Abstract
A 1 year carcinogenicity bioassay was conducted in rats treated with three short cycles of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)/high-fat (HF) diet, followed by 2% white tea (wt/vol), 0.05% epigallocatechin-3-gallate (EGCG) or 0.065% caffeine as sole source of fluid intake. Thirty-two percent of the PhIP/HF controls survived to 1 year, compared with 50, 48.7 and 18.2% in groups given white tea, EGCG and caffeine, respectively. After 1 year, PhIP/HF controls had tumors in the colon, skin, small intestine, Zymbal’s gland, salivary gland and pancreas. For all sites combined, excluding the colon, tumor incidence data were as follows: PhIP/HF 69.5%, PhIP/HF + EGCG 48.7%, PhIP/HF + white tea 46.9% and PhIP/HF + caffeine 13.3%. Unexpectedly, a higher incidence of colon tumors was detected in rats post-treated with white tea (69%) and caffeine (73%) compared with the 42% incidence in PhIP/HF controls. In the colon tumors, β-catenin mutations were detected at a higher frequency after caffeine posttreatment, and there was a shift toward more tumors harboring substitutions of Gly34 with correspondingly high protein and messenger RNA expression seen for both β-catenin and c-Myc. c-Myc expression exhibited concordance with tumor promotion, and there was a concomitant increase in cell proliferation versus apoptosis in colonic crypts. A prior report described suppression of PhIP-induced colonic aberrant crypts by the same test agents, but did not incorporate a HF diet. These findings are discussed in the context of epidemiological data which do not support an adverse effect of tea and coffee on colon tumor outcome—indeed, some such studies suggest a protective role for caffeinated beverages.
Introduction
It is now well established that human colorectal cancers contain mutations that stabilize the β-catenin protein, causing constitutive activation of β-catenin/T-cell factor (Tcf) signaling and overexpression of downstream targets, such as c-Myc, c-Jun and cyclin D1 (1). Similar findings have been reported in preclinical models of colon cancer. For example, carcinogen-induced rat colon tumors contain oncogenic mutants of β-catenin that are resistant to degradation via the phosphorylation/ubiquitination/proteosome pathway (2), and β-catenin stabilization constitutively activates c-myc, c-jun and cyclin D1 expression in the corresponding tumors (3,4). However, the frequency and spectrum of β-catenin mutations depends on the dosing protocol employed. Continuous feeding of the heterocyclic amine 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) produced colon tumors with high levels of β-catenin expression (5), and the tumors harbored mutations in either β-catenin or adenomatous polyposis coli (6). When dietary PhIP was given via three short cycles followed by a high-fat (HF) diet, β-catenin protein again was expressed at high levels, but only 55% of the colon tumors harbored β-catenin or adenomatous polyposis coli mutations (7). The spectrum and frequency of β-catenin mutations also can be influenced by post-initiation exposure to phytochemicals. For example, a higher proportion of tumors containing direct substitutions in critical Ser/Thr residues of the β-catenin protein was seen following treatment with indole-3-carbinol and chlorophyllin (3,4).
In the present investigation, we sought to test the post-initiation tumor-suppressing effects of white tea and two of its major constituents, namely caffeine and the polyphenolic compound epigallocatechin-3-gallate (EGCG), in rats treated with PhIP/HF diet. White tea is the least processed type of tea; leaves of Camellia sinensis contain polyphenols that undergo increasing oxidation in the conversion of white to green to oolong and finally to black tea (8). Prior studies with white tea showed (i) potent antimutagenic effects against PhIP and other heterocyclic amines in the Salmonella assay; (ii) inhibition of β-catenin/Tcf activity in vitro; (iii) suppression of intestinal polyps in the Apcmin mouse and (iv) protection against PhIP-induced colonic aberrant crypt foci (ACF) in the rat (8–12). In the present report, white tea and caffeine given post-initiation unexpectedly promoted rather than suppressed PhIP-induced colon tumors. Caffeine increased β-catenin and c-Myc expression, altered the spectrum and frequency of β-catenin mutations and augmented cell proliferation while inhibiting apoptosis in colonic crypts.
Materials and methods
Test agents
PhIP was purchased from Toronto Research Chemicals (Ontario, Canada) and prepared as an 8 mg/ml solution in test vehicle (0.8% dimethylsulfoxide in milliQ water, adjusted to pH 3.5 with 0.1 N HCl). AIN-93G, AIN-93M and AIN-93G supplemented with 23% (wt/wt) hydrogenated vegetable oil (HF diet) were obtained from Dyets (Bethlehem, PA). Mutan White tea (referred to hereafter as ‘white tea’) was provided by Stash Tea Co. (Portland, OR). A solution of 2% (wt/vol) white tea was prepared by brewing loose leaf tea (2 g/100 ml) for 3 min in just-boiled 0.5% citric acid buffer (26 mM, pH 2.7). EGCG (TEAVIGO™, DSM Nutritional Products, Basel, Switzerland) and caffeine (Sigma–Aldrich, St Louis, MO) also were dissolved in 0.5% citric acid buffer, which enhances the stability of tea constituents compared with milliQ water alone. Solutions were prepared fresh every 2–3 days, and the stability of tea constituents was verified by high-performance liquid chromatography, as reported (8). 5-Bromo-2′-deoxyuridine (BrdU; Sigma–Aldrich) was prepared as a 20 mM solution in phosphate-buffered saline (PBS).
