Abstract
Background
Immunologic responses at birth likely relate to subsequent risks for allergic diseases and wheezing in infancy; however, the influences of parental characteristics and prenatal factors on neonatal immune responses are incompletely understood.
Objective
This study investigates potential correlations between urban parental, prenatal and perinatal factors on innate and adaptive stimuli-induced cytokine responses.
Methods
560 and 49 children of parents with and without allergic disease or asthma, respectively, were enrolled into a prospective birth cohort study (Urban Environment and Childhood Asthma [URECA]). Cord blood mononuclear cells were incubated with innate and adaptive immune stimuli, and cytokine responses (ELISA) were compared to season of birth, parental characteristics, in-utero stressors, and fetal growth.
Results
Many cytokine responses varied by season of birth, including 2–3 fold fluctuations with specific IFN-α and IFN-γ responses. Birthweight was inversely associated with IFN-γ responses to RSV (R=−0.16), but positively associated with IL-8 responses to a variety of innate stimuli (R=0.08–0.12). Respiratory syncytial virus (RSV) induced cytokine responses were 21–54% lower in children of mothers with asthma. Cytokine responses were generally lower in babies born to parents with allergy/asthma.
Conclusions
Innate cytokine responses are associated with parental allergic or airway disease, somatic fetal growth, ethnicity, and season of birth. Collectively, these findings suggest that urban prenatal exposures and familial factors affect the development of the fetal immune system.
Clinical Implications
Prenatal influences that disturb normal immune development might establish the patterns that are associated with recurrent wheezing in infants and preschoolers, and later as allergy and asthma in school-age children.
Keywords: Atopy, immune response, cord blood, birth cohort, gestational age, cytokines
Introduction
Immune development is an active process in the prenatal and perinatal periods, and is potentially influenced by genetic factors as well as the intrauterine and perinatal environment. Consequently, at birth the cytokine responses of cord blood mononuclear cells (CBMC) to innate and adaptive stimuli may reflect these influences. These relationships are of interest because there is evidence that neonatal cord blood cytokine responses not only reflect perinatal exposures, but also may predict later risk of wheezing with viral illnesses,1–3 atopic dermatitis, allergy4 and possibly asthma.5
Several characteristics related to parents, prenatal exposures, and birth are also related to the subsequent risk of allergic diseases and asthma. For example, maternal asthma6 and African American or Puerto Rican ethnicity7 predict increased risk of early childhood asthma. In addition, pre-term birth,8, 9 low birth weight for gestational age (somatic growth),10 and infant adiposity11 have all been linked to increased early life wheeze and asthma risk, and in separate studies, to skewed neonatal cytokine responses.12–14
Moreover, season of birth has been linked to allergy and asthma risk.15 Each of these factors could represent a complex of in utero environmental exposures, epigenetic effects, and for parental influences, genetics. The timing during pregnancy of intrauterine influences, which may be linked to seasonality of viral and other prenatal exposures,16 may be important in determining the effects of these influences on immune development.17
Collectively, these findings support the hypothesis that certain prenatal exposures modify immune development in utero to influence immune responses in the newborn, and ultimately the risk of atopic outcomes in early childhood. Prenatal and perinatal exposures may have their greatest influence on innate (rather than adaptive) immune responses. These responses, which are present at birth and continue to develop in childhood, are likely to be major influences on the subsequent establishment of adaptive immune responses.18, 19 Factors that influence the prenatal development of innate immune responses are incompletely understood.
Prenatal modification of developmental immunity may be especially relevant to children who grow up in urban environments, where there are a host of unfavorable environmental exposures (e.g. maternal smoking ), and health conditions (e.g. maternal asthma, prematurity, poor growth) that could increase the risk of subsequent asthma. Furthermore, most studies on neonatal cytokine patterns and their relation to hereditary and environmental influences have included only Caucasian, or predominantly Caucasian and Mexican American populations.5, 17, 20, 21 To address these gaps in knowledge, we evaluated an urban cohort of newborns of predominantly African American and Hispanic ethnicity to define the relationship of innate and adaptive cord blood mononuclear cell cytokine responses to parental allergic disease and ethnicity, in-utero stressors, birth measures of fetal growth, and season of birth. We conceived of these rather broad categories of exposures as representing larger groups of potential in-utero or perinatal stressors which could ultimately skew immune function towards an allergic or asthmatic phenotype.
Methods
Study population
The Urban Environment and Childhood Asthma (URECA) birth cohort study is designed to identify environmental, lifestyle and genetic factors that influence immunologic development in early childhood and consequently modify the risk of developing recurrent wheezing and ultimately asthma.22 The study protocol was approved by Institutional Review Boards at each of the participating institutions, and written, informed consent was obtained prior to enrollment.
Expectant families were recruited during the prenatal period in 4 large cities: Baltimore, Boston, New York, and St. Louis. Selection criteria include residence in an area with >20% residents below the poverty level, mother or father with allergic rhinitis, eczema, and/or asthma, and birth at ≥ 34 weeks gestation. Exclusion criteria included conditions and congenital anomalies that could potentially affect lung or immune system development or function. Recruitment occurred between February 2005 and March 2007: 1853 families were screened, 779 met the eligibility criteria, and 560 were enrolled (Table I).
Table I.
Study population.
| Allergic Families | Non-allergic Families | |||
|---|---|---|---|---|
| n | % | n | % | |
| Total Mothers | 557 | 100.0 | 49 | 100.0 |
| Mother’s age in years at child’s birth (median, range) | 23 | 13–42 | 24 | 15–38 |
| Race or ethnicity of mother | ||||
| Hispanic of any race | 107 | 19.4 | 9 | 18.4 |
| Black alone | 390 | 70.9 | 39 | 79.6 |
| White alone | 22 | 4.0 | 0 | 0.0 |
| More than one race | 20 | 3.6 | 1 | 2.0 |
| All others | 11 | 2.0 | 0 | 0.0 |
| Missing | 7 | -- | 0 | -- |
| Maternal asthma | 307 | 55.3 | 0 | 0.0 |
| Maternal BMI (pre-pregnancy) | ||||
| <20 | 35 | 7.2 | 6 | 14.3 |
| 20-<25 | 144 | 29.8 | 15 | 35.7 |
| 25-<30 | 131 | 27.1 | 10 | 23.8 |
| 30+ | 173 | 35.8 | 11 | 26.2 |
| Pregnancy | ||||
| Inhaled corticosteroid use | 54 | 9.7 | 0 | 0.0 |
| Diabetes | 51 | 9.2 | 3 | 6.1 |
| Maternal smoking | 97 | 17.6 | 6 | 12.2 |
| Hypertensive disorders | 62 | 11.1 | 9 | 18.4 |
| Infection | 222 | 39.9 | 21 | 42.9 |
| Prolonged labor | 32 | 7.2 | 1 | 3.4 |
| C Section delivery | 172 | 30.9 | 14 | 28.6 |
| Number of previous live births | ||||
| 0 | 220 | 39.5 | 17 | 34.7 |
| 1 | 147 | 26.4 | 19 | 38.8 |
| 2 | 96 | 17.2 | 5 | 10.2 |
| ≥ 3 | 94 | 16.9 | 8 | 16.3 |
| Total Newborns | 560 | 100.00 | 49 | 100.00 |
| Newborn characteristics | ||||
| Gender (% male) | 288 | 51.4 | 25 | 51.0 |
| Birthweight, g (median, range) | 3220 | 1815–4850 | 3220 | 2280–4425 |
| Gestational age (median, range) | 39 | 34–42 | 39 | 36–41 |
| Quarter of birth | ||||
| January - March | 137 | 24.5 | 7 | 14.3 |
| April - June | 142 | 25.4 | 2 | 4.1 |
| July - September | 160 | 28.6 | 25 | 51.0 |
| October - December | 121 | 21.6 | 15 | 30.6 |
A separate group of families without allergies or asthma were recruited into the nonatopic comparison group. The eligibility and exclusion criteria were the same except for the absence of parental allergic diseases or asthma. 240 were screened, 70 met the eligibility criteria and consented to the study, and 49 families were enrolled (Table I).
