Abstract
The objective of our study was to determine GM-CSF activity in the brain following GM-CSF induction. We injected recombinant mouse GM-CSF into the brains of 8-month-old C57BL6 mice via intracerebroventricular injections and studied the activities of microglia, astrocytes, and neurons. We also sought to determine whether an anti-GM-CSF antibody could suppress endogenous microglial activity in the C57BL6 mice and could also suppress microglial activity induced by the recombinant mouse GM-CSF in another group of C57BL6 mice. Using quantitative real-time RT-PCR, we assessed microglial, astrocytic, and neuronal activity by measuring mRNA expression of pro-inflammatory cytokines, GFAP, and the neuronal marker NeuN in the cerebral cortex tissues from C57BL6 mice. We performed immunoblotting and immunohistochemistry of activated microglia in different regions of the brains from control (PBS-injected C57BL6 mice) and experimental mice (recombinant GM-CSF-injected C57BL6 mice, GM-CSF antibody-injected C57BL6 mice, and recombinant mouse GM-CSF plus anti-GM-CSF antibody-injected C57BL6 mice). We found increased mRNA expression of CD40 (9.75 fold), TNFα (2.1 fold), CD45 (1.73 fold), and CD11c (1.70 fold) in the cerebral cortex of C57BL6 mice that were induced with recombinant GM-CSF, compared to control mice. Further, the anti-GM-CSF antibody suppressed microglia in mice that were induced with recombinant GM-CSF. Our immunoblotting and immunohistochemistry findings of GM-CSF associated cytokines in C57BL6 mice induced with recombinant GM-CSF, in C57BL6 mice injected with the anti-GM-CSF antibody, and in C57BL6 mice injected with recombinant mouse GM-CSF plus anti-GM-CSF antibody concurred with our real-time RT-PCR findings. These findings suggest that GM-CSF is critical for microglial activation and that anti-GM-CSF antibody suppresses microglial activity in the CNS. The findings from this study may have implications for anti-inflammatory effects of Alzheimer's disease (AD) and experimental autoimmune encephalomyelitis mice (a multiple sclerosis mouse model).
Introduction
The granulocyte-macrophage colony-stimulating factor (GM-CSF), a monomeric glycoprotein secreted by activated vascular endothelial cells, is a hematopoietic factor and an inflammatory cytokine that is expressed in a wide variety of cells, including T-cells, monocytes, macrophages, fibroblasts, and endothelial cells (Whetton and Dexter 1989; Fleetwood et al. 2005; Hamilton 2008; Hamilton 2002; Franzen et al. 2004). GM-CSF stimulates the proliferation and maturation of myeloid progenitors, precursors of neutrophils, monocytes, macrophages, and eosinophils. GM-CSF receptors are present in haematopoietic cells of the peripheral nervous system; in microglia, astrocytes, and oligodendrocytes; and, to a lesser extent, in neurons of the central nervous system (CNS) (Sawada et al. 1993). Astrocytes are the unique source of GM-CSF. Importantly, GM-CSF regulates the functions of microglia via several cytokines (Malipiero et al. 1990). GM-CSF modulates the function of glial cells and contributes to a unique cytokine network in the CNS. GM-CSF is reported to cross the blood-brain, blood-spinal cord, and blood-testis barriers (McLay et al. 1997; McLay et al. 1997).
The activation of GM-CSF is related to inflammatory responses, such as injury to the CNS (Frazen et al. 2004; Hamilton 2008). Injury to the CNS leads to complex inflammatory responses involving an influx of blood-derived monocytes and macrophages, and the activation of astrocytes and microglia. These events are mediated by the release of pro-inflammatory cytokines. Microglial activation is a key cellular response in many infectious, inflammatory, traumatic, neoplastic, ischaemic, and degenerative disease conditions in the CNS, such as Alzheimer's disease (AD) and multiple sclerosis (MS) (Manczak et al. 2009, Mao and Reddy 2009).
GM-CSF is involved in several important and beneficial cellular functions (Fleetwood et al. 2005). GM-CSF induces, proliferates, and changes microglial morphology; it is these microglial cells that are involved in removing myelin debris after CNS injury. GM-CSF is one of the key factors promoting axonal regeneration. GM-CSF activates and proliferates microglial cells, which in turn helps to successfully repair injured axons. It has been reported that 3 to 4 weeks after spinal cord injury, the deactivation of macrophages coincides with the involution of spontaneous axonal regeneration (Brooks et al. 1998). In addition, GM-CSF has been found to display a neurotrophic action by stimulating the growth of neurites in cultures (Kannan et al. 2000). Further, GM-CSF may influence the survival and functioning of neighboring neurons (Giulian et al. 1994; Franzen et al. 2004). Secreted by activated vascular endothelial cells, GM-CSF acts as an anti-apoptotic factor that delays cell death from recruited neutrophils (Franzen et al. 2004, Schäbitz et al. 2008).
In contrast to the positive effects of GM-CSF activation, GM-CSF activation is responsible for the excessive production of reactive glial cells, astrocytes, and microglia (Franzen et al. 2004), all of which are considered to be barriers to axonal regeneration and neuroprotection (Franzen et al. 2004). GM-CSF regulates the composition of the glial scar, which is a reactive cellular process involving astrogliosis that occurs after injury to the CNS. As a pro-inflammatory cytokine, GM-CSF is considered to be a critical mediator in the development of chronic inflammation.
Several recent studies found increased levels of GM-CSF in the cerebrospinal fluid of AD patients (Tarkowski et al. 2001 and 2003), which may be due to the pathophysiology of AD, which involves inflammatory responses (Banati and Beyreuther 1995; McGeer and McGeer 2003). Proliferations of reactive astrocytes and microglia have also been observed in brains of AD patients (McGeer and McGeer 2003) and AD transgenic mice (Mehlhorn et al. 2000; Apelt et al. 2001; Matsuoka et al. 2001; Abbas et al. 2002; Wirths et al. 2008; Maier et al. 2008; Manczak et al. 2009). Furthermore, AD transgenic mice lines that over-express APP and amyloid beta (Aβ) have been found to exhibit significant cerebrovascular inflammation and microgliosis around areas of Aβ plaque deposition (Frautschy et al. 1998; Stalder et al. 1999; Wegiel et al. 2001). In AD patients, GM-CSF levels were correlated with Fas/APO-1 and Tau concentrations, suggesting a role for GM-CSF in neuronal damage and apoptosis.
GM-CSF is a cytokine that also functions as a white blood-cell growth factor. GM-CSF stimulates stem cells to produce granulocytes – neutrophils, eosinophils, and basophils and monocytes. Monocytes exit the circulating blood and migrate into tissue where they mature into macrophages. Monocytes are, thus, part of the immune/inflammatory cascade, in which the activation of a small number of macrophages can rapidly lead to an increase in numbers – an important process in fighting infection in AD patients.