Animals and treatments
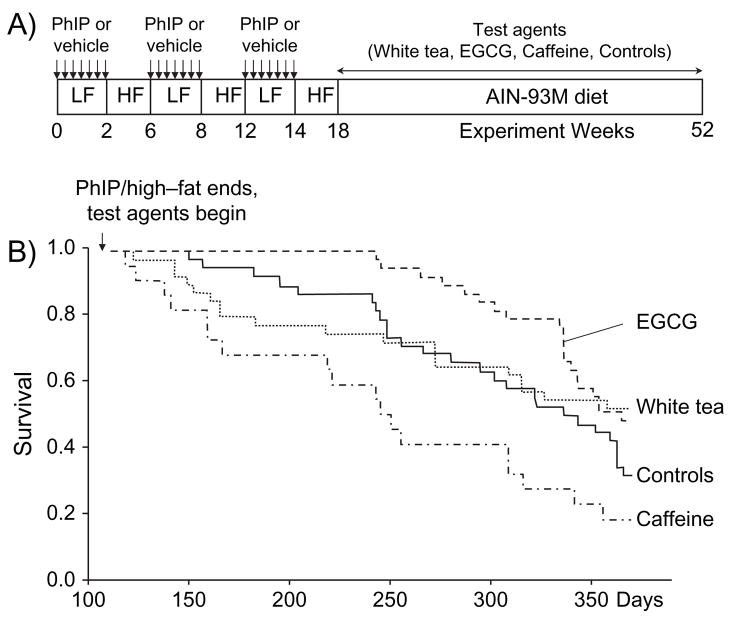
These studies received prior approval from Oregon State University Institutional Animal Care and Use Committee. Male F344 rats, 3–4 weeks of age, were purchased from the National Cancer Institute and housed in a ventilated, temperature-controlled room at 25°C with a 12 h light–dark cycle. After acclimatization to the basal diet (AIN-93G) for 3 days, rats were treated with three cycles of PhIP/HF diet using a protocol modified from Ubagai et al. (7). Specifically, PhIP (50 mg/kg) or vehicle alone was given by oral gavage every day for 2 weeks and rats were fed standard AIN-93G (low fat) diet, and this was followed by 4 weeks on AIN-93G HF diet, during which time no PhIP was administered (Figure 1A). After three such cycles of PhIP/HF treatment, rats were switched to standard AIN-93M diet for the remainder of the study. At this time, rats were randomly assigned to the various treatment groups, namely 2% white tea, 0.05% EGCG, 0.065% caffeine or citrate buffer alone (controls), administered as before (12) as sole source of drinking fluid. Test and vehicle groups initially comprised 40 and 10 rats each, respectively, and survival was followed until the study was terminated at 1 year. Rats were euthanized early due to one of the following signs of morbidity: (i) sudden loss of body weight, appetite or fluid intake; (ii) bleeding in the stools over several days and (iii) overt discomfort and breathing difficulty or other signs of malaise. In all cases, rats were euthanized by CO2 inhalation and a thorough necropsy was performed. One hour before killing, four rats in each group were selected at random to receive an intraperitoneal injection of 200 μmol BrdU/kg body wt.
Fig. 1.
Dosing protocol and survival curves of rats given PhIP/HF diet followed by white tea, EGCG or caffeine. (A) Male F344 rats were fed standard AIN-93G (low fat, LF) diet and given vehicle or PhIP (50 mg/kg) by oral gavage every day for 2 weeks, followed by 4 weeks on AIN-93G supplemented with 23% (wt/wt) hydrogenated vegetable oil (HF diet), during which time no PhIP was administered. After three such cycles, rats were switched to standard AIN-93M diet and given 2% white tea, 0.05% EGCG, 0.065% caffeine or citrate buffer alone (controls) as sole source of drinking fluid. (B) Survival curves of animals given PhIP/HF followed by white tea, EGCG or caffeine. The first sign of morbidity was at day 128 in the group post-treated with caffeine (dotted–dashed line). Data shown in the figure are cumulative and include animals that were euthanized before the study was terminated at 1 year.
Histopathology and immunohistochemistry
The following tissues were collected at necropsy: colon, small intestine, pancreas, prostate, lung, liver, kidney, bladder, skin and Zymbal’s gland. The colon and small intestine were cleaned with cold PBS and opened longitudinally so as to record the position and size of tumors. Small tumors (<10 mm3) were fixed whole in 10% formalin, whereas larger tumors were sectioned longitudinally into two or three portions, one of which was fixed in 10% formalin, stained with hematoxylin and eosin and analyzed by light microscopy. Other portions were stored at −80°C for molecular analyses. After tumors were removed, colons from rats treated with BrdU were fixed in 10% buffered formalin and embedded longitudinally in paraffin, from which serial sections were cut for immunostaining.
Immunostaining used the BrdU in situ detection kit (BD Bioscience, San Diego, CA) according to the manufacturer’s instructions. Sections were dewaxed and rehydrated, and after antigen retrieval with BD™ Retrieval A working solution in a microwave oven (80°C, 10 min) and slow cooling >20 min, slides were blocked for 10 min with 3% H2O2, rinsed three times in PBS and incubated with biotinylated anti-BrdU monoclonal antibody (1:40 dilution) for 1 h in a humidified chamber. After reaction with streptavidin peroxidase, sections were stained with Nova Red (Vector Laboratories, Burlingame, CA) and counterstained with hematoxylin.
Cleaved caspase-3 was determined using the EnVision+System–HRP Kit (Dako, Carpinteria, CA). Sections were dewaxed, rehydrated, rinsed with Dako AR buffer and then heated in a pressure cooker (121°C) for 5 min, followed by 20 min cooling at room temperature. After washing with water, endogenous peroxidases were blocked by incubating sections in 3% H2O2 in Dako TBST for 10 min, followed by Tris-buffered saline Tween-20 solution (TBST) alone. Sections were blocked with Dako serum-free protein for 10 min and then covered with polyclonal rabbit anticleaved caspase-3 (1:50 dilution in PBS), which specifically detects the endogenous large fragment of cleaved caspase-3 resulting from cleavage adjacent to Asp175 (Cell Signaling Technology, Danvers, MA). Sections were developed with Nova Red for 7 min, rinsed in water and counterstained with hematoxylin.