Measurement of cytokine responses
Collection of cord blood
Cord blood samples were collected in the delivery room using sterile needles and syringes, and all personnel were trained to use identical collection techniques as previously described.23 After collection, the blood was transferred from syringes to sterile 50 mL tubes, diluted 1:1 with RPMI 1640 medium containing heparin, and kept at room temperature pending cell separation.
Cell stimulation and cytokine assays
At each research center, mononuclear cells were separated by density gradient centrifugation using Accuspin tubes (Sigma) within 16 hrs of collection, and cells (1 × 106 cells/1 mL) were incubated in the presence of medium and specific immune stimulants or medium alone (Table II), as previously reported.23 After incubation for 24 hrs (innate and polyclonal stimuli) or 5 days (antigens), cell supernatant fluids were collected, divided into aliquots, frozen at −80°C, and shipped to a central laboratory for analysis. Supernatants were analyzed for cytokines with a bead-based multiplex assay (Beadlyte, Upstate Biotechnology, Lake Placid, NY). Cytokines (Table II) were selected based on involvement with specific innate and adaptive immune responses that have been related to allergic inflammation and the immune response to respiratory viruses. Detection limits are shown in Table II. For analysis purposes, results below detection were assigned a value just below the limit (e.g. 6.8 if the detection limit was 6.9 pg/ml).
Table II.
Stimulants used and cytokines measured in the cytokine secretion assays
| Stimulants* | Final Concentration | Cytokines measured with all stimulants | Lower Limit of Detection (pg/ml) |
|---|---|---|---|
| Innate Responses | |||
| Lipopolysaccharide1 | 0.1 μg/mL | IFN-α | 8.2 |
| Polyinosinic-polycytidylic acid2 | 25 μg/mL | IFN-γ | 6.9 |
| Peptidoglycan3 | 1.25 μg/mL | IL-10 | 6.9 |
| CpG-C4 | 1 μg/mL | IL-12p40 | 11.0 |
| Respiratory syncytial virus5 | 500 sfu/mL† | TNF-α | 6.9 |
| Medium alone | -- | IL-8 | 15600.0 |
| Adaptive and Polyclonal Responses | |||
| Phytohemagglutinin6 | 15 μg/mL | IFN-γ | 6.9 |
| Cockroach extract7 | 10 μg/mL | IL-10 | 6.9 |
| Dust mite (D. pteronyssinus) extract8 | 10 μg/mL | IL-13 | 6.9 |
| Tetanus toxoid9 | 10 μg/mL | IL-4 | 6.9 |
| Medium alone | -- | ||
Sources for reagents:
Associates of Cape Cod (Falmouth, MA),
Amersham Biosciences (Piscataway, NJ),
InvivoGen (San Diego, CA),
Coley Pharmaceuticals (Wellesley, MA),
Ann Mosser (University of Wisconsin-Madison),
Sigma (St. Louis, MO),
Greer Inc. (Lenoir NC),
Massachusetts Biologics (Jamaica Plain, MA)
sfu = syncytium-forming units
Several procedures were followed to minimize assay variability and monitor quality control.23 All tissue culture reagents were purchased from a single source, and stimulants were purchased in bulk and then divided into single use aliquots that were stored at −80°C. Technicians from all 4 clinical sites attended a centralized training session. The reproducibility of the assays was monitored approximately yearly by sending aliquots of a single sample of fresh blood to each of the clinical sites, and the detailed procedures and reproducibility results of these quality control tests have been previously published.23
Maternal characteristics and obstetrical data
Information on allergic history, smoking and alcohol use before and during pregnancy, household and personal stress during the prenatal period, and basic demographic information was collected from the mothers during a prenatal interview (Table I).
Information regarding the pregnancy and delivery were abstracted from the medical record. This information included parity, pregnancy complications and comorbidities, medication use, duration of labor and type of delivery. The child’s gestational age, birthweight, and Apgar score were also recorded. In addition, to estimate fetal growth, the percentile (z-value) of birthweight adjusted for gestational age was calculated for each infant, using national reference data.24 Information about systemic infections during pregnancy was obtained from the maternal obstetrical charts. Included in this definition were pneumonia (n=2), pyelonephritis (n=14), chorioamnionitis (n=22), and other respiratory and systemic infections (n=20).
Statistical analysis
Pearson correlations between predictors of interest and log-transformed (base 10) cytokine levels were examined. Spearman correlations were examined to verify that the correlations were not severely affected by the distribution of the responses. These results were not markedly different, so Pearson correlations are presented. Due to the large number of cytokine outcomes and predictors, these correlations are presented visually using matrix plots with colors indicating the direction and intensity of correlation between a given predictor and cytokine. The structure of the data contained high measures of association within the set of predictors as well as within the cytokine response values. A false discovery rate with α=0.10 was used to investigate the impact of multiple comparisons for each cytokine.25 Almost every association determined significant at the α=0.05 level was found to retain significance after adjustment. With the high level of association between stimulant responses and analyses being exploratory in nature, the initial α=0.05 cutoff was maintained as the significance criterion. To adjust for responses in unstimulated samples, semi-partial correlations were performed. The semi-partial correlation was defined as the correlation between the predictor variable and the residual obtained from fitting a linear model with the media control value as the predictor and the stimulated cytokine as the response. Further complex associations were evaluated with multiple regression models adjusting for different covariates. To determine whether children with atopic parents have a distinct pattern of cord blood cytokine responses, we compared results from the 49 newborns with no parental history of allergic diseases or asthma (non-atopic cohort) to those from an equal number of infants in the main URECA study who were matched for season of birth and study center. Analyses were performed using SAS Version 9.1.3 (Cary, NC) and the R system for statistical computing (version 2.7.0).26
Results
Cord blood cytokine responses
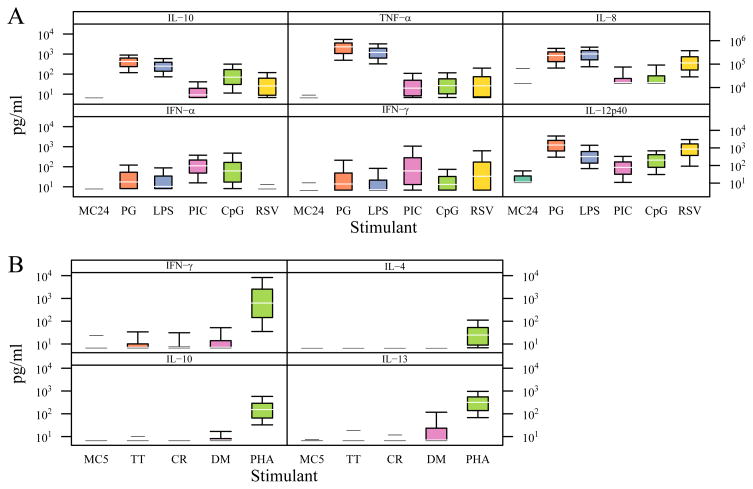
Cord blood mononuclear cells were incubated with innate, polyclonal, and adaptive immune stimuli, and cytokine responses were examined. Responses to innate stimuli were generally vigorous, and patterns of cytokine responses were stimulus-dependent (Figure 1A). For example, PG and LPS stimulation of cells induced high levels of IL-8, IL-10, and TNF-α, and lesser amounts of IFN-α and IFN-γ secretion whereas, PIC and CpG stimulation induced greater amounts of IFN-α, and less IL-8, IL-10, and TNF-α. RSV induced a unique pattern of cytokine responses, with high IL-12p40, IFN-γ, and IL-8, and low to absent IFN-α. PHA induced IFN-γ, IL-10, and IL-13, but relatively little IL-4 secretion (Figure 1B). Measurable cytokine responses to protein antigens were less frequent than innate responses, and dust mite extract was most likely to elicit cytokine responses. Unstimulated cells secreted little or no cytokine.
