cytokine genes. Data were processed according to 2−ΔΔCT method [49] and expressed as fold induction compared with values detected in medium-treated control monocytes taken as 1. (C, D) Cell-free supernatants were collected and secretion levels of the indicated cytokines and chemokines were analyzed by ELISA. Data (mean ± sd) of 5 experiments are shown. *, P < 0.05; **, P < 0.01.
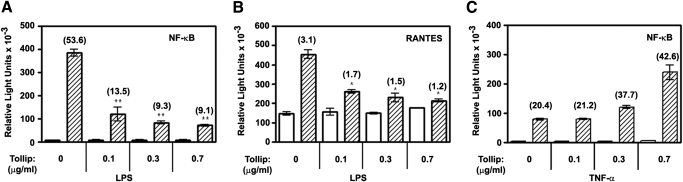
Overexpression of Tollip inhibits LPS-mediated NF-κB and RANTES reporter activation but does not affect TNF-α-induced NF-κB reporter activity
We next wanted to elucidate functional consequences of increased Tollip expression in endotoxin-tolerant cells. To mimic this process, we studied the effect of Tollip overexpression in 293/TLR4/CD14/MD-2 cells on LPS-mediated activation of NF-κB- (pELAM-luciferase) and RANTES-dependent luciferase reporters. LPS stimulation of 293/TLR4/CD14/MD-2 cells transfected with a control empty vector (pCDNA3) led to 53.6- and 3.1-fold activation of pELAM-luciferase and pGL3-RANTES-luciferase reporters, respectively (Fig. 7A, B). Overexpression of Tollip caused a strong suppressive effect on LPS-inducible NF-κB reporter activation in a dose-dependent manner, resulting in 74–83% inhibition at Tollip input doses of 0.1–0.3 μg (Fig. 7A). Introduction of Tollip also led to a less pronounced, yet marked dose-dependent inhibition of RANTES reporter activation in response to LPS, giving rise to 45–62% inhibition (Fig. 7B). Stimulation of 293/TLR4/CD14/MD-2 cells with TNF-α produced 20.4-fold activation of NF-κB reporter, and cotransfection of Tollip at 0.1 μg and 0.3 μg input levels did not affect this response, whereas the highest dose of Tollip (0.7 μg) even increased TNF-α-mediated NF-κB reporter activation (Fig. 7C). These results demonstrate specific inhibition of TLR4-inducible NF-κB and RANTES reporter activation by Tollip, whereas TLR-unrelated, TNF-α-mediated NF-κB response was not suppressed.
Figure 7.
The effect of Tollip overexpression on LPS-mediated activation of NF-κB- and RANTES-driven luciferase reporters. HEK293T cells were plated in 24-well tissue culture plates (105 cells per well), grown overnight, and transiently transfected with pCDNA3-TLR4 (50 ng per well), pCDNA3-CD14 (20 ng per well), pEFBOS-MD2 (2 ng per well), pRL-TK (50 ng per well), pELAM-luciferase or pGL3-RANTES-luciferase (200 ng per well each), and the indicated doses of pCDNA3-Flag-Tollip. Total level of plasmid DNA was adjusted to 1 μg per well with empty (pCDNA3) vector. After recovery, cells were stimulated for 5 h with medium (open bars), 100 ng/ml LPS (hatched bars, A and B) or 20 ng/ml TNF-α (hatched bars, C). Cell lysates were assayed for firefly vs. Renilla luciferase activities. Shown are data of a representative (n=3) experiment.
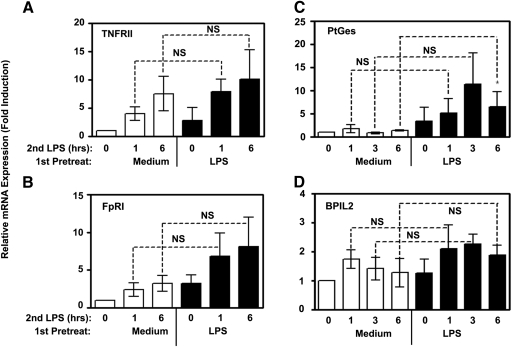
LPS tolerization does not inhibit expression of TNFRII, formyl peptide receptor I, PGE synthase and bactericidal/permeability-increasing protein-like 2 (BPIL2)
Previously published data obtained in mouse macrophages indicate that endotoxin tolerance does not globally inhibit macrophage functional activities, as expression of several anti-inflammatory cytokines and antimicrobial effectors is not affected [37]. In our next series of experiments, we wanted to extend these results to human monocytes and demonstrate that endotoxin tolerization cannot be considered as profound suppression of all TLR4-inducible responses. To this end, the same samples of RNA obtained from control and endotoxin-tolerant monocytes that showed inhibition of LPS-mediated cytokine and chemokine gene expression (Fig. 6 and S3) were examined for expression of several genes reported not to be tolerized in mouse macrophages (nontolerizable genes) [37]. We observed similar ability of LPS to up-regulate TNFRII, formyl peptide receptor (FpR)I mRNA (Fig. 8A, B), as well as PGE synthase (PtGes) and bactericidal/permeability-increasing protein-like 2 (BPIL2) genes (Fig. 8C, D) in control and endotoxin-tolerant human monocytes, indicating that endotoxin tolerance does not affect LPS-mediated expression of TNFRII, FpRI, PtGes, and BPIL2.