Labeling indices were determined based on the number of positive cells/total cells per crypt, using at least 45 well-distinguished crypts per rat; that is at least 15 crypts in the distal, middle and proximal regions of the colon. Only complete, well-oriented, longitudinally sectioned crypts with lumen at the top and muscularis mucosae at the base were evaluated.
Immunoblotting
Western analyses of β-catenin and c-Myc, normalized to β-actin, were examined as described before (3,4). In brief, frozen samples of tumors and adjacent normal-looking tissue were thawed and homogenized in lysis buffer, then centrifuged at 15 000 r.p.m. for 5 min and the supernatant (20 μg protein) was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis on a 4–12% bis–Tris gel (Novex, Invitrogen, Carlsbad, CA), followed by transfer to nitrocellulose membrane (Invitrogen). Equal protein loading was confirmed by Amido Black staining. The membrane was blocked for 1 h with 2% bovine serum albumin, followed by incubation with primary antibody overnight at 4°C, ending with secondary antibody conjugated with horseradish peroxidase (Bio-Rad, Hercules, CA). Primary antibodies were as reported (3).
Quantitative real-time polymerase chain reaction
Isolation of messenger RNA (mRNA) from tissues and quantification of β-catenin gene (Ctnnb1) (rat), cyclin D1, c-myc, c-jun and glyceraldehyde-3-phosphate dehydrogenase were performed using the methodology reported by Wang et al. (13).
Single-strand conformation polymorphism and direct sequencing
Screening for β-catenin mutations by polymerase chain reaction (PCR)-based single-strand conformation polymorphism analysis coupled with direct sequencing was performed using the methodology reported elsewhere (3,4,13).
Statistics
Tumor incidence and multiplicity (number of tumors/tumor-bearing animal) were compared by logistic regression and non-parametric rank tests, respectively. Student’s t-test and analysis of variance were used, respectively, for pairwise and group comparisons of molecular data.
Results
Survival was enhanced by EGCG but reduced by caffeine
The concentrations of EGCG and caffeine used here were similar to those present in brewed white tea beverage and did not adversely affect body weights for the first few weeks after the start of their administration; indeed, a slight gain in body weights was detected for the three treatment groups versus the control group, but this was not statistically significant (data not presented). Some of the animals were euthanized early due to the presence of large Zymbal’s gland or skin tumors, whereas others suddenly lost appetite and/or body weight and at necropsy presented with colon or other tumors. In the PhIP/HF control group, the first sign of morbidity appeared at day 154, and less than one-third of the animals (32%) survived to the end of 1 year (Figure 1). In the group post-treated with EGCG, the first sign of morbidity was delayed significantly, until day 243, and the overall survival rate at 1 year was 49% (P = 0.033 versus PhIP/HF controls, Wilcoxon test). In the group post-treated with caffeine, initial signs of morbidity were seen as early as day 128, and only 18% survived to 1 year (P = 0.013 versus PhIP/HF controls). In rats post-treated with white tea, the first sign of morbidity was on day 132, fully 3 weeks before PhIP/HF controls showed morbidity, but 52% survived to 1 year; there was no significant difference overall compared with PhIP/HF controls (P = 0.580, trend analysis).
Unexpected promotion of colon tumors by white tea and caffeine
No tumors were detected in any of the vehicle/HF groups when the study was terminated after 1 year, but a broad spectrum of tumors was seen in animals given PhIP/HF diet (Table I). As expected, the colon was the most common target organ, and 41.6% of the PhIP/HF controls developed adenocarcinomas of the colon (Table I). In the same group, the incidence of tumors at other sites after 1 year was as follows: skin 27.8%, small intestine adenocarcinomas 19.4%, Zymbal’s gland sebaceous squamous cell carcinomas 11.1%, acinary pancreatic adenocarcinomas 5.6% and salivary gland adenocarcinomas 5.6%. A similar range of tumors was reported before in rats given PhIP, including pancreatic tumors (14), although to our knowledge this is the first observation of PhIP-induced salivary gland tumors. Histopathology studies identified a wide spectrum of lesions in the skin, including sebaceous adenoma, sebaceous epithelioma, squamous cell carcinoma, papilloma, keratoacanthoma, cystic basal cell tumor and basal cell carcinoma. PhIP was reported as a prostate carcinogen when fed continuously in the diet for 1 year (15), but no prostate lesions were detected in the present investigation or in a prior study that cycled PhIP/HF (7).
Table I.
Cumulative incidence of colon and non-colon tumors induced in the rat by three short cycles of PhIP/HF diet, and the effects of EGCG, white tea and caffeine given post-initiation
| Tumor site/target organ | PhIP/HF controls (%) | PhIP/HF + EGCG (%) | PhIP/HF + white tea (%) | PhIP/HF + caffeine (%) |
|---|---|---|---|---|
| Colon (primary target organ) | 41.6 | 43.6 | 68.5* | 73.3* |
| Non-colon (other tumor sites) | ||||
| Skin | 27.8 | 23.1 | 21.9 | 6.7 |
| Small intestine | 19.4 | 7.7 | 12.5 | 6.7 |
| Zymbal’s gland | 11.1 | 7.7 | 3.1 | 0 |
| Pancreas | 5.6 | 0 | 3.1 | 0 |
| Salivary gland | 5.6 | 0 | 3.1 | 0 |
| Bladder | 0 | 2.6 | 3.1 | 0 |
| Mammary gland | 0 | 0 | 3.1 | 0 |
| All non-colon combineda | 69.5 | 48.7 | 46.9 | 13.3*** |
The data shown in the table are cumulative and include animals that were euthanized early before the study was finally terminated at 1 year.
