Abstract
The cement-bone interface plays an important role in load transfer between cemented implant systems and adjacent bone, but little is known about the micro-mechanical behavior of this interface following in vivo service. Small samples of post-mortem retrieved cement-bone specimens from cemented total hip replacements were prepared and mechanically loaded to determine the mechanical response to tensile and compressive loading. The morphology of the cement-bone interface was quantified using a CT-based stereology approach. Laboratory prepared specimens were used to represent immediate post-operative conditions for comparison. The stiffness and strength of the cement-bone interface from post-mortem retrievals was much lower than that measured from laboratory prepared specimens. The cement-bone interfaces from post-mortem retrievals were very compliant (under tension and compression) and had a very low tensile strength (0.21 ± 0.32 MPa). With a linear regression model, including interface contact fraction and intersection fraction between cement and bone could explain 71% (p<0.0001) of the variability in experimental response. Bony remodeling following an arthroplasty procedure may contribute to reduced contact between cement and bone and this is associated with weaker and more compliant interfaces.
Introduction
Polymethylmethacrylate (PMMA) bone cements are routinely used to provide fixation between orthopaedic implants and bone. The interface between cement and bone is relied on for fixation and it is well recognized that there is a biologic response to the presence of PMMA. Bleeding of the bone bed during the arthroplasty procedure (10), thermal necrosis from the polymerization process (16), and bone remodeling (6) have been described as factors affecting the integrity of the cement-bone interface. In well-functioning femoral components there are regions of direct apposition between cement and bone (8), although there are also reports of extensive fibrous tissue formation between the cement and bone, oftentimes in the same construct (4). How these biological changes affect the structural performance of the cement-bone interface is, however, not clear.
The mechanics of the cement-bone interface using laboratory-prepared specimens has been studied extensively (1, 2, 11, 12). These efforts have been fruitful in identifying surgical and design factors that contribute to stronger cement-bone interfaces including pulsatile lavage of the femoral canal, use of a distal plug, retrograde cement filling of the canal with pressurization, and use of cement in a doughy state (17). Recent work with laboratory-prepared specimens has also indicated that micro-motion due to cyclic loading localizes at the interface between the cement and bone and that the interface is relatively compliant when compared to the adjacent bone and cement (14). However, these laboratory prepared specimens represent the immediate post operative condition and cannot capture the consequences of the biological adaptation that will occur in-vivo. It remains unclear whether the morphology and mechanical response of the cement-bone interface retrieved from well-functioning cemented femoral components is comparable to laboratory-prepared specimens.
The goals of the present study were to determine the interfacial micro-mechanics of small samples of post-mortem retrieved cement-bone specimens and to determine if there was a relationship between micro-mechanical response to loading and the morphology of the cement-bone interface. A secondary goal was to contrast the micro-mechanics of post-mortem retrieved specimens with those prepared using laboratory-prepared techniques. With these goals in mind we asked four research questions: (1) what is the stiffness and strength of the cement-bone interface of retrieved femoral components?; (2) are these substantially different from laboratory prepared specimens?; (3) can interface morphology explain differences in strength for the post-mortem retrieved specimens?; and finally (4) is the relationship between interface morphology and interface strength the same for post-mortem retrievals and lab-prepared specimens?
Methods
Proximal femurs with cemented hip replacement retrievals (n=8) were obtained en bloc from the Anatomical Donor Programs at SUNY Upstate Medical University and the University of Alabama at Birmingham (Table 1). The number of years in service ranged from 0.2 to 14 years (mean 5.5±4.6 yrs) with subject age at death from 67 to 92 years. All stems had a satin finished surface and had collars (n=6) or were of a calcar replacement design (n=2). Four of the stems were hemi-arthroplasties (donors A, B, C) and four were total hip replacements (donors D, E, F). Donors C and E had bilateral hip replacements. From review of post-mortem planar radiographs, there was no indication of osteolytic lesions although several of the cemented femurs did appear to have endosteal thinning and the development of a neo-cortex. Prior to physical sectioning, the cemented hip was mounted in a mechanical test frame and the axial micro-motion between the stem collar and the calcar was measured with an extensometer during application of a 400 N distally directed femoral head load. Micro-motions between 4.3 and 58 microns were measured. The axial motions from two femora (donor C) were not available; but these appeared well-fixed based on radiographic inspection.
Table 1.
Specifications of donor bones with cemented femoral hip components used to create cement-bone test specimens used in this study. Collar-calcar motion was measured during application of a 400 N axially directed load on the head of the implant.
| Donor | Age | Gender | Years in Service |
Collar-calcar motion (microns) |
Number of test specimens |
Cause of death |
Implant Type – Manufacturer |
|---|---|---|---|---|---|---|---|
| A | 88 | M | 0.2 | 4.3 | 8 | Cardiac arrest |
Perfecta – Wright Medical Technology |
| B | 87 | F | 0.9 | 59 | 7 | Cardiac arrest |
Perfecta PDA calcar replacement – Wright Medical Technology |
| C | 76 | F | 2 | Not available | 3 | Breast cancer |
Modular calcar replacement – Zimmer |
| C | 76 | F | 5 | Not available | 9 | Breast cancer |
Precision long stem – Howmedica Orthopaedics |
| D | 92 | F | 6 | 38 | 5 | Renal insufficiency |
Endurance – Depuy Orthopaedics |
| E | 85 | F | 8 | 24 | 5 | Bacterial endocarditis |
Versys cemented – Zimmer |
| E | 85 | F | 8 | 59 | 4 | Bacterial endocarditis |
Versys cemented – Zimmer |
| F | 67 | F | 14 | 13 | 8 | Alzheimer’s disease |
Harris precoat – Zimmer |
The cemented implants containing stem, cement, and bone were sectioned transversely in 10 mm intervals using a water irrigated saw (Isomet 2000, Buehler, Lake Bluff, IL). To stabilize the cement-bone interface before further cutting, a Plexiglas side-plate was attached between the cement and bone using cyanoacrylate glue (Figure 1). Care was taken to fix the side plate only on the cement and bone and not on the interface. Rectangular prism shaped specimens containing the bone and cement with 4mm×8mm cross sections were created. Prior to testing, specimens were micro-CT scanned at 12 micron isotropic resolution to document cement-bone morphology (Scanco 40, SCANCO Medical AG, Brüttisellen, Switzerland). A total of 49 specimens were created from the distal half of the cemented-stem constructs with near even distributions from four quadrants (anterior: 13, lateral: 14, medial: 11, and posterior: 11) of the transverse sections. Specimens were taken from the distal half of the cemented femoral construct because there was ample cortical bone available for mechanical gripping.
