Abstract
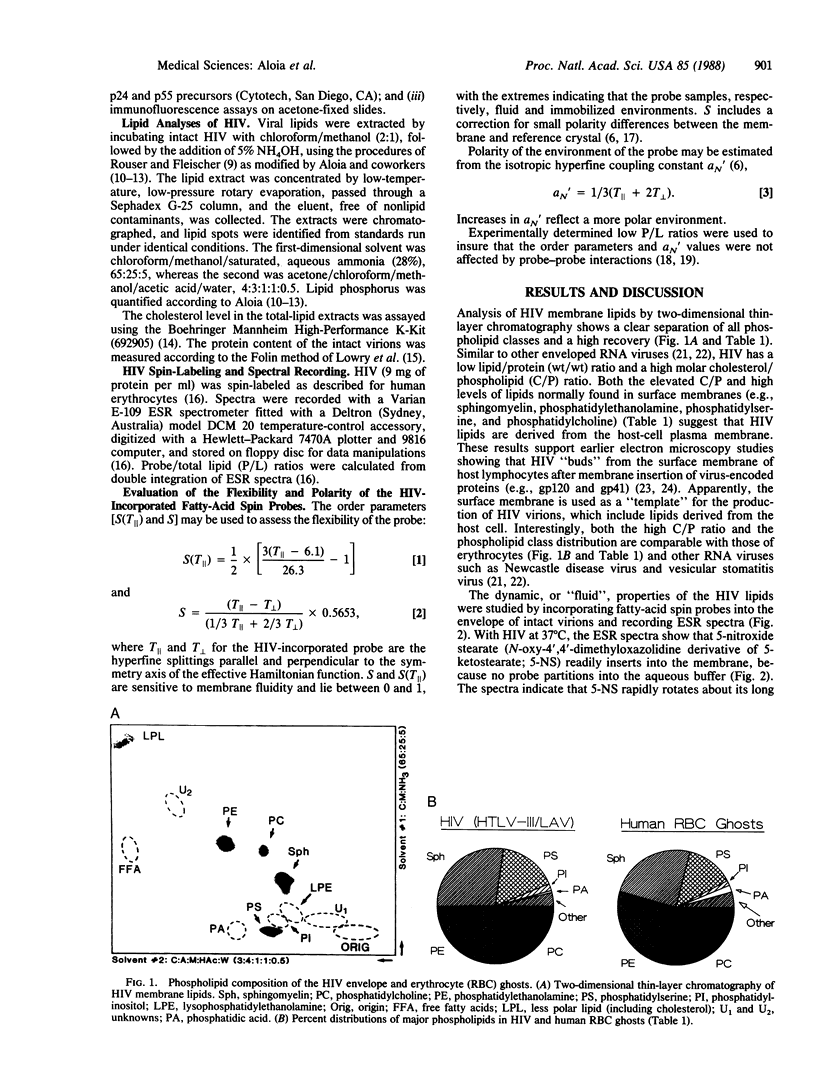
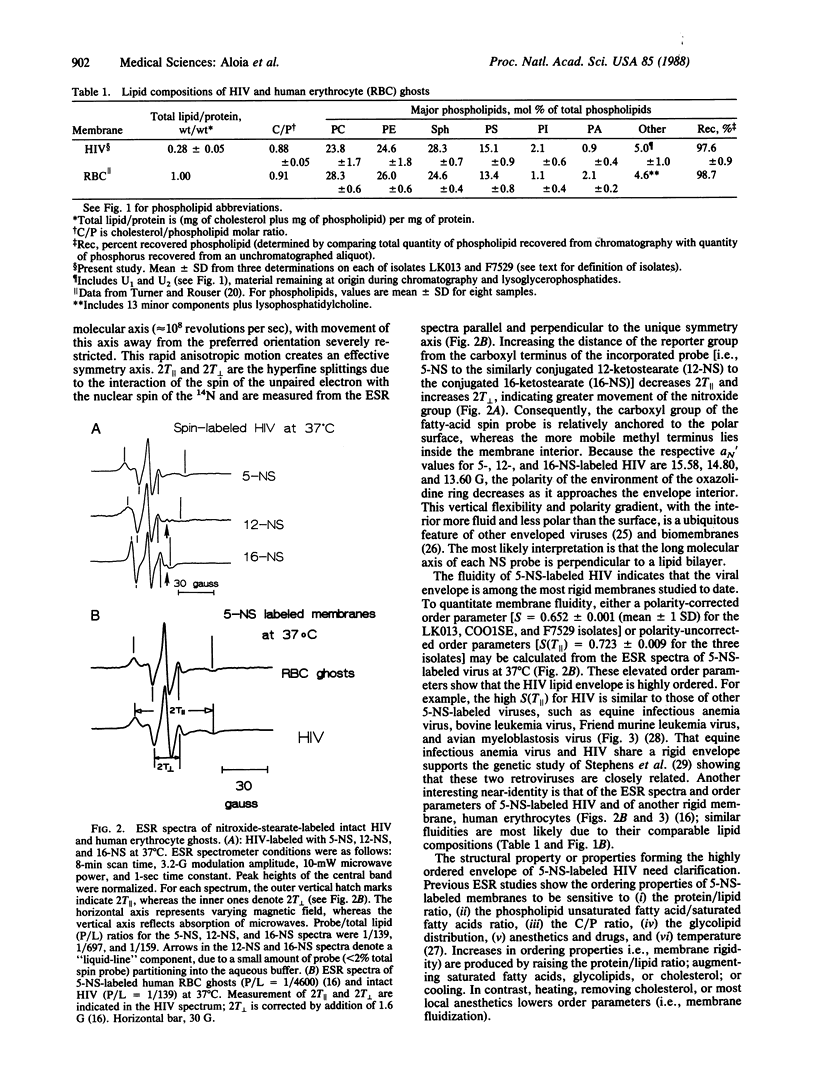
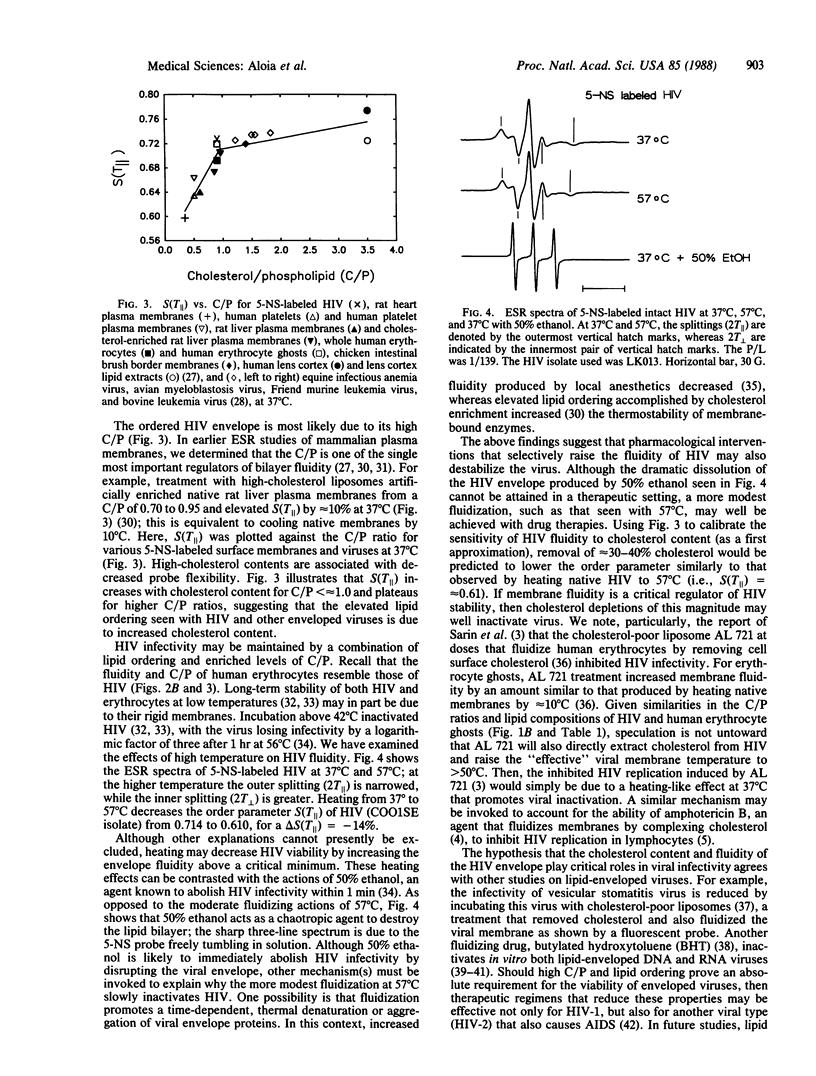
Lipid analyses of the human immunodeficiency virus (HIV) propagated in Hut 78 cells indicated a low total lipid/protein ratio, a high cholesterol/phospholipid molar ratio, and major phospholipids consisting of phosphatidylcholine, phosphatidylethanolamine, sphingomyelin, and phosphatidylserine; comparable lipid profiles were noted for human erythrocytes and other RNA viruses. Electron spin resonance (ESR) studies of HIV labeled with 5-nitroxide stearate (N-oxy-4',4'-dimethyloxazolidine derivative of ketostearate) showed a low "fluidity" at 37 degrees C, similar to other enveloped RNA viruses and erythrocytes and probably due to the high cholesterol/phospholipid ratio. Ethanol (50%) completely disrupts the envelope, contributing to the rapid inactivation of HIV by ethanol. Contrarily, heating to 57 degrees C causes much less fluidization, and this heating may play a role in the slower viral inactivation at high temperatures. Should a critical minimum ordering in the HIV envelope be necessary for viral stability and infectivity, manipulating the lipid composition or fluidizing the HIV membrane, or both, may provide an untried therapeutic approach.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloia R. C., Paxton J., Daviau J. S., van Gelb O., Mlekusch W., Truppe W., Meyer J. A., Brauer F. S. Effect of chronic alcohol consumption on rat brain microsome lipid composition, membrane fluidity and Na+-K+-ATPase activity. Life Sci. 1985 Mar 11;36(10):1003–1017. doi: 10.1016/0024-3205(85)90398-4. [DOI] [PubMed] [Google Scholar]
- Blough H. A., Lawson D. E. The lipids of paramyxoviruses: a comparative study of Sendai and Newcastle disease viruses. Virology. 1968 Oct;36(2):286–292. doi: 10.1016/0042-6822(68)90146-3. [DOI] [PubMed] [Google Scholar]