Figure 8.
Comparable LPS inducibility of TNFRII, FpRI, BPIL2, and PtGes mRNA in control and endotoxin-tolerant human monocytes. Cells were incubated with medium or 10 ng/ml LPS for 20 h, washed and treated with medium or restimulated with 100 ng/ml LPS for the indicated time periods. RNA was isolated and reverse transcribed; and expression levels of HPRT, TNFRII, FpRI, BPIL2, and PtGes mRNA were examined by real-time PCR. Data were processed according to 2−ΔΔCT method [49] and expressed as fold induction compared with values detected in medium-treated control monocytes taken as 1. Results of three (TNFRII, PtGes, and BPIL2) and six (FpRI) independent experiments (mean ± sd) are shown.
DISCUSSION
Endotoxin tolerance reprograms cellular responses to LPS, suppressing induction of proinflammatory cytokines, while not affecting or even enhancing anti-inflammatory cytokines and antimicrobial effectors [32, 35, 37]. This phenomenon has been suggested to play an important role for the prevention of excessive “cytokine storm” and tissue damage but at the same time, to predispose to secondary infections often occurring in septic shock survivors [32, 35, 37]. Previous reports from our group and other investigators have revealed several changes in endotoxin-tolerant cells at the level of signalosome assembly of MyD88-dependent intermediates. These include deficient LPS-mediated tyrosine phosphorylation of TLR4 and the “bridging” adaptor protein MAL [39, 40], disrupted recruitment of MyD88 to TLR4 [41], altered IRAK1-MyD88 association [42], and inhibited IRAK1 activation [39, 41,42,43]. This study uncovers several important features of MyD88- and TRIF-dependent signaling affected by endotoxin tolerization and shows increased expression of several negative regulators of TLR4 signaling that interfere with signalosome formation and activation of IRAKs and TBK1.
We show several molecular hallmarks of endotoxin tolerance at the level of the TRIF signaling pathway that have not been reported previously. To the best of our knowledge, our manuscript represents the first demonstration of LPS-inducible interactions of endogenous TLR4, TRIF, and TBK1 in human monocytes and the first observation that endotoxin tolerization leads to inhibited LPS-mediated association of the “signaling” adaptor TRIF with TLR4, and impaired TRIF-TBK1 activation. Consistent with the important role of the TRIF-TBK1 axis, inhibited TLR4-TRIF-TBK1 assembly translates into impaired LPS-mediated activation of TBK1 and IRF3 in human monocytes, underlying reduced expression of TRIF-dependent cytokines. These results, combined with our previous finding of inhibited LPS-mediated MyD88 recruitment to TLR4 in endotoxin-tolerant monocytes [41], strongly suggest that dysregulation of recruitment of adaptor proteins to TLR4 represents a central paradigm of endotoxin tolerance. Second, consistent with an essential role of TLR signalosome assembly in mediating kinase activation [1, 15], we observed impaired LPS-inducible phosphorylation and activation of kinase activity of TBK1 in endotoxin-tolerant human monocytes. Third, in line with importance of TBK1 in IRF3 activation [48], suppressed LPS-induced activation of TBK1 in endotoxin-tolerant monocytes translated into impaired IRF3 dimerization, phosphorylation, and nuclear translocation, in contrast to robust responses observed in control cells. These results extend data on deficient IRF3 activation in response to LPS challenge earlier reported in endotoxin-tolerant mouse macrophages [43]. Furthermore, experiments in 293/TLR4/MD-2 cells demonstrated inhibited transactivation potential of IRF3 in tolerized cells upon LPS challenge. Fourth, this paper shows elevated levels of negative regulators of TLR4 signaling affecting the TRIF pathway, SARM and SIKE, in endotoxin-tolerized human monocytes upon second treatment with medium or challenge with LPS, compared with those observed in control cells subjected to the same restimulation procedures. SARM was identified as the fifth adaptor protein specifically inhibiting TRIF-mediated signaling cascade [56], and SIKE was found to associate with TBK1 and inhibit TBK1 and IRF3 activation [57]. Hence, an increase in expression levels of SARM may lead to competition with TRIF for association with TLR4, resulting in inhibited TLR4-TRIF association noted in endotoxin-tolerant monocytes, and up-regulated SIKE may contribute to overall inhibition by suppressing TBK1 activation, blunting TRIF-mediated signal transduction. Because the TRIF-dependent TLR4 signaling cascade elicits production of proinflammatory cytokines, type I IFN and IFN-inducible genes [25, 64], sustained increased expression of SARM and SIKE noted in endotoxin-tolerant cells may represent an anti-inflammatory mechanism to blunt deleterious excessive inflammation in sepsis and shock.
