Abstract
Objectives
MKK4 is a metastasis suppressor that is downregulated in some ovarian cancers. We sought to investigate whether promoter methylation, loss of heterozygosity, or changes in phosphorylation are involved in MKK4 dysregulation during ovarian carcinogenesis.
Methods
Bisulfite sequencing was used to determine MKK4 promoter methylation. PCR analysis of tumor/normal DNA was performed to determine LOH at the MKK4 locus. Normal human ovarian surface epithelium (HOSE) and SKOV-3 cells were serum starved and treated with EGF, TGFβ, or wortmannin. Western blotting was performed using antibodies that detect total and phosphorylated MKK4.
Results
No MKK4 promoter hypermethylation was detected in 21 ovarian cancers. LOH was detected at the MKK4 intragenic marker D17S969 in 35% of cases and at D17S1303 in 20%. MKK4 protein was detected in 97% of ovarian tumors. The inactivated phosphoserine-80 (ser-80) form comprised 62% of phosphorylated MKK4 protein in ovarian tumors. Treatment of HOSE or SKOV-3 cells with EGF induced a 1.7 to 4.2-fold increase in phosphorylation of ser-80 MKK4 without altering total MKK4 protein. TGFβ increased MKK4 ser-80 phosphorylation by 5.4 fold above baseline. The PI3K/Akt pathway inhibitor wortmannin decreased the amount of ser-80 MKK4 by 50%, and inhibited EGF stimulation of MKK4 ser-80 phosphorylation by 60%.
Conclusions
LOH of MKK4 occurs in some ovarian cancers, but without loss of MKK4 protein. MKK4 expression does not appear to be downregulated by promoter methylation. Peptide growth factors induce MKK4 ser-80 phosphorylation, which downregulates its activity. PI3K/Akt pathway inhibitors can partially block ser-80 phosphorylation and this may have therapeutic implications.
Keywords: MKK4, metastasis, AKT, ovarian cancer
Introduction
Approximately 24,000 new cases of ovarian epithelial cancer occur annually in the United States, and 13,600 women die of ovarian cancer every year. Although cure rates for advanced stage ovarian cancer have not changed appreciably, median survival has improved substantially. This is generally attributed to more complete surgical cytoreduction and the development of new chemotherapy agents.[1] Whereas the superior efficacy of new chemotherapy regimens has been demonstrated in prospective randomized trials, the benefit of surgical debulking has been inferred from retrospective analyses.[2, 3] Currently, median survival is about 5 years in patients in whom residual tumor nodules are all equal to or less than 1 cm in maximal diameter (optimally debulked) [4] compared to only about 3 years in those with larger tumors.[1]
Resection of metastatic tumor masses may be efficacious in ovarian cancer because disease is usually confined to the abdominal cavity at presentation – despite an extraordinarily large tumor burden. Although optimal debulking has been associated with improved survival in observational studies, it is unclear whether this is directly attributable to removal of the primary tumor and metastases. It is conceivable that outcome may be predetermined by the underlying tumor biology and that optimal debulking is more likely to be technically feasible in cancers that are inherently less virulent and/or more sensitive to chemotherapy.
We have previously investigated the hypothesis that optimally debulked and suboptimally debulked ovarian tumors exhibit distinct molecular signatures.[5] Microarray analysis of serous ovarian cancers revealed patterns of gene expression that retrospectively predicted optimal versus suboptimal debulking with 73% accuracy.[5] These data were supportive of the hypothesis that underlying tumor biology is a determinant of the ability to cytoreduce ovarian cancers. Two genes that are members of the mitogen activated protein (MAP) kinase pathway, MKK4 and M3K7, were downregulated in suboptimally debulked ovarian cancers.[5] The MAP kinase pathways are comprised of a three tiered system of kinases that successively phosphorylate each other on serine and threonine residues. These pathways have been implicated in regulation of diverse cellular processes including growth, cellular stress, differentiation and apoptosis. The MKK4 gene, located on chromosome 17p11.2 [6], ultimately exerts its affects via regulation of the activity of transcription factors such as c-jun. [7] There is evidence that MKK4 acts as a metastasis suppressor gene [8] and progressive loss of expression occurs in prostate[9] and pancreatic [10], and although mutations in MKK4 are rare, homozygous deletions and loss of one allele of MKK4 gene are not uncommon.
Yamada et. al. have demonstrated that MKK4 expression is higher in normal ovarian epithelium compared to metastatic ovarian cancer and that transfection of the MKK4 gene into the SKOV-3 ovarian cancer cell line inhibited formation of peritoneal metastases by 95%.[6] This suggests that MKK4 acts as a metastasis suppressor gene in ovarian cancer. The consequences of downregulation of MKK4 could include the development of more extensive metastatic disease that is relatively difficult to optimally debulk. In view of the potential importance of MKK4 in regulating metastasis of ovarian cancer, we have further characterized its expression and regulation.
Materials and Methods
Tissues
Normal ovaries and ovarian cancer specimens were collected at the time of initial surgery under an IRB approved protocol at Duke University Medical Center. The tissues were aliquoted into Nunc tubes, snap frozen in liquid nitrogen, and stored in a −70°C freezer.
Loss of Heterozygosity (LOH)
Informative STS markers at the MKK4 locus on chromosome 17 were examined, including D17S969 within the MKK4 gene and D17S1303 distal to the MKK4 locus. One hundred nanograms of genomic DNA from ovarian cancers and corresponding normal lymphocytes were amplified under standard STS amplification conditions with a Tm of 55°C. D17S969 primers were as follows: F 5’ ATCTAATCTGTCATTCATCTATCCA and R 5’ AACTGCAGTGCTGCATCATA. D17S1303 primers were: F 5’ CTCTCCAAGGCTCACTCAAA; and R 5’ TGGTCTTTTTCCATTCCAAA. Products were resolved on ethidium bromide stained 1% TBE agarose gels.