- Brugh M., Jr Butylated hydroxytoluene protects chickens exposed to Newcastle disease virus. Science. 1977 Sep 23;197(4310):1291–1292. doi: 10.1126/science.897670. [DOI] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Clavel F., Guétard D., Brun-Vézinet F., Chamaret S., Rey M. A., Santos-Ferreira M. O., Laurent A. G., Dauguet C., Katlama C., Rouzioux C. Isolation of a new human retrovirus from West African patients with AIDS. Science. 1986 Jul 18;233(4761):343–346. doi: 10.1126/science.2425430. [DOI] [PubMed] [Google Scholar]
- Curtain C. C., Looney F. D., Gordon L. M. Electron spin resonance spectroscopy in the study of lymphoid cell receptors. Methods Enzymol. 1987;150:418–446. doi: 10.1016/0076-6879(87)50098-2. [DOI] [PubMed] [Google Scholar]
- DAY A. J., JOHNSON A. R., O'HALLORAN M. W., SCHWARTZ C. J. The effect of the antioxidant butylated hydroxy toluene on serum lipid and glycoprotein levels in the rat. Aust J Exp Biol Med Sci. 1959 Jun;37:295–305. doi: 10.1038/icb.1959.31. [DOI] [PubMed] [Google Scholar]
- Dipple I., Houslay M. D. Amphoptericin B has very different effects on the glucagon and fluoride-stimulated adenylate cyclase activities of rat liver plasma membranes. FEBS Lett. 1979 Oct 1;106(1):21–24. doi: 10.1016/0014-5793(79)80686-9. [DOI] [PubMed] [Google Scholar]
- Eletr S., Williams M. A., Watkins T., Keith A. D. Perturbations of the dynamics of lipid alkyl chains in membrane systems: effect on the activity of membrane-bound enzymes. Biochim Biophys Acta. 1974 Mar 15;339(2):190–201. doi: 10.1016/0005-2736(74)90317-4. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Sarngadharan M. G., Popovic M., Shaw G. M., Hahn B., Wong-Staal F., Robert-Guroff M., Zaki Salahuddin S., Markham P. D. HTLV-III and the etiology of AIDS. Prog Allergy. 1986;37:1–45. [PubMed] [Google Scholar]
- Gordon L. M., Looney F. D., Curtain C. C. Spin probe clustering in human erythrocyte ghosts. J Membr Biol. 1985;84(1):81–95. doi: 10.1007/BF01871650. [DOI] [PubMed] [Google Scholar]