Includes other occasional rare tumors—sarcoma, lymphoma, osteosarcoma and fibrous histocytoma.
P < 0.05,
P < 0.001 versus PhIP/HF controls.
In contrast to our previous findings on PhIP-induced ACF (12), no protective effects were seen for EGCG against the development of colon tumors at 1 year (43.6% final incidence, Table I). Moreover, in rats post-treated with white tea and caffeine, the colon tumor incidence was increased significantly to 68.5 and 73.3%, respectively, compared with 41.6% in the PhIP/HF controls (P = 0.025 and 0.039, using the Pearson’s chi-square test). Interestingly, however, caffeine posttreatment reduced the tumor incidence in several other target organs; for all non-colon tumors combined, the final cumulative incidence was 13.3% (Table I) compared with 69.5% in the PhIP/HF controls (P = 0.0003). The corresponding incidence data for EGCG (48.7%) and white tea (46.9%) were not statistically different from the controls for all non-colon tumors combined.
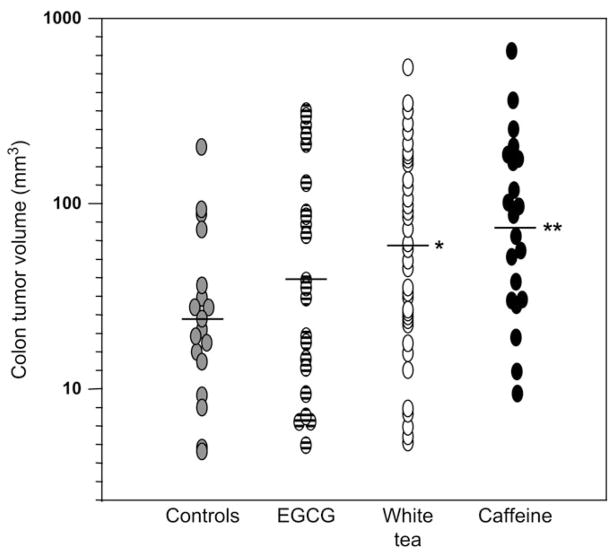
In addition to the increase in colon tumor incidence, white tea and caffeine (but not EGCG) also increased the colon tumor volume (P = 0.012 and 0.009, respectively, versus controls, Figure 2). No significant differences were seen for tumor multiplicity, but the trends were similar; the corresponding data were as follows (colon tumors/colon tumor-bearing animal, mean ± standard error): PhIP/HF controls 1.47 ± 0.19, PhIP/HF + EGCG 1.27 ± 0.14, PhIP/HF + white tea 1.91 ± 1.15 and PhIP/HF + caffeine 2.18 ± 0.50 (P > 0.05, for all group comparisons, Wilcoxon test).
Fig. 2.
White tea and caffeine increase colon tumor volume in the rat. Each data point represents a single colon tumor induced by PhIP/HF treatment (see Figure 1A for the dosing protocol). *P < 0.05 and **P < 0.01 compared with controls. Note that the data include colon tumors from animals that were euthanized early before the study was terminated at 1 year.
Altered frequency and spectrum of β-catenin mutations
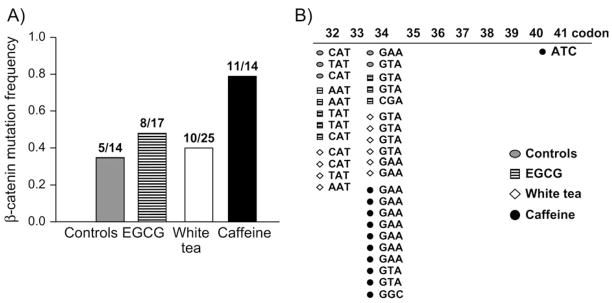
We next examined β-catenin mutations in colon tumors using PCR-based single-strand conformation polymorphism analysis followed by direct sequencing. The frequency of β-catenin mutations was similar for controls and groups given EGCG or white tea, but significantly higher in rats post-treated with caffeine (Figure 3A). Specifically, in PhIP/HF controls, 36% (5/14) of the colon tumors harbored β-catenin mutations compared with 79% (11/14) after caffeine posttreatment (P = 0.022, Pearson’s chi-square test). Although slightly elevated at 47.1% (8/17) and 40% (10/25) in the colon tumors from the rats post-treated with EGCG and white tea, respectively, these mutation frequencies were not significantly different from controls.
Fig. 3.
Altered frequency and spectrum of β-catenin mutations in colon tumors. (A) β-Catenin mutations were screened by PCR-based single-strand conformation polymorphism analysis and direct sequencing, as before (3,4,13). Incidence data show the number of confirmed mutations/total samples analyzed by single-strand conformation polymorphism, above each corresponding bar. (B) Mutations were localized almost exclusively to codons 32 and 34 of the Ctnnb1, except for a codon 41 mutation in the caffeine group, which also completely lacked the codon 32 mutants detected in other groups. Data shown in the figure include colon tumors from animals that were euthanized before the study was terminated at 1 year.
In addition to the increase in β-catenin mutation frequency, caffeine also produced a shift in the β-catenin mutation spectrum (Figure 3B). Thus, whereas β-catenin mutations were distributed more or less equally between codons 32 and 34 in controls and in groups given EGCG or white tea, in the colon tumors from rats post-treated with caffeine all but one of the mutations was detected in codon 34, and none were seen in codon 32 (black symbols, Figure 3B). Statistical analyses revealed that caffeine altered the spectrum of β-catenin mutations significantly compared with controls (P = 0.017, Fisher’s exact test). Codons 32 and 34 represent ‘hot spots’ in Ctnnb1 (4) and the corresponding mutations substitute Asp32 and Gly34, which flank Ser33, a key site for phosphorylation/ubiquitination in human and rat β-catenin (1,2).