Quantitative RT- PCR
Total RNA was extracted using the RNeasy RNA extraction kit (Qiagen) and reverse transcribed using the Roche First Strand cDNA kit (Roche) using random primers. MKK4 primers (F 5’-AGT GGA CAG CTT GTG GAC TCT-3’ and R 5’-AAC TCC AGA CAT CAG AGC GGA-3’) specifically amplified cDNA. Quantitative RT-PCR was performed using the Roche LightCycler system using the QuantiTect SYBR Green PCR Kit (Qiagen).
Promoter methylation analysis
Bisulfite-treated genomic DNA was amplified by PCR with primers specific to the MKK4 promoter region surrounding the transcription start site (BSF 5’-GGT TTT GTA GTT TAG TAT TTG GTT-3’ and BSR 5’-GTT CCT TAC CCT ACA TAC TAC TAA C-3’). The 311-bp products were isolated from agarose gels and cycle sequenced using Thermo Sequenase Radiolabeled Terminator Cycle Sequence Kit (Amersham Biosciences). The sequencing products were resolved on 5% denaturing polyacrylamide gels followed by exposure to radiographic film (BioMax MR; Kodak).
Immunohistochemistry
Frozen tissue samples collected as described above were embedded in OCT medium. Sections were subsequently cut by microtome and mounted on glass slides.[5] These frozen sections of normal ovaries and ovarian carcinomas were subjected to Hematoxylin and Eosin staining to confirm greater than 60% tumor content. Sequential slides from the same block were used for MKK4 immunostaining. The slides were incubated overnight at 4°C using rabbit-anti-MKK4/MEK4 H98 antibody (5µg/mL; sc-13070 Santa Cruz Biotechnologies) or isotype control (5µg/mL; whole rabbit IgG) in protein blocking solution. Slides were subsequently incubated with goat antirabbit biotin-conjugated IgG, (5µg/mL; Santa Cruz Biotechnologies) followed by incubation with ABC Vectastain kit (Vector Labs). Immunostaining was detected using 3,3’-diaminobenzidine peroxidase substrate kit (Vector Labs), and slides were counterstained with Methyl Green (Sigma M-5015). The fraction of cancer cells expressing MKK4 was scored as absent, low, (<50% cells stained) or high (>50% cells stained). Samples were scored by two independent reviewers and differences resolved by consensus. Discordant scores were noted for approximately 15% of the immunohistochemical sections.
Western Analysis
Protein extraction was performed using an SDS based buffer with the addition of protease (Roche Complete Protease inhibitor cocktail 1–835–153) and kinase inhibitors (Sigma cocktail 1 P2850 and cocktail 2 P5726 ). Insoluble proteins were pelleted by centrifugation and the supernantants were spectrometrically quantified by modified Bradford assay (BioRad). One hundred micrograms of total protein from each tumor or cell line specimen was resolved on 10–12.5% SDS-PAGE gels (BioRad Criterion). Western transfer and blocking was performed according to established protocols. Duplicate blots were made for each sample and hybridized with one primary antibody. Primary antibodies obtained from Cell Signaling Technology included: Phospho-SEK1/MKK4(Ser80) (#9155 used at 1:1000); Phospho-SEK1/MKK4(Ser257/Thr261) (# 9156, used at 1:1000); SEK1/MKK4 (#9152 used at 1:1000); p38 (#9212 used at 1:1000); and JNK (#9152, used at 1:1000). Beta-actin antibody was obtained from Sigma (A4700) and used at 1:10,000. TAK1 antibody was obtained from Abcam (#ab1554) and used at 2 µg/ml. Protein expression was detected with horseradish peroxidase-conjugated IgG secondary antibody (Jackson Labs), followed by Western Lightning (Perkin Elmer) chemiluminescence and Kodak film. Expression signals were quantitated using Scion ImageQuant or Image J software.
Statistical Methods
Raw MKK4 expression data was normalized to the beta actin signal in each separate lane of the Western blots. Differences in the mean normalized MKK4 expression between optimal and suboptimal ovarian cancers were compared by Student’s t test using the SAS Enterprise Guide statistical software package, version 8.2.
Cell culture
SKOV-3 ovarian cancer cells were cultured in RPMI 1640 media (GibcoBRL) containing 10% FCS (BRL) in 5% CO2 humidified chambers. Cells were passaged by trypsinization and split into 100 mm replicate plates. At approximately 60% confluence, cells were rinsed twice with PBS solution and placed into RPMI media lacking fetal calf serum. After overnight serum starvation, cells were stimulated by defined concentrations of growth factors. All growth factors and inhibitors were obtained from Sigma: EGF (E9644); TGFα (T7924), TGFβ (T7039) and wortmannin (W1628).