Even though GM-CSF is known to play a role in inflammatory responses in the CNS and is known to impair cellular functions, current knowledge of GM-CSF, particularly its mechanism of functioning is still not clear. Further, the precise connection between the GM-CSF induction of microglia and the types of inflammatory cytokines that are activated in the CNS upon GM-CSF induction are not fully understood. In addition, it is still unclear whether an anti-GM-CSF anti-mouse antibody can suppress excessive microglia in the CNS. The objective of our study was to determine activity in the brain of C57BL6 mice (a well-studied mouse strain for several human diseases) upon GM-CSF induction. We investigated the following in the present study: 1) We induced recombinant mouse GM-CSF by injecting recombinant mouse GM-CSF into the brains of 8-month-old C57BL6 mice via intracerebroventricular (ICV) injections and studied the activities of microglia, astrocytes, and neurons. 2) We also investigated whether the anti-GM-CSF antibody could suppress endogenous microglial activity in C57BL6 mice and microglial activity induced by recombinant GM-CSF in the brains of C57BL6 mice. 3) We assessed microglial, astrocytic, and neuronal activity by measuring mRNA expression of cytokines, GFAP, and neuronal markers (NeuN) using Sybr-Green chemistry-based quantitative real-time RT-PCR in the cerebral cortex tissues of C57BL6 mice. 4) We also conducted immunoblotting analysis in order to determine protein levels of cytokines are affected by recombinant GM-CSF and anti-GM-CSF antibody. 5) We also performed immunohistochemistry of activated microglial markers, and astrocytes, in the brains from all animals studied.
Materials and Methods
The C57BL6 mice
Twenty, 8-month-old C57BL6 male mice (25 to 40 grams) were purchased from Taconic Farms (New York, NY) and housed at the animal facility of the Oregon National Primate Center at Oregon Health & Science University (OHSU). We divided the mice into 4 groups: 3 experimental and 1 control (n = 5 per group) (see Table 1). The OHSU Institutional Animal Care and Use Committee approved all procedures for animal care according to guidelines set forth by NIH.
Table 1. Summary of stereotaxic intracerebroventricular injections of in control and experimental groups of 8-month-old C57BL6 mice.
| Control and experimental groups | Intracerebroventricular injections | Number of mice |
|---|---|---|
| 1. C57BL6 mice +PBS vehicle | 5-7 μL PBS vehicle | 5 |
| 2. C57BL6 mice + recombinant mouse GM-CSF | 5-7 μL containing 30 units of recombinant mouse GM-CSF | 5 |
| 3. C57BL6 mice + anti-GM-CSF antibody | 5-7 μL containing 11 μg of anti-mouse anti-GM-CSF antibody | 5 |
| 4. C57BL6 mice + recombinant mouse GM-CSF + anti-GM-CSF antibody | 5-7 μL containing 30 units of recombinant mouse GM-CSF plus 11 μg ant-GM-CSF anti-mouse antibody | 5 |
The recombinant mouse GM-CSF and anti-mouse GM-CSF antibody
The recombinant mouse GM-CSF antigen was purchased from Thermo Scientific (Waltham, MA), and the anti-GM-CSF antibody was supplied by KaloBios Pharmaceuticals (Palo Alto, CA). The GM-CSF antibody was originally developed from a murine hybridoma elicited from a rat immunized with a purified, recombinant mouse GM-CSF antigen.
The reagents for real-time RT-PCR analysis
RNA isolation and cDNA synthesis reagents, including TriZol, reverse transcriptase superscript, and other chemicals, were purchased from Invitrogen (Carlsbad, CA), and real-time RT-PCR reagents were purchased from Applied Biosystems (Foster City, CA). Using primer express Software (Applied Biosystems), we designed the oligonucleotide primers for the housekeeping genes β-actin; the mitochondrial-encoded gene NDAH-subunit 1; the cytokines TNFα, IL1β, IL6, gp91, CD11b, CD11c, CD40, CD45, and MHCII; the astrocytic marker GFAP; and the neuronal marker NeuN. The primer sequences and amplicon sizes are listed in Table 2.
Table 2. Summary of oligonucleotide primers used for real-time RT-PCR in measuring mRNA expression of cytokine, astrocytic, and neuronal markers in experimental groups and control group of C57BL6 mice.
| Marker | DNA sequence (5′-3′) | PCR product size in base-pair | |
|---|---|---|---|
| TNFα | Forward Primer | TTCGGCTACCCCAAGTTCAT | 55 |
| Reverse Primer | CGCACGTAGTTCCGCTTTC | ||
| IL1β | Forward Primer | CCATGGCACATTCTGTTCAAA | 55 |
| Reverse Primer | GCCCATCAGAGGCAAGGA | ||
| IL6 | Forward Primer | CCACGGCCTTCCCTACTTC | 60 |
| Reverse Primer | TTGGGAGTGGTATCCTCTGTGA | ||
| CD11b | Forward Primer | AAACCACAGTCCCGCAGAGA | 57 |
| Reverse Primer | CGTGTTCACCAGCTGGCTTA | ||
| CD11C | Forward Primer | CCTGAGGGTGGGCTGGAT | 50 |
| Reverse Primer | GCCAATTTCCTCCGGACAT | ||
| CD40 | Forward Primer | TTCGAGTCAACGCCCATTC | 53 |
| Reverse Primer | GATCCACTGCTGGGCTTCA | ||
| CD45 | Forward Primer | GAACATGCTGCCAATGGTTCT | 70 |
| Reverse Primer | TGTCCCACATGACTCCTTTCC | ||
| gp91 | Forward Primer | CCCTCGGACTTTGGCAAA | 55 |
| Reverse Primer | CCAGACTCGAGTATCGCTGACA | ||
| MHCII-2 | Forward Primer | CTATGATGGCCGCGATTACA | 56 |
| Reverse Primer | GCTGCTGTCCACGTTTTCAG | ||
| GFAP | Forward Primer | CCAGCTTCGAGCCAAGGA | 55 |
| Reverse Primer | GAAGCTCCGCCTGGTAGACA | ||
| NeuN | Forward Primer | GGCAATGGTGGGACTCAAAA | 65 |
| Reverse Primer | GGGACCCGCTCCTTCAAC | ||
| Housekeeping Genes | |||
| Beta Actin | Forward Primer | ACGGCCAGGTCATCACTATTC | 65 |
| Reverse Primer | AGGAAGGCTGGAAAAGAGCC | ||
| NADH-subunit 1 | Forward Primer | CGGGCCCCCTTCGAC | 72 |
| Reverse Primer | GGCCGGCTGCGTATTCT | ||
Optimization of the recombinant mouse GM-CSF and anti-GM-CSF antibody
It has been suggested that microglia can be induced in rodent brains through the administration of 10 to 50 units GM-CSF (Giulian et al. 1988). Further, we had previously optimized recombinant mouse GM-CSF in C57BL6 mice by injecting GM-CSF in a range of 10 to 50 units, and found a maximum microglial induction at 30 units without side and adverse effects (data not shown). Beyond 50 units, however, the C57BL6 mice died within 48 hrs. As shown in Table 1, we injected 30 units GM-CSF into the brains of C57BL6 mice using stereotaxic intracerebroventricular (ICV) injections. To determine whether the GM-CSF antibody could suppress endogenous glial activity and microglia (induced by recombinant mouse GM-CSF), we injected 11 μg anti-GM-CSF anti-mouse antibody (Table 1) using stereotaxic ICV injections.