Figure 1.
Cytokine responses of cord blood cells (n = 558) to innate immune stimuli (A) and specific proteins and PHA (B). Box plots represent median values with 10th, 25th, 75th, and 90th percentiles. IL-8 responses were much higher than those for the other cytokines and are plotted on a different scale.
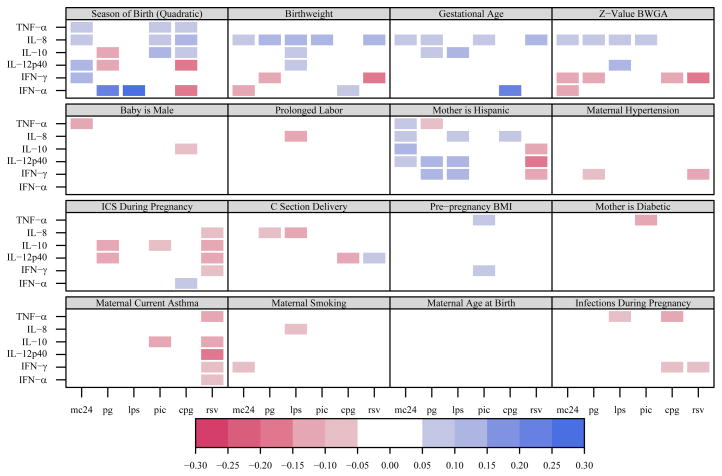
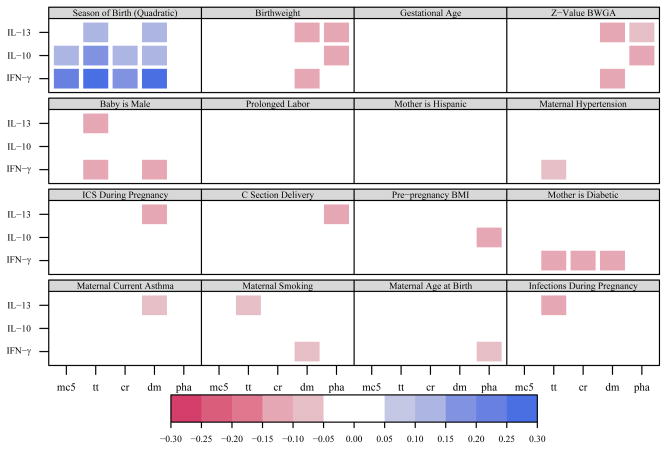
Correlations between predictors and cytokine responses
Several selected maternal, perinatal, and newborn characteristics were examined as predictors of cytokine responses (Figures 2 and 3). The predictors with the most associations with innate immune responses included season of birth, ethnicity, birthweight/gestational age, and maternal asthma/use of inhaled corticosteroids (Figure 2). Although cytokine responses to protein antigens were low, it was nevertheless possible to evaluate maternal and child characteristics influencing protein antigen responses in cord blood cells. Cytokine responses to PHA and specific proteins were associated with season of birth, maternal diabetes, and birthweight (Figure 3).
Figure 2.
Correlation matrix for innate immune responses. Statistically significant (p<0.05) correlations between perinatal factors and innate immune cytokine responses are represented with colored squares; blue represents a positive correlation and red represents a negative correlation. Darker colors represent larger absolute values for correlation coefficients.
Figure 3.
Correlation matrix for adaptive and PHA-induced responses. Statistically significant (p<0.05) correlations between perinatal factors and cytokine responses to protein extracts and PHA are represented with colored squares; blue represents a positive correlation and red represents a negative correlation. Darker colors represent larger absolute values for correlation coefficients. There were no significant IL-4 responses to antigens, and so they were not included in the matrix.
Season of birth
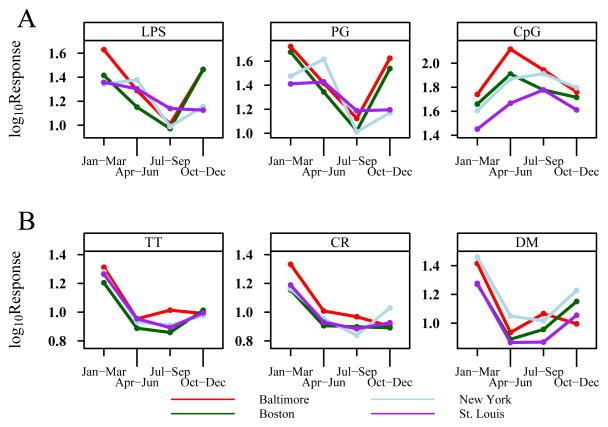
Season of birth had the strongest associations with cytokine responses to both innate and adaptive stimuli, and when the responses were examined collectively, several patterns were apparent (Figure 2). CpG-induced responses were influenced by season, and the direction of the association differed by cytokine. IFN-α and IL-12p40 responses were greatest in the spring and summer months, while IL-8, IL-10, and TNF-α responses were greatest in the fall and winter. In addition, the seasonality of IFN-α responses was stimulus dependent; there was a 2-fold variability in CpG-induced IFN-α with greatest responses in the spring and summer, while IFN-α responses to PG and LPS varied 2-fold and were greatest in the fall and winter (Figure 4A). These patterns were remarkably similar among subjects enrolled in the 4 study centers (Figure 4A). IFN-α responses to PIC and RSV did not follow a seasonal pattern.
Figure 4.
Seasonal patterns of selected cytokine mean responses. A) IFN-α responses to LPS, PG, and CpG according to season (3 month intervals). B) IFN-γ responses to cockroach (CR) and dust mite (DM) extracts, and tetanus toxoid (TT). Seasonal patterns from the 4 individual study sites are depicted by the colored lines. The depicted cytokine mean responses are those with the strongest seasonality patterns (see also Figure 2). All figures demonstrate a significant quadratic effect with each panel having p<0.001.
Season of birth was also associated with cytokine responses to all 3 specific antigens and in unstimulated cells, but not with cytokine responses to PHA (Figure 3). These effects were most evident in relation to IFN-γ responses, which were highest from January to March (geometric mean values: TT 18.7 pg/ml, CR 16.4 pg/mL, DM 22.4 pg/mL), and bordered on undetectable for the spring and summer months (Figure 4B). Again, there was consistency among the 4 clinical study sites (Figure 4B). After adjusting for IFN-γ secretion in cells incubated in medium alone, the seasonal variation remained statistically significant for dust mite- and tetanus-toxoid-induced IFN-γ (p<0.001).
Ethnicity
Most of the URECA mothers were either Hispanic or African American (Table I), and so associations with ethnicity are largely comparisons between these two groups. Ethnicity appeared to have the greatest influence on innate immune responses (Figure 2). Babies born to Hispanic mothers had enhanced IFN-γ and IL-12p40 responses to PG and LPS (Table III); these ranged 1.4 to 2.5-fold higher than corresponding responses in babies born to Black mothers. In contrast, RSV-induced IFN-γ and IL-12p40 responses were 2.2 and 1.5-fold higher in babies born to Black mothers (Table III). Finally, PHA-induced IL-4 responses were reduced in babies of Hispanic mothers (median 14.9 pg/ml vs. 28.0, p<0.001). These differences by ethnicity persist after accounting for control values.
Table III.