Results
Loss of Heterozygosity
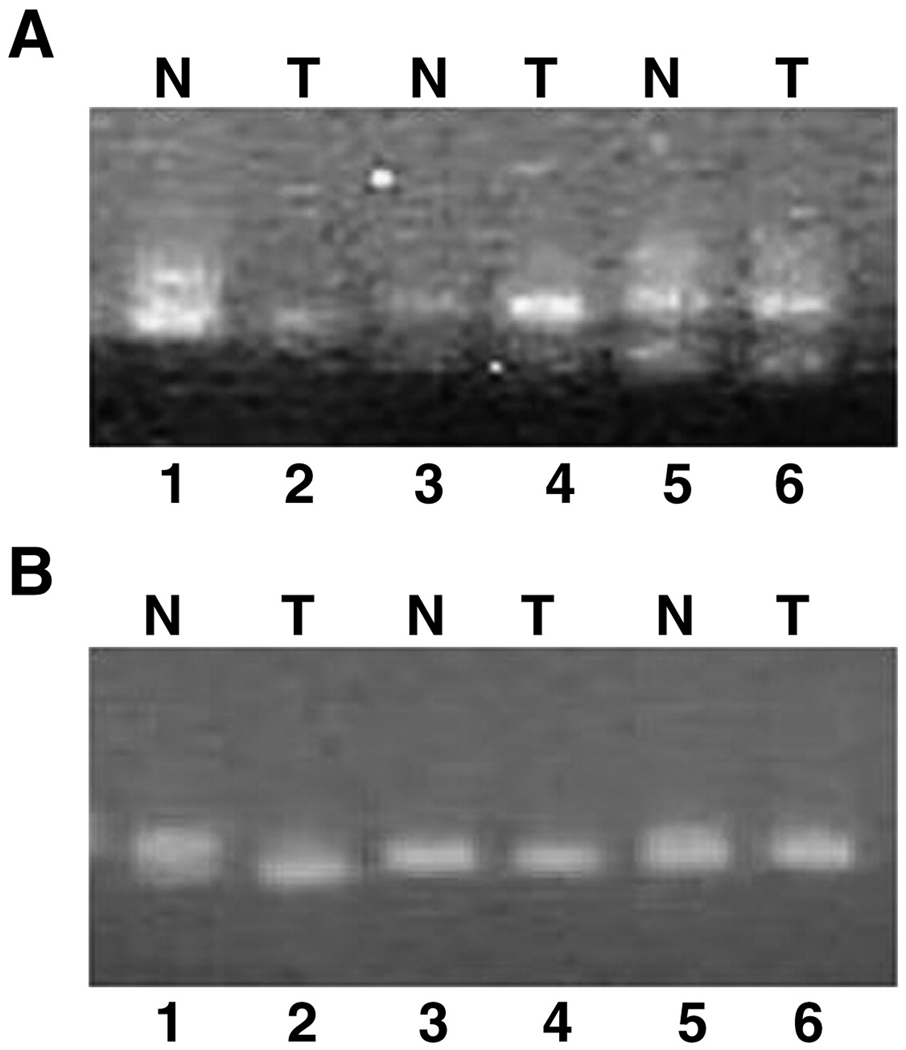
To determine whether genomic deletions of the MKK4 gene contribute to downregulation, twenty matched normal peripheral blood lymphocyte DNA and ovarian tumor samples were examined for LOH at the D17S9696 and D17S1303 loci (figure 1). The intragenic D17S969 marker (figure 1A) was informative in 14 out of 20 samples, with 36% (5/14) of the informative samples demonstrating LOH. For the distal D17S1303 marker (figure 1B) 10 of 20 samples were informative, and two (20%) exhibited LOH. No ovarian cancer contained a homozygous deletion of the MKK4 locus Eleven samples were from suboptimally debulked cancers, 6 from optimally debulked cancers, and 3 had an unknown debulking status. LOH at the MKK4 locus did not correlate with debulking status. In addition, DNA from the SKOV-3 ovarian cancer cell line and the spontaneously immortalized normal ovarian surface epithelial cell line NOSE006 was amplified to confirm the presence of at least one genomic copy of the MKK4 gene. All cell lines contained one allele for the MKK4 locus, and none of the ovarian cell lines demonstrated a homozygous deletion for the MKK4 locus.
Figure 1. Loss of heterozygosity for matched lymphocyte (N) and ovarian tumor (T) DNA at the MKK4 locus on chromosome 17.
Representative data for the D17S969 intragenic locus (A) and D17S1303 distal locus (B) is shown. The sample pairs in lanes 1,2 and 5,6 are informative at both loci. The normal/tumor pair in lanes 1 and 2 demonstrates LOH for both markers. The other samples in lanes 3–6 do not demonstrate LOH at either locus.
Promoter methylation
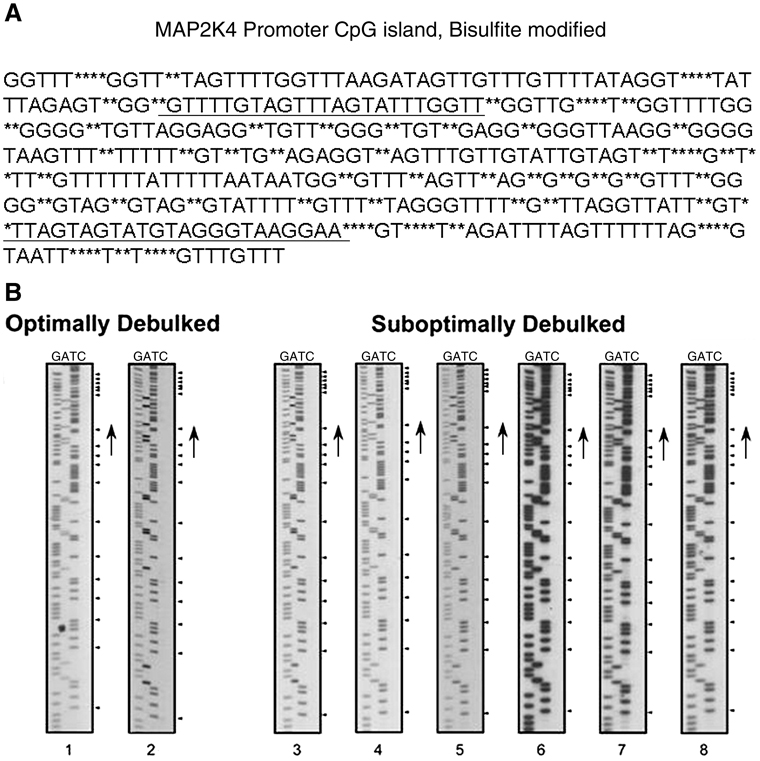
Another mechanism of gene silencing is through differential methylation at CpG islands in gene promoters[11] within the transcription start sites. The promoter sequence of MKK4 is encompassed within a 920 bp CpG island (figure 2A). Primers were designed to flank the transcription start site of bisulfite modified genomic DNA. PCR amplicons were purifed and sequenced to identify potentially methylated CpG dinucleotides (figure 2B). Promoter methylation was not detected in the 21 primary ovarian cancers subjected to bisulfite sequencing.
Figure 2. MKK4 promoter methylation.
A. CpG Island Map MKK4 Promoter. MKK4 promoter DNA sequence with bisulfite modification sites annotated as asterisks within the sequence. Primers used for sequencing are marked by underlining and flank the transcription start site.