β-Catenin and its downstream targets—role of c-myc in tumors promoted by tea and caffeine
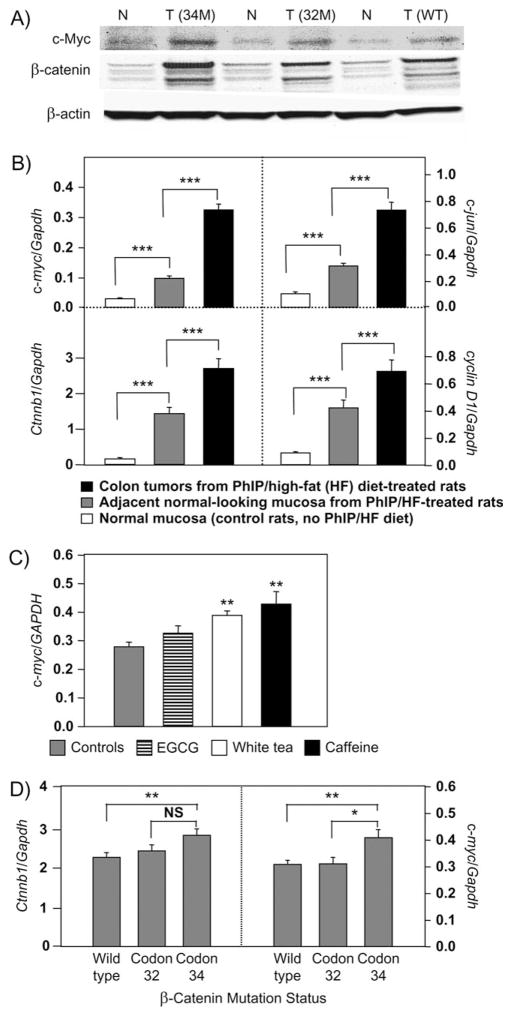
Immunoblots revealed that β-catenin and c-Myc were more highly expressed in PhIP-induced colon tumors than in adjacent normal-looking tissue (Figure 4A). In addition, mRNA levels of Ctnnb1, c-myc, c-jun and cyclin D1 were highly overexpressed in colon tumors compared with adjacent normal-looking tissue and in adjacent normal-looking tissue compared with normal colonic mucosa from untreated rats (P < 0.001 using Student’s t-test, see pairwise comparisons indicated in Figure 4B).
Fig. 4.
Overexpression of β-catenin and β-catenin/Tcf target genes in rat colon tumors induced by PhIP/HF treatment. (A) Immunodetection of β-catenin and c-Myc in colon tumors (T) and adjacent normal-looking tissue (N). The loading control was β-actin. Blots shown are representative data from more than a dozen tumors, each with mutations in Ctnnb1 codon 32 (32M), codon 34 (34M) or wild-type (WT), respectively. (B) Quantitative real-time PCR was used to examine Ctnnb1, c-myc, c-jun and cyclin D1 mRNA levels, normalized to glyceraldehyde-3-phosphate dehydrogenase (Gapdh). Data = mean ± standard error, ***P < 0.001. (C) Quantitative real-time PCR data for c-myc (mean ± standard error) plotted as a function of posttreatment regimen; **P < 0.01 versus controls. No significant differences were seen for the corresponding data on c-jun, cyclin D1 or Ctnnb1 (data not shown). (D) Quantitative real-time PCR data for Ctnnb1 and c-myc (mean ± standard error) plotted as a function of β-catenin mutation status. *P < 0.05, **P < 0.01; NS, not significant. No significant changes were seen for corresponding data on c-jun and cyclin D1 (data not shown). Data in the figure are cumulative and include colon tumors from animals that were euthanized before the study was terminated at 1 year.
The quantitative real-time PCR data were analyzed further, and we observed that colon tumors from rats given white tea or caffeine, but not EGCG, had significantly higher mRNA expression levels of c-myc (P < 0.01 versus controls, Figure 4C). No such association was seen for Ctnnb1, c-jun or cyclin D1 and the posttreatment regimen (data not shown). When plotted as function of the β-catenin mutation status (Figure 4D), mRNA levels of c-myc were more highly expressed in colon tumors harboring codon 34 mutations than in tumors with wild-type β-catenin (P < 0.01) or codon 32 mutations (P < 0.05), and Ctnnb1 mRNA expression also was higher in tumors with codon 34 mutations than wild-type β-catenin (P < 0.01).
Enhanced cell proliferation and reduced apoptosis during colon tumor promotion by white tea and caffeine
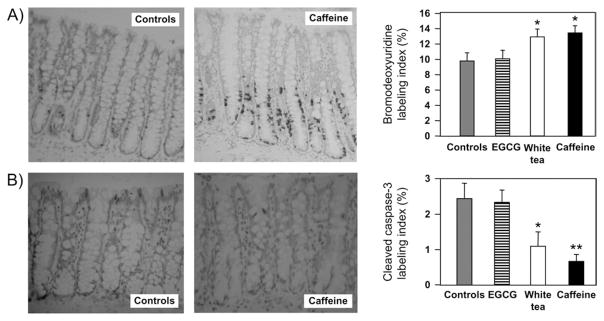
Immunostaining for BrdU incorporation revealed an expansion of the colonic crypt zone of cell proliferation in groups post-treated with caffeine and white tea, but not EGCG. The BrdU-labeling index, determined for the entire crypt column, was 12.1 ± 1.1% and 13.3 ± 0.5% in rats post-treated with white tea and caffeine, respectively, and augmented significantly versus 9.7 ± 0.9% in the PhIP/HF controls (P < 0.05, Figure 5A). The corresponding index for cleaved caspase-3, a measure of apoptosis, was 1.11 ± 0.38% and 0.68 ± 0.19% in rats post-treated with white tea and caffeine, respectively, which was significantly <2.44 ± 0.43% in the controls (P < 0.05 and P < 0.01, respectively, Figure 5B). Upon closer inspection, apoptotic cells were located mainly in the extreme apical crypt region for controls, but further down the crypt in rats post-treated with caffeine (Figure 5B). Cell proliferation and apoptosis indices were unaffected by posttreatment with EGCG. It is noteworthy that in rats given vehicle (no PhIP) and post-treated with white tea or caffeine, but not EGCG, the apoptosis index also was attenuated significantly versus the corresponding controls (data not presented).