Intracerebroventricular injections
To inject the PBS vehicle, the recombinant mouse GM-CSF, and/or the GM-CSF antibody, we performed survival surgeries for all animals. For injections of the recombinant mouse anti-GM-CSF antibody into brain, the animals were anesthetized with 7% chloral hydrate (280 mg/kg) using IP injections. The fur was shaved, and animal was placed in a stereotactic apparatus with a mouse adaptor. A small burr whole was made in the skull overlying the lateral ventricle. As shown in Table 1, the following were injected into the lateral ventricle of the C57BL6 mouse brains in each experimental and control groups, through a 33-gauge injector attached to a 10-microliter Hamilton syringe (Hamilton Co., Reno NV): 7 μl PBS vehicle (in control C57BL6 mice - group 1) 30 units recombinant mouse GM-CSF (in experimental mice - group 2), 11 μg GM-CSF antibody (in experimental mice - group 3), and 30 units recombinant mouse GM-CSF plus 11 μg anti-GM-CSF anti-mouse antibody (in experimental mice -group 4). We previously tested the total volume of PBS vehicle or mouse GM-CSF and/or GM-CSF antibody integration into the brains of the C57BL6 mice, and found that up to 7 μl of GM-CSF can be easily integrated into their brains. Thus, we used 7 μl of GM-CSF in our experiments.
The recombinant GM-CSF injection was administered over 5 min, after which the cannula was left in place for an additional 5 min to allow for diffusion. The incision was then closed with sutures or surgical staples, and was covered with Xylocaine. Mice were placed in individual cages, kept warm, and carefully monitored during recovery. Five days after the ICV injections, the mice were sacrificed by cervical dislocation since maximum immunoreactivity of microglia was reported at 4-5 days after GM-CSF induction (Raivich et al. 1991). The brains were collected from all animals, and the cerebral cortex and cerebellum/brainstem were dissected from each brain.
Quantification of mRNA expression of cytokines and neuronal markers using real-time RT-PCR
Total RNA was isolated from dissected cerebral cortex tissues from each group of experimental and control mice using the TriZol reagent (Invitrogen, Carlsbad, CA). As shown in Table 2, mRNA expression of several cytokines, GFAP, and neuronal markers was measured using SYBR-Green chemistry-based quantitative real-time RT-PCR, as described by Manczak et al. (2004). Briefly, 1 μg of DNAse-treated total RNA was used as starting material, to which we added 1 μl of oligo (dT), 1 μl of 10 mM dNTPs, 4 μl of 5× first strand buffer, 2 μl of 0.1 M DTT, and 1 μl of RNAse out. The reagents RNA, oligo (dT), and dNTPs were mixed first, heated at 65 °C for 5 min, and then chilled on ice until other components were added. The samples were incubated at 42 °C for 2 min. Then 1 μl of Superscript II (40 U/μl) was added, and the samples were incubated at 42 °C for 50 min. The reaction was inactivated by heating at 70 °C for 15 min.
Quantitative real-time PCR amplification reactions were carried out in an ABI Prism 7900 sequence detection system (Applied Biosystems), in a 25-μl volume of total reaction mixture. The reaction mixture consisted of 1× PCR buffer containing SYBR-Green; 3 mM MgCl2; 100 nm of each primer; 200 nm each of dATP, dGTP, and dCTP; 400 nm of dUTP; 0.01 U/μl of AmpErase UNG; and 0.05 U/μl of AmpliTaq Gold. Fifty ng cDNA template was added to each reaction mixture.
To determine the unregulated endogenous reference gene in C57BL6 mice, the CT-values of β-actin and the NADH-subunit (mitochondrial-encoded gene) were tested. In this case, the CT-value was the cycle number at which the fluorescence generated within the reaction crossed the threshold within the linear phase of the amplification profile. The CT-value is an important quantitative parameter in real-time PCR analysis (Gutala and Reddy 2004; Manczak et al. 2004). All reactions were carried out in duplicate with no template control. The PCR conditions were: 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. The fluorescent spectra were recorded during the elongation phase of each PCR cycle. To distinguish specific amplicons from non-specific amplifications, a dissociation curve was generated. The CT-values were calculated with sequence-detection system (SDS) software V1.7 (Applied Biosystems) and an automatic setting of base line, which was the average value of PCR, cycles 3–15, plus CT generated 10 times its standard deviation. The amplification plots and CT-values were exported from the exponential phase of PCR directly into a Microsoft Excel worksheet for further analysis.
The mRNA transcript level was normalized against β-actin and the mitochondrial-encoded complex I gene NADH-subunit 1 at each dilution. The standard curve was the normalized mRNA transcript level plotted against the log-value of the input cDNA concentration at each dilution. To compare β-actin, the NADH-subunit 1, and cytokine markers, relative quantification was performed according to the CT method from Applied Biosystems (Manczak et al. 2004, 2009). Briefly, the comparative CT method involved averaging duplicate samples which were taken as the CT values for β-actin, the NADH-subunit 1, and cytokine markers. We used β-actin normalization in the present study because β-actin CT values were similar for the control mice, the recombinant GM-CSF-injected C57BL6 mice, and the anti-GM-CSF antibody-injected C57BL6 mice. The ΔCT-value was obtained by subtracting the average β-actin CT value from the average CT-value of cytokine markers. We used the average ΔCT of 5 animals as the calibrator. The fold change was calculated according to the formula 2−(ΔΔCT), where ΔΔCT was the difference between ΔCT and the ΔCT calibrator value.
Immunoblotting analysis of microglial markers
To determine whether the GM-CSF antibody suppresses the protein levels of microglial markers that showed decreased mRNA expression in our real-time RT-PCR, we studied immunoblotting analysis of protein lysates from mice injected with PBS (control), mice injected with recombinant GM-CSF, mice injected with GM-CSF antibody, and mice injected with recombinant GM-CSF plus the GM-CSF antibody. Twenty mg protein lysate from cerebral cortex was resolved on a 12% SDS-PAGE gel, and the resolved proteins were transferred to nylon membranes (Novax Inc., San Diego, CA, USA) that were then incubated at room temperature with a blocking buffer (5% dry milk dissolved in a TBST buffer) for 1 h. The nylon membranes were incubated overnight with the primary antibodies TNFα (1:200; mouse-monoclonal Abcam, Cambridge, MA), CD40 (1:600; rabbit-polyclonal Abcam, Cambridge, MA), CD11c (1:500; rabbit-polyclonal Abcam, Cambridge, MA), and β-actin (1:500; mouse-monoclonal Chemican-Millipore, Temicula, CA). The membranes were washed with a TBST buffer 3 times at 10-min intervals and were then incubated with appropriate secondary antibodies (anti-rabbit 1:10,000; β-actin, sheep-anti-mouse 1:10,000) for 2 h, followed by washing again 3 times with a TBST buffer. Microglial proteins were detected with chemiluminescent reagents (Pierce Biotechnology), and the bands from immunoblots were quantified on a Kodak Scanner (ID Image Analysis Software, Kodak Digital Science, Kennesaw, GA). Briefly, image analysis was used to analyze gel images captured with a Kodak Digital Science CD camera. The lanes were marked to define the positions and specific regions of the bands. ID fine-band command was used to locate the bands in each lane, to scan the bands, and to record the readings.