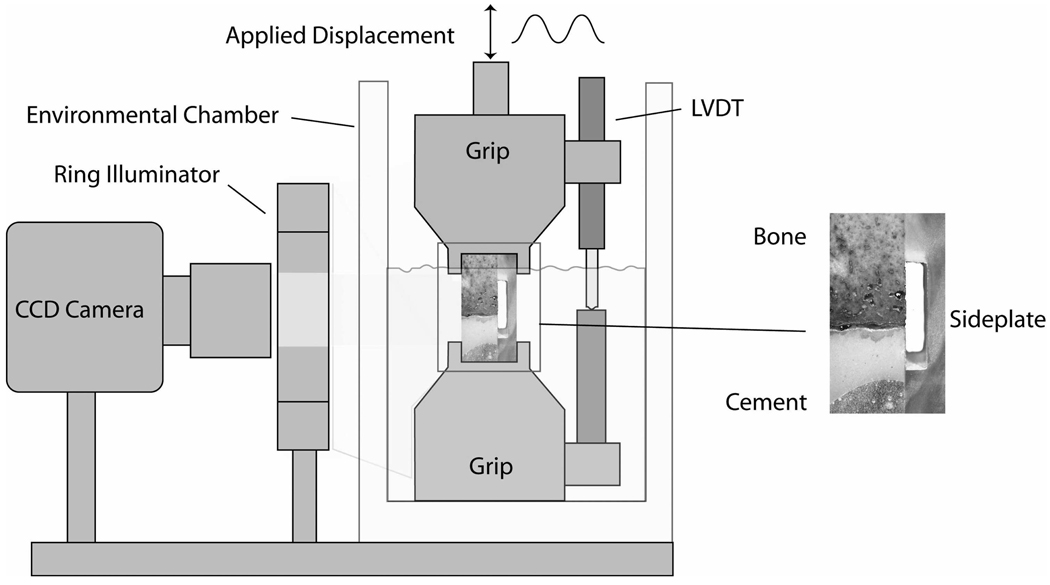
Figure 1.
Schematic of experimental apparatus used to load cement-bone specimens in tension and compression. The side plate was severed prior to mechanical loading.
A digital image correlation (DIC) technique was used to measure interface motion between the cement and bone during uni-axial tension-compression loading of the cement bone specimens. With this imaging technique, an overspray of black acrylic paint was applied to one surface of the cement-bone specimen to provide image texture for the DIC measurements. A CCD camera (0.0089 pixel resolution) with telecentric lens was used to capture images of the specimen during loading (Figure 1). Relative axial motion at the interface between the cement and bone was measured by quantifying the displacements at sampling points immediately adjacent to the interface. Details of the DIC measurement system are described elsewhere (14). The RMS error of the DIC system in this configuration was 0.000395 mm.
Three mechanical loading sequences were performed: (1) Initial tension and compressive global excursions of ±0.01 mm; (2) mechanical response to 0.5 MPa compression; (3) tension test to failure (Figure 2). Loading was provided by a screw-driven mechanical test frame at 0.5 mm/min and tests were conducted in calcium buffered saline at 37° C. Global motion of the specimen grips were measured by a Linear Variable Differential Transformer (LVDT) while the above described DIC method was used to quantify cement-bone interface motion during the tests. After gripping the specimen, but prior to any mechanical loading, the side plate was carefully severed using a hot scalpel blade. This procedure did not load nor damage the interface because the support grips were locked to prevent any relative motion.
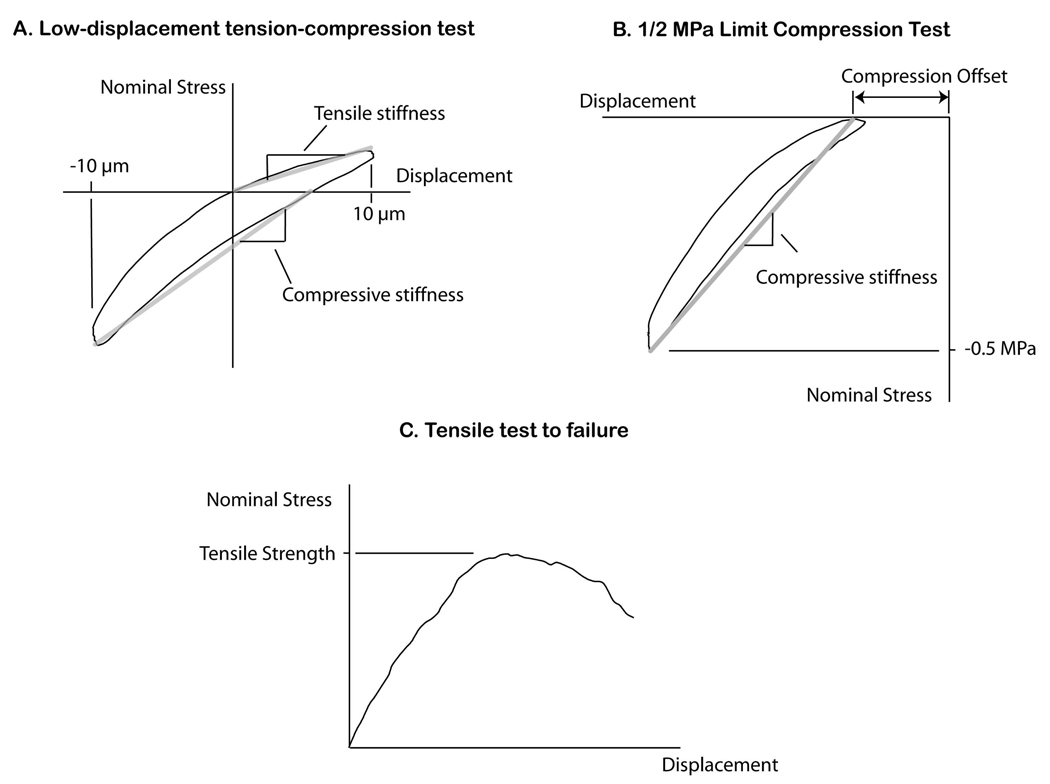
Figure 2.