Fig. 5.
Cell proliferation and apoptosis in rat colonic mucosa after posttreatment with white tea, EGCG and caffeine. Immunodetection of (A) bromodeoxyuridine incorporation and (B) cleaved caspase-3, and the corresponding labeling indices determined for the entire colonic crypt. The number of positively stained cells was divided by the total number of cells and multiplied by 100 to obtain ‘percent labeling’ for the entire crypt. Data = mean ± standard error, *P < 0.05. **P < 0.01 versus controls (Note: color versions of these images are avaliable as supplementary data on the Carcinogenesis website).
Discussion
Ubagai et al. (7) described a protocol for the efficient induction of large intestine tumors in the rat by intermittent administration of PhIP and a HF diet. Specifically, 2 weeks of PhIP treatment at 400 p.p.m. in the diet were followed by 4 weeks of HF diet, which was repeated three times, ending with continuous feeding of HF diet for 42 weeks—this regimen produced a final incidence of large intestine tumors similar to that seen with continuous dietary PhIP treatment for an entire year (7). Although it reduced dramatically the amount of PhIP needed to produce colon tumors in the rat, we modified the protocol in order to further optimize carcinogen use. We also sought to eliminate HF exposure in the latter part of the study to facilitate post-initiation experiments with phytochemicals. Thus, PhIP was given by oral gavage at a dose (50 mg/kg body wt/day) that was matched to the daily carcinogen intake from 400 p.p.m. PhIP in the diet, and after three such cycles of PhIP/HF treatment, standard AIN-93M diet rather than HF diet was given until the study was terminated at 1 year. Using this modified protocol, the colon tumor incidence was 41.6%, comparable with the 45% incidence reported by Ubagai et al. (7). Animals in the PhIP/HF control group developed a wide spectrum of tumors (Table I) and less than one-third survived tumor free for 1 year (Figure 1). Rats were euthanized early due to the presence of Zymbal’s gland tumors and/or skin lesions or after the appearance of blood in the stools, which was usually indicative of bleeding into the gastrointestinal tract from one or more tumors in the large or small intestine.
We reported previously (12) that white tea, EGCG and caffeine protected in the rat against PhIP-induced ACF, which are putative preneoplastic lesions and used as biomarkers of final colon tumor outcome (16–18). However, in the present investigation, EGCG had no inhibitory effect, and white tea and caffeine promoted rather than suppressed colon tumors. It is unclear whether this reflects a basic limitation of ACF as biomarkers of tumor outcome, as reported by others for genistein and cholic acid (19–21), or alternatively that caffeine and white tea exert a true biphasic response in the rat colon, being beneficial (protective) during short-term treatment but deleterious (promotional) after prolonged exposure. Importantly, however, we observed that caffeine offered significant protection in other target organs of PhIP-induced tumorigenesis (Table I). The cumulative results include animals that died early, but the overall trends were similar in each of the treatment groups for rats that survived to 1 year (data not shown). We interpret this as evidence that protection by caffeine in other target organs was real, and not due to fewer rats surviving to 1 year. Previously (22), caffeine was reported to protect against PhIP-induced mammary tumors in female rats but also promoted tumor formation in the colon.
Consistent with their tumor-promoting activities, caffeine and white tea altered colonic crypt homeostasis by enhancing cell proliferation versus apoptosis (Figure 5). Similar findings were obtained before in rats given chlorophyllin or indole-3-carbinol, and colon tumor promotion was associated with a shift in the β-catenin mutation spectrum (3,4). Thus, we rationalized that a likely mechanism for tumor promotion by white tea and caffeine might be via the dysregulation of β-catenin/Tcf signaling since β-catenin mutation and increased β-catenin expression are clearly associated with tumor progression (1). An increase in β-catenin mutations thus would be consistent with enhanced tumor growth by caffeine and white tea.
An increase in β-catenin mutation frequency coupled with a shift in the β-catenin mutation spectrum indeed was observed in colon tumors from rats post-treated with caffeine, but not white tea, indicating that β-catenin mutation status alone could not explain the promotional activities seen here. The colon tumors from rats given caffeine harbored codon 34 β-catenin mutants at a higher frequency than codon 32 mutants, suggesting that one or more promotional mechanisms ‘selected’ certain oncogenic forms of β-catenin to progress in preference to others. We do not know the reason for this apparent selection pressure, given that oncogenic β-catenin mutants with substitutions at either Asp32 or Gly34 were equally effective in activating a β-catenin/Tcf-responsive reporter construct (3,23).
Downstream targets of β-catenin/Tcf were highly expressed in colon tumors, namely c-myc, c-jun and cyclin D1 and so too was Ctnnb1 mRNA itself. Overexpression of β-catenin protein therefore might arise due to mutational events that stabilize the β-catenin protein (1) or via dysregulation of Ctnnb1 expression at the transcriptional level, as reported for 1,2-dimethylhydrazine-induced colon tumors (13). Importantly, among the three β-catenin/Tcf target genes examined, only c-myc showed any concordance with tumor promotion by white tea and caffeine (Figure 4C). Thus, rather than β-catenin, c-Myc overexpression may be important in the promotional mechanism of white tea and caffeine. In its capacity as an ataxia telangiectasia mutated/ATM and Rad3-related kinase inhibitor, caffeine was reported to prevent p53 accumulation upon activation of c-Myc or E2F1 (24). We recently described a pathway in which overexpression of c-Myc elevated E2F1 and enhanced Bcl-2 levels, resulting in suppression of apoptosis (25). Suppression of apoptosis in the present study was evident for caffeine and white tea using cleaved caspase-3 as a biomarker, in animals treated with PhIP/HF diet and also in the negative controls given three cycles of vehicle/HF diet. However, we did not examine the possible role of E2F1, Bcl-2 or p53 in the promotional mechanism of caffeine and white tea in the rat colon, and this is worthy of future study.
