Abstract
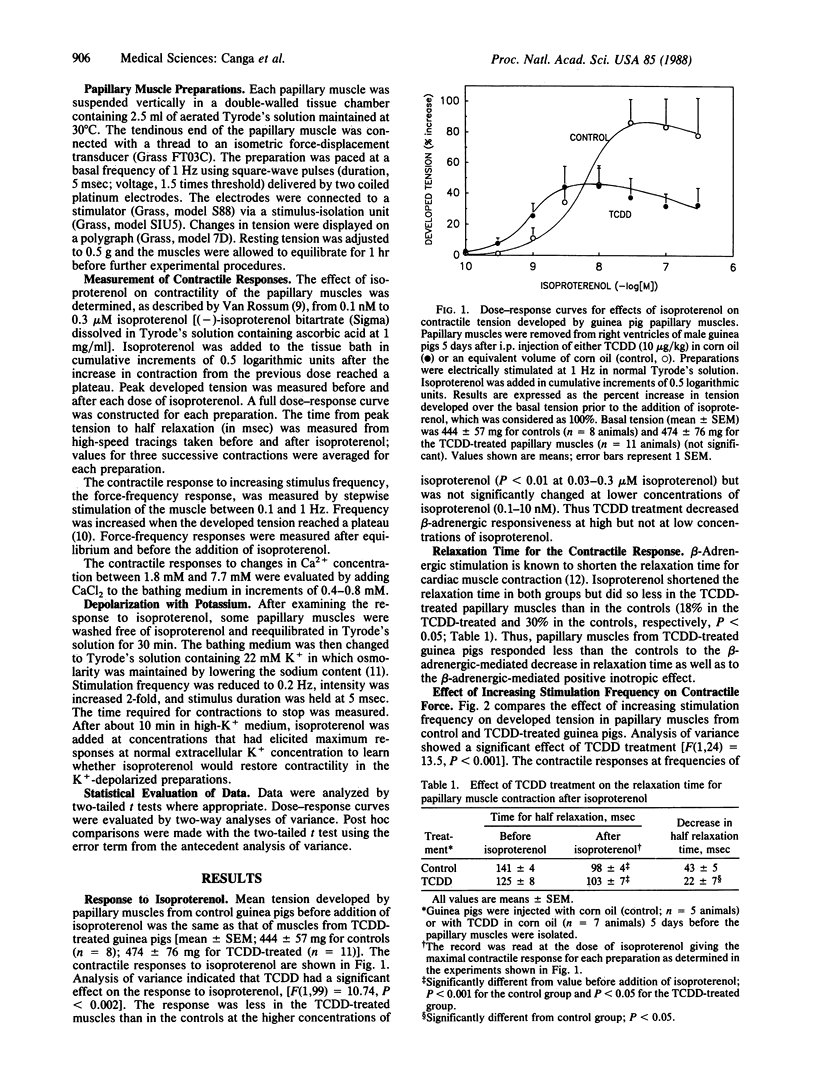
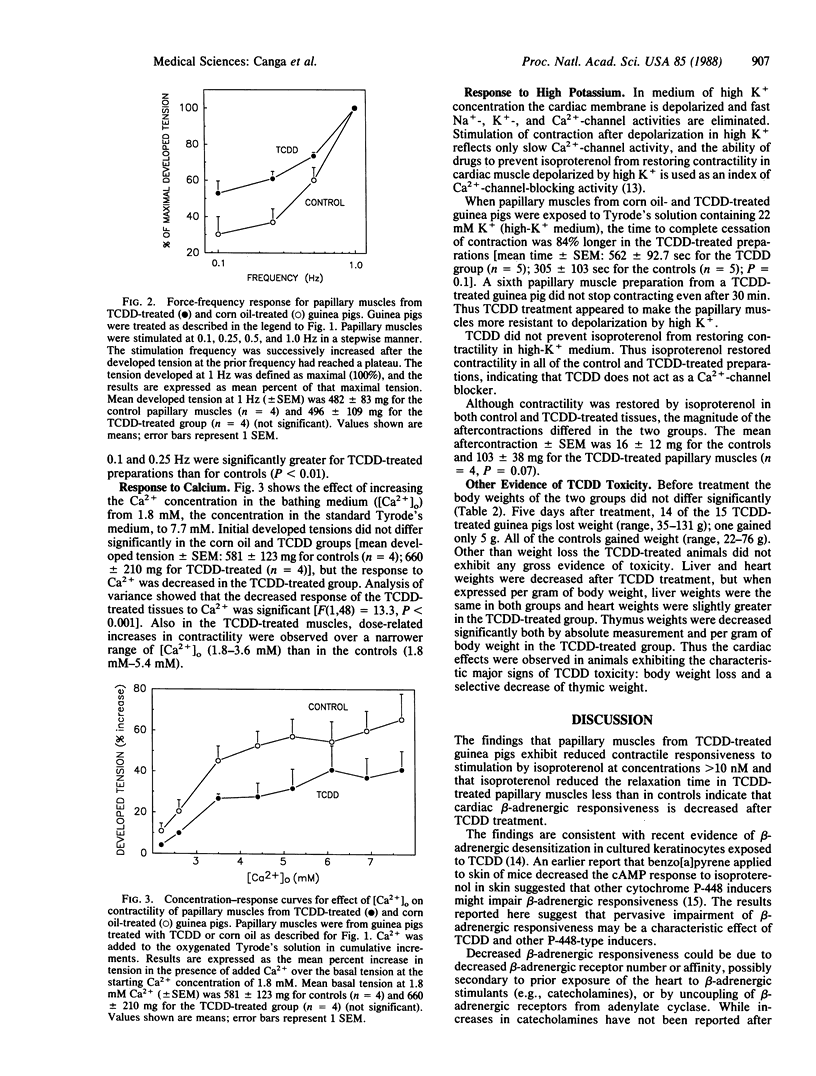
The heart has not been regarded as a major target organ of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) toxicity notwithstanding that lethal cardiac dysfunction can occur in the absence of histopathological changes. To assess possible TCDD cardiotoxicity, we studied the effect of TCDD five days after treatment (10 micrograms/kg of body weight; single dose given i.p. in corn oil) on the contractility of guinea pig right ventricular papillary muscle. Controls were treated with corn oil. TCDD treatment significantly decreased beta-adrenergic responsiveness. In papillary muscles from TCDD-treated guinea pigs, the positive inotropic effect of isoproterenol (0.03-0.3 microM) was decreased by a mean of 65% (P less than 0.001), and the enhancement in the velocity of relaxation was 60% less than in the controls (P less than 0.05). On the other hand, TCDD treatment did not alter the positive inotropic effect of lower concentrations of isoproterenol (0.1-10 nM). After TCDD, responsiveness to low-frequency stimulation (0.1 and 0.25 Hz) was enhanced, responsiveness to increases in extracellular Ca2+ concentration was attenuated, and isoproterenol-elicited aftercontractions in K+-depolarized preparations were increased in magnitude. Collectively, the latter findings suggest that in addition to decreasing beta-adrenergic responsiveness, TCDD increases the intracellular Ca2+ concentration in papillary muscle. Finally, slow Ca2+ channels were not blocked after TCDD treatment, inasmuch as isoproterenol restored contractility equally effectively in K+-depolarized TCDD-treated and control papillary muscles. Our findings indicate that TCDD causes a specific pattern of cardiac dysfunction in a mammalian species, selectively augmenting or decreasing different cardiac responses. The cardiac changes are consistent with reported membrane effects of TCDD; further, they suggest that the heart may be a major target organ for TCDD toxicity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akera T., Brody T. M. The role of Na+,K+-ATPase in the inotropic action of digitalis. Pharmacol Rev. 1977 Sep;29(3):187–220. [PubMed] [Google Scholar]
- Allen J. R., Carstens L. A. Light and electron microscopic observations in Macaca mulatta monkeys fed toxic fat. Am J Vet Res. 1967 Sep;28(126):1513–1526. [PubMed] [Google Scholar]