Mechanical tests of the cement-bone specimens included low-displacement tension-compression loading (A), compression loading to a set 0.5 MPa compression level (B), and a tensile test to failure (C).
The initial low-displacement tension-compression test was performed to document the low-force micromechanics of the cement bone interface. For the first loading sequence, specimens were loaded in tension and compression to ±0.01 mm as measured by the LVDT for 10 cycles with DIC and force data collected on the 10th cycle. The tension and compressive stiffness (nominal applied stress / displacement) was used as the primary outcome measure. Specimens were then loaded in compression to 0.5 MPa for 10 cycles with DIC data collection on the 10th cycle. The purpose of the larger compressive loading level was to quantify interface mechanics during a loading condition that could be experienced in vivo (3). The compressive stiffness and compressive offset (zero-force x-intercept) were chosen as the primary outcome measures for this experiment (Figure 2). The final loading step consisted of a single cycle tension test that was loaded beyond the peak strength until there was a 25% drop in applied load. Here apparent tensile strength (peak load / specimen cross sectional area) served as the primary outcome measure.
For comparison purposes, results from a previous study (14) using laboratory-prepared cemented femoral constructs were included (n=21). The laboratory-prepared specimens were created using conditions that mimicked the surgical procedure of cemented hip replacement using 2nd generation cementing techniques into cadaver femurs that were warmed to 37° C and simulated medullary bleeding with a blood analog solution (15). Note that the 0.5 MPa limit compression test was not performed for the laboratory-prepared specimens, so comparison with post-mortem retrievals was not available for this loading case.
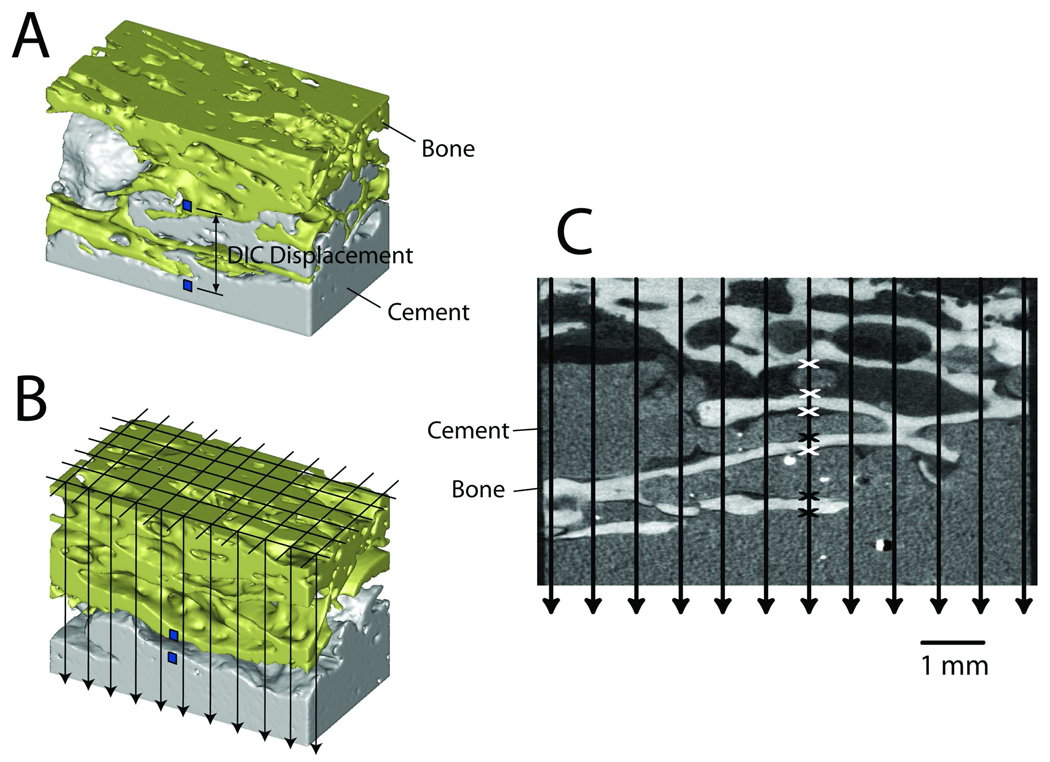
A CT-based stereology approach was used to document the morphology of the cement-bone interface (Figure 3). A grid with 0.65 mm spacing was randomly placed over the CT-data set and lines were projected vertically through the specimen. At points where the projected lines crossed an interface between cement and bone, the status of the interface was designated as either in apposition, in proximity, or gapped (greater than 0.25 mm). From this, a contact fraction was calculated as the total number of points of apposition divided by the number of projection lines. Points that were either in apposition or proximity were summed and divided by total number of projection line to generate an intersection fraction. A gap between the cement and bone of more than 0.25 mm was not considered as an intersection because it was unlikely that the interface would come in contact during loading. The contact fraction was used as a measure of the apposition between cement and bone while the intersection fraction was an indicator of the cement-bone interlock. The interface gap thickness between cement and bone was measured at nine sampling points for each specimen using the vertical projection lines in the CT scans. The measurements were then averaged for each specimen to provide a measure of the magnitude of the interface gap for each specimen.