B. Bisulfite Modified Methylation Specific Sequence of MKK4 Promoter. Bisulfite modified MKK4 promoter DNA was sequenced, resolved by denaturing polyacrylamide gel electrophoresis, and detected by exposure to radiographic film. The panels on the left are representative sequences from 2 optimally debulked ovarian tumors. The panels on the right are representative sequences from 6 suboptimally debulked ovarian tumors. Methylated residues would be detected in the ‘C’ lane at the positions marked by the arrowheads. No methylated cytosine residues were detected in the samples.
Immunohistochemistry
Immunohistochemical staining detected strong expression of MKK4 in normal ovarian surface epithelium (Figure 3A). Immunohistochemical staining for MKK4 was performed in 24 primary ovarian cancers (12 optimal, 12 suboptimal) (Figure 3B). MKK4 staining was seen in both the nuclei and cytoplasm of tumor cells. This is consistent with other studies of the cellular localization of MKK4 in both human and rodent. MKK4 was enriched in the cytosolic fraction of whole rat brain lysates, but also seen in the nuclear fraction.[12]
Figure 3. A: MKK4 expression in normal human ovary and suboptimally debulked ovarian carcinoma.
A representative section of normal human ovary with a single cuboidal layer of surface epithelium was stained for MKK4 expression in the left panel. In the right panel, a representative section of suboptimally debulked serous ovarian carcinoma was stained for MKK4 expression.
B: Loss of MKK4 expression in suboptimally debulked ovarian cancer. Representative sections of suboptimally (left) and optimally (right) debulked ovarian cancers were stained for MKK4 expression (lower panels). The negative control IgG stain is shown in the top panels.
Immunostaining was characterized as high (>50% of cells staining), low (<50% staining) or absent. Among the 12 optimally debulked tumors 4 (33%) had high expression, whereas high expression was not seen in any of the suboptimally debulked cancers. An additional three optimally debulked cancers (25%) exhibited low expression and 5 (42%) optimally debulked tumors exhibited no detectable expression. Most suboptimally debulked cancers, 83% (10/12), had no detectable MKK4 expression. Only 2 (16%) suboptimally debulked cancers exhibited low expression of MKK4.
MAP Kinase Pathway Proteins in Ovarian Carcinoma
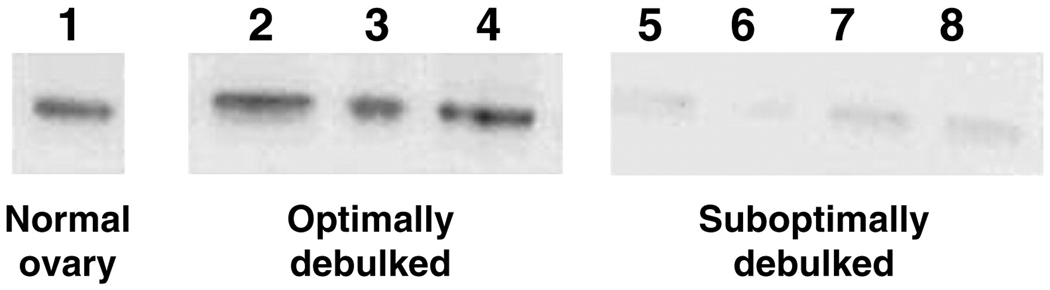
Small increases in the steady-state pool of mRNA may be amplified into large increases in the total protein product of those transcripts. We explored the hypothesis that suboptimally debulked ovarian cancers have lower levels of MKK4 protein. Protein lysates from 14 subotimally debulked and 12 optimally debulked cancers were subjected to Western blotting (figure 4). The MKK4 protein migrated as a 44 kDa band and was seen in 97% of cases.
Figure 4. Expression of MKK4 in primary human ovarian tumor samples.
Representative total MKK4 protein expression detected by Western blots of normal ovary, suboptimally and optimally debulked ovarian tumors.
The mean normalized expression of total MKK4 in suboptimally debulked cancers (0.11, 95% CI 0.05 – 0.29) was 35% lower than that seen in optimally debulked cancers (0.17, 95% CI 0.02 – 0.21 (p=0.4). We also examined expression of phosphoserine 80 MKK4 and found that expression in suboptimally debulked tumors (0.19, 95% CI 0.11 – 0.27) was 49% lower than in optimally debulked tumors (0.37, 95% CI 0.2 – 0.54) (p = 0.04).
Confirmation of antibody specificity and identification of the MKK4 product was accomplished by the inclusion of protein lysates from a negative control cell line, AsPC-1, which harbors a homozygous deletion of the MKK4 gene.[6] Lysates from the NIH 3T3 cell line were the positive control, and beta-actin served as a control for equal protein loading and as an internal normalization control.
Regulation of MKK4 Phosphorylation In Human Tumors
MKK4 is a member of a MAP kinase cascade. In order for MKK4 to signal effectively, the upstream and down stream components of the MAP kinase pathway must also be present. Expression of the upstream kinase M3K7(TAK1)(figure 5, row 1), total MKK4 (figure 5, row 4), as well as the downstream effectors p38 (figure 5, row 5), and JNK (figure 5, row 6) were examined in primary ovarian cancers using Western analysis.
Figure 5. Western analysis of MAP kinase pathway in primary ovarian cancers.
TAK1 (MAP3K7) was detected as an 80 kDa protein (top row). Phosphorylated forms of MKK4 were detected by phosphoserine257/ phosphothreonine262 anti-MKK 4 (2nd row), and phosphoserine 80 MKK4 (3rd row). The total amount of MKK4 protein was detected in row 4. The p38 protein migrated at the expected 38 kDa size (row 5) while total JNK migrated as a doublet of 54 and 46 kDa (6th row). Beta-actin (42 kDa) serves as a loading control (bottom row).