- Anderson W. B., Estival A., Tapiovaara H., Gopalakrishna R. Altered subcellular distribution of protein kinase C (a phorbol ester receptor). Possible role in tumor promotion and the regulation of cell growth: relationship to changes in adenylate cyclase activity. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1985;19:287–306. [PubMed] [Google Scholar]
- Bombick D. W., Madhukar B. V., Brewster D. W., Matsumura F. TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) causes increases in protein kinases particularly protein kinase C in the hepatic plasma membrane of the rat and the guinea pig. Biochem Biophys Res Commun. 1985 Feb 28;127(1):296–302. doi: 10.1016/s0006-291x(85)80158-3. [DOI] [PubMed] [Google Scholar]
- Buu-Hoï N. P., Chanh P. H., Sesque G., Azum-Gelade M. C., Saint-Ruf G. Organs as targets of "dioxin" (2,3,7,8-tetrachlorodibenzo-p-dioxin) intoxication. Naturwissenschaften. 1972 Apr;59(4):174–175. doi: 10.1007/BF00637374. [DOI] [PubMed] [Google Scholar]
- DeCaprio A. P., McMartin D. N., O'Keefe P. W., Rej R., Silkworth J. B., Kaminsky L. S. Subchronic oral toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in the guinea pig: comparisons with a PCB-containing transformer fluid pyrolysate. Fundam Appl Toxicol. 1986 Apr;6(3):454–463. doi: 10.1016/0272-0590(86)90219-8. [DOI] [PubMed] [Google Scholar]
- Hattori Y., Levi R. Negative inotropic effect of leukotrienes: leukotrienes C4 and D4 inhibit calcium-dependent contractile responses in potassium-depolarized guinea-pig myocardium. J Pharmacol Exp Ther. 1984 Sep;230(3):646–651. [PubMed] [Google Scholar]
- Homcy C. J., Graham R. M. Molecular characterization of adrenergic receptors. Circ Res. 1985 May;56(5):635–650. doi: 10.1161/01.res.56.5.635. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Lederer W. J., Tsien R. W., Weingart R. Role of calcium ions in transient inward currents and aftercontractions induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978 Aug;281:187–208. doi: 10.1113/jphysiol.1978.sp012416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy R. H., Akera T., Brody T. M. The effect of stimulation frequency and calcium concentration on maintenance of developed tension in isolated heart muscle preparations. J Pharmacol Methods. 1987 Apr;17(2):95–110. doi: 10.1016/0160-5402(87)90021-0. [DOI] [PubMed] [Google Scholar]