Figure 3.
MicroCT scans were created of each specimen prior to mechanical loading (A). The small squares placed on image A and B indicate location of digital image correlation (DIC) measurement locations at the cement-bone interface. The relative vertical displacement between the two squares was used as the measure of interface micromotion. A grid was constructed and projected vertically through the image sets (B) and stereology was used to quantify the contact fraction and intersection fraction at the cement-bone interface (C). Points of apposition (black x) and points of proximity (white x) are shown for one of the projection lines.
A representative micro-CT image set was used with a large number (n=10) of repeated measures to determine the reproducibility of the stereology technique (7). Confidence intervals (95% CI) were used to indicate the variability of a single measurement, while the standard error of the mean (SEM) was used to describe errors in estimating the sample mean. Confidence intervals and standard error of the mean were found to be lower for the contact fraction measurement (mean: 0.123, 95% CI: 0.021, SEM: 0.0092) when compared to the intersection fraction measurement (mean: 0.805, 95% CI: 0.075, SEM: 0.033).
Two-sample t-tests were used to compare the mechanical response (tensile and compressive stiffness, and failure strength) and interface morphology measures (interface contact fraction, intersection fraction, and interface gap thickness) for the post-mortem retrievals and laboratory-prepared specimens. Bonferroni corrections were made for multiple tests (18). Linear regression models were used to determine if contact fraction (CF), intersection fraction (IF), and interface gap thickness (GT) contributed to interface strength. This took the form:
| Eqn 1 |
where b1 to b4 are regression coefficients.
Results
During the mechanical loading of the test specimens, the majority of motion occurred at the interface between cement and bone with little deformation within the adjacent bulk cement and bone. As such, all micro-motions presented here are taken using digital image correlation (DIC) sampling points in the bone and cement immediately adjacent to the interface. Interface compressive stiffness was greater than interface tensile stiffness (paired t-test, p=0.01), but both exhibited substantial motion with small applied loads. The interface tensile and compressive stiffness of the post-mortem retrieval specimens were, on average, an order of magnitude lower (two-sample t-test, p<0.0001) than those measured with laboratory-prepared specimens (Table 2). It is noted that for the post-mortem specimens, the range of responses from the low-displacement tension-compression tests was very large and a minority of specimens exhibited responses within the range found for laboratory-prepared specimens (4 of 49 in tension, 5 of 49 in compression).
Table 2.
Mechanical test and stereology results for cement-bone specimens prepared from post-mortem retrievals and laboratory prepared constructs (previously reported). Compression test results were not performed in the previous laboratory prepared specimens. Test parameters were all significantly lower (p<0.0001) for the post-mortem retrievals as tested using two-sample t-tests. There were 49 post-mortem retrieval specimens and 21 laboratory prepared specimens.
| Post-mortem Retrievals | Laboratory Prepared | |||||
|---|---|---|---|---|---|---|
| Parameter | Mean | Standard Deviation |
Range | Mean | Standard Deviation |
Range |
| Interface tensile stiffness (MPa/mm) | 16 | 35 | 0.01 – 180 | 208 | 131 | 61.7 – 587 |
| Interface compressive stiffness (MPa/mm) | 28 | 58 | 0.06 – 346 | 277 | 158 | 84 – 630 |
| Compression test stiffness (MPa/mm) | 47 | 61 | 0.27 – 332 | |||
| Compression test offset (mm) | 0.039 | 0.045 | 0 – 0.16 | |||
| Failure strength (MPa) | 0.21 | 0.32 | 0 – 1.02 | 3.0 | 1.4 | 1.2 – 6.4 |
| Stereology contact fraction (CF) | 0.11 | 0.17 | 0 – 0.72 | 1.6 | 0.97 | 0.5 – 4.1 |
| Stereology intersection fraction (IF) | 0.72 | 0.62 | 0.01 – 3.3 | 2.7 | 1.24 | 1.2 – 6.3 |
| Interface gap thickness (mm) | 0.33 | 0.28 | 0.024–1.38 | 0.013 | 0.023 | 0 – 0.08 |
When loaded in compression to 0.5 MPa, the post-mortem retrieval specimens exhibited a modest increase in stiffness when compared to the initial low displacement test (Table 2). The offset in displacement for the compression test was 0.039mm with a range of 0 to 0.16mm. This indicates that the cement-bone interface would need to compress 0.039mm (on average) before the interface could support even modest compressive loads.
The tensile strength of the cement-bone interface from post-mortem retrievals was also an order of magnitude smaller (two-sample t-test, p<0.0001) than that measured from laboratory-prepared specimens (Table 2). Of 49 specimens tested, 15 had no measurable tensile strength (<0.01 MPa), and each donor bone had at least one specimen with no tensile strength. Post-mortem retrieval specimens derived from the posterior quadrant (0.49±0.41 MPa, mean ± standard deviation) of the femur were generally stronger than those produced from the other quadrants (anterior: 0.20±0.31 MPa, lateral: 0.03±0.07 MPa, medial: 0.16±0.27 MPa). There was no correlation (r2=0.004, p=0.67) between axial position and interface strength.
The contact fraction and intersection fraction between cement and bone was significantly lower (two sample t-test, p<0.0001) for the post-mortem retrievals when compared to the laboratory-prepared specimens. The interface gap thickness for the post-mortem retrievals was large, with a mean of 0.33 mm for all specimens, and a range from 0.024 to 1.38 mm. In contrast, lab-prepared specimens had a mean interface gap thickness of 0.013 mm with a range of 0 to 0.080 mm.