MKK4 is regulated by phosphorylation at specific serine and threonine residues. Phosphorylation at serine-80 is thought to be an inactivating event, downregulating MKK4’s effect on downstream JNK/p38 signaling.[13] In contrast, dual phosphorylation at serine 257 and threonine 262 is an activating signal which increases MKK4 signaling to JNK/p38.[12] Monoclonal phosphoantibodies to Serine-80 MKK4 (figure 5, row 3) and serine257/threonine262 MKK4 (figure 5, row 2) were hybridized to tumor lysates. The dual-phosphorylated activated MKK4 form was rarely detected. In contrast, the inactivated phosphoserine 80 MKK4 isoform represented 62% of the total MKK4 protein found in all ovarian tumor samples.
Regulation of MKK4 Phosphorylation in In Vitro Cell Cultures
Peptide growth factors are potent activators of the MAP kinase cascade. In figure 6, EGF (epidermal growth factor) stimulation of SKOV-3 cells induces a rapid 2.5-fold induction of MKK4 phosphorylation on the serine 80 residue as indicated in the top panel (lanes 4–6). The induction is present by five minutes, and persists for at least 15 minutes of stimulation. The control SKOV-3 cells in lanes 1–3 were treated with media without serum and harvested at the indicated time points. In the control cells, the fraction of phosphorylated MKK4 remained constant. The total amount of MKK4 did not change (middle panel) under control (lanes 1–3) or EGF stimulated (lanes 4–6) conditions. Beta-actin served as a loading control (bottom panel).
Figure 6. EGF induces MKK4 phosphorylation in the SKOV-3 ovarian cancer cell line.
Control SKOV-3 cells were serum starved, and then treated with fresh media without serum (lanes 1–3). Epidermal growth factor (EGF) was added at a concentration of 10ng/ml media (lanes 4–6) for experimental cells. Samples were harvested at 5, 10 and 15 minute time points and subjected to Western blotting. Phosphoserine 80 MKK4 was detected in the top panel, total unphosphorylated MKK4 in the middle panel, and beta-actin in the bottom panel. EGF stimulation of cells induced phosphorylation of MKK4, while the total amount of MKK4 remained constant. After five minutes of EGF stimulation, the fraction of the phosphorylated MKK4 normalized to total MKK4 was 1.7 fold above baseline (lane 1 and 4); at 10 minutes, EGF stimulation increased the phosphorylation by 2 fold (lanes 2 and 5) and at 15 minutes, the phosphorylated isoform of MKK4 was 2.5 fold higher than control (lanes 3 and 6).
Figure 7 shows the EGF-mediated induction of serine 80 phosphorylation of MKK4 (top panel, lanes 3–5) in the normal ovarian surface epithelial cell line NOSE006. EGF stimulation of the normal ovarian surface epithelial cells induced a 2.5-fold increase in phosphoserine 80 MKK4 signal after 5 minutes; a 3–fold increase after 10 minutes; and 4.2 fold increase after 20 minutes. The sample in lane 2 is a control serum starved sample. The 3T3 cell line serves as a positive control for MKK4 (lane 1). Equal protein loading was confirmed with beta-actin (bottom panel).
Figure 7. EGF induces MKK4 Phosphorylation in normal human ovarian surface epithelial cells.
NOSE006, a normal human ovarian surface epithelial cell line was serum starved. The control cells in lane 2 were serum starved at baseline. Fresh media containing 10 ng/ ml EGF without serum was added to the experimental cells, and harvested at 5 minutes (lane 3), 10 minutes (lane 4), and 20 minutes (lane 5). Lysates were subjected to Western blotting to detect phosphoserine 80 MKK4 (top panel). A duplicate aliquot of lysate was used for the actin control (bottom panel). 3T3 cell protein (lane 1) serves as a positive control for MKK4 phosphoserine 80 expression. The expression of phosphoserine 80 MKK4 was quantitated and normalized to the actin control. When compared to the normalized signal from lane 1, the phosphoserine 80 MKK4 signal was increased by 2.5 fold at 5 minutes (lane 3), 3 fold at 10 minutes (lane 4) and 4.2 fold at 20 minutes (lane 5).
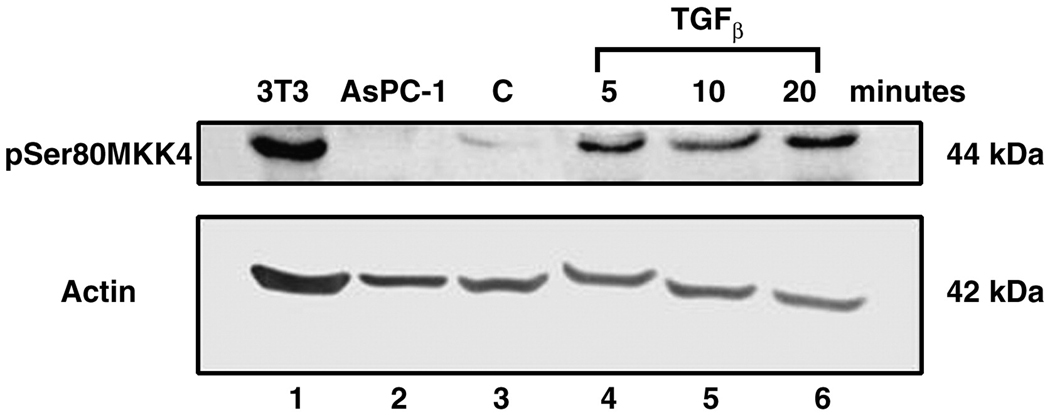
MAP3K7, also known as the TGF-β associated kinase (TAK1), is an immediate upstream effector of the MKK4 pathway. MAP3K7 was also associated with optimal debulking status on the cDNA microarray analysis ovarian tumors [5] and was detected in primary ovarian tumor cell lysates (figure 5, top panel). TGF-β acts as a negative peptide growth factor in many tumors.[14] We hypothesized that TGF-β treatment of ovarian cancer cells would also induce phosphorylation of MKK4.