Controversial results have been reported with respect to whether endotoxin tolerance affects expression of TRIF-dependent cytokines. Dobrovolskaia et al. [66] reported that endotoxin tolerance in mouse macrophages is manifested by inhibited expression of LPS-mediated, TRIF-dependent IFN-β mRNA expression. In line with these data, Sato et al. [43] showed decreased LPS-mediated IRF3 activation and deficient expression of IFN-inducible genes, such as IP-10, GARG16, and Best5 in mouse macrophages upon induction of an endotoxin-tolerant state. In contrast, other groups found normal RANTES and IFN-β expression levels and functional TRIF-dependent STAT-1 signaling in LPS-stimulated mouse macrophages tolerized by prior exposure to endotoxin [37, 65]. The reasons for these conflicting observations are unknown but could be related to species-specific differences (mouse vs. human cells), the cell type (bone marrow-derived vs. peritoneal macrophages) or macrophage activation state (unstimulated or thyoglycollate-elicited). Our results in human monocytes support the hypothesis that LPS tolerance dysregulates LPS-elicited, TRIF-dependent signaling pathways suggested previously by data obtained in mouse cells [43, 66].
Consistent with published data [34, 36,37,38, 41, 43], we show that endotoxin-tolerant human monocytes exhibited decreased LPS-inducible expression of proinflammatory cytokines (TNF-α and IL-6) and chemokines (e.g., IL-8) whose expression is controlled via both MyD88- and TRIF-dependent signaling cascades, as evidenced by studies in mice deficient for these adaptor proteins [17, 26]. Experiments in SOCS-1−/−, IRAK-M−/−, and SHIP−/− mice showed significantly elevated production of proinflammatory cytokines in response to LPS and a partial loss of the capacity of mouse macrophages to become endotoxin-tolerant [60, 61, 67], revealing their functions as negative regulators of TLR signaling involved in tolerance induction. It is important to verify data obtained in the mouse system for human cells, given known species-specific differences and contrasting reports published in the literature. One example of these differences is how MyD88 deficiency affects innate immune responses: while MyD88−/− mice show impaired responses to a variety of pathogens, MyD88-deficient human patients are only susceptible to certain pyogenic bacteria, while preserving responses to other pathogens [rev. in 4]. Thus, it is essential to confirm data obtained in the mouse system for human cells, especially primary human monocytes. Endotoxin tolerization of human monocytes was accompanied by increased expression of SOCS-1, IRAK-M, and SHIP-1, extending data obtained in LPS-tolerant mouse macrophages [60, 61, 67]. Notably, IRAK-M was shown to suppress dissociation of IRAK-4 and IRAK-1 from MyD88 [60], providing a mechanistic insight into molecular mechanisms of IRAK-M suppressive effects. SOCS-1 is an E3-ubiquitin ligase involved in ubiquitination and degradation of Mal [59], and its increased expression in endotoxin-tolerant cells may modify MAL and interfere with Bruton tyrosine kinase-mediated MAL tyrosine phosphorylation [59] or caspase-1-mediated cleavage [68]. Besides its capacity to affect the MyD88-dependent pathway through MAL targeting, SOCS-1 regulates paracrine IFN-β signaling by affecting the JAK-STAT pathway, and fine-tune the sensitivity toward IFN-γ and GM-CSF, thereby modulating antimicrobial activity of macrophages and differentiation of DCs [69].
We also show that endotoxin-tolerant human monocytes exhibited increased expression of Tollip mRNA and proteins, confirming and extending earlier reports on increased Tollip expression found in endotoxin-tolerant THP-1 and epithelial intestinal cells [47, 70]. In unstimulated cells, Tollip associates with IRAK1 and, upon stimulation, is phosphorylated by IRAK1 and dissociates from the receptor complex [58, 71]. We confirm earlier findings on the ability of Tollip to inhibit LPS-mediated NF-κB activation upon overexpression, and show for the first time that it also mediates inhibition of LPS-inducible activation of RANTES-dependent luciferase reporter. RANTES expression is controlled by the transcription factors NF-κB and IRF3 in a cooperative manner, and disruption of either pathway dramatically abolishes the ability of the other to bind and activate RANTES expression [72]. Hence, suppressed expression of RANTES activation by Tollip could be due to its ability to inhibit NF-κB activation. Notably, because Tollip sequesters IRAK1 [58] and IRAK1 has been implicated in TLR-mediated IFN-β expression [27], inhibited expression of RANTES may be due to sequestration of IRAK1 by Tollip, resulting in inhibited IRAK1 activation. In addition, Tollip interacts with phosphatidylinositol-3-phosphate and phosphatidylinositol-3,4,5-phosphate, suggesting the involvement of phosphotidyl inositol-3 kinase (PI3K), and inhibition of PI3K activity by wortmannin alleviates Tollip-mediated inhibition [47]. Interestingly, activation of PI3K-Akt-GSK3-β pathway mediates hepatocyte growth factor inhibition of RANTES expression in renal tubular epithelial cells [73], further supporting a role for PI3K in Tollip-mediated suppression of RANTES expression.