There was a positive correlation between interface stiffness and tensile strength (linear regression: r2=0.57, p<0.0001) for the post-mortem retrieval specimens indicating that specimens with less motion at the contact interface between cement and bone resulted in higher strength. Using an Analysis of Covariance (ANCOVA), it was determined that the influence of interface tensile stiffness on failure strength was not different (same slope) for post-mortem retrievals and lab-prepared specimens (p=0.374).
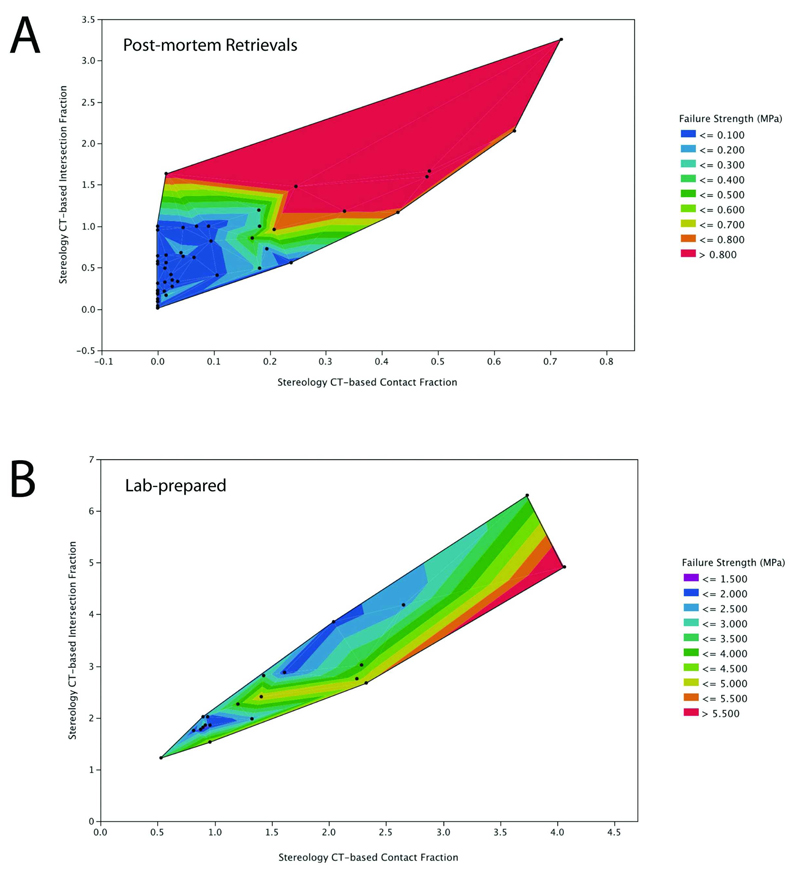
From the regression model of tensile strength relating morphological variables (Table 3), there were contributions of contact fraction and intersection fraction to bone strength, but cement-bone gap thickness did not contribute significantly to the regression relationship. Interestingly, the effect of intersection fraction had a different sign for the post-mortem retrieval and laboratory-prepared specimens. This interaction can be seen in contour plots of the post-mortem retrievals (Figure 4A) and lab-prepared specimens (Figure 4B). For the post-mortem retrievals, for a constant contact fraction, increasing the intersection fraction contributed positively to interface strength. In contrast, for a constant contact fraction in laboratory-prepared specimens, an increased intersection fraction was associated with lower interface strength. Overall, the strength-morphology regression model was capable of explaining a large fraction of the experimental variability for the post-mortem retrievals (r2=0.71, p<0.0001) and a more moderate regression model for the laboratory-prepared specimens (r2=0.49, p=0.0022).
Table 3.
Regression models for the post-mortem retrievals (adjusted R2=0.71, p<0.0001, RMS error=0.174 MPa) and laboratory-prepared specimens (adjusted R2=0.49, p=0.0022, RMS error=0.992 MPa) relating the dependent variable tensile strength of the cement-bone interface and independent variables including morphology parameters contract fraction (CF), intersection fraction (IF), and cement-bone gap thickness (GT).
| Regression Model Term | Estimate | Standard error of mean | P value |
|---|---|---|---|
| Post-Mortem Retrievals | |||
| Constant (b1) | −0.066 | 0.072 | 0.365 |
| CF (b2) | 0.884 | 0.261 | 0.0015 |
| IF (b3) | 0.223 | 0.082 | 0.0090 |
| GT (b4) (mm) | 0.040 | 0.110 | 0.717 |
| Laboratory-Prepared | |||
| Constant (b1) | 2.93 | 0.561 | <0.0001 |
| CF (b2) | 2.64 | 0.651 | 0.0008 |
| IF (b3) | −1.5555 | 0.521 | 0.0083 |
| GT (b4) (mm) | −4.52 | 10.32 | 0.667 |
Figure 4.
Contour plot of cement-bone tensile strength as a function of intersection fraction and contact fraction for post-mortem retrievals (A) and lab-prepared specimens (B). Individual data points are illustrated as dots on the contour plot.
When comparing average interface strength as a function of donor bone source, there was no correlation (r2=0.09, p=0.47) between years of service and interface strength. However, there was a negative correlation between donor age and interface strength (r2=0.75, p=0.0053); younger donor bones had stronger interfaces.