Immunohistochemistry and immunofluorescence analysis of CD40, TNFα, and GFAP
To determine microglial activity in the brains of all 4 groups of mice as shown in Table 1, we used immunohistochemistry and immunofluorescence analysis of most up-regulated genes (CD40, TNFα, and GFAP) in 8-month-old C57BL6 mice injected with recombinant GM-CSF relative to the age-matched PBS-injected mice in our real-time RT-PCR assay. Briefly, we fixed the midbrain sections from the PBS-injected mice, the recombinant mouse GM-CSF-injected mice, the anti-GM-CSF anti-mouse antibody-injected mice, and the recombinant mouse GM-CSF plus anti-GM-CSF antibody-injected mice. We then incubated the sections overnight with a CD40 antibody (rabbit-polyclonal 1:100; Abcam, Cambridge, MA) and TNFα (rabbit-polyclonal 1:200; Abcam, Cambridge, MA), and GFAP (mouse polyclonal 1:500; Abcam, Cambridge, MA) at room temperature, according to the manufacturers' recommended dilutions. On the next day, we incubated the sections with appropriate secondary antibodies conjugated with the HRP and further incubated the tyramide-tagged fluorescent dye or Alexa 488 (green) or Alexa 594 (red) (Molecular Probes, Eugene, OR) for 10 min at room temperature. Photographs were taken using a confocal microscope.
To quantify the immunoreactivity of microglial marker CD40, for each animal 3 to 4 sections of the midbrain covering the hippocampus and cortex were stained as described above, and quantified as follows: From immunostained sections, several photographs were taken at 20× and at (the original) magnification covering different regions of the brain, including cortex and hippocampus of the brain. The signal intensity of the positive immunoreactive fluorescence of microglia was measured using the red-green and blue (RGB) method (Wilcock et al. 2006; Manczak et al. 2009). We quantified the signal intensity of immunoreactivity for several randomly selected images for each animal and assessed statistical significance using a Student's T-test for each microglial marker between control mice and experimental mice, as described in Table 1.
Double-labeling analysis of CD40 and GFAP
To determine whether CD 40 is expressed in astrocytes, we performed double-labeling immunohistochemistry and immunofluorescence analyses using an anti-CD40 polyclonal antibody and an anti-GFAP monoclonal antibody. We fixed the midbrain sections with the anti-CD40 antibody (rabbit-polyclonal 1:100) and incubated them overnight at room temperature. On the next day, the sections were incubated with the HRP-labeled secondary antibody for 1 hr and were then labeled with the tyramide-labeled fluorescent dye Alexa 484 (green) (Molecular Probes) for 10 min at room temperature. For the second labeling, the sections were incubated overnight with the anti-GFAP antibody (monoclonal 1:500; Abcom, Cambridge, MA). On the third day, the sections were incubated with a secondary antibody conjugated with fluorescent dye Alexa 594 (red). Photographs were taken with a fluorescence microscope.
Double-labeling analysis of TNFα and GFAP
To determine whether TNFα is expressed in astrocytes, we performed double-labeling immunohistochemistry and immunofluorescence analyses using an anti-TNFα polyclonal antibody and an anti-GFAP monoclonal antibody. We fixed the midbrain sections with anti-TNFα antibody (rabbit-polyclonal 1:200) and incubated them overnight at room temperature. On the next day, the sections were incubated with the HRP-labeled secondary antibody for 1 hr and were then labeled with the tyramide-labeled fluorescent dye Alexa 594 (red) (Molecular Probes) for 10 min at room temperature. For a second labeling, the sections were incubated overnight with the anti-GFAP antibody (monoclonal 1:500; Abcom, Cambridge, MA). On the third day, the sections were incubated with the secondary antibody conjugated with fluorescent dye Alexa 498 (green). Photographs were taken with a fluorescence microscope.
Statistical considerations
Statistical analyses were conducted for immunoreactivity of the microglial marker CD40 between the control (C57BL6 mice injected with PBS) and experimental groups (C57BL6 mice injected with recombinant GM-CSF, C57BL6 mice injected with the GM-CSF antibody, and C57BL6 mice injected with the recombinant GM-CSF plus the GM-CSF antibody), using a Student's T-test.
Results
mRNA expression of cytokines in C57BL6 mice injected with recombinant mouse GM-CSF
The primary objective of this study was to assess microglial activation in the cerebral cortex of 8-month-old C57BL6 mice by administering recombinant mouse GM-CSF into the brains of mice via ICV injections and measuring mRNA expression of several cytokines using quantitative real-time RT-PCR. As shown in Table 3, we found a 9.75-fold increase in mRNA levels for CD40, followed by a 2.1-fold increase for TNFα, a 1.73-fold increase for CD45, a 1.7-fold increase for CD11C, and a 1.22-fold increase for MHCII in the mice injected with recombinant GM-CSF compared to the PBS vehicle-injected control mice. We also found mRNA levels unchanged or slightly decreased for several other cytokine markers, such as IL1, IL6, CD11b, and gp91, in the recombinant GM-CSF-injected C57BL6 mice compared to the PBS vehicle-injected control mice (Table 3). We found unchanged the mRNA expression of NeuN in recombinant GM-CSF-injected mice compared to the PBS-injected control mice, suggesting that recombinant GM-CSF does not affect neuronal expression.
Table 3. mRNA expression difference in fold change of cytokine and neuronal markers between control and experimental animals.
| Marker | mRNA difference between recombinant GM-CSF-injected C57BL6 mice/control mice (PBS injected C57BL6 mice) | mRNA difference between anti-GM-CSF antibody-injected C57BL6 mice/control mice (PBS injected C57BL6 mice) | mRNA difference between recombinant GM-CSF plus antibody-injected C57BL6 mice/control mice (PBS injected C57BL6 mice) |
|---|---|---|---|
| TNFα | 2.1 | 1.3 | 0.28 |
| IL-1β | 0.63 | 0.30 | 0.94 |
| IL-6 | 0.36 | 0.35 | 2.89 |
| Gp91 | 0.08 | 0.08 | 0.41 |
| CD11b | 0.29 | 0.13 | 0.91 |
| CD11c | 1.70 | 2.19 | 0.60 |
| CD40 | 9.75 | 2.0 | 0.48 |
| CD45 | 1.73 | 1.38 | 1.04 |
| MHCII -2 | 1.22 | 0.81 | 0.66 |
| GFAP | 1.5 | 0.79 | 1.11 |
| NeuN | 0.9 | 1.1 | 0.60 |
Note: mRNA fold change was calculated based on comparative cycle threshold (CT) values of real-time RT-PCR between experimental mice/control mice for the following: 1). recombinant GM-CSF-injected C57BL6 mice/control mice (PBS injected C57BL6 mice), 2) anti-GM-CSF antibody-injected C57BL6 mice/control mice (PBS injected C57BL6 mice), and 3). recombinant GM-CSF plus antibody-injected C57BL6 mice/control mice (PBS injected C57BL6 mice).