Overall, our results on sustained increased expression of IRAK-M, SHIP-1, SOCS-1 and Tollip in endotoxin-tolerant human monocytes support functional roles for these molecules in the induction and maintenance of the LPS-tolerant phenotype by blunting activation of the TLR4-MyD88-IRAK4/1 signaling cascade that elicits production of proinflammatory cytokines. Our real-time PCR analyses demonstrated Tollip, IRAK-M, and SHIP-1 as late LPS-inducible genes whose mRNA expression was not noticeably modulated by LPS within 1-6 h poststimulation but was increased and sustained in monocytes rendered endotoxin tolerant by pretreatment with LPS for 20 h. Since experiments in gene-deficient mice showed these molecules act as negative regulators of proinflammatory TLR signaling, it is plausible they also perform similar functions in human monocytes. It is tempting to speculate that late induction of IRAK-M and Tollip proteins in endotoxin-tolerant monocytes serves an important biological function to limit excessive inflammation in inflammatory conditions such as endotoxin shock and sepsis by inhibiting expression of proinflammatory mediators and shifting the balance toward anti-inflammatory milieu. Gene silencing experiments are under way to directly address this hypothesis.
Interestingly, endotoxin tolerance does not globally suppress cell functions, as unchanged or even enhanced expression of anti-inflammatory mediators and antimicrobial effectors has been reported in LPS-tolerant mouse macrophages [32, 35, 37]. We extend these results to human cells and demonstrate that endotoxin-tolerant human monocytes show a tendency to increase expression of mRNA encoding TNFRII and FpRI and preserved expression of BPIL-2 and PtGes genes. While studies in gene-deficient mice demonstrated that expression of proinflammatory cytokines, such as TNF-α and IL-6 is dependent on MyD88 and TRIF [17, 26], as well as IRAK4 [74], molecular mechanisms controlling expression of nontolerizable genes TNFRII, FPR1, PtGes, and BPIL-2 are poorly characterized. LPS inducibility of a number of NF-κB-dependent genes, such as TNF-α, SOCS1, and PtGes, are differentially regulated in endotoxin-tolerant monocytes, showing either inhibition (TNF-α) or up-regulation (SOCS-1 and PtGes). Tolerization mediates reprogramming of cytokine expression at several levels, including chromatin remodeling by histone trimethylation and acetylation, transcriptional control by a complex interplay of various transcription factors, including NF-κB, AP-1, ATF-2 and others, post-transcriptional regulation (e.g., mRNA stabilization), and at the post-translational level. At the level of chromatin remodeling, Foster et al. [37] showed a differential control of LPS tolerizable and nontolerizable genes by chromatin modifications, such as LPS-induced histone acetylation and trimethylation. Furthermore, these studies uncovered differential recruitment of ATP-dependent chromatin remodeling complexes, Brg1 and Mi-2, to promoters of tolerizable and nontolerizable genes in cells challenged with LPS [37]. It is plausible that endotoxin-tolerant human monocytes exhibit preserved chromatin remodeling and recruitment of Brg1 and Mi2 at the promoter of nontolerizable (e.g., SOCS-1, PtGes) vs. tolerizable (e.g., TNF-α) genes. In addition, despite inhibited LPS-induced p65 nuclear translocation, studies by us and others revealed increased nuclear expression of p50 NF-κB subunit in endotoxin-tolerant cells [36, 44, 66]. Despite transcriptional deficiency due to the lack of the transactivation domain (TAD), p50 can associate with Bcl-3 and other TAD-containing transcriptional factors, and these complexes can bind to promoters of certain genes in a sequence-specific manner, activating their transcription [75]. Hence, differences in chromatin remodeling at promoters of nontolerizable genes (e.g., SOCS-1, PtGes, permissible) vs. tolerizable genes (e.g., TNF-α, nonpermissible) would allow for binding of p50 associated with transacting-competent transcription factors to the promoter of SOCS-1 and PtGes, but not TNF-α, leading to enhanced expression of nontolerizable genes. Interestingly, proximal TLR4 signaling events mediating differential chromatin remodeling/modification processes at nontolerizable vs. tolerizable genes are unknown. Because of the existence of signaling pathways operating in the absence of both IRAK4 and IRAK1 that mediate a unique pattern of NF-κB activation without activation of IKKs and phosphorylation of IκB-α and induce expression of certain proinflammatory mediators (e.g., TNF-α) [64], we propose a nonredundant, differential control of nontolerizable genes via yet unknown modules of adapters and kinases.
