Abstract
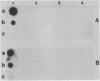
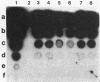
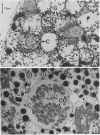
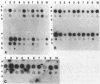
Anaplasmosis is the most widely distributed of several important tick-borne diseases that constrain cattle production throughout much of the world. Evaluation of the effectiveness of disease control strategies that integrate vaccination with tick control requires the ability to monitor tick and cattle infection rates. To detect Anaplasma marginale in ticks and bovine erythrocytes, a 2-kilobase DNA fragment from a cloned A. marginale gene coding for a surface protein having a Mr of 105,000 was prepared and evaluated as a probe. The probe was species specific and detected A. marginale DNA derived from infected bovine erythrocytes and adult Dermacentor ticks infected either as nymphs or adults. Tick infection was confirmed by microscopy and test feeding on a susceptible calf. The sensitivity of the probe is suitable for detecting infected ticks in experimental and field epizootiology studies.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGDORFER W., LACKMAN D. Identification of Rickettsia rickettsii in the wood tick, Dermacentor andersoni, by means of fluorescent antibody. J Infect Dis. 1960 Sep-Oct;107:241–244. doi: 10.1093/infdis/107.2.241. [DOI] [PubMed] [Google Scholar]
- Barbet A. F., Palmer G. H., Myler P. J., McGuire T. C. Characterization of an immunoprotective protein complex of Anaplasma marginale by cloning and expression of the gene coding for polypeptide Am105L. Infect Immun. 1987 Oct;55(10):2428–2435. doi: 10.1128/iai.55.10.2428-2435.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff W. L., Johnson W. C., Kuttler K. L. Development of an indirect fluorescent antibody test, using microfluorometry as a diagnostic test for bovine anaplasmosis. Am J Vet Res. 1985 May;46(5):1080–1084. [PubMed] [Google Scholar]
- Goff W. L., Yunker C. E. Babesia bovis: increased percentage parasitized erythrocytes in cultures and assessment of growth by incorporation of [3H]hypoxanthine. Exp Parasitol. 1986 Oct;62(2):202–210. doi: 10.1016/0014-4894(86)90024-x. [DOI] [PubMed] [Google Scholar]
- Howley P. M., Israel M. A., Law M. F., Martin M. A. A rapid method for detecting and mapping homology between heterologous DNAs. Evaluation of polyomavirus genomes. J Biol Chem. 1979 Jun 10;254(11):4876–4883. [PubMed] [Google Scholar]
- Kocan K. M., Hair J. A., Ewing S. A., Stratton L. G. Transmission of Anaplasma marginale Theiler by Dermacentor andersoni Stiles and Dermacentor variabilis (Say). Am J Vet Res. 1981 Jan;42(1):15–18. [PubMed] [Google Scholar]
- Kocan K. M., Hair J. A., Ewing S. A. Ultrastructure of ANaplasma marginale Theiler in Dermacentor andersoni Stiles and Dermacentor variabilis (Say). Am J Vet Res. 1980 Dec;41(12):1966–1976. [PubMed] [Google Scholar]
- Kocan K. M., Oberst R. D., Ewing S. A., Hair J. A., Barron S. J. Demonstration of Anaplasma marginale in hemolymph of Dermacentor andersoni by animal inoculation and by fluorescent-antibody technique. Am J Vet Res. 1983 May;44(5):798–801. [PubMed] [Google Scholar]
- Lawrie J. M., Jackson P. R., Stiteler J. M., Hockmeyer W. T. Identification of pathogenic Leishmania promastigotes by DNA: DNA hybridization with kinetoplast DNA cloned into E. coli plasmids. Am J Trop Med Hyg. 1985 Mar;34(2):257–265. doi: 10.4269/ajtmh.1985.34.257. [DOI] [PubMed] [Google Scholar]
- Maas J., Lincoln S. D., Coan M. E., Kuttler K. L., Zaugg J. L., Stiller D. Epidemiologic aspects of bovine anaplasmosis in semiarid range conditions of south central Idaho. Am J Vet Res. 1986 Mar;47(3):528–533. [PubMed] [Google Scholar]
- Magonigle R. A., Eckblad W. P., Lincoln S. D., Frank F. W. Anaplasma ovis in Idaho sheep. Am J Vet Res. 1981 Feb;42(2):199–201. [PubMed] [Google Scholar]
- McGuire T. C., Palmer G. H., Goff W. L., Johnson M. I., Davis W. C. Common and isolate-restricted antigens of Anaplasma marginale detected with monoclonal antibodies. Infect Immun. 1984 Sep;45(3):697–700. doi: 10.1128/iai.45.3.697-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., Barbet A. F., Davis W. C., McGuire T. C. Immunization with an isolate-common surface protein protects cattle against anaplasmosis. Science. 1986 Mar 14;231(4743):1299–1302. doi: 10.1126/science.3945825. [DOI] [PubMed] [Google Scholar]
- Palmer G. H., McGuire T. C. Immune serum against Anaplasma marginale initial bodies neutralizes infectivity for cattle. J Immunol. 1984 Aug;133(2):1010–1015. [PubMed] [Google Scholar]
- Zaugg J. L., Stiller D., Coan M. E., Lincoln S. D. Transmission of Anaplasma marginale Theiler by males of Dermacentor andersoni Stiles fed on an Idaho field-infected, chronic carrier cow. Am J Vet Res. 1986 Oct;47(10):2269–2271. [PubMed] [Google Scholar]