mRNA expression of cytokines in C57BL6 mice injected with the anti-GM-CSF antibody
We determined whether the anti-GM-CSF antibody can suppress endogenous glial activity in the brains of 8-month-old C57BL6 mice. As shown in Table 3, we found decreased mRNA levels of IL1, IL6, gp91, CD11b, and MHCII in the anti-GM-CSF antibody-injected mice compared to the control mice. However, mRNA levels were slightly increased for CD40 (2.0-fold increase), CD11C (2.19-fold increase), and CD45 in the C57BL mice injected with the anti-GM-CSF antibody compared to the control mice. We found unchanged the mRNA expression of NeuN in the anti-GM-CSF antibody-injected C57BL6 mice compared to the control mice (Table 3), suggesting that the anti-GM-CSF antibody does not affect neuronal expression. Overall, these observations suggest that the anti-GM-CSF antibody suppresses endogenous microglial activity in the cerebral cortex of 8-month-old C57BL6 mice.
mRNA expression of cytokines in C57BL6 mice injected with recombinant mouse GM-CSF plus the anti-GM-CSF antibody
We determined whether the anti-GM-CSF antibody can suppress microglia induced by recombinant GM-CSF. We administered recombinant GM-CSF with the anti-GM-CSF antibody into the brains of C57BL6 the mice. Five days after the ICV injections, we assessed the mRNA expression of microglial cytokines. Interestingly, we found normal or decreased levels of CD40 (0.48 fold) in the recombinant GM-CSF plus anti-GM-CSF antibody-injected mice compared to the control mice. Previously, we had found increased levels of mRNA of CD40 (9.57 fold) in the C57BL6 mice induced with recombinant GM-CSF, and we had found that the anti-GM-CSF antibody suppressed microglial activation dramatically in C57BL6 mice that were injected with the recombinant GM-CSF and anti-GM-CSF antibody (see Table 3). All of these results suggest that the anti-GM-CSF antibody is a potent suppressor of activated microglia in the CNS. The anti-GM-CSF antibody also decreased mRNA expression of other cytokines, such as TNFα, CD45, and MHCII, further support for the anti-GM-CSF antibody suppressing microglial activity in the CNS.
We also found that mRNA expression of IL6 increased by 2.89 fold in the mice injected with recombinant GM-CSF and the anti-GM-CSF antibody compared to the control mice, indicating the combination recombinant mouse GM-CSF and the anti-GM-CSF antibody may increase IL6 mRNA expression in the mouse brain.
Immunoblotting analysis of proteins of microglial markers
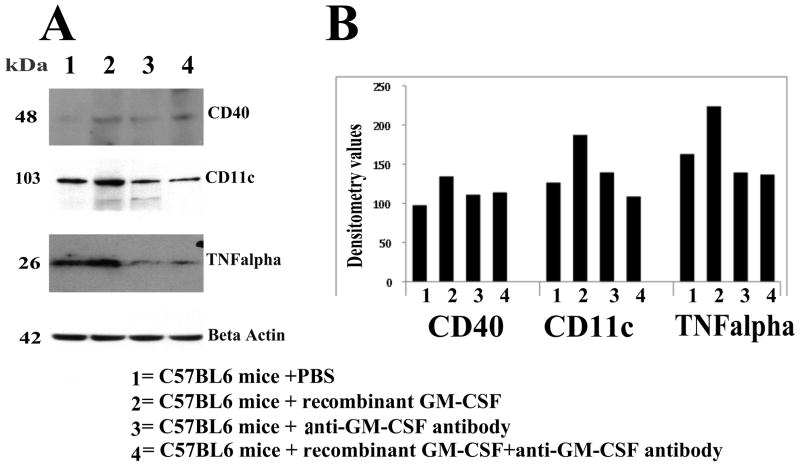
To determine whether the anti-GM-CSF antibody reduces protein levels of microglial markers that are associated with GM-CSF in mice, we performed immunoblotting analysis of markers of microglia, TNFα, CD40, and CD11c in protein lysates prepared from cerebral cortices of mice injected with recombinant GM-CSF, mice injected with the anti-GM-CSF antibody, mice injected with the recombinant GM-CSF and anti-GM-CSF antibody, and PBS-injected control mice. As shown in Fig. 1 and Table 4, we found increased protein levels for CD11c (by 48%), CD40 (by 38%), and TNFα (by 37%) in the C57BL6 mice injected with recombinant GM-CSF compared to the control mice, suggesting that, in C57BL6 mice, recombinant GM-CSF induces microglial proteins associated with GM-CSF. These findings agreed with the results of our real-time RT-PCR and immunohistochemistry analysis (below).
Figure 1.
Immunoblotting analysis of microglial proteins in C57BL6 mice injected with PBS, C57BL6 mice injected with recombinant mouse GM-CSF, and C57BL6 mice injected with recombinant GM-CSF plus GM-CSF antibody. A. Twenty micrograms of protein lysate was used from each sample and immunoblotting analysis was performed using an antibodies of NTFα, CD11c, CD40 and β-actin. Bottom panel represents the immunoblotting of β-actin for equal loading. B. Densitometry values for microglial proteins TNFα, CD11c and CD40 in C57BL6 mice injected with PBS, C57BL6 mice injected with recombinant mouse GM-CSF, and C57BL6 mice injected with recombinant mouse GM-CSF plus GM-CSF antibody. As shown, we found increased protein levels for CD11c (by 48%), followed by CD40 38% and TNFα, 37% in C57BL6 mice injected with recombinant GM-CSF compared to C57BL6 mice injected with PBS.
Table 4. Densitometry values of CD40, CD11c and TNFα in control and experimental groups of C57BL6 mice using immunoblotting analysis.
| Cytokine marker | Control and experimental groups | Densitometry values | % of Difference control and experimental groups |
|---|---|---|---|
| CD40 | C57BL6 mice injected with PBS (control) | 97.6 | 38 % up |
| C57BL6 mice injected with recombinant GM-CSF | 134.4 | ||
| C57BL6 mice injected with GM-CSF antibody | 111.1 | ||
| C57BL6 mice injected with recombinant GM-CSF and GM-CSF antibody | 114.0 | ||
| CD11c | C57BL6 mice injected with PBS (control) | 126.5 | 48% up |
| C57BL6 mice injected with recombinant GM-CSF | 187.3 | ||
| C57BL6 mice injected with GM-CSF antibody | 139.5 | ||
| C57BL6 mice injected with recombinant GM-CSF and GM-CSF antibody | 108.8 | ||
| TNFα | C57BL6 mice injected with PBS (control) | 162.9 | 37% up |
| C57BL6 mice injected with recombinant GM-CSF | 223.8 | ||
| C57BL6 mice injected with GM-CSF antibody | 139.4 | ||
| C57BL6 mice injected with recombinant GM-CSF and GM-CSF antibody | 136.8 | ||
However, mice injected with recombinant GM-CSF plus the anti-GM-CSF antibody showed decreased immunoblotting densitometry values compared to the immunoblotting densitometry values of mice injected with recombinant GM-CSF, indicating that the GM-CSF antibody is a potent suppressor of microglial activity (Table 4).