Neonatal cytokine responses (median and interquartile range levels in pg/ml) of IFN-γ and IL-12 by maternal race/ethnicity.
| Stimulant | Cytokine | Hispanic or Hispanic Mixed (n=108) | Black or Black Mixed (n=410) | p-value* |
|---|---|---|---|---|
| PG | IFN-γ | 28 (6.8, 85) | 11 (6.8, 40) | <0.001 |
| IL-12(p40) | 1787 (800, 3795) | 1270 (600, 2310) | <0.001 | |
| LPS | IFN-γ | 10 (6.8, 35) | 6.8 (6.8, 18) | 0.005 |
| IL-12(p40) | 395 (190, 770) | 291 (130, 559) | 0.003 | |
| RSV | IFN-γ | 18 (6.8, 85) | 39 (6.8, 189) | 0.025 |
| IL-12(p40) | 571 (110, 1167) | 870 (403, 1772) | <0.001 | |
| CpG | IFN-γ | 15 (6.8, 37) | 12 (6.8, 31) | 0.13 |
| IL-12(p40) | 154 (66, 326) | 211 (83, 415) | 0.04 | |
| PIC | IFN-γ | 60 (18, 241) | 58 (13, 273) | 0.80 |
| IL-12(p40) | 75 (31, 201) | 73 (32, 145) | 0.54 |
Mann-Whitney U test
Birthweight and gestational age
Birthweight was positively associated with IL-8 responses to most innate stimuli (R=0.08–0.12), but negatively associated with IFN-γ responses to PG (R=−0.11) and RSV (R=−0.16, Figure 2). In addition, birthweight was negatively associated with IL-13 responses to both PHA and dust mite protein (R=−0.11 –0.10, Figure 3). All of these relationships persisted after adjustment for gestational age and there were additional inverse correlations with IFN-γ responses (Figures 2 and 3). The effects for CpG and RSV (but not PG) persist after accounting for IFN-γ secretion by unstimulated cells.
Our data showed that the median birthweight was significantly lower among women who smoked during pregnancy (3090 g vs. 3270 g; p=0.004). In order to determine whether the effect for birthweight on IFN-γ was confounded by smoking, we adjusted for smoking in a separate set of models. For an increase in birthweight from 2920 g to 3564 g (25th to 75th percentile) there was a decrease in PG-induced IFN-γ of 8.7% (95% CI: 15.4% to 2.0%) in the unadjusted model, compared to a 9.3% (95% CI: 16.0% to 2.5%) decrease in the model adjusted for smoking.
Maternal asthma
We used two markers of maternal asthma: maternal report of a current diagnosis of asthma, and use of inhaled corticosteroid during pregnancy. RSV-induced cytokine responses were 21–54% lower in the presence of maternal asthma (Figure 2, Table IV). A similar pattern was evident for associations with the use of inhaled corticosteroids (Figure 2). There were no associations between maternal asthma and the responses to proteins or PHA (Figure 3).
Table IV.
Associations between maternal asthma and newborn RSV-induced cytokine responses (median and interquartile range, pg/mL).
| Maternal Asthma | |||
|---|---|---|---|
| No (N=332) | Yes (N=216) | p-value* | |
| IFN-α | 8.1 (8.1, 8.1) | 8.1 (8.1, 8.1) | 0.130 |
| IFN-γ | 46 (6.8, 208) | 21 (6.8, 108) | 0.015 |
| IL-12p40 | 918 (474, 1920) | 637 (212, 1295) | <0.001 |
| IL-10 | 27 (10, 66) | 19 (6.8, 53) | 0.016 |
| IL-8 | 114000 (62850, 209500) | 90050 (46700, 193750) | 0.053 |
| TNF-α | 28 (8.3, 84) | 18 (6.8,56) | 0.007 |
Mann-Whitney U test
Maternal diabetes
Maternal diabetes was associated with reduced interferon responses to specific antigens (Figure 3). However, neither PHA-induced IFN-γ responses nor spontaneous IFN-γ secretion in unstimulated cells were associated with diabetes.
Cytokine responses in newborns from parents with and without a history of allergic diseases or asthma
In general, cytokine responses to innate stimuli were diminished in children with a parental history of allergy/asthma (Table V). These trends were especially apparent for IL-8 (33–63% lower) and IL-12p40 (16–71% lower), and also included TNF-α, IFN-γ, and IFN-α responses to some stimuli. Of the stimulants, PIC-induced responses were most strongly blunted (38–71% lower). One exception to this trend was that PHA-induced IL-13, but not IL-4, responses were enhanced in children with a parental history of allergy/asthma (349 vs. 204 pg/mL, p = 0.022).
Table V.
Difference in cytokine responses among newborns of allergic parents vs. non-allergic parents.
| Innate Stimulant Panel | ||||||
|---|---|---|---|---|---|---|
| Allergic Family History N=49 | Non-Allergic Family History N=49 | |||||
| Cytokine | Stimulant | Median (pg/ml) | Interquartile Range | Median (pg/ml) | Interquartile Range | p-value* |
| Innate Stimulants | ||||||
| IFN-α | MC24 | 8.1 | (8.1, 8.1) | 8.1 | (8.1, 8.1) | 0.65 |
| PG | 8.1 | (8.1, 18.1) | 8.1 | (8.1, 9.9) | 0.016 | |
| LPS | 8.1 | (8.1, 22) | 8.1 | (8.1, 10.2) | 0.074 | |
| PIC | 84 | (42, 160) | 155 | (48, 300) | 0.049 | |
| CpG | 30 | (11, 122) | 84 | (30, 138) | 0.187 | |
| RSV | 8.1 | (8.1, 8.1) | 8.1 | (8.1, 8.1) | 0.135 | |
| IFN-γ | MC24 | 6.8 | (6.8, 6.8) | 6.8 | (6.8, 6.8) | 0.065 |
| PG | 8.6 | (6.8, 24) | 21 | (6.8, 105) | 0.037 | |
| LPS | 6.8 | (6.8, 12) | 6.8 | (6.8, 33) | 0.089 | |
| PIC | 60 | (15, 186) | 199 | (38, 1110) | 0.004 | |
| CpG | 14 | (6.8, 25) | 19 | (9.1, 55) | 0.041 | |
| RSV | 10 | (6.8, 52) | 43 | (6.8, 134) | 0.058 | |
| IL-12p40 | MC24 | 11 | (11, 26) | 44 | (13, 80) | < 0.001 |
| PG | 1554 | (600, 2598) | 3390 | (1530, 5738) | < 0.001 | |
| LPS | 333 | (203, 1050) | 750 | (278, 2305) | 0.008 | |
| PIC | 89 | (29, 251) | 310 | (104, 644) | < 0.001 | |
| CpG | 196 | (77, 336) | 232 | (152, 420) | 0.091 | |
| RSV | 726 | (334, 1660) | 1380 | (715, 3145) | 0.003 | |
| IL-10 | MC24 | 6.8 | (6.8, 6.8) | 6.8 | (6.8,6.8) | 0.161 |
| PG | 534 | (285, 829) | 474 | (317, 915) | 0.819 | |
| LPS | 247 | (137, 411) | 219 | (105, 314) | 0.21 | |
| PIC | 10 | (6.8, 18) | 16 | (7.1, 26) | 0.064 | |
| CpG | 75 | (31, 190) | 91 | (51, 189) | 0.164 | |
| RSV | 24 | (6.8, 83) | 28 | (12, 63) | 0.622 | |
| TNF-α | MC24 | 6.8 | (6.8, 6.8) | 6.8 | (6.8, 29) | 0.003 |
| PG | 2612 | (1060, 3916) | 2560 | (1110, 4840) | 0.657 | |
| LPS | 1081 | (749, 2360) | 1010 | (644, 2240) | 0.518 | |
| PIC | 16 | (8.3, 52) | 49 | (19, 244) | 0.003 | |
| CpG | 16 | (7.8, 61) | 47 | (11, 146) | 0.019 | |
| RSV | 112 | (6.8, 36) | 38 | (11, 101) | 0.006 | |
| IL-8 | MC24 | 15599 | (15599, 31200) | 16400 | (15599,86100) | 0.059 |
| PG | 246000 | (142000, 375000) | 370000 | (257000,474000) | 0.013 | |
| LPS | 259000 | (144000, 504000) | 441000 | (301000,590000) | 0.004 | |
| PIC | 15599 | (15599, 31100) | 38600 | (15599,88400) | 0.01 | |
| CpG | 16900 | (15599, 52300) | 46000 | (15599,106000) | 0.031 | |
| RSV | 105000 | (49000, 240000) | 284500 | (132750,457500) | < 0.001 | |
p-value from Mann-Whitney U test of difference between medians
Discussion
In this study, we examined cytokine production from cord blood mononuclear cells in newborns of parents with a history of allergic disease or asthma. We observed that responses to a variety of immune stimuli were significantly influenced by maternal characteristics and gestational events. Prior studies of cord blood mononuclear cells have emphasized the potential of prenatal priming in response to specific antigens, such as β-lactoglobulin, ovalbumin and Der p1.27–33 We identified specific characteristics such as season of birth, ethnicity, birthweight/gestational age, and parental medical conditions (maternal diabetes and parental allergy or asthma) that also may influence newborn immune function. Since newborn cytokine responses have been linked to the subsequent risk of postnatal diseases, prenatal influences that disturb normal immune development might establish the patterns that are associated with recurrent wheezing in infants and preschoolers, and later as allergy and asthma in school-age children. 1, 3, 5, 21, 34–37