In figure 8, SKOV-3 cell lysates were subjected to Western blotting after treatment with TGFβ. In lane 1, 3T3 lysate serves as a positive control, while AsPC-1 lysate (lane 2) serves as a negative MKK4 control. Lane 3 contains SKOV-3 control serum starved lysate, demonstrating a low endogenous phosphoserine 80 MKK4 signal. In the top panel, the phosphoserine 80 MKK4 isoform is detected at 5 minutes (lane 4), 10 minutes (lane 5) and 20 minutes (lane 6) after initial TGFβ application. A 5.4 fold increase in serine 80 MKK4 signal was averaged across the 20 minute time course and normalized to the beta-actin signal. Beta-actin serves as a protein loading control (bottom panel).
Figure 8. TGF-β induces MKK4 Phosphorylation in the SKOV-3 ovarian cancer cell line.
SKOV-3 cells were serum starved (control, lane 3) or treated with TGFβ (10 ng/ ml). Samples were harvested at the indicated time points (lanes 4–6). Duplicate Western blots were probed for either phosphoserine 80 MKK4 (top panel) or beta-actin (bottom panel). 3T3 cells are the MKK4 positive control (lane 1) and AsPc-1 cells are the negative control (lane 2). A 5.4 fold increase in serine 80 MKK4 signal was averaged across the 20 minute time course and normalized to the beta-actin signal. Beta-actin serves as a protein loading control (bottom panel).
Cross-talk between signaling pathways
Wortmannin is a natural fungal-derived inhibitor of the PI3/Akt pathway. Figure 9 is a dose response experiment of wortmannin in the presence and absence of a constant amount of EGF in SKOV-3 cells. Lane 1 is the positive control, 3T3, and lane 2 is the negative control, AsPC-1. Lane 3 is a serum starved control. In lane 4, an induction of phosphoserine 80 MKK4 is mediated by treatment of the cells with 10 ng/ml of EGF. In lanes 5–7, increasing amounts of wortmannin are added to the cells. The wortmannin diminished detectable steady-state phosphoserine 80 MKK4 signal by 50%, when control cell signal in lane 3 is compared to the average signal across the three dosages of wortmannin in lanes 5–7. In lanes 8–10, the cells were treated with the same amount of EGF as the cells in lane 4; however, increasing amounts of wortmannin were also added to the cells. The addition of wortmannin effected a 60% decrease in the EGF stimulated amount of phosphoserine 80 MKK4, averaged across the three dosage points (lanes 4, 8–10)
Figure 9. PI3K inhibitor wortmannin inhibits phosphorylation of MKK4.
SKOV-3 cells were serum starved (control cells lane 3). Cells were pretreated for one hour with wortmannin at increasing concentrations: 100nM (lanes 5, 8); 500 nM (lanes 6, 9); 1 µM (lanes 7, 10). EGF (10 ng/ml) was added in lanes 4, 8–10. Duplicate Western blots were made and probed with phosphoserine 80 MKK4 (top panel) or beta-actin (bottom panel). 3T3 cells are the MKK4 positive control and AsPC-1 are the negative control. Wortmannin, a PI3K inhibitor, decreased the steady state level of phosphoserine 80 MKK4 by 50% (comparing the control lane 3 to the average phosphoserine MKK4 expression in lanes 5–7). Pretreatment with wortmannin blocked the EGF stimulated increase in phosphoserine 80 MKK4 by 60%. (comparing EGF stimulated lanes to the average phosphoserine MKK4 expression in lanes 8–10).
Discussion
Previously, we had found using microarray analysis that the MKK4 (MAP2K4) gene was among the most significantly differentially expressed genes between optimally and suboptimally debulked ovarian cancers.[5] Although the absolute difference in MKK4 mRNA expression was small (1.3 fold) [5] this gene had already been implicated as an important metastasis suppressor in ovarian cancer.[6] In addition, a related MAP kinase gene (MAP3K7) also was among the most differentially expressed genes between optimally and suboptimally debulked ovarian cancers in our microarray analyses.[5] In view of this, we sought to further characterize the MKK4 gene in ovarian cancers.
In addition to its role in the MAP kinase pathway, MKK4 has been identified as a metastasis suppressor for ovarian carcinoma.[6] Overexpression of activated MKK4 reduced the number of metastatic lesions in a nude mouse model of ovarian carcinoma. In the same mouse model, overexpression of MKK4 extended the survival time of the mice after injection with ovarian carcinoma.[6] The action of high levels of MKK4 as a metastasis suppressor when artificially overexpressed predicts that metastatic ovarian cancers should have very low levels of MKK4 protein. We found, however, that 97% of primary ovarian tumors expressed detectable total MKK4 protein by western analysis. This high level of MKK4 expression is similar to findings in pancreatic cancer, where 86.7% of tumors were found to express MKK4.[10] The proportion of tumors expressing MKK4 by western blotting was higher than the proportion expressing MKK4 by immunohistochemistry. This may be explained by differences in the antibodies utilized for each method, or by extended incubation times during immunohistochemical staining. Although the stability of MKK4 and its phosphorylated isoforms is not known, the methods used for the initial banking of both the suboptimal and optimal tumor specimens was the same.
Traditional mechanisms of inactivation of metastasis suppressor genes include loss of heterozygosity, methylation, mutation or deletion. We found no evidence of MKK4 promoter hypermethylation in ovarian cancer cells. The absence of hypermethylation suggests that both alleles of the MKK4 locus are open for transcription. The LOH rate we detected for MKK4 in ovarian carcinoma was similar to that seen in metastatic prostate cancer (35%). LOH at the D17S969 locus did not appear to correlate with decreased expression of MKK4 in metastatic prostate cancer samples.[9] In gastric carcinoma, LOH was detected at both D17S969 and D17S1303; however, this did not appear to correlate with mean expression of MKK4 mRNA.[15] In breast carcinomas, a homozygous deletion of MKK4 was detected in 1/22 samples; similarly, only 1/16 biliary adenocarcinomas had a homozygous deletion in MKK4.[16] Homozygous deletions of the MKK4 locus were not seen our primary ovarian cancer samples or cell lines.