Immunohistochemistry and immunofluorescence analyses of cytokines induced by recombinant GM-CSF
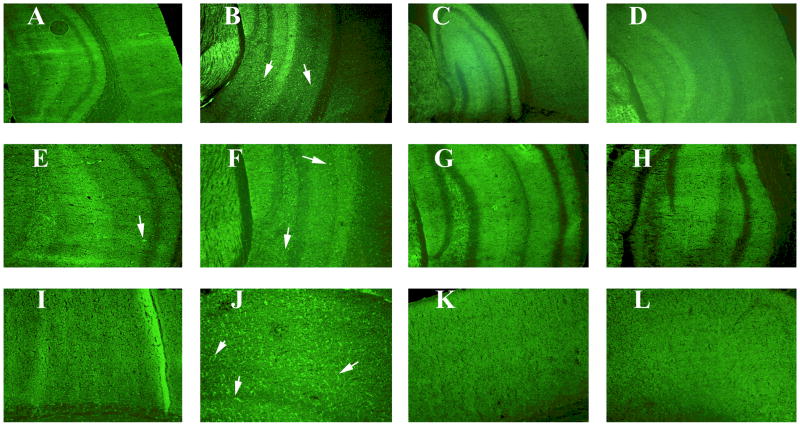
To determine whether increased mRNA expression of cytokines translates to proteins, we also conducted immunohistochemistry analysis of most of the up-regulated cytokines, CD40, and TNFα in different regions of the mouse brains, in order to assess regional differences of cytokine expression and microglial activity, if any, induced by recombinant mouse GM-CSF. Immunohistochemistry and immunofluorescence analyses of CD40 revealed increased immunoreactivity in several brain regions, including the cerebral cortex and hippocampus (Figs. 2 and 3) in mice injected with recombinant GM-CSF compared to the control mice injected with PBS vehicle. Further, our data showing the increased immunoreactivity of CD40 concurred with our real-time RT-PCR data (Table 3).
Figure 2.
Immunoreactivity of CD40 in different brain regions of representative sections from 8-month-old mice. Accumulated immunoreactivity was found in the cerebral cortex, and hippocampus in C57BL6 mice injected with recombinant GM-CSF. Arrows indicate the increased immunoreactivity of CD40 in the hippocampus and cortex. Images A-D were photographed at 5× the original magnification (represents cortex and hippocampus). Images E-H were photographed at 10× the original magnification (represents hippocampus). Images I –L photographed at 10× the original magnification (represents cerebral cortex). Images A, E and I are from C57BL6 mouse injected with PBS (control group). Images B, F and J are from C57BL6 mouse injected with recombinant GM-CSF (increased immunoreactivity of CD40), Images C, G and K are from C57BL6 mouse injected with GM-CSF antibody, and images D, H and L are from C57BL6 mouse injected with recombinant GM-CSF plus GM-CSF antibody.
Figure 3.
Immunoreactivity of CD40 in the cerebral cortex sections from 8-month-old C57BL6 mice. Arrows indicate increased immunoreactivity. Image A is from C57BL6 mouse injected with PBS (control group). B from C57BL6 mouse injected with recombinant GM-CSF (increased immunoreactivity of CD40), C from C57BL6 mouse injected with GM-CSF antibody and D from C57BL6 mouse injected with recombinant GM-CSF plus GM-CSF antibody. Photographs were taken 20× the original magnification.
Our quantitative immunohistochemistry analysis of CD40 in the mice injected with recombinant mouse GM-CSF and the PBS vehicle-injected control mice revealed significantly increased GM-CSF immunoreactivity in the brain regions, including cortex and hippocampus of mice (P<0.003) (Table 5), suggesting that recombinant mouse GM-CSF is a potent inducer of microglia in mice. These observations agreed with real-time RT-PCR (Table 3) and immunoblotting analysis (Fig. 1).
Table 5. Immuno-reactive signal intensity of CD40 in control and experimental groups of C57BL6 mice.
| Experimental and control groups of C57BL6 mice (comparison) | Signal intensity values Mean ± SD | P value |
|---|---|---|
| C57BL6 mice +PBS (control group) | 96.28 ± 4.02 | <0.003* |
| C57BL6 mice + recombinant mouse GM-CSF (experimental group) | 100.73 ±5.82 | |
| C57BL6 mice +PBS (control group) | 96.28 ± 4.02 | <0.45 |
| C57BL6 mice + anti-GM-CSF antibody (experimental group) | 97.26 ± 4.9 | |
| C57BL6 mice (control group) | 96.28 ± 4.02 | < 0.51 |
| C57BL6 mice + recombinant mouse GM-CSF and anti-GM-CSF antibody (experimental group) | 95.44 ± 2.95 | |
| Mean ± SD | P value | |
| C57BL6 mice +recombinant mouse GM-CSF | 100.73 ±5.82 | <0.03* |
| C57BL6 mice + anti-GMCSF antibody | 97.26 ± 4.9 | |
| C57BL6 mice +recombinant mouse GM-CSF | 100.73 ±5.82 | < 0.006* |
| C57BL6 mice +recombinant mouse GM-CSF + anti-GM-CSF antibody | 95.44 ± 2.95 | |
As shown in Fig. 4, we found increased immunoreactivity of TNFα in brains of C57BL6 mice injected with recombinant GM-CSF compared to the PBS vehicle-injected control mice, indicating that recombinant GM-CSF is a potent inducer of microglia marked by TNFα.
Figure 4.
Immunoreactivity of TNFα in the cerebral cortex sections from 8-month-old C57BL6 mice. Image A is from C57BL6 mouse injected with PBS (control group). B from C57BL6 mouse injected with recombinant GM-CSF (increased immunoreactivity of TNFα), C from C57BL6 mouse injected with GM-CSF antibody and D from C57BL6 mouse injected with recombinant GM-CSF plus GM-CSF antibody. Photographs were taken 20× the original magnification. Arrows indicate increased immunoreactivity of TNFα.
Immunohistochemistry and immunofluorescence analyses of cytokines suppressed by the anti-GM-CSF antibody in C57BL6 mice
We investigated whether the anti-GM-CSF antibody can suppress endogenous microglial activity in mice and whether it can suppress microglial activity induced by recombinant mouse GM-CSF antigen in mice. We conducted immunohistochemistry and immunofluorescence analysis of cytokines that showed decreased mRNA expression in our real-time RT-PCR (Table 3), in mice injected with the anti-GM-CSF antibody. Immunohistochemistry of CD40 and TNFα revealed decreased immunoreactivity in the cerebral cortex and hippocampus of these mice (Figs. 2 and 3 for CD40, and TNFα for Fig. 4) compared to the control mice injected with PBS. These results are in agreement with results from our real-time RT-PCR analysis, suggesting that the anti-GM-CSF antibody suppresses endogenous microglial activity in different regions of the mouse brain.