Specifically, associations with season of birth, maternal diabetes and asthma show that the fetal immune system interacts with maternal experiences. For instance, the correlation of season of birth with cytokine response patterns covered a wide spectrum of both innate and adaptive stimuli. In response to innate stimuli, IFN-α exhibited notable correlation with season, and dependent on the specific stimulus, the results were reproducible between all four sites indicating that this was not a random finding. IFN-γ responses also showed a clear seasonal pattern across multiple adaptive stimuli. Considering reports of delayed production of this cytokine in infants and preschoolers with recurrent wheezing episodes, this finding may be of special importance.4, 37–40 Season of birth has indeed been studied as a risk factor for allergy and asthma, but primarily as an opportunity for concurrent postnatal viral infections, allergen exposures and sensitization of the newborn and infant related to the time of year.16, 41–46 Our findings suggest that there are intrauterine considerations as well. One study reporting prenatal priming to pollen allergens, suggested that the susceptibility of the fetal immune system to be primed varied during the gestation period.47 Obviously, there are a number of factors that are seasonal (e.g. viral infections, allergen exposure, vitamin D levels), and additional studies are needed to link specific seasonal exposures to immunologic effects on the newborn.
Further evidence of prenatal immune conditioning is expressed in the correlations between maternal race/ethnicity and cytokine responses. We found a multiplicity of correlations related to whether the mother was Black or Hispanic; the extent to which this represents differences in environmental vs hereditary influences is unknown. Compared to neonates of Caucasian ethnicity, neonatal black race/ethnicity has been shown to be associated with increased cord blood lymphoproliferative response to Bla g 2 and/or Der f 1.48 In later childhood and adulthood, compared to Caucasian ethnicity, black ethnicity is also linked to elevated risk of allergic sensitization and elevated IgE.49 In this cohort, approximately one-fifth of participants declared themselves to be Hispanic, but the relation of Hispanic ethnicity to allergy risk varies widely depending on country of birth and duration of time in the United States.50 Ethnic differences in neonatal cytokine responses and in subsequent allergic or respiratory disease expression likely result from a combination of heredity and prenatal and postnatal environmental differences.51, 52 Although the implications of these findings are unknown, they do raise the possibility that different immune set points at birth could contribute to observed ethnic differences in the prevalence of asthma and perhaps other diseases.
Measures of fetal growth and development were also associated with specific cytokine responses, particularly in the positive association with IL-8 and negative association with IFN-γ related to both birthweight and gestational age. IL-8 is a chemotactic and activating factor for neutrophils, and is elevated with chorioamnionitis and neonatal sepsis.53–55 It is constituently produced by the placenta during pregnancy; one other study by Mondestin-Sorrento supports the hypothesis that the increase in IL-8 levels with gestational age may be a normative finding.55
Among other significant maternal-newborn associations was the relationship between current asthma in the mother and reduced cytokine responses to RSV. This finding resonates with reports of decreased innate immune responses to viral infection in infants and toddlers with wheezing.1, 21, 56 Factors that we considered but found no consistent associations with cytokine responses included maternal infections, prolonged labor, maternal smoking and maternal weight (BMI).
One of the most consistent findings that may be relevant for future immune behavior was that newborns with at least one parent with allergic disease or asthma had blunted mononuclear cell cytokine responses to innate immune stimulation by TLR2, TLR3, TLR4 and TLR9 agonists, (PG, PIC, LPS, CpG, respectively) as well as to in vitro stimulation by RSV. A suggestion of reduced anti-inflammatory cytokine and T-regulatory function is evidenced in another birth cohort study, where PG-induced IL-10 production and induction of FoxP3 were lower in CBMCs of children with maternal atopy.20 In our study, while cytokine levels were generally reduced in response to the TLR stimulants, differences in IL-10 responses between children of families with and without allergic disease or asthma were not prominent. In contrast, Prescott and colleagues recently reported that maternal allergy was associated with higher, instead of reduced, TLR-initiated responses in CBMC.57 The different findings could be due to a number of differences with our study, including unique features of the urban environment, ethnicity, and the use of freshly isolated vs. cryopreserved cells. Nonetheless, our findings suggest the possibility that newborns of parents with allergy/asthma in the urban environment could be globally less responsive to innate immune stimuli in early life. We are monitoring these children to determine whether these differences persist, and whether group-specific differences in cytokine responses will be associated with distinct atopic outcomes.
Strengths of our study include a large sample size, and multiple sites that enabled the identification of relationships that were consistent across 4 different urban areas. In addition, there was careful attention to quality control of the immunologic assays, which included periodic processing of standardized specimens.23 This enabled us to evaluate cytokine responses to a variety of specific innate and adaptive stimuli and their association with various prenatal and birth factors. Consequently, this study involved multiple comparisons between maternal and perinatal characteristics and newborn cytokine responses. To minimize the possibility of Type I statistical errors, we focused on the identification of associations that were consistent across multiple stimuli or cytokine responses. It should be noted that this study focused on the inner-city environment and population, which limits the generalizability of our results and conclusions. However, the intent of the study was to investigate a population that demonstrates a high prevalence and morbidity due to allergic diseases and asthma.
Many of the associations that we observed were of relatively low magnitude; however, from an immunoepidemiologic point of view, such correlations between environmental predictors and cytokine responses have potential biologic significance. Stronger correlations would not be expected, given the biological complexities involved in cytokine regulation. Many different factors may contribute to the cord blood cytokine responses, including genetic effects, environmental exposures, and the actual process of birth, and these effects may be additive. We have not measured all the possible predictors, nor do we have a complete picture of all the biological pathways that link environmental predictors (e.g. smoking) to cytokine responses. Finally, cord blood mononuclear cells were used in the cytokine response assays, and it is possible that individual variations in cell subsets could contribute to differences in cytokine responses.