MKK4 fits within a well-defined, but complex, MAP kinase pathway that serves to integrate cellular stresses and extracellular signaling to drive cell fate. More than ten kinases are able to activate MKK4 by phosphorylation in vitro including MEKKs, MLKs, TAK1, ASK2.[12] Activated MKK4 phosphorylates downstream partners in the stress-activated portion of the MAP kinase cascade, including JNK and p38. In this paper, we have demonstrated that the upstream and downstream MKK4 stress activated MAP kinase pathway members are expressed in primary ovarian tumor specimens. The expression of these upstream and downstream effectors did not appear to be significantly different between suboptimally and optimally debulked ovarian tumors. While the presence of the proteins is important, the functional status of the pathway depends upon the ability of the proteins to phosphorylate partners. This pathway appears to be active in primary ovarian tumors, because we were able to detect phosphorylated forms of MKK4 in frozen tumor specimens.
The finding that the majority of the phosphorylated form of MKK4 in primary ovarian tumors was the inactivated phosphoserine 80 isoform was intriguing. Expression of the MKK4 phosphoserine 80 isoform correlated with optimal debulking status in primary cancers (p = 0.04). This suggests that the proportion of the phosphorylated isoform of MKK4 in ovarian cancer cells may be more important in metastasis regulation than the absolute amount of MKK4 protein. Confirmation of these results in an additional set of ovarian tumors will be important.
Under controlled in vitro conditions, treatment of normal human ovarian epithelial cells or the SKOV-3 ovarian cancer cell line with EGF or TGFβ also appears to induce MKK4 phosphorylation on the inactivate phosphoserine 80 residue. This is a rapid event, occurring within 5 minutes of stimulation. Phosphorylation appears to occur on previously synthesized MKK4 protein, while the total amount of MKK4 protein remains stable.
In the mouse, MKK4 appears to be primarily inactivated by a phosphorylation mechanism that involves the Akt pathway. Phosphorylation of mouse MKK4/SEK1 on serine 78 (ortholog of serine 80 in human) has been demonstrated in vitro by Akt (protein kinase B).[13] The phosphorylation of serine 78 inactivated mouse MKK4/SEK1. A mutant of mouse MKK4/SEK1 that changed the serine 78 residue was resistant to Akt inactivation.
In this paper, we have demonstrated a potential interaction between the Akt pathway and MKK4 pathways in human ovarian carcinoma. Wortmannin, a PI3K/Akt pathway inhibitor, diminishes the steady state level of phosphoserine 80 MKK4 in ovarian cancer cells in culture. In addition, we demonstrate that wortmannin is able to partially inhibit the EGF stimulation of ser-80 phosphorylation of MKK4. Inhibition of the Akt pathway in the SK-OV-3 ovarian cancer cell line appears to restore the functional non-phosphorylated pool of MKK4 protein. Future studies will examine the ability of wortmannin to inihibit MKK4 negative phosphorylation in other established ovarian cancer cell lines.
Additional evidence for cross-talk between the PI3K/Akt pathways is found in lung cancer cells, where PI3K and MKK4 cooperate to enhance cell survival and reduce apoptosis.[17] Akt itself functions as a cell survival factor, by functionally inactivating proteins involved in apoptosis.[18] Studies from the mouse confirm that constitutively activate Akt inhibited MKK4/SEK1 mediated apoptosis in vitro.[13]
Vander Griend et al., recently demonstrated that the active kinase function of JNKK1/MKK4 was required for the suppression of metastases in the AT6.1 model of spontaneous metastasis.[19] In addition, they found a cooperation between JNKK1/MKK4 and MKK7 in suppression of AT6.1 cell metastases. Metastasis suppression was specific for those two kinases, as they were unable to demonstrate a similar reduction with MKK6. The cooperation between MKK4 and MKK7 is notable in view of our finding from microarray experiments that identified both of these genes as differentially expressed in optimally versus suboptimally debulked ovarian carcinomas.[5] Future studies will examine the possibility of interaction of the Akt pathway with MKK7.
We have demonstrated in this paper that the traditional mechanisms of inactivation of metastasis suppressor genes do not appear to be the primary regulatory mechanisms for MKK4 in ovarian cancers. In this paper, we have presented the first demonstration of differential phosphorylation of MKK4 in human ovarian tumors. MKK4 metastasis suppressor inactivation by phosphorylation fits well with previously predicted hypotheses. In a review of MKK4[12], Cuenda predicted that inhibition of MKK4 might have a paradoxical effect. Instead of inhibiting proliferation of tumor cells, MKK4 inactivation might promote survival of cancer cells through lack of activation of JNK-mediated apoptosis pathways. Steeg also hypothesized that metastasis suppressors such as NM23, MKK4 and KAI1 are not generally mutated, but rather “turned off.”[20] The regulation of MKK4 may also be tissue and site-specific, as suggested by Cunningham.[21]
While wortmannin is an older, less specific inhibitor of the PI3K/Akt pathway, new potent inhibitors of Akt are under development by pharmaceutical companies. [22] These new inhibitors of the Akt pathway may prove to be appealing candidates for ovarian cancer therapeutics by restoring the functional active pool of MKK4 protein.
