Abstract
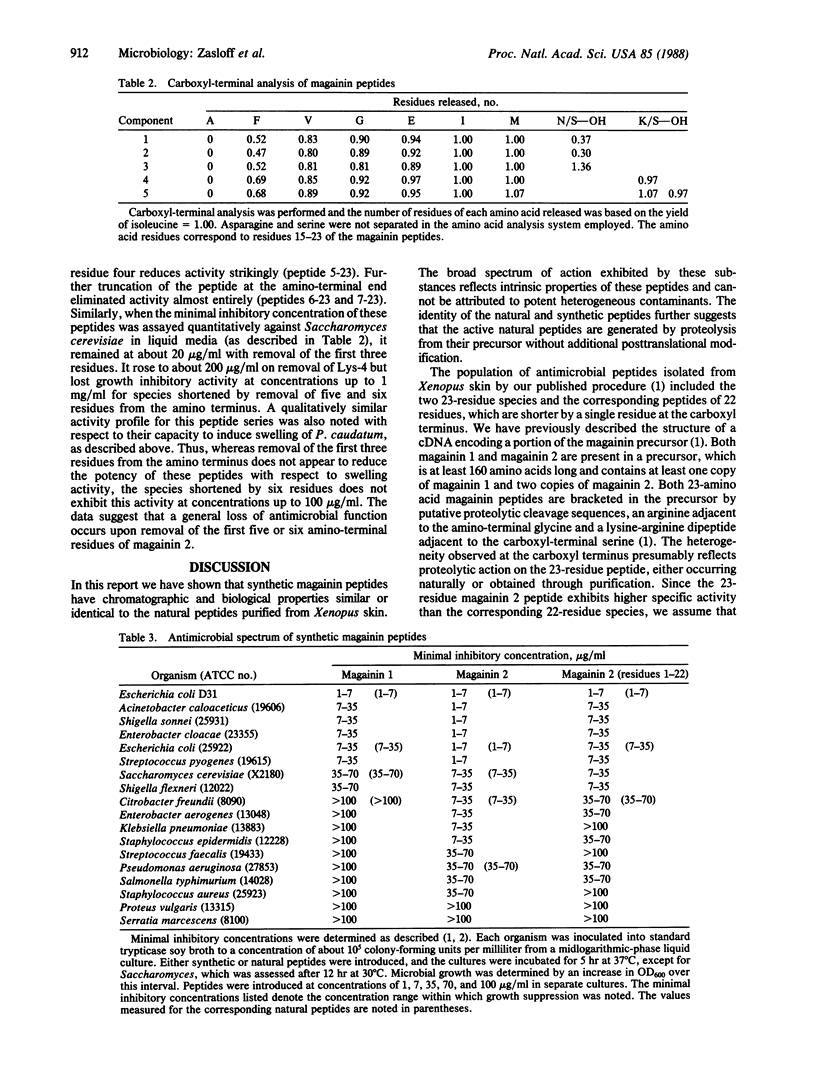
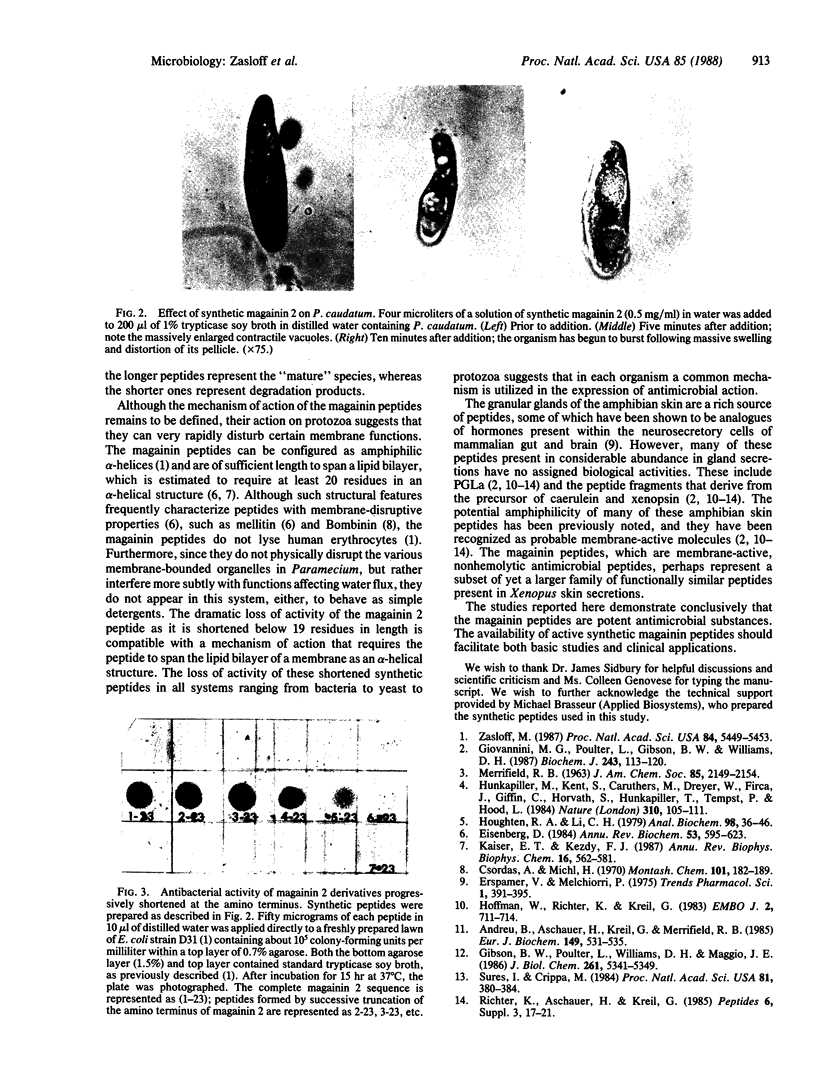
We have previously reported the isolation of two broad-spectrum antimicrobial peptides ("magainins") from the skin of the African clawed frog Xenopus laevis. These natural peptides are active against many species of bacteria and fungi and also induce osmotic lysis of protozoa. In this report we demonstrate that synthetic magainin peptides appear to be indistinguishable from the natural products with respect to chromatographic properties and biological activity. These studies demonstrate conclusively that the magainin peptides are potent antimicrobial substances.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreu D., Aschauer H., Kreil G., Merrifield R. B. Solid-phase synthesis of PYLa and isolation of its natural counterpart, PGLa [PYLa-(4-24)] from skin secretion of Xenopus laevis. Eur J Biochem. 1985 Jun 18;149(3):531–535. doi: 10.1111/j.1432-1033.1985.tb08957.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- Gibson B. W., Poulter L., Williams D. H., Maggio J. E. Novel peptide fragments originating from PGLa and the caerulein and xenopsin precursors from Xenopus laevis. J Biol Chem. 1986 Apr 25;261(12):5341–5349. [PubMed] [Google Scholar]