- Gordon L. M., Mobley P. W. Thermotropic lipid phase separations in human erythrocyte ghosts and cholesterol-enriched rat liver plasma membranes. J Membr Biol. 1984;79(1):75–86. doi: 10.1007/BF01868528. [DOI] [PubMed] [Google Scholar]
- Gordon L. M., Sauerheber R. D. Studies on spin-labelled egg lecithin dispersions. Biochim Biophys Acta. 1977 Apr 1;466(1):34–43. doi: 10.1016/0005-2736(77)90206-1. [DOI] [PubMed] [Google Scholar]
- Keith A. D., Arruda D., Snipes W., Frost P. The antiviral effectiveness of butylated hydroxytoluene on herpes cutaneous infections in hairless mice. Proc Soc Exp Biol Med. 1982 Jun;170(2):237–244. doi: 10.3181/00379727-170-41425. [DOI] [PubMed] [Google Scholar]
- Kim K. S., Moon H. M., Sapienza V., Carp R. I., Pullarkat R. Inactivation of cytomegalovirus and Semliki Forest virus by butylated hydroxytoluene. J Infect Dis. 1978 Jul;138(1):91–94. doi: 10.1093/infdis/138.1.91. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landsberger F. R., Compans R. W. Effect of membrane protein on lipid bilayer structure: a spin-label electron spin resonance study of vesicular stomatitis virus. Biochemistry. 1976 Jun 1;15(11):2356–2360. doi: 10.1021/bi00656a017. [DOI] [PubMed] [Google Scholar]
- Lyte M., Shinitzky M. A special lipid mixture for membrane fluidization. Biochim Biophys Acta. 1985 Jan 10;812(1):133–138. doi: 10.1016/0005-2736(85)90530-9. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Martin L. S., Cort S. P., Mozen M., Heldebrant C. M., Evatt B. L. Thermal inactivation of the acquired immunodeficiency syndrome virus, human T lymphotropic virus-III/lymphadenopathy-associated virus, with special reference to antihemophilic factor. J Clin Invest. 1985 Aug;76(2):875–877. doi: 10.1172/JCI112045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnier L. Lymphadenopathy associated virus: its role in the pathogenesis of AIDS and related diseases. Prog Allergy. 1986;37:46–64. [PubMed] [Google Scholar]