Immunohistochemistry and immunofluorescence analyses of cytokines induced with recombinant mouse GM-CSF and the anti-GM-CSF antibody in C57BL6 mice
We investigated whether the anti-GM-CSF antibody can suppress microglial activity induced by the recombinant GM-CSF in the brains of mice. Our immunohistochemistry of CD40 and TNFα revealed decreased immunoreactivity in the cerebral cortex and hippocampus in mice treated with anti-GM-CSF antibody, and also in mice treated with recombinant GM-CSF plus anti-GM-CSF antibody compared to mice induced with recombinant GM-CSF (Figs. 2 and 3 for CD40 and Fig.4 for TNFα), suggesting that the anti-GM-CSF antibody suppresses microglial activity induced by recombinant GM-CSF.
Immunohistochemistry and immunofluorescence analysis of GFAP
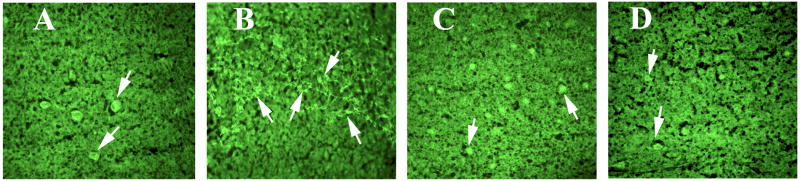
To determine recombinant GM-CSF activate astrocytes, we conducted immunohistochemistry and immunofluorescence analyses of astrocytes in the brain sections from C57BL6 mice induced with recombinant GM-CSF compared to other groups (Table 1). We found increased immunoreactivity of GFAP in several regions of the brains of mice treated with GM-CSF (Fig. 5), suggesting that recombinant GM-CSF activates astrocytes.
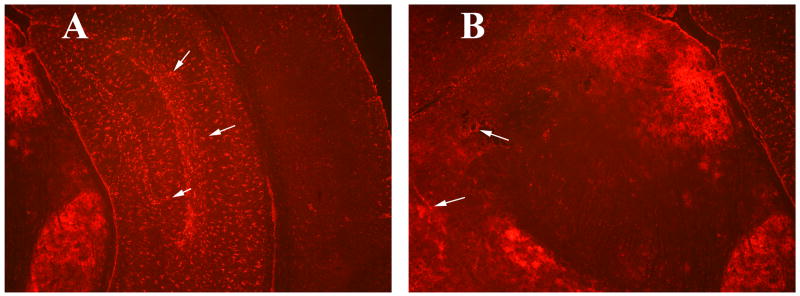
Figure 5.
Immunoreactivity of GFAP in several regions of the brain in a representative 8-month-old C57BL6 mouse induced with mouse recombinant GM-CSF. Image A is showing GFAP immunoreactivity in the hippocampus and cerebral cortex. B is from C57BL6 mouse showing immunoreactivity of GFAP in the midbrain. Arrows indicate GFAP immunoreactivity.
Double-labeling analysis of CD40 and GFAP
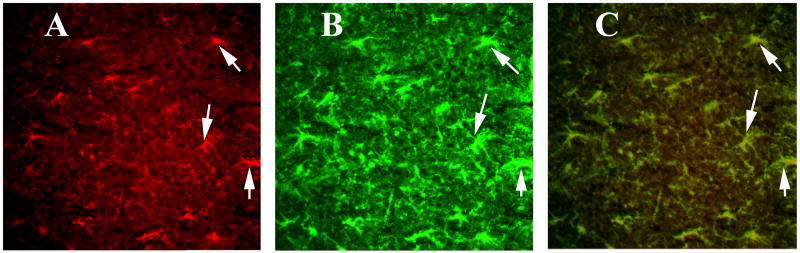
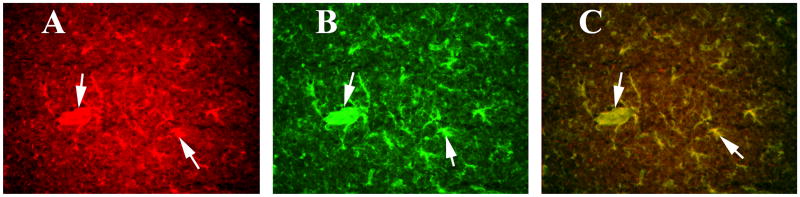
To determine whether activated GFAP is localized to CD40, we performed double-labeling analysis of CD40 and GFAP. We found activated astrocytes are mostly co-localized/co-expressed with CD40 expression (Supplemental Figs. 1 and 2). We found CD40 expression in several regions of the brain, including the cerebral cortex, hippocampus (image A of Supplemental Fig. 1), and the mid brain (striatum; image A of Supplemental Fig. 2). However, astrocytes were activated in the hippocampus and midbrain, and to a lesser extent in the cerebral cortex (image B of supplemental Fig. 1) and in the midbrain regions, including the striatum (image B of Supplemental Fig. 2). On high magnification, we found that CD40 is mostly co-localized with astrocytes in the hippocampus (Fig. 6) and ventricles (Fig. 7), suggesting that the induction of GM-CSF is activated by both CD40 and GFAP. Our high magnification images also revealed that CD40 is expressed in pyramidal neurons of hippocampus (Fig. 6).
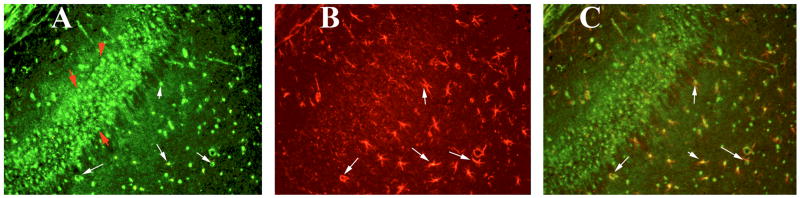
Figure 6.
Double labeling immunofluorescence analysis of CD40 and GFAP in the hippocampus of a representative 8-month-old C57BL mouse injected with recombinant GM-CSF. (A) represents immunoreactivity of CD40 in the hippocampus (in green), (B) GFAP (in red) of the same section and (C) Overlay of CD40 and GFAP. Red arrows indicate CD40 expression in pyramidal neurons of hippocampus. White arrows indicate localization of CD40 and GFAP. Sections were photographed at 40× the original magnification.
Figure 7.
Double labeling immunofluorescence analysis of CD40 and GFAP in ventricular region of a representative 8-month-old C57BL mouse injected with recombinant GM-CSF. (A) represents immunoreactivity of CD40 in the mid ventricle (in green), (B) GFAP (in red) of the same section and (C) Overlay of CD40 and GFAP. Arrows indicate localization of CD40 and GFAP. Sections were photographed at 40× the original magnification.