Discussion
The mechanical response of post-mortem retrieved specimens containing cement-bone interfaces was characterized by a highly compliant interface in both tensile and compressive loading directions. The post-mortem retrievals also exhibited a very low tensile strength. When compared to laboratory-prepared specimens, the relatively weak and compliant cement-bone interfaces from the post-mortem retrievals could be explained by the paucity of direct apposition between the cement and bone and the lack of cement-bone interlock. With compressive loading to 0.5 MPa, the interfaces produced a bi-linear response with a zero-force displacement offset before substantial compressive loads were supported. Given that all of the specimens could support 0.5 MPa of compressive loading without failure, while the tensile strength was much lower (0.2 MPa on average), suggests that these interfaces transmitted in vivo loads much more effectively in compression when compared to tension.
The morphology of the interface between the cement and bone could be correlated with the strength of the interface using the stereology indices of contact fraction and intersection fraction. Overall, a greater contact fraction between the cement and bone was associated with a stronger and stiffer interface. There was an interesting interaction between interface strength and intersection fraction that depended on bone source (either laboratory-prepared or post-mortem retrieval). For the post-mortem retrievals, both contact fraction and intersection fraction contributed positively to creating a stronger interface. But for the lab-prepared specimens, increasing intersection fraction (for the same contact fraction) resulted in a weaker interface. At this time, this relationship is only correlative, but it would appear that having increased intersections at the cement-bone interface for the post-mortem retrievals even with low contact fraction (perhaps due to bony resorption) would be desirable in terms of increasing interface strength. For the laboratory prepared specimens, having additional intersections at the cement-bone interface would appear to weaken the interface. It is possible that once there is sufficient cement-bone contact, such as with the laboratory prepared specimens, additional intersections act as stress risers for loads transmitting from the cement to bone. This could diminish the tensile strength of the specimens. Finite element modeling techniques would be helpful in understanding this phenomenon more fully.
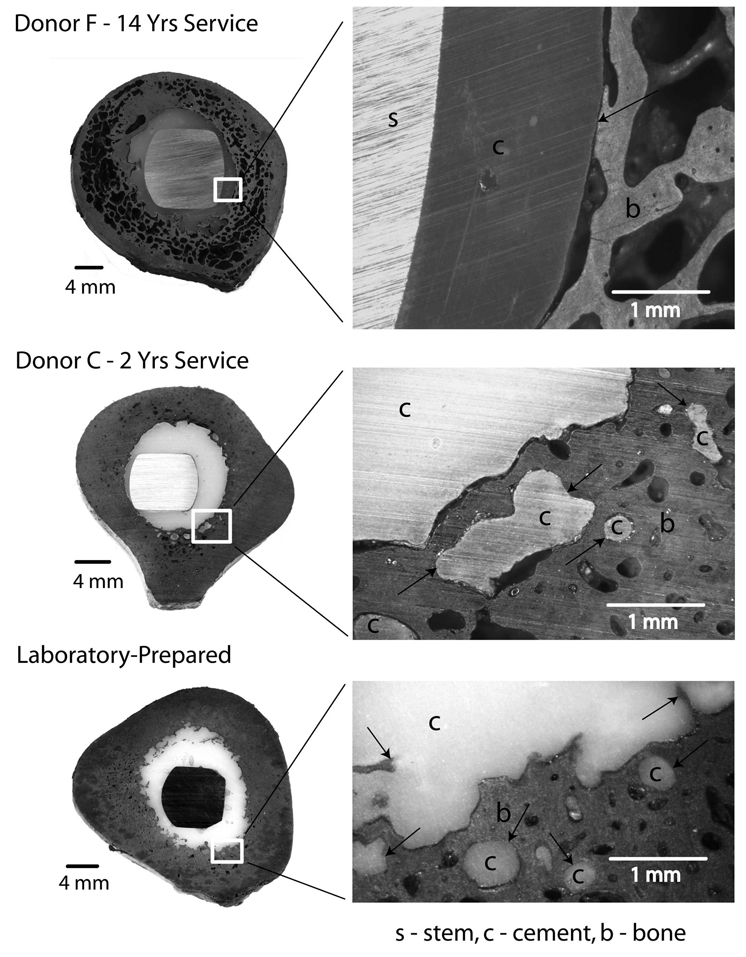
To our knowledge, the biomechanical response of the cement-bone interface obtained from post-mortem retrievals has not been previously investigated. However, there has been long-standing interest in the histology of the cement-bone interface in both well-fixed and revision total joint replacements. Charnley (4) performed extensive studies with well-fixed total hip replacements where he found that “in load bearing areas, no tissue of any kind intervenes between the cement surface and caps of changed bone on the ends of the load-bearing trabeculae”. He found that these “caps of changed bone” were rather sparsely distributed around the cement-bone interface. Other areas of the cement-bone interface were filled with either thin fibrous tissue or were fluid-filled cavities. Draenert (5) illustrated with an animal model that gap formation from polymerization shrinkage can be filled with lamellar bone and that a secondary medullary cavity can form with regions of direct cement-bone apposition and regions in which gaps are evident. More recently, radiolucencies seen on x-ray were found to be due to remodeling of the bone with an appositional neo-cortex of bone (9, 13). Although direct quantitative comparison is not possible between these different studies, the post-mortem retrievals used in this study were consistent with those described in the literature, where we commonly found regions of sparsely distributed bone around the cement (Figure 5).
Figure 5.
Transverse section from donor bone F (70 mm distal to collar) illustrates extensive remodeling of the cortical bone and generation of a neocortex at the cement-bone interface. There are regions of cement-bone apposition along the interface. A section from donor bone C (120 mm distal to collar) shows much less cortical bone remodeling. There was cement flow into trabecular spaces and cortical bone lacunae and there were regions of apposition at the cement-bone interface. A section from a laboratory-prepared construct illustrates regions of cement-bone apposition and a very narrow gap between the cement and bone. Black arrows indicate areas of cement-bone apposition.