In summary, we demonstrate several novel features of endotoxin tolerance in human monocytes, including suppressed LPS-inducible TLR4-TRIF-TBK1 interactions, activation of TBK1 and IRF3, and up-regulated SARM and SIKE levels. These data indicate impaired TLR4-TRIF-TBK1 signalosome assembly/TBK1 activation and increased expression of SARM and SIKE as principal molecular events leading to reprogramming of the TRIF pathway in endotoxin-tolerant monocytes. Our data, revealing that induction of endotoxin tolerance in human monocytes inhibits LPS-mediated association of the “signaling” adaptor TRIF with TLR4 and impaired TRIF-TBK1 activation, combined with previous findings of inhibited MyD88 recruitment to TLR4 and IRAK-1-MyD88 association [41, 42], supports the paradigm that dysregulated recruitment of adaptor proteins to TLR4 and altered adaptor-kinase assembly underlies endotoxin tolerization. We extend reports on increased expression of Tollip, IRAK-M, SHIP-1, and SOCS-1 earlier observed in LPS-tolerant mouse macrophages or human monocytic cell lines to endotoxin-tolerized primary human monocytes that show elevated levels of these negative regulators. Experiments are in progress to uncover molecular mechanisms by which negative regulators of TLR signaling interfere with LPS induction of the MyD88- and TRIF-dependent signaling cascades in endotoxin-tolerant cells.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health RO1 grants AI-059524 (A. E. M.), AI067497 (K. A. F.), and AI64414 (L. L.).
Supplementary Material
Footnotes
Abbreviations: BPIL2=bactericidal/permability-increasing protein, HEK= human embryonic kidney, IB=immunoblotting, IP=immunoprecipitation, PAMPs=pathogen-associated patterns, PtGes=PGE synthase, SARM= sterile-α and Armadillo motif containing protein, SIKE=suppressor of IκB-ε, SOCS=suppressor of cytokine signaling, TAD=transactivation domain, TIR=Toll-IL-IR, TIRAP=TIR domain-containing adaptor protein, Tollip=Toll-interacting protein, TRIF=TIR domain-containing adaptor, inducing IFN-β
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
References
- O'Neill L A. How Toll-like receptors signal: what we know and what we don’t know. Curr Opin Immunol. 2006;18:3–9. doi: 10.1016/j.coi.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway C A., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu M Y, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- O'Neill L A. When signaling pathways collide: positive and negative regulation of toll-like receptor signal transduction. Immunity. 2008;29:12–20. doi: 10.1016/j.immuni.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Lien E, Sellati T J, Yoshimura A, Flo T H, Rawadi G, Finberg R W, Carroll J D, Espevik T, Ingalls R R, Radolf J D, Golenbock D T. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J Biol Chem. 1999;274:33419–33425. doi: 10.1074/jbc.274.47.33419. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Smith K D, Ozinsky A, Hawn T R, Yi E C, Goodlett D R, Eng J K, Akira S, Underhill D M, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- Gewirtz A T, Navas T A, Lyons S, Godowski P J, Madara J L. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–1885. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Holt A C, Medzhitov R, Flavell R A. Recognition of double-stranded RNA and activation of NFκB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- Vabulas R M, Ahmad-Nejad P, Ghosh S, Kirschning C J, Issels R D, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- Leadbetter E A, Rifkin I R, Hohlbaum A M, Beaudette B C, Schlomchik M J, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- Park J S, Svetkauskaite D, He Q, Kim J Y, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol. 2006;117:979–987. doi: 10.1016/j.jaci.2006.02.023. [DOI] [PubMed] [Google Scholar]
- O'Neill L A, Bowie A G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- Burns K, Martinon F, Esslinger C, Pahl H, Schneider P, Bodmer J L, Di Marco F, French L, Tschopp J. MyD88, an adaptor protein involved in IL-1 signaling. J Biol Chem. 1998;273:12203–12209. doi: 10.1074/jbc.273.20.12203. [DOI] [PubMed] [Google Scholar]
- Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway C A., Jr MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- Wesche H, Henzel W J, Shillinglaw W, Li S, Cao Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7:837–847. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- Fitzgerald K A, Palsson-McDermott E M, Bowie A G, Jefferies C A, Mansell A S, Brady G, Brint E, Dunne A, Gray P, Harte M T, McMurray D, Smith D E, Sims J E, Bird T A, O'Neill L A. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- Horng T, Barton G M, Medzhitov R. TIRAP: an adapter molecule in the Toll signaling pathway. Nat Immunol. 2001;2:835–841. doi: 10.1038/ni0901-835. [DOI] [PubMed] [Google Scholar]
- Biragyn A, Ruffini P A, Leifer C A, Klyushnenkova E, Shakhov A, Chertov O, Shirakawa A K, Farber J M, Segal D M, Oppenheim J J, Kwak L W. Toll-like receptor 4-dependent activation of dendritic cells by β-defensin 2. Science. 2002;298:1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- Muzio M, Ni J, Feng P, Dixit V. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science. 1997;278:1612–1615. doi: 10.1126/science.278.5343.1612. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Ninomiya-Tsuji J, Qian Y, Matsumoto K, Li X. Interleukin-1 (IL-1) receptor-associated kinase-dependent IL-1-induced signaling complexes phosphorylate TAK1 and TAB2 at the plasma membrane and activate TAK1 in the cytosol. Mol Cell Biol. 2002;22:7158–7167. doi: 10.1128/MCB.22.20.7158-7167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Sugiyama M, Yamamoto M, Watanabe Y, Kawai T, Takeda K, Akira S. Toll/IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-κB and IFN-regulatory factor-3, in the Toll-like receptor signaling. J Immunol. 2003;171:4304–4310. doi: 10.4049/jimmunol.171.8.4304. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- Honda K, Yanai H, Mizutani T, Negishi H, Shimada N, Suzuki N, Ohba Y, Takaoka A, Yeh W C, Taniguchi T. Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in Toll-like receptor signaling. Proc Natl Acad Sci USA. 2004;101:15416–15421. doi: 10.1073/pnas.0406933101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Ohba Y, Yanai H, Negishi H, Mizutani T, Takaoka A, Taya C, Taniguchi T. Spatiotemporal regulation of MyD88-IRF-7 signaling for robust type-I interferon induction. Nature. 2005;434:1035–1040. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- Uematsu S, Sato S, Yamamoto M, Hirotani T, Kato H, Takeshita F, Matsuda M, Coban C, Ishii K J, Kawai T, Takeuchi O, Akira S. Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-α induction. J Exp Med. 2005;201:915–923. doi: 10.1084/jem.20042372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaoka A, Yanai H, Kondo S, Duncan G, Negishi H, Mizutani T, Kano S, Honda K, Ohba Y, Mak T W, Taniguchi T. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature. 2005;434:243–249. doi: 10.1038/nature03308. [DOI] [PubMed] [Google Scholar]
- Carpenter S, O'Neill L A. How important are Toll-like receptors for antimicrobial responses? Cell Microbiol. 2007;9:1891–1901. doi: 10.1111/j.1462-5822.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- Medvedev A E, Sabroe I, Hasday J D, Vogel S N. Tolerance to microbial TLR ligands: molecular mechanisms and relevance to disease. J Endotoxin Res. 2006;12:133–150. doi: 10.1179/096805106X102255. [DOI] [PubMed] [Google Scholar]
- Ertel W, Kremer J P, Kenney J, Steckholzer U, Jarrar D, Trentz O, Schildberg F W. Downregulation of proinflammatory cytokine release in whole blood from septic patients. Blood. 1995;85:1341–1347. [PubMed] [Google Scholar]
- Haupt W, Zirngibl H, Riese J, Stehr A, Linde H J, Hohenberger W. Depression of tumor necrosis factor-α, interleukin-6, and interleukin-10 production: a reaction to the initial systemic hyperactivation in septic shock. J Invest Surg. 1997;10:349–355. doi: 10.3109/08941939709099598. [DOI] [PubMed] [Google Scholar]
- Cavaillon J M, Adib-Conquy M. Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit Care. 2006;10:233–245. doi: 10.1186/cc5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev A E, Kopydlowski K M, Vogel S N. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and toll-like receptor 2 and 4 gene expression. J Immunol. 2000;164:5564–5574. doi: 10.4049/jimmunol.164.11.5564. [DOI] [PubMed] [Google Scholar]
- Foster S L, Hargreaves D C, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- Nomura F, Akashi S, Sakao Y, Sato S, Kawai T, Matsumoto M, Nakanishi K, Kimoto M, Miyake K, Takeda K, Akira S. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J Immunol. 2000;164:3476–3479. doi: 10.4049/jimmunol.164.7.3476. [DOI] [PubMed] [Google Scholar]