- Langer G. A. Sodium exchange in dog ventricular muscle. Relation to frequency of contraction and its possible role in the control of myocardial contractility. J Gen Physiol. 1967 May;50(5):1221–1239. doi: 10.1085/jgp.50.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappano A. J. Calcium-dependent action potentials produced by catecholamines in guinea pig atrial muscle fibers depolarized by potassium. Circ Res. 1970 Sep;27(3):379–390. doi: 10.1161/01.res.27.3.379. [DOI] [PubMed] [Google Scholar]
- Peterson R. E., Madhukar B. V., Yang K. H., Matsumura F. Depression of adenosine triphosphatase activities in isolated liver surface membranes of 2,3,7,8-tetrachlorodibenzo-p-dioxin-treated rats: correlation with effects on ouabain biliary excretion and bile flow. J Pharmacol Exp Ther. 1979 Aug;210(2):275–282. [PubMed] [Google Scholar]
- Poland A., Knutson J. C. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Rifkind A. B., Muschick H. Benoxaprofen suppression of polychlorinated biphenyl toxicity without alteration of mixed function oxidase function. Nature. 1983 Jun 9;303(5917):524–526. doi: 10.1038/303524a0. [DOI] [PubMed] [Google Scholar]
- Ringer R. K. PBB fed to immature chickens: its effect on organ weights and function and on the cardiovascular system. Environ Health Perspect. 1978 Apr;23:247–255. doi: 10.1289/ehp.7823247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman M., Ferreri N. R., Carroll M. A., Songu-Mize E., McGiff J. C. Renal cytochrome P450-related arachidonate metabolite inhibits (Na+ + K+)ATPase. Nature. 1985 Apr 18;314(6012):620–622. doi: 10.1038/314620a0. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Nambi P., Peters J. R., Lefkowitz R. J. Phorbol diesters promote beta-adrenergic receptor phosphorylation and adenylate cyclase desensitization in duck erythrocytes. Biochem Biophys Res Commun. 1984 Jun 29;121(3):973–979. doi: 10.1016/0006-291x(84)90772-1. [DOI] [PubMed] [Google Scholar]
- Thyrum P. T. Inotropic stimuli and systolic transmembrane calcium flow in depolarized guinea-pig atria. J Pharmacol Exp Ther. 1974 Jan;188(1):166–179. [PubMed] [Google Scholar]
- Tsien R. W. Cyclic AMP and contractile activity in heart. Adv Cyclic Nucleotide Res. 1977;8:363–420. [PubMed] [Google Scholar]
- VAN ROSSUM J. M. Cumulative dose-response curves. II. Technique for the making of dose-response curves in isolated organs and the evaluation of drug parameters. Arch Int Pharmacodyn Ther. 1963;143:299–330. [PubMed] [Google Scholar]
- Verma A. K., Murray A. W. The effect of benzo(alpha)pyrene on the basal and isoproterenol-stimulated levels of cyclic adenosine 3',5'-monophosphate in mouse epidermis. Cancer Res. 1974 Dec;34(12):3408–3413. [PubMed] [Google Scholar]