- Moore N. F., Patzer E. J., Shaw J. M., Thompson T. E., Wagner R. R. Interaction of vesicular stomatitis virus with lipid vesicles: depletion of cholesterol and effect on virion membrane fluidity and infectivity. J Virol. 1978 Aug;27(2):320–329. doi: 10.1128/jvi.27.2.320-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham L., Whetton A. D., Houslay M. D. The local anaesthetic and bilayer fluidising agent, benzyl alcohol decreases the thermostability of the integral membrane protein adenylate cyclase. FEBS Lett. 1982 Apr 5;140(1):85–88. doi: 10.1016/0014-5793(82)80526-7. [DOI] [PubMed] [Google Scholar]
- Omodeo Salè F., Marchesini S., Fishman P. H., Berra B. A sensitive enzymatic assay for determination of cholesterol in lipid extracts. Anal Biochem. 1984 Nov 1;142(2):347–350. doi: 10.1016/0003-2697(84)90475-5. [DOI] [PubMed] [Google Scholar]
- Patzer E. J., Moore N. F., Barenholz Y., Shaw J. M., Wagner R. R. Lipid organization of the membrane of vesicular stomatitis virus. J Biol Chem. 1978 Jul 10;253(13):4544–4550. [PubMed] [Google Scholar]
- Reimund E. Envelope perturbation and AIDS. Lancet. 1986 Nov 15;2(8516):1159–1159. doi: 10.1016/s0140-6736(86)90564-7. [DOI] [PubMed] [Google Scholar]
- Resnick L., Veren K., Salahuddin S. Z., Tondreau S., Markham P. D. Stability and inactivation of HTLV-III/LAV under clinical and laboratory environments. JAMA. 1986 Apr 11;255(14):1887–1891. [PubMed] [Google Scholar]
- Rho H. M., Poiesz B., Ruscetti F. W., Gallo R. C. Characterization of the reverse transcriptase from a new retrovirus (HTLV) produced by a human cutaneous T-cell lymphoma cell line. Virology. 1981 Jul 15;112(1):355–360. doi: 10.1016/0042-6822(81)90642-5. [DOI] [PubMed] [Google Scholar]
- Sarin P. S., Gallo R. C., Scheer D. I., Crews F., Lippa A. S. Effects of a novel compound (AL 721) on HTLV-III infectivity in vitro. N Engl J Med. 1985 Nov 14;313(20):1289–1290. doi: 10.1056/NEJM198511143132011. [DOI] [PubMed] [Google Scholar]
- Sauerheber R. D., Gordon L. M., Crosland R. D., Kuwahara M. D. Spin-label studies on rat liver and heart plasma membranes: do probe-probe interactions interfere with the measurement of membrane properties? J Membr Biol. 1977 Feb 24;31(1-2):131–169. doi: 10.1007/BF01869402. [DOI] [PubMed] [Google Scholar]