The newborn period has been characterized as one in which there is rapid development of both innate and adaptive immune responses. Previous studies have related variations in newborn responses to the development of atopic diseases in childhood.37, 58–60 Yet, the results of this study provide evidence that the variability of newborn immune responses is not random, and appears to be responsive to maternal characteristics and experiences, indicating a continuity of immune development beginning with the fetus into the perinatal and postnatal periods. Our findings also suggest that a newborn of parents with allergy/asthma is primed to respond differently to post-natal environmental experiences. This maternal influence may be partially mediated by genetics; however, seasonal variations suggest that either environmental or epigenetic mechanisms may also affect the developing immune system. This cohort of infants will be followed for several years to determine the long-term effect of these maternal-fetal interactions on immunity and atopic outcomes in early life, including recurrent wheezing and asthma.
Acknowledgments
Funding
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health, under Contracts number NO1-AI-25496 and NO1-AI-25482, and from the National Center for Research Resources, National Institutes of Health, under grants RR00052, M01RR00533, 1UL1RR025771, M01RR00071, 1UL1RR024156, and 5UL1RR024992-02. The NIAID participated in the development of the study protocol and monitors its implementation. Dr. Alkis Togias participated in the writing of this manuscript.
The authors thank members of the Scientific Advisory Committee (Rebecca Adkins, Patrick Holt, Sebastian Johnston, Marcella Sarzotti-Kelsoe, Lloyd Mayer, and Donata Vercelli) for advice and guidance related to the conduct of the study, and Jeff Bluestone and Vicky Seyfert-Margolis of the Immune Tolerance Network for help related to the design and standardization of the immunologic studies.
The Urban Environment and Childhood Asthma Study is a collaboration of the following institutions and investigators (principal investigators are indicated by an asterisk; protocol chair is indicated by double asterisks): Johns Hopkins University, Baltimore, MD – R Wood*, E Matsui, H Lederman, F Witter, J Logan, B Adams, D Scott, V Colson, L Daniels, A Swift; Boston University School of Medicine, Boston, MA – G O’Connor*, W Cruikshank, M Sandel, A Lee-Parritz, C Jordan, C Ledger, E Gjerasi, C Longfellow, H Fernandez, K Pollenz, H Yamasaki, M Tuzova; Harvard Medical School, Boston, MA – D Gold, R Wright; Columbia University, New York, NY – M Kattan*, J D’Agostino, L Stokes, A Chen, J Edelson, S Roche, R Maril, C Sanabia; Mount Sinai School of Medicine, New York, NY – H Sampson, W Shreffler, R Sperling, B Kim, R Castro; Washington University School of Medicine, St Louis, MO – G Bloomberg*, L Bacharier, M Grayson, M de la Morena, Y Sadovsky, E Tesson, L Dents, L Henson, C Koerkenmeier, T Starks, R Sharp, J Durrange, I Bauer-Sardina; Statistical and Clinical Coordinating Center - Rho, Inc, Chapel Hill, NC – H Mitchell*, P Zook, C Visness, G David, S Gerzsenyi, S Arbes, M Walter, R Bailey, C Larsen; Scientific Coordination and Administrative Center – University of Wisconsin, Madison, WI – W Busse*, J Gern**, P Heinritz, C Sorkness, WM Lee, M Burger, K Grindle, N Christianson, A Dresen, T Pappas; National Institute of Allergy and Infectious Diseases, Bethesda, MD – P Gergen, A Togias, E Smartt, K Thompson.
Abbreviations
- Bla g 2
Blatella germanica 2 (cockroach allergen)
- BMI
Body mass index
- CBMC
Cord blood mononuclear cells
- CpG
Cytosine-phosphate-guanosine
- FoxP3
Forkhead box P3
- Der f 1
Dermatophagoides farina 1 (dust mite allergen)
- Der p 1
Dermatophagoides pteronyssinus 1 (dust mite allergen)
- LPS
Lipopolysaccharide
- PG
Peptidoglycan
- PHA
Phytohaemagglutinin
- PIC
Polyinosinic-polycytidylic acid
- RSV
Respiratory syncytial virus
- TLR2
Toll-like receptor 2
- TLR3
Toll-like receptor 3
- TLR4
Toll-like receptor 4
- TLR9
Toll-like receptor 9
Footnotes
Author Contributions
DG helped with interpretation of the data and writing the manuscript, GB leads the St. Louis clinical site and helped to write the manuscript, WC helped to design the immunologic assays and helped with interpretation of the data and writing the manuscript, CV is the lead analyst for the study and helped to write the manuscript, JS is a statistician who analyzed and helped to interpret the data, MK leads the New York clinical site, GO leads the Boston clinical site, RW leads the Baltimore clinical site, MB performed the immunologic assays, RW designed the stress assessments for the study, FW helped to design study procedures related to the maternal database and delivery room procedures, AP helped to design study procedures related to the maternal database and delivery room procedures, RS helped to design study procedures related to the maternal database and delivery room procedures, JS helped to design study procedures related to the maternal database and delivery room procedures, AT is a sponsor of the study, and JG conceived of the study, is the lead investigator for the project, and helped to interpret the data and write the manuscript. All authors assisted with revision of the final draft of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gern JE, Brooks GD, Meyer P, Chang A, Shen K, Evans MD, et al. Bidirectional interactions between viral respiratory illnesses and cytokine responses in the first year of life. J Allergy Clin Immunol. 2006;117:72–8. doi: 10.1016/j.jaci.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Copenhaver CC, Gern JE, Li Z, Shult PA, Rosenthal LA, Mikus LD, et al. Cytokine response patterns, exposure to viruses, and respiratory infections in the first year of life. Am J Respir Crit Care Med. 2004;170:175–80. doi: 10.1164/rccm.200312-1647OC. [DOI] [PubMed] [Google Scholar]
- 3.Ly NP, Rifas-Shiman SL, Litonjua AA, Tzianabos AO, Schaub B, Ruiz-Perez B, et al. Cord blood cytokines and acute lower respiratory illnesses in the first year of life. Pediatrics. 2007;119:e171–8. doi: 10.1542/peds.2006-0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neaville W, Tisler CJ, Bhattacharya A, Anklam K, Gilbertson-White S, Hamilton R, et al. Developmental cytokine response profiles and the clinical and immunologic expression of atopy during the first year of life. Journal Allergy Clin Immunol. 2003;112:740–6. doi: 10.1016/s0091-6749(03)01868-2. [DOI] [PubMed] [Google Scholar]
- 5.Macaubas C, de Klerk NH, Holt BJ, Wee C, Kendall G, Firth M, et al. Association between antenatal cytokine production and the development of atopy and asthma at age 6 years. Lancet. 2003;362:1192–7. doi: 10.1016/s0140-6736(03)14542-4. [DOI] [PubMed] [Google Scholar]
- 6.Litonjua AA, Carey VJ, Burge HA, Weiss ST, Gold DR. Parental history and the risk for childhood asthma. Does mother confer more risk than father? Am J Respir Crit Care Med. 1998;158:176–81. doi: 10.1164/ajrccm.158.1.9710014. [DOI] [PubMed] [Google Scholar]