There are several limitations to the present study. First, a detailed surgical report was not available for these post-mortem retrievals because they were obtained from willed body programs. However, it is known that a distal plug was used in 7 of the 8 donor bones (donor B uncertain because the femur was sectioned just distal to the stem tip) and 7 of 8 donor bones (donor B was hand mixed) used vacuum mixing as evidenced by very low mid-mantle porosity. This suggests that second generation cementing techniques were used on at least 7 of the 8 donor bones. Second, while the axial stability of the constructs was quantified, it is not known if all of the hips could be classified clinically as well-functioning. All were very stable in response to axial loading and the lowest axial stiffness measured in this study (6780 N/mm) was greater than that estimated in another study of well-functioning femoral components (lowest estimated to be 4000N/mm based on data provided) (8). Quasistatic loading in tension and compression was the only loading case used in this study. Shear loading and fatigue loading are also important loading conditions to be considered. Quasistatic loading was chosen because we anticipated that these interface might be very weak and performing a fatigue study with these specimens would have been very challenging, particularly in load control.
The specimens used in this study were taken from the distal half of the cemented stem constructs. As such, the mechanical behavior of specimens from more proximal regions (where there would be more abundant trabecular bone for interdigitation) was not determined. Preliminary measurements of the interface morphology from transverse sections of the post-mortem retrievals indicates that gap fractions at the cement-bone interface (gap area divided by cement area) were higher for proximal sections (16.9%) when compared to distal sections (12.5%). In contrast, for a series of six laboratory-prepared constructs, there were much lower gap fractions at the cement-bone interface (3.6% proximal and 2.6% distal). Larger gap fractions could be associated with weaker interfaces; this suggests that the weak interfaces found in the post-mortem retrievals would be present in both proximal and distal regions. Further work is needed to elucidate the changes in cement-bone micro-mechanics for sections from the proximal portion of the cement mantle.
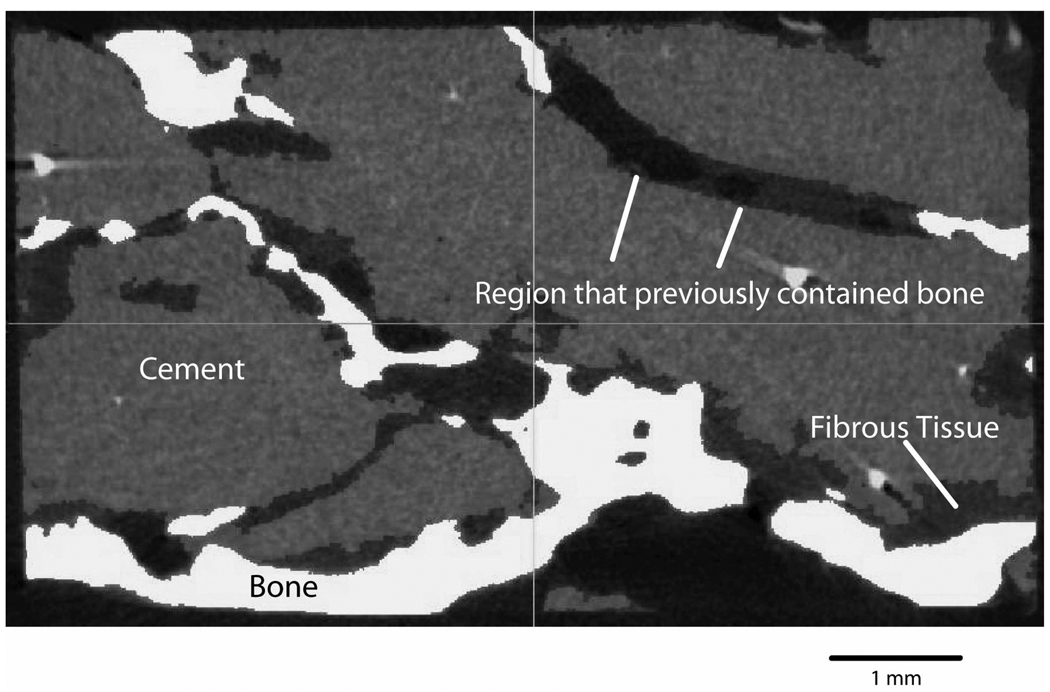
When compared to the laboratory-prepared specimens, there are likely several factors that contribute to the weaker interfaces found with the post-mortem retrievals. First, there was clear evidence (Figure 6) of bony resorption at the interface between cement and bone for some of the post-mortem retrieval specimens. Bone resorption at the interface would reduce the contact fraction and intersection fraction at the cement-bone interface and subsequently result in weaker interfaces. If remodeling occurs such that cement is supported in small, discrete locations as suggested previously (4, 5), then our samples could be missing these connections because they are small relative to the scale of remaining appositional bone. Testing with larger sections would seem warranted to understand how these “caps of changed bone” support load transfer. Second, it was not clear that all the post-mortem retrievals were pressurized as well as can be achieved in the lab and there was some indication of polymerization shrinkage of the PMMA away from the bone in some of the short-term retrievals (0.2 and 0.9 yrs). Further analysis comparing the morphology of the cemented stem constructs from lab-prepared and post-mortem sources is warranted.
Figure 6.
Micro-CT image of an interdigitated cement-bone region from donor F (14 years in service) at 12 micron resolution. Regions of cement-bone apposition are evident as are cavities that appeared to have previously contained bone.
From a clinical perspective, these results suggest that the original morphology of the cement-bone interface with high apposition and interlock at the time of surgery (as simulated in the lab-prepared specimens) become less well connected with short-term reaction to the surgical procedure, presence of cement, and long-term bony remodeling at the cement-bone interface. The reduced apposition results in a more compliant interface that has only a limited capacity to transfer tensile loads. As such, the load transfer mechanism between stem, cement and bone would change over time in a way that is not yet understood. It is important to note that most finite element models of cemented implant systems assume the cement-bone interface is bonded (no interfacial motion and a very high interfacial strength). Inclusion of new information from post-mortem retrieval mechanics would be helpful in understanding changes in load transfer between implant components and bone. The results also indicate that the strength and stiffness of these specimens vary widely, and this does not seem to be a direct function of time in service. This is not surprising given the range of donor bone ages, years in service, likely differences in cementation technique, and differences in in-vivo loading. When grouped by donor, the strength of the cement-bone interface was greater for low donor age, but was not a function of years of service. More donor specimens are required to assess whether there are any significant correlations between the micromechanical parameters and the donor age and time of service.