- Sauerheber R. D., Zimmermann T. S., Esgate J. A., VanderLaan W. P., Gordon L. M. Effects of calcium, lanthanum, and temperature on the fluidity of spin-labeled human platelets. J Membr Biol. 1980;52(3):201–219. doi: 10.1007/BF01869190. [DOI] [PubMed] [Google Scholar]
- Schaffner C. P., Plescia O. J., Pontani D., Sun D., Thornton A., Pandey R. C., Sarin P. S. Anti-viral activity of amphotericin B methyl ester: inhibition of HTLV-III replication in cell culture. Biochem Pharmacol. 1986 Nov 15;35(22):4110–4113. doi: 10.1016/0006-2952(86)90037-7. [DOI] [PubMed] [Google Scholar]
- Slosberg B. N., Montelaro R. C. A comparison of the mobilities and thermal transitions of retrovirus lipid envelopes and host cell plasma membranes by electron spin resonance spectroscopy. Biochim Biophys Acta. 1982 Jul 28;689(2):393–402. doi: 10.1016/0005-2736(82)90274-7. [DOI] [PubMed] [Google Scholar]
- Spire B., Dormont D., Barré-Sinoussi F., Montagnier L., Chermann J. C. Inactivation of lymphadenopathy-associated virus by heat, gamma rays, and ultraviolet light. Lancet. 1985 Jan 26;1(8422):188–189. doi: 10.1016/s0140-6736(85)92026-4. [DOI] [PubMed] [Google Scholar]
- Stephens R. M., Casey J. W., Rice N. R. Equine infectious anemia virus gag and pol genes: relatedness to visna and AIDS virus. Science. 1986 Feb 7;231(4738):589–594. doi: 10.1126/science.3003905. [DOI] [PubMed] [Google Scholar]
- Turner J. D., Rouser G. Precise quantitative determination of human blood lipids by thin-layer and triethylaminoethylcellulose column chromatography. I. Erythrocyte lipids. Anal Biochem. 1970 Dec;38(2):423–436. doi: 10.1016/0003-2697(70)90467-7. [DOI] [PubMed] [Google Scholar]
- Whetton A. D., Gordon L. M., Houslay M. D. Elevated membrane cholesterol concentrations inhibit glucagon-stimulated adenylate cyclase. Biochem J. 1983 Feb 15;210(2):437–449. doi: 10.1042/bj2100437. [DOI] [PMC free article] [PubMed] [Google Scholar]