Acknowledgements
This work was supported in part by a Program of Excellence grant to MAS from the Ovarian Cancer Research Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McGuire W, Hoskins W, Brady M, Kucera P, Partridge E, Look K, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–5. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 2.Hoskins W, McGuire W, Brady M, Homesley H, Creasman W, Berman M, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol. 1994;170:974–979. doi: 10.1016/s0002-9378(94)70090-7. [DOI] [PubMed] [Google Scholar]
- 3.Omura G, Brady M, Homesley H, Yordan E, Major F, Buchsbaum H, et al. Long-term follow-up and prognostic factor analysis in advanced ovarian carcinoma: the Gynecologic Oncology Group experience. J Clin Oncol. 1991;9:1138–1150. doi: 10.1200/JCO.1991.9.7.1138. [DOI] [PubMed] [Google Scholar]
- 4.Markman M, Bundy B, Alberts D, Fowler J, Clark-Pearson D, Carson L, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001–1007. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- 5.Berchuck A, Iversen E, Lancaster J, Dressman H, West M, Nevins J, et al. Prediction of optimal versus suboptimal cytoreduction of advanced-stage serous ovarian cancer with the use of microarrays. Am J Obstet Gynecol. 2004;190(4):910–925. doi: 10.1016/j.ajog.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Yamada S, Hickson J, Hrobowski Y, Vander Griend D, Benson D, Montag A, et al. Mitogen-activated protein kinase 4 (MKK4) acts as a metastasis suppressor gene in human ovarian carcinoma. Cancer Research. 2002;62:6717–6723. [PubMed] [Google Scholar]
- 7.Yang D, Tournier C, Wysk M, Ku H-T, Xu J, Davis RJ, et al. Targeted disruption of the MKK4 gene causes embryonic death, inhibition of c-Jun NH2-terminal kinase activation, and defects in AP-1 transcriptional activity. Proc Natl Acad Sci USA. 1997;94:3004–3009. doi: 10.1073/pnas.94.7.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson V, Hickson J, Vander Griend D, Dubauskas Z, Rinker-Schaeffer C. MKK4 and metastasis suppression: a marriage of signal transduction and metastasis research. Clinical & Experimental Metastasis. 2003;20:25–30. doi: 10.1023/a:1022586318678. [DOI] [PubMed] [Google Scholar]
- 9.Kim H, Vander Griend D, Yang X, Benson D, Dubauskas Z, Yoshida B, et al. Mitogen-activated protein kinase kinase 4 metastasis suppressor gene expression in inversely related to histological pattern in advancing human prostatic cancers. Cancer Research. 2001;61:2833–2837. [PubMed] [Google Scholar]
- 10.Xin W, Yun KJ, Ricci F, Zahurak M, Qiu W, Su GH, et al. MAP2K4/MKK4 expression in pancreatic cancer: genetic validation of immunohistochemistry and realtionship to disease course. Clinical Cancer Research. 2004;10:8516–8520. doi: 10.1158/1078-0432.CCR-04-0885. [DOI] [PubMed] [Google Scholar]
- 11.Murphy S, Jirtle R. Imprinted genes as potential genetic and epigenetic toxicologic targets. Environ Health Perspect. 2000;108 Suppl 1:5–11. doi: 10.1289/ehp.00108s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuenda A. Mitogen-activated protein kinase kinase 4 (MKK4) International Journal of Biochemistry and Cell Biology. 2000;32:581–587. doi: 10.1016/s1357-2725(00)00003-0. [DOI] [PubMed] [Google Scholar]
- 13.Park H-S, Kim M-S, Huh S-H, Park J, Chung J, Kang S, et al. Akt (Protein kinase B) negatively regulates SEK1 by means of protein phosphorylation. Journal of Biological Chemistry. 2002;277(4):2573–2578. doi: 10.1074/jbc.M110299200. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez GC, Haisley C, Hurteau J, Moser T, Whitaker R, Bast RJ, et al. Regulation of invasion of epithelial ovarian cancer by transforming growth factor-beta. Gynecol Oncol. 2001;80(2):245–253. doi: 10.1006/gyno.2000.6042. [DOI] [PubMed] [Google Scholar]
- 15.Chae K-S, Ryu B-K, Lee M-G, Byun D-S, Chi S-G. Expression and mutation analyses of MKK4, a candidate tumour suppressor gene encoded by chromosome 17p, in human gastric adenocarcinoma. European Journal of Cancer. 2002;38:2048–2057. doi: 10.1016/s0959-8049(02)00147-8. [DOI] [PubMed] [Google Scholar]
- 16.Su G, Hilgers W, Shekher M, Tang D, Yeo C, Hruban R, et al. Alterations in pancreatic, biliary, and breast carcinomas support MKK4 as a genetically targeted tumor suppressor gene. Cancer Res. 1998;58:2339–2342. [PubMed] [Google Scholar]
- 17.Lee H-Y, Srinivas H, Xia D, Lu Y, Superty R, LaPushin R, et al. Evidence that phosphatidylinositol 3-kinase and mitogen-activated protein kinase-4/c-Jun NH2-terminal kinase-dependent pathways cooperate to maintain lung cancer cell survival. Journal of Biological Chemistry. 2003;278(26):23630–23638. doi: 10.1074/jbc.M300997200. [DOI] [PubMed] [Google Scholar]
- 18.Franke T, Hornik C, Segev L, Shostak G, Sugimoto C. Pi3K/Akt and apoptosis: size matters. Oncogene. 2003;22:8983–8998. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- 19.Vander Griend D, Kocherginsky M, Hickson J, Stadler W, Lin A, Rinker-Schaeffer C. Suppression of metastatic colonization by the context-dependent activation of the c-Jun NH2-terminal kinase kinases JNKK1/MKK4 and MKK7. Cancer Res. 2005;65(23):10984–10991. doi: 10.1158/0008-5472.CAN-05-2382. [DOI] [PubMed] [Google Scholar]
- 20.Steeg P. Metastasis suppressors alter the signal transduction of cancer cells. Nature Reviews Cancer. 2003;3:55–63. doi: 10.1038/nrc967. [DOI] [PubMed] [Google Scholar]
- 21.Cunningham S, Gallmeier E, Hucl T, Dezentje D, Calhoun E, Falco G, et al. Targeted deletion of MKK4 in cancer cells: a detrimental phenotype manifests as decreased experimental metastasis and suggests a counterweight to the evolution of tumor-suppressor loss. Cancer Res. 2006;66(11):5560–5564. doi: 10.1158/0008-5472.CAN-06-0555. [DOI] [PubMed] [Google Scholar]
- 22.Sawyers C. Will kinase inhbitors have a dark side? N Engl J Med. 2006;355:313–315. doi: 10.1056/NEJMcibr062354. [DOI] [PubMed] [Google Scholar]