- 7.Gold DR, Wright R. Population disparities in asthma. Annu Rev Public Health. 2005;26:89–113. doi: 10.1146/annurev.publhealth.26.021304.144528. [DOI] [PubMed] [Google Scholar]
- 8.Raby BA, Celedon JC, Litonjua AA, Phipatanakul W, Sredl D, Oken E, et al. Low-normal gestational age as a predictor of asthma at 6 years of age. Pediatrics. 2004;114:e327–32. doi: 10.1542/peds.2003-0838-L. [DOI] [PubMed] [Google Scholar]
- 9.Dombkowski KJ, Leung SW, Gurney JG. Prematurity as a predictor of childhood asthma among low-income children. Ann Epidemiol. 2008;18:290–7. doi: 10.1016/j.annepidem.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz J, Gold DR, Dockery DW, Weiss ST, Speizer FE. Predictors of asthma and persistent wheeze in a national sample of children in the United States. Association with social class, perinatal events and race. Am Rev Resp Dis. 1990;142:555–62. doi: 10.1164/ajrccm/142.3.555. [DOI] [PubMed] [Google Scholar]
- 11.Taveras EM, Rifas-Shiman SL, Camargo CA, Jr, Gold DR, Litonjua AA, Oken E, et al. Higher adiposity in infancy associated with recurrent wheeze in a prospective cohort of children. J Allergy Clin Immunol. 2008;121:1161–6. e3. doi: 10.1016/j.jaci.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers BB, Alexander JM, Head J, McIntire D, Leveno KJ. Umbilical vein interleukin-6 levels correlate with the severity of placental inflammation and gestational age. Hum Pathol. 2002;33:335–40. doi: 10.1053/hupa.2002.32214. [DOI] [PubMed] [Google Scholar]
- 13.Laskowska M, Laskowska K, Leszczynska-Gorzelak B, Oleszczuk J. Comparative analysis of the maternal and umbilical interleukin-8 levels in normal pregnancies and in pregnancies complicated by preeclampsia with intrauterine normal growth and intrauterine growth retardation. J Matern Fetal Neonatal Med. 2007;20:527–32. doi: 10.1080/14767050701412719. [DOI] [PubMed] [Google Scholar]
- 14.Lo HC, Tsao LY, Hsu WY, Chen HN, Yu WK, Chi CY. Relation of cord serum levels of growth hormone, insulin-like growth factors, insulin-like growth factor binding proteins, leptin, and interleukin-6 with birth weight, birth length, and head circumference in term and preterm neonates. Nutrition. 2002;18:604–8. doi: 10.1016/s0899-9007(01)00811-5. [DOI] [PubMed] [Google Scholar]
- 15.Lendor C, Johnson A, Perzanowski M, Chew GL, Goldstein IF, Kelvin E, et al. Effects of winter birth season and prenatal cockroach and mouse allergen exposure on indoor allergen-specific cord blood mononuclear cell proliferation and cytokine production. Ann Allergy Asthma Immunol. 2008;101:193–9. doi: 10.1016/S1081-1206(10)60209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knudsen TB, Thomsen SF, Ulrik CS, Fenger M, Nepper-Christensen S, Backer V. Season of birth and risk of atopic disease among children and adolescents. J Asthma. 2007;44:257–60. doi: 10.1080/02770900701246832. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan Dillie KT, Tisler CJ, Dasilva DF, Pappas TE, Roberg KA, Carlson-Dakes KT, et al. The influence of processing factors and non-atopy-related maternal and neonate characteristics on yield and cytokine responses of cord blood mononuclear cells. Clin Exp Allergy. 2008;38:298–304. doi: 10.1111/j.1365-2222.2007.02891.x. [DOI] [PubMed] [Google Scholar]
- 18.Prescott SL, Taylor A, King B, Dunstan J, Upham JW, Thornton CA, et al. Neonatal interleukin-12 capacity is associated with variations in allergen-specific immune responses in the neonatal and postnatal periods. Clin Exp Allergy. 2003;33:566–72. doi: 10.1046/j.1365-2222.2003.01659.x. [DOI] [PubMed] [Google Scholar]
- 19.Yerkovich ST, Wikstrom ME, Suriyaarachchi D, Prescott SL, Upham JW, Holt PG. Postnatal development of monocyte cytokine responses to bacterial lipopolysaccharide. Pediatr Res. 2007;62:547–52. doi: 10.1203/PDR.0b013e3181568105. [DOI] [PubMed] [Google Scholar]
- 20.Schaub B, Campo M, He H, Perkins D, Gillman MW, Gold DR, et al. Neonatal immune responses to TLR2 stimulation: influence of maternal atopy on Foxp3 and IL-10 expression. Respir Res. 2006;7:40. doi: 10.1186/1465-9921-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stern DA, Guerra S, Halonen M, Wright AL, Martinez FD. Low IFN-gamma production in the first year of life as a predictor of wheeze during childhood. J Allergy Clin Immunol. 2007;120:835–41. doi: 10.1016/j.jaci.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 22.Gern JE, Visness CM, Gergen PJ, Wood RA, Bloomberg GR, O’Connor GT, et al. The Urban Environment and Childhood Asthma (URECA) birth cohort study: design, methods, and study population. BMC Pulm Med. 2009;9:17. doi: 10.1186/1471-2466-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shreffler WG, Visness CM, Burger M, Cruikshank WW, Lederman HM, de la Morena M, et al. Standardization and performance evaluation of mononuclear cell cytokine secretion assays in a multicenter study. BMC Immunol. 2006;7:29. doi: 10.1186/1471-2172-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;57:289. Series B. [Google Scholar]
- 26.R Development Core Team. R: A language and environment for statistical computing. 2008 http://www.R-project.org.
- 27.Piastra M, Stabile A, Firoranvanti G, Castagnola M, Pani G, Ria F. Cord blood mononuclear cell responsiveness to beta-lactoglobulin: T-cell activity in “atopy-prone” and “non-atopy-prone” newborns. International Archives of Allergy & Immunology. 1994;104:358–65. doi: 10.1159/000236692. [DOI] [PubMed] [Google Scholar]
- 28.Piccini M, Mecacci F, Sampognaro S, Manetti R, Parronchi P, Maggi E, et al. Aeroallergen senisitization can occur during fetal life. Int Arch Allergy immunol. 1993;102:301. doi: 10.1159/000236541. [DOI] [PubMed] [Google Scholar]
- 29.Prescott S, Macaubas C, Yabuhara A, Venaille TJ, Holt BJ, Habre W, et al. Developing patterns of T cell memory to environmental allergens in the first two years of life. International Archives of Allergy & Immunology. 1997;113:75–9. doi: 10.1159/000237512. [DOI] [PubMed] [Google Scholar]
- 30.Prescott SL, Macaubas C, Holt BJ, Smallacombe TB, Loh R, Sly PD, et al. Transplacental priming of the human immune system to environmental allergens: Universal skewing of initial T cell responses toward the Th2 cytokine profile. Journal of Immunology. 1998;160:4730–7. [PubMed] [Google Scholar]
- 31.Prescott S, Holt P, Jenmalm M, Bjorksten B. Effects of maternal allergen-specific IgG in cord blood on early postnatal development of allergen-specific T-cell immunity. Allergy. 2000;55:470–5. doi: 10.1034/j.1398-9995.2000.00286.x. [DOI] [PubMed] [Google Scholar]
- 32.Szepfalusi Z, Pichler J, Elsasser S, van Duren K, Ebner C, Bernaschek G, et al. Transplacental priming of the human immune system with environmental allergens can occur early in gestation. J Allergy Clin Immunol. 2000;106:530–6. doi: 10.1067/mai.2000.108710. [DOI] [PubMed] [Google Scholar]
- 33.Miller RL, Chew GL, Bell CA, Biedermann SA, Aggarwal M, Kinney PL, et al. Prenatal exposure, maternal sensitization, and sensitization in utero to indoor allergens in an inner-city cohort. American Journal of Respiratory and Critical Care Medicine. 2001;164:995–1001. doi: 10.1164/ajrccm.164.6.2011107. [DOI] [PubMed] [Google Scholar]