Finally, it is important to note that a substantial literature exists on tea and coffee consumption in humans, which does not indicate an adverse effect on tumor outcome—indeed, some studies suggest a protective role for these caffeinated beverages. For example, Tavani et al. (26) reviewed epidemiological studies for the period 1990–2003 on coffee, decaffeinated coffee and tea and cancer of the colon and rectum. For coffee consumption, most case–control studies found risk estimates below unity for colon cancer, but no relation for rectal cancer. A meta-analysis of 5 cohort and 12 case–control studies found a pooled relative risk of 0.75 for colon cancer, which was significant. No such relationship was seen for tea or decaffeinated coffee. A more recent meta-analysis (27) concluded that ‘despite the strong evidence from in vitro and non-human in vivo studies in support of green and black tea as potential chemopreventive agents against colorectal cancer, available epidemiologic data are insufficient to conclude that either tea type may protect against colorectal cancer in humans’. In light of the findings from the present investigation and the increased risk for colorectal adenoma associated with meat cooked at high temperature (28), it would be interesting to reassess the epidemiological evidence for possible increased risk of colon cancer in a subset of individuals consuming tea (or coffee) plus large amounts of cooked meat and a HF diet.
In summary, we report here that in rats treated with three short cycles of PhIP/HF diet, post-initiation exposure to white tea and caffeine promoted rather than suppressed colon tumorigenesis, whereas EGCG had no effect. Caffeine increased the frequency of β-catenin mutations, and the colon tumors harbored almost exclusively β-catenin mutants with direct substitutions of Gly34. One important conclusion from these studies is that the final spectrum of β-catenin mutants in colon tumors is clearly influenced by exposure to dietary phytochemicals, as reported before for chlorophyllin and indole-3-carbinol (3,4). There was no direct concordance between the changes in tumor outcome and the expression of β-catenin or its downstream target genes. However, c-Myc was identified as a possible key player in the promotional mechanism of white tea and caffeine. Caffeine protected in other target organs of PhIP-induced tumorigenesis, suggesting a complex pattern of tumor modulation, in line with the general heterogeneity noted in human epidemiology studies of caffeinated beverages.
Acknowledgments
Funding
Inko’s White Tea (New York, NY) and National Institutes of Health (CA90890, CA65525 and CA122959); Foundation for Promotion of Cancer Research, Tokyo, Japan to R.H.D.
We thank Barbara Delage, Qingjie Li, Tianwei Yu, Gayle Orner, Valerie Elias, Kate Cleveland and Christine Larsen for assistance with necropsy examinations. Laboratory Animal Service staff are gratefully acknowledged for their help with animal care and maintenance. C. Riegger of DSM Nutritional Products kindly provided the EGCG (TEAVIGO™). Mutan White tea was a generous gift from Stash Tea Co.
Abbreviations
- ACF
aberrant crypt foci
- BrdU
5-bromo-2′-deoxyuridine
- Ctnnb1
β-catenin gene (rat)
- EGCG
epigallocatechin-3-gallate
- HF
high fat
- mRNA
messenger RNA
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- PhIP
2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine
- Tcf
T-cell factor
Footnotes
For Permissions, please journals.permissions@oxfordjournals.org
Supplementary material
The colour version of figure 5 can be found at http://carcin.oxfordjournals.org/
Conflict of Interest Statement: None declared.
References
- 1.Schneikert J, et al. The canonical Wnt signalling pathway and its APC partner in colon cancer development. Gut. 2007;56:417–425. doi: 10.1136/gut.2006.093310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Fageeh M, et al. Phosphorylation and ubiquitination of oncogenic mutants of beta-catenin containing substitutions at Asp32. Oncogene. 2004;23:4839–4846. doi: 10.1038/sj.onc.1207634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blum CA, et al. Beta-Catenin mutation in rat colon tumors initiated by 1,2-dimethylhydrazine and 2-amino-3-methylimidazo[4,5-f]quinoline, and the effects of post-initiation treatment with chlorophyllin and indole-3-carbinol. Carcinogenesis. 2001;22:315–320. doi: 10.1093/carcin/22.2.315. [DOI] [PubMed] [Google Scholar]
- 4.Blum CA, et al. Mutational analysis of Ctnnb1 and Apc in tumors from rats given 1,2-dimethylhydrazine and 2-amino-3-methylimidazo[4,5-f]-quinoline: mutational ‘hotspots’ and the relative expression of beta-catenin and c-jun. Mol Carcinog. 2003;36:195–203. doi: 10.1002/mc.10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugimura T, et al. Heterocyclic amines: mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004;95:290–299. doi: 10.1111/j.1349-7006.2004.tb03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dashwood RH, et al. High frequency of beta-catenin (Ctnnb1) mutations in the colon tumors induced by two heterocyclic amines in the F344 rat. Cancer Res. 1998;58:1127–1129. [PubMed] [Google Scholar]