- Medvedev A E, Piao W, Shoenfelt J, Rhee S H, Chen H, Basu S, Wahl L M, Fenton M J, Vogel S N. Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance. J Biol Chem. 2007;282:16042–16053. doi: 10.1074/jbc.M606781200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao W, Song C, Chen H, Wahl L M, Fitzgerald K A, O'Neill L A, Medvedev A E. Tyrosine phosphorylation of MyD88 adapter-like (Mal) is critical for signal transduction and blocked in endotoxin tolerance. J Biol Chem. 2008;283:3109–3119. doi: 10.1074/jbc.M707400200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev A E, Lentschat A, Wahl L M, Golenbock D T, Vogel S N. Dysregulation of LPS-induced Toll-like receptor 4-MyD88 complex formation and IL-1 receptor-associated kinase 1 activation in endotoxin-tolerant cells. J Immunol. 2002;169:5209–5216. doi: 10.4049/jimmunol.169.9.5209. [DOI] [PubMed] [Google Scholar]
- Li L, Cousart S, Hu J, McCall C E. Characterization of interleukin-1 receptor-associated kinase in normal and endotoxin-tolerant cells. J Biol Chem. 2000;275:23340–23345. doi: 10.1074/jbc.M001950200. [DOI] [PubMed] [Google Scholar]
- Sato S, Takeuchi O, Fujita T, Tomizawa H, Takeda K, Akira S. A variety of microbial components induce tolerance to lipopolysaccharide by differentially affecting MyD88-dependent and -independent pathways. Int Immunol. 2002;14:783–791. doi: 10.1093/intimm/dxf046. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H W, Wedel A, Schraut W, Strobel M, Wendelgass P, Sternsdorf T, Bauerle P A, Haas J G, Riethmuller G. Tolerance to lipopolysaccharide involves mobilization of nuclear factor-κB with predominance of p50 homodimers. J Biol Chem. 1994;269:17001–17004. [PubMed] [Google Scholar]
- El Gazzar M, Yoza B K, Hu J Y, Cousart S L, McCall C E. Epigenetic silencing of tumor necrosis factor α during endotoxin tolerance. J Biol Chem. 2007;282:26857–26864. doi: 10.1074/jbc.M704584200. [DOI] [PubMed] [Google Scholar]
- Hirschfeld M, Ma Y, Weis J H, Vogel S N, Weis J J. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine Toll-like receptor 2. J Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- Li T, Hu J, Li L. Characterization of Tollip protein upon lipopolysaccharide challenge. Mol Immunol. 2004;41:85–92. doi: 10.1016/j.molimm.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Fitzgerald K A, McWhirter S M, Faia K L, Rowe D C, Latz E, Golenbock D T, Coyle A J, Liao S M, Maniatis T. IKKε and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- Livak K J, Schmittgen T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(–Δ Δ C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Roberts Z J, Goutagny N, Perera P Y, Kato H, Kumar H, Kawai T, Akira S, Savan R, van Echo D, Fitzgerald K A, Young H A, Ching L M, Vogel S N. The chemotherapeutic agent DMXAA potently and specifically activates the TBK1-IRF-3 signaling axis. J Exp Med. 2007;204:1559–1569. doi: 10.1084/jem.20061845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, Akira S. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-β promoter in the Toll-like receptor signaling. J Immunol. 2002;169:6668–6672. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- Ikeda F, Hecker C M, Rozenknop A, Nordmeier R D, Rogov V, Hofmann K, Akira S, Dötsch V, Dikic I. Involvement of the ubiquitin-like domain of TBK1/IKK-i kinases in regulation of IFN-inducible genes. EMBO J. 2007;26:3451–3462. doi: 10.1038/sj.emboj.7601773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes B, Lubyova B, Pitha P M. On the role of IRF in host defense. J Interferon Cytokine Res. 2002;22:59–71. doi: 10.1089/107999002753452665. [DOI] [PubMed] [Google Scholar]
- Fitzgerald K A, Rowe D C, Barnes B J, Caffrey D R, Visintin A, Latz E, Monks B, Pitha P M, Golenbock D T. LPS-TLR4 signaling to IRF-3/7 and NF-κB involves the toll adapters TRAM and TRIF. J Exp Med. 2003;198:1043–1055. doi: 10.1084/jem.20031023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew F Y, Xu D, Brint E K, O'Neill L A. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- Carty M, Goodbody R, Schröder M, Stack J, Moynagh P N, Bowie A G. The human adaptor SARM negatively regulates adaptor protein TRIF-dependent Toll-like receptor signaling. Nat Immunol. 2006;7:1074–1081. doi: 10.1038/ni1382. [DOI] [PubMed] [Google Scholar]
- Huang J, Liu T, Xu L G, Chen D, Zhai Z, Shu H B. SIKE is an IKKε/TBK1-associated suppressor of TLR3- and virus-triggered IRF-3 activation pathways. EMBO J. 2005;24:4018–4028. doi: 10.1038/sj.emboj.7600863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Ghosh S. Negative regulation of Toll-like receptor-mediated signaling by Tollip. J Biol Chem. 2002;277:7059–7065. doi: 10.1074/jbc.M109537200. [DOI] [PubMed] [Google Scholar]