- 34.Tang MLK, Kemp AS, Hill DJ, Thorburn J. Reduced interferon-[gamma] secretion in neonates and subsequent atopy. The Lancet. 1994;344:983–5. doi: 10.1016/s0140-6736(94)91641-1. [DOI] [PubMed] [Google Scholar]
- 35.Holt PG. Primary allergic sensitization to environmental antigens: perinatal T cell priming as a determinant of responder phenotype in adulthood. Journal of Experimental Medicine. 1996;183:1297–301. doi: 10.1084/jem.183.4.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown M, Halonen M, Martinez FD. Cutting the cord: is birth already too late for primary prevention of allergy? Clinical & Experimental Allergy. 1997;27:4–6. [PubMed] [Google Scholar]
- 37.Prescott S, Holt P. Abnormalities in cord blood mononuclear cytokine production as a predictor of later atopic disease in childhood. Clinical & Experimental Allergy. 1998;28:1313–6. doi: 10.1046/j.1365-2222.1998.00427.x. [DOI] [PubMed] [Google Scholar]
- 38.Kondo N, Kobayashi Y, Shinoda S, Takenaka R, Teramoto T, Kaneko H, et al. Reduced interferon gamma production by antigen-stimulated cord blood mononuclear cells is a risk factor of allergic disorders----6-year follow-up study. Clinical & Experimental Allergy. 1998;28:1340–4. doi: 10.1046/j.1365-2222.1998.00418.x. [DOI] [PubMed] [Google Scholar]
- 39.Heaton T, Rowe J, Turner S, Aalberse R, de Klerk NH, Suriyaarachchi D, et al. An immunoepidemiological approach to asthma: identification of in-vitro T-cell response patterns associated with different wheezing phenotypes in children. Lancet. 2005;365:142–9. doi: 10.1016/S0140-6736(05)17704-6. [DOI] [PubMed] [Google Scholar]
- 40.Umetsu DT. Revising the immunological theories of asthma and allergy. Lancet. 2005;365:142–3. doi: 10.1016/S0140-6736(05)17714-9. [DOI] [PubMed] [Google Scholar]
- 41.Kuzume K, Kusu M. Before-birth climatologic data may play a role in the development of allergies in infants. Pediatr Allergy Immunol. 2007;18:281–7. doi: 10.1111/j.1399-3038.2006.00526.x. [DOI] [PubMed] [Google Scholar]
- 42.Guerra S, Sherrill DL, Cottini M, Michetti G, Allegra L. On the association between date of birth and pollen sensitization: is age an effect modifier? Allergy Asthma Proc. 2002;23:303–10. [PubMed] [Google Scholar]
- 43.Yoo Y, Yu J, Kang H, Kim DK, Koh YY, Kim CK. Birth month and sensitization to house dust mites in asthmatic children. Allergy. 2005;60:1327–30. doi: 10.1111/j.1398-9995.2005.00856.x. [DOI] [PubMed] [Google Scholar]
- 44.Nilsson L, Bjorksten B, Hattevig G, Kjellman B, Sigurs N, Kjellman NIM. Season of birth as predictor of atopic manifestations. British Medical Journal. 1997;76:341–4. doi: 10.1136/adc.76.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carroll KN, Wu P, Gebretsadik T, Griffin MR, Dupont WD, Mitchel EF, et al. Season of infant bronchiolitis and estimates of subsequent risk and burden of early childhood asthma. J Allergy Clin Immunol. 2009;123:964–6. doi: 10.1016/j.jaci.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu P, Dupont WD, Griffin MR, Carroll KN, Mitchel EF, Gebretsadik T, et al. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med. 2008;178:1123–9. doi: 10.1164/rccm.200804-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Duren-Schmidt K, Pichler J, Ebner C, Bartmann P, Forster E, Urbanek R, et al. Prenatal contact with inhalant allergens. Pediatr Res. 1997;41:128–31. doi: 10.1203/00006450-199701000-00020. [DOI] [PubMed] [Google Scholar]
- 48.Willwerth BM, Schaub B, Tantisira KG, Gold DR, Palmer LJ, Litonjua AA, et al. Prenatal, perinatal, and heritable influences on cord blood immune responses. Ann Allergy Asthma Immunol. 2006;96:445–53. doi: 10.1016/S1081-1206(10)60912-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Litonjua AA, Celedon JC, Hausmann J, Nikolov M, Sredl D, Ryan L, et al. Variation in total and specific IgE: effects of ethnicity and socioeconomic status. J Allergy Clin Immunol. 2005;115:751–7. doi: 10.1016/j.jaci.2004.12.1138. [DOI] [PubMed] [Google Scholar]
- 50.Gold DR, Acevedo-Garcia D. Immigration to the United States and acculturation as risk factors for asthma and allergy. J Allergy Clin Immunol. 2005;116:38–41. doi: 10.1016/j.jaci.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 51.Raby BA, Van Steen K, Lazarus R, Celedon JC, Silverman EK, Weiss ST. Eotaxin polymorphisms and serum total IgE levels in children with asthma. J Allergy Clin Immunol. 2006;117:298–305. doi: 10.1016/j.jaci.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 52.Choudhry S, Taub M, Mei R, Rodriguez-Santana J, Rodriguez-Cintron W, Shriver MD, et al. Genome-wide screen for asthma in Puerto Ricans: evidence for association with 5q23 region. Hum Genet. 2008;123:455–68. doi: 10.1007/s00439-008-0495-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shimoya K, Moriyama A, Matsuzaki N, Ogata I, Koyama M, Azuma C, et al. Human placental cells show enhanced production of interleukin (IL)-8 in response to lipopolysaccharide (LPS), IL-1 and tumour necrosis factor (TNF)-alpha, but not to IL-6. Mol Hum Reprod. 1999;5:885. doi: 10.1093/molehr/5.9.885. [DOI] [PubMed] [Google Scholar]
- 54.Dembinski J, Behrendt D, Martini R, Heep A, Bartmann P. Modulation of pro- and anti-inflammatory cytokine production in very preterm infants. Cytokine. 2003;21:200–6. doi: 10.1016/s1043-4666(02)00498-2. [DOI] [PubMed] [Google Scholar]
- 55.Mondestin-Sorrentino M, Smulian JC, Vintzileos AM, Sorrentino D, Ananth CV, Sharma S, et al. Variations in cervical IL-10 and IL-8 concentrations throughout gestation in normal pregnancies. Am J Reprod Immunol. 2007;57:482–7. doi: 10.1111/j.1600-0897.2007.00468.x. [DOI] [PubMed] [Google Scholar]
- 56.Guerra S, Lohman C, Halonen M, Martinez FD, Wright AL. Reduced Interferon g Production and Soluble CD 14 levels in Early Life Predict Recurrent Wheezing by 1 Year of Age. Am J Respir Crit Care Med. 2004;169:70–6. doi: 10.1164/rccm.200304-499OC. [DOI] [PubMed] [Google Scholar]
- 57.Prescott SL, Noakes P, Chow BW, Breckler L, Thornton CA, Hollams EM, et al. Presymptomatic differences in Toll-like receptor function in infants who have allergy. J Allergy Clin Immunol. 2008;122:391–9. 9, e1–5. doi: 10.1016/j.jaci.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 58.Ly NP, Li Y, Sredl DL, Perkins DL, Finn PW, Weiss ST, et al. Elevated allergen-induced IL-13 secretion predicts IgE elevation in children ages 2–5 years. J Clin Immunol. 2005;25:314–20. doi: 10.1007/s10875-005-4699-5. [DOI] [PubMed] [Google Scholar]
- 59.Allam JP, Zivanovic O, Berg C, Gembruch U, Bieber T, Novak N. In search for predictive factors for atopy in human cord blood. Allergy. 2005;60:743–50. doi: 10.1111/j.1398-9995.2005.00815.x. [DOI] [PubMed] [Google Scholar]
- 60.Chung EK, Miller RL, Wilson MT, McGeady SJ, Culhane JF. Antenatal risk factors, cytokines and the development of atopic disease in early childhood. British Medical Journal. 2007;92:F68–73. doi: 10.1136/adc.2006.106492. [DOI] [PMC free article] [PubMed] [Google Scholar]