- 7.Ubagai T, et al. Efficient induction of rat large intestinal tumors with a new spectrum of mutations by intermittent administration of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in combination with a high fat diet. Carcinogenesis. 2002;23:197–200. doi: 10.1093/carcin/23.1.197. [DOI] [PubMed] [Google Scholar]
- 8.Santana-Rios G, et al. Potent antimutagenic activity of white tea in comparison with green tea in the Salmonella assay. Mutat Res. 2001;495:61–74. doi: 10.1016/s1383-5718(01)00200-5. [DOI] [PubMed] [Google Scholar]
- 9.Dashwood WM, et al. Inhibition of β-catenin/Tcf activity by white tea, green tea, and epigallocatechin-3-gallate (EGCG): minor contribution of H2O2 at physiologically relevant EGCG concentrations. Biochem Biophys Res Commun. 2002;296:584–588. doi: 10.1016/s0006-291x(02)00914-2. [DOI] [PubMed] [Google Scholar]
- 10.Orner GA, et al. Suppression of tumorigenesis in the Apcmin mouse: down-regulation of β-catenin signaling by a combination of tea plus sulin-dac. Carcinogenesis. 2003;24:263–267. doi: 10.1093/carcin/24.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santana-Rios G, et al. Inhibition by white tea of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine-induced colonic aberrant crypts in the F344 rat. Nutr Cancer. 2001;41:98–103. doi: 10.1080/01635581.2001.9680618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carter O, et al. Comparison of white tea, green tea, epigallocatechin-3-gallate and caffeine as inhibitors of PhIP-induced colonic aberrant crypts in the rat. Nutr Cancer. 2007;58:60–65. doi: 10.1080/01635580701308182. [DOI] [PubMed] [Google Scholar]
- 13.Wang R, et al. Tumors from rats given 1,2-dimethylhydrazine plus chlorophyllin or indole-3-carbinol contain transcription changes in β-catenin that are independent of the β-catenin mutation status. Mutat Res. 2006;601:11–18. doi: 10.1016/j.mrfmmm.2006.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogawa K, et al. Modification by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) of 3,2′-dimethyl-4-aminobiphenyl (DMAB)- induced rat pancreatic and intestinal tumorigenesis. Cancer Lett. 1998;124:31–37. doi: 10.1016/s0304-3835(97)00441-2. [DOI] [PubMed] [Google Scholar]
- 15.Shirai T, et al. The prostate: a target organ for carcinogenicity of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) derived from cooked foods. Cancer Res. 1997;57:195–198. [PubMed] [Google Scholar]
- 16.Bird RP, et al. The significance of aberrant crypt foci for understanding the pathogenesis of colon cancer. Toxicol Lett. 2000;112–113:395–402. doi: 10.1016/s0378-4274(99)00261-1. [DOI] [PubMed] [Google Scholar]
- 17.Stevens RG, et al. Epidemiology of colonic aberrant crypt foci: review and analysis or existing studies. Cancer Lett. 2007;252:171–183. doi: 10.1016/j.canlet.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corpet DE, et al. Most effective colon cancer chemopreventive agents in rats: a systematic review of aberrant crypt foci and tumor data, ranked by potency. Nutr Cancer. 2002;43:1–21. doi: 10.1207/S15327914NC431_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pereira MA, et al. Use of azoxymethane-induced foci of aberrant crypts in rat colon to identify potential cancer chemopreventive agents. Carcinogenesis. 1994;15:1049–1054. doi: 10.1093/carcin/15.5.1049. [DOI] [PubMed] [Google Scholar]
- 20.Rao CV, et al. Enhancement of experimental colon cancer by genistein. Cancer Res. 1997;57:3717–3722. [PubMed] [Google Scholar]
- 21.Shirtliff N, et al. Growth features of aberrant crypt foci that resist modulation by cholic acid. Carcinogenesis. 1996;17:2093–2096. doi: 10.1093/carcin/17.9.2093. [DOI] [PubMed] [Google Scholar]
- 22.Hagiwara A, et al. Organ- dependent modifying effects of caffeine, and two naturally occurring antioxidants alpha-tocopherol and n-tritriacon-tane-16,18-dione, on 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-induced mammary and colonic carcinogenesis in female F344 rats. Jpn J Cancer Res. 1999;90:399–405. doi: 10.1111/j.1349-7006.1999.tb00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porfiri E, et al. Induction of a beta-catenin-LEF-1 complex by wnt-1 and transforming mutants of beta-catenin. Oncogene. 1997;15:2833–2839. doi: 10.1038/sj.onc.1201462. [DOI] [PubMed] [Google Scholar]
- 24.Lindstrom MS, et al. Myc and E2F1 induce p53 through p14ARF-independent mechanisms in human fibroblasts. Oncogene. 2003;22:4993–5005. doi: 10.1038/sj.onc.1206659. [DOI] [PubMed] [Google Scholar]
- 25.Li Q, et al. Bcl-2 over-expression in PhIP-induced colon tumors: cloning of the rat Bcl-2 promoter and characterization of a pathway involving β-catenin, c-Myc and E2F1. Oncogene. 2007;26:6194–6202. doi: 10.1038/sj.onc.1210438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tavani A, et al. Coffee, decaffeinated coffee, tea and cancer of the colon and rectum: a review of epidemiological studies, 1999–2003. Cancer Causes Control. 2004;15:743–757. doi: 10.1023/B:CACO.0000043415.28319.c1. [DOI] [PubMed] [Google Scholar]
- 27.Sun CL, et al. Green tea, black tea and colorectal cancer risk: a meta-analysis of epidemiologic studies. Carcinogenesis. 2006;27:1301–1309. doi: 10.1093/carcin/bgl024. [DOI] [PubMed] [Google Scholar]
- 28.Sinha R, et al. Meat, meat cooking methods and preservation, and risk of colorectal adenoma. Cancer Res. 2005;65:8034–8041. doi: 10.1158/0008-5472.CAN-04-3429. [DOI] [PubMed] [Google Scholar]