- Mansell A, Smith R, Doyle S L, Gray P, Fenner J E, Crack P J, Nicholson S E, Hilton D J, O'Neill L A, Hertzog P J. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat Immunol. 2006;7:148–155. doi: 10.1038/ni1299. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Hernandez L D, Galán J E, Janeway C A, Jr, Medzhitov R, Flavell R A. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- Sly L M, Rauh M J, Kalesnikoff J, Song C H, Krystal G. LPS-induced upregulation of SHIP is essential for endotoxin tolerance. Immunity. 2004;21:227–239. doi: 10.1016/j.immuni.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- Shim J H, Xiao C, Paschal A E, Bailey S T, Rao P, Hayden M S, Lee K Y, Bussey C, Steckel M, Tanaka N, Yamada G, Akira S, Matsumoto K, Ghosh S. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagoe T, Sato S, Matsushita K, Kato H, Matsui K, Kumagai Y, Saitoh T, Kawai T, Takeuchi O, Akira S. Sequential control of Toll-like receptor-dependent responses by IRAK1 and IRAK2. Nat Immunol. 2008;9:684–691. doi: 10.1038/ni.1606. [DOI] [PubMed] [Google Scholar]
- Biswas S K, Bist P, Dhillon M K, Kajiji T, Del Fresno C, Yamamoto M, Lopez-Collazo E, Akira S, Tergaonkar V. Role for MyD88-independent, TRIF pathway in lipid A/TLR4-induced endotoxin tolerance. J Immunol. 2007;179:4083–4092. doi: 10.4049/jimmunol.179.6.4083. [DOI] [PubMed] [Google Scholar]
- Dobrovolskaia M A, Medvedev A E, Thomas K E, Cuesta N, Toshchakov V, Ren T, Cody M J, Michalek S M, Rice N R, Vogel S N. Induction of in vitro reprogramming by Toll-like receptor (TLR)2 and TLR4 agonists in murine macrophages: effects of TLR “homotolerance” versus “heterotolerance” on NF-κB signaling pathway components. J Immunol. 2003;170:508–519. doi: 10.4049/jimmunol.170.1.508. [DOI] [PubMed] [Google Scholar]
- Nakagawa R, Naka T, Tsutsu H I, Fujimoto M, Kimura A, Abe T, Seki E, Sato S, Takeuchi O, Takeda K, Akira S, Yamanishi K, Kawase I, Nakanishi K, Kishimoto T. SOCS-1 participates in negative regulation of LPS responses. Immunity. 2002;17:677–687. doi: 10.1016/s1074-7613(02)00449-1. [DOI] [PubMed] [Google Scholar]
- Miggin S M, Pålsson-McDermott E, Dunne A, Jefferies C, Pinteaux E, Banahan K, Murphy C, Moynagh P, Yamamoto M, Akira S, Rothwell N, Golenbock D, Fitzgerald K A, O'Neill L A. NF-κB activation by the Toll-IL-1 receptor domain protein MyD88 adapter-like is regulated by caspase-1. Proc Natl Acad Sci USA. 2007;104:3372–3377. doi: 10.1073/pnas.0608100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalpke A, Heeg K, Bartz H, Baetz A. Regulation of innate immunity by suppressor of cytokine signaling (SOCS) proteins. Immunobiology. 2008;213:225–235. doi: 10.1016/j.imbio.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Melmed G, Thomas L S, Lee N, Tesfay S Y, Lukasek K, Michelsen K S, Zhou Y, Hu B, Arditi M, Abreu M T. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J Immunol. 2003;170:1406–1415. doi: 10.4049/jimmunol.170.3.1406. [DOI] [PubMed] [Google Scholar]
- Kollewe C, Mackensen A C, Neumann D, Knop J, Cao P, Li S, Wesche H, Martin M U. Sequential autophosphorylation steps in the interleukin-1 receptor-associated kinase-1 regulate its availability as an adapter in interleukin-1 signaling. J Biol Chem. 2004;279:5227–5236. doi: 10.1074/jbc.M309251200. [DOI] [PubMed] [Google Scholar]
- Génin P, Algarté M, Roof P, Lin R, Hiscott J. Regulation of RANTES chemokine gene expression requires cooperativity between NF-κB and IFN-regulatory factor transcription factors. J Immunol. 2000;164:5352–5361. doi: 10.4049/jimmunol.164.10.5352. [DOI] [PubMed] [Google Scholar]
- Gong R, Rifai A, Dworkin L D. Activation of PI3K-Akt-GSK3β pathway mediates hepatocyte growth factor inhibition of RANTES expression in renal tubular epithelial cells. Biochem Biophys Res Commun. 2005;330:27–33. doi: 10.1016/j.bbrc.2005.02.122. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Suzuki S, Duncan G S, Millar D G, Wada T, Mirtsos C, Takada H, Wakeham A, Itie A, Li S, Penninger J M, Wesche H, Ohashi P S, Mak T W, Yeh W C. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature. 2002;416:750–756. doi: 10.1038/nature736. [DOI] [PubMed] [Google Scholar]
- Hayden M S, Ghosh S. Shared principles of NF-κB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.