In summary, the stiffness and strength of the cement-bone interface from retrieved femoral components was much lower than that measured from laboratory prepared specimens. The cement-bone interfaces from post-mortem retrievals were very compliant (under tension and compression) and had a very low strength in tensile loading. Interface morphology including the contact fraction and intersection fraction between cement and bone could explain the differences in strength. The relationship between interface morphology and interface strength was not the same for post-mortem retrievals and lab-prepared specimens. For a constant contact fraction, increasing the intersection fraction was associated with a stronger interface for the post-mortem retrievals, while increasing the intersection fraction was associated with a weaker interface for the laboratory prepared specimens.
Acknowledgement
This work was financially supported by NIH AR42017 (KAM) and NIH EB001715 (AWE). The authors also acknowledge Caroline Mann for help with the stereology analysis and Preston Beck and Dan Yaeger for help with specimen procurement.
References
- 1.Askew MJ, Steege JW, Lewis JL, Ranieri JR, Wixson RL. Effect of cement pressure and bone strength on polymethylmethacrylate fixation. J Orthop Res. 1984;1:412–420. doi: 10.1002/jor.1100010410. [DOI] [PubMed] [Google Scholar]
- 2.Balu GR, Noble PC, Alexander JW, Vela VL. The effect of intramedullary reaming on the strength of the cement/bone interface. Trans Orthop Res Soc. 1994;19:797. [Google Scholar]
- 3.Chang PB, Mann KA, Bartel DL. Cemented Femoral Stem Performance: Effects of Proximal Bonding, Geometry, and Neck Length. Clin Orthop Rel Res. 1998;355:57–69. [PubMed] [Google Scholar]
- 4.Charnley J. Low friction arthroplasty of the hip. New York: Springer-Verlag; 1979. p. 376. [Google Scholar]
- 5.Draenert KK. The John Charnley Award Paper. Histomorphology of the bone-to-cement interface: remodeling of the cortex and revascularization of the medullary canal in animal experiments. In: Salvato EA, editor. The Hip: Proceedings of the ninth open scientific meeting of The Hip Society; The C. V. Mosby Company; 1981. pp. 71–110. [PubMed] [Google Scholar]
- 6.Goodman SB, Ma PH, Song Y, Lee K, Doshi A, Rushdieh B, Woolson S, Maloney W, Schurman D, Sibley R. Loosening and osteolysis of cemented joint arthroplasties: A biologic spectrum. Clin Ortho Rel Res. 1997;337:149–163. doi: 10.1097/00003086-199704000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Howard CV, Reed MG. Unbiased Stereology: Three-dimensional measurement in microscopy. New York: Springer-Verlag; 1998. [Google Scholar]
- 8.Jasty M, Maloney WJ, Bragdon CR, Haire T, Harris WH. Histomorphological studies of the long-term skeletal responses to well fixed cemented femoral components. J. Bone Jt Surg. 1990;72-A:1220–1229. [PubMed] [Google Scholar]
- 9.Jasty M, Maloney WJ, Bragdon CR, O'Conner DO, Haire T, Harris WH. The initiation of failure in cemented femoral components of hip arthroplasties. J Bone Jnt Surg. 1991;73-B:551–558. doi: 10.1302/0301-620X.73B4.2071634. [DOI] [PubMed] [Google Scholar]
- 10.Juliusson R, Flivik J, Nilsson J, Ryd L, Onnerfält R. Circulating blood diminishes cement penetration into cancellous bone. Acta Orthop Scand. 1995;66:234–238. doi: 10.3109/17453679508995531. [DOI] [PubMed] [Google Scholar]
- 11.Krause WR, Krug W, Miller J. Strength of the Cement-Bone Interface. Clin Orthop. 1982;163:290–299. [PubMed] [Google Scholar]
- 12.MacDonald W, Swarts E, Beaver R. Penetration and shear strength of cement-bone interfaces in-vivo. Clin Ortho Rel Res. 1993;286:283–288. [PubMed] [Google Scholar]
- 13.Maloney WJ, Schmalzried T, Harris WH. Analysis of long-term cemented total hip arthroplasty retrievals. Clin Orthop Relat Res. 2002:70–78. doi: 10.1097/00003086-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Mann KA, Miller MA, Cleary RJ, Janssen D, Verdonschot N. Experimental micromechanics of the cement-bone interface. J Orthop Res. 2008;26:872–879. doi: 10.1002/jor.20575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller MA, Race A, Gupta S, Higham P, Clarke MT, Mann KA. The role of cement viscosity on cement-bone apposition and strength an in vitro model with medullary bleeding. J Arthroplasty. 2007;22:109–116. doi: 10.1016/j.arth.2006.02.076. [DOI] [PubMed] [Google Scholar]
- 16.Mjoberg B. Loosening of the cemented hip prosthesis. The importance of heat injury. Acta Orthop Scand Suppl. 1986;221:1–40. [PubMed] [Google Scholar]
- 17.Smith SW, Estok DM, Harris WH. Total hip arthrolplasty with use of second-generation cementing techniques: An eighteen-year-average follow-up study. J Bone Jnt Surg Am. 1998;80:1632–1640. doi: 10.2106/00004623-199811000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Zar JH. Biostatistical Analysis. Upper Saddle River, New Jersey: Prentice-Hall, Inc.; 1999. [Google Scholar]