- Giovannini M. G., Poulter L., Gibson B. W., Williams D. H. Biosynthesis and degradation of peptides derived from Xenopus laevis prohormones. Biochem J. 1987 Apr 1;243(1):113–120. doi: 10.1042/bj2430113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann W., Richter K., Kreil G. A novel peptide designated PYLa and its precursor as predicted from cloned mRNA of Xenopus laevis skin. EMBO J. 1983;2(5):711–714. doi: 10.1002/j.1460-2075.1983.tb01489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghten R. A., Li C. H. Reduction of sulfoxides in peptides and proteins. Anal Biochem. 1979 Sep 15;98(1):36–46. doi: 10.1016/0003-2697(79)90702-4. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M., Kent S., Caruthers M., Dreyer W., Firca J., Giffin C., Horvath S., Hunkapiller T., Tempst P., Hood L. A microchemical facility for the analysis and synthesis of genes and proteins. Nature. 1984 Jul 12;310(5973):105–111. doi: 10.1038/310105a0. [DOI] [PubMed] [Google Scholar]
- Kaiser E. T., Kézdy F. J. Peptides with affinity for membranes. Annu Rev Biophys Biophys Chem. 1987;16:561–581. doi: 10.1146/annurev.bb.16.060187.003021. [DOI] [PubMed] [Google Scholar]
- Richter K., Aschauer H., Kreil G. Biosynthesis of peptides in the skin of Xenopus laevis: isolation of novel peptides predicted from the sequence of cloned cDNAs. Peptides. 1985;6 (Suppl 3):17–21. doi: 10.1016/0196-9781(85)90345-6. [DOI] [PubMed] [Google Scholar]
- Sures I., Crippa M. Xenopsin: the neurotensin-like octapeptide from Xenopus skin at the carboxyl terminus of its precursor. Proc Natl Acad Sci U S A. 1984 Jan;81(2):380–384. doi: 10.1073/pnas.81.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]