Double-labeling analysis of TNFα and GFAP
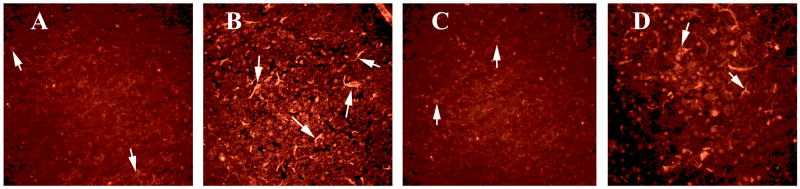
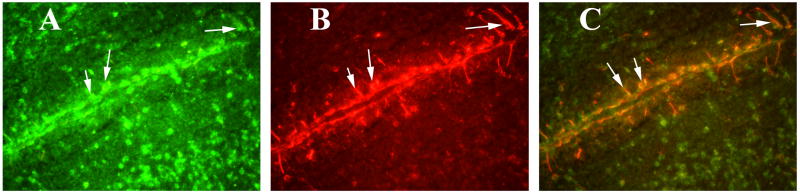
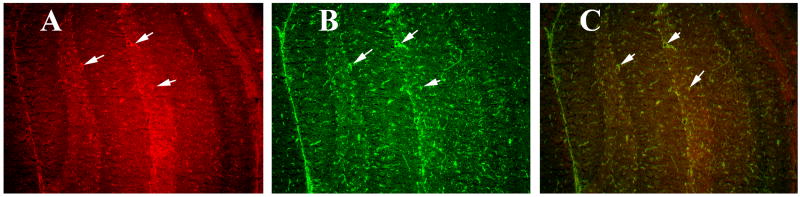
To determine whether activated TNFα is localized to CD40, we performed double-labeling analysis of TNFα and GFAP. We found increased GFAP expression in the hippocampus (image B in Fig. 8); this increased GFAP was mostly co-localized with TNFα (image A in Fig. 8). GM-CSF-induced TNFα was overexpressed in several regions of the brain, including the cerebral cortex and the hippocampus (image A of Supplemental Fig. 3). Our high magnification sections revealed TNFα co-localized with astrocytes in the hippocampus (Fig. 9) and the cortex (Fig. 10), suggesting that the induction of GM-CSF is activated by TNFα and GFAP, and TNFα expressed in astrocytes.
Figure 8.
Double labeling immunofluorescence analysis of TNFα and GFAP in the hippocampus of a representative 8-month-old C57BL mouse injected with recombinant GM-CSF. (A) represents immunoreactivity of TNFα in the hippocampus (in red), (B) GFAP (in green) of the same section and (C) Overlay of TNFα and GFAP. Arrows indicate localization of TNFα and GFAP. Sections were photographed at 20× the original magnification.
Figure 9.
Double labeling immunofluorescence analysis of TNFα and GFAP in the hippocampus of a representative 8-month-old C57BL mouse injected with recombinant GM-CSF. (A) represents immunoreactivity of TNFα in the hippocampus (in red), (B) GFAP (in green) of the same section and (C) Overlay of TNFα and GFAP. Arrows indicate localization of TNFα and GFAP. Sections were photographed at 40× the original magnification.
Figure 10.
Double labeling immunofluorescence analysis of TNFα and GFAP in the cerebral cortex of a representative 8-month-old C57BL mouse injected with recombinant GM-CSF. (A) represents immunoreactivity of TNFα in cerebral cortex (in red), (B) GFAP (in green) of the same section and (C) Overlay of TNFα and GFAP. Arrows indicate localization of TNFα and GFAP. Sections were photographed at 40× the original magnification.
Discussion
The objective of this study was to determine microglial activity in the CNS of C57BL6 mice after delivering GM-CSF directly into their brains. Using ICV injections, we successfully injected the PBS vehicle, recombinant mouse GM-CSF, the anti-GM-CSF antibody, and the recombinant mouse GM-CSF and anti-GM-CSF antibody into the brains of C57BL6 mice. Using quantitative real-time RT-PCR, we assessed microglial activity by measuring mRNA in several GM-CSF-associated cytokines. Further, we used immunoblotting, immunohistochemistry, and immunofluorescence analyses of cytokines that were up-regulated in our real-time RT-PCR assay. Using double-labeling analyses of most up-regulated cytokines (CD40, TNFα, and GFAP), we determined co-expressions of CD40 and TNFα in astrocytes, in brain sections from C57BL6 mice induced with mouse recombinant GM-CSF. Our real-time RT-PCR assays revealed increased mRNA expression of CD40, TNFα, CD45, and CD11C in the cerebral cortices of C57BL6 mice induced with recombinant mouse GM-CSF. Our immunoblotting and immunohistochemistry findings concurred with these real-time RT-PCR findings. Further, we found that the anti-GM-CSF antibody suppressed both endogenous and GM-CSF-induced glial activity in the C57BL6 mouse brains.
Recombinant GM-CSF induces microglia in CNS
We found increased mRNA expression of CD40, TNFα, CD45, CD11C, and MHCII in the cerebral cortices of the C57BL6 mice injected with recombinant mouse GM-CSF, compared to the C57BL6 mice injected with PBS. There are few published studies that directly investigated microglial induction using ICV injections of recombinant mouse GM-CSF into C57BL6 mouse brains. However, Giulian et al. (1988) investigated the immunomodulators that alter brain inflammatory responses, following infusion of recombinant forms of GM-CSF, multi-CSF, macrophage-CSF, and G-CSF into the cerebral cortex of rats. After a 48-hr infusion, multi-CSF or GM-CSF stimulated the appearance of large numbers of mononuclear phagocytes at the injection site and accelerated the clearance of polystyrene microspheres from the brain. Giulian et al. concluded that some classes of immunomodulators are potent mitogens and activators of microglia in the CNS. Findings from our study support their observations.
Several other recent cell-culture studies also support our findings, that GM-CSF induction can activate pro-inflammatory cytokines (Hornell et al. 2003; Yamanishi et al. 2007; Derigs et al. 1994; Suh et al. 2005). Hornell et al. (2003) found increased expression of CD40 and MHCII in primary human monocytes treated with GM-CSF for 24 to 48 hrs. Further, increasing evidence suggests that CD45, a transmembrane protein tyrosine phosphatase, is an important modulator of macrophage activationref. Microglia, which are resident brain macrophages, have been found to express CD45 and to proliferate under pathologic and GM-CSF-induced conditions (Cosenza et al. 2002; Masliah et al. 1991). Transition needed here Our present study also supports the hypothesis that GM-CSF is a potent inducer of microglia, as evidenced by the increased expression of CD45 in the CNS that we found.
In addition, Dergis et al. (1994) reported that the promoter of the GM-CSF gene is a critical factor for increased TNFα expression in the CNS, and they speculated that GM-CSF induction would increase TNFα expression in general and in the CNS, in particular, which we observed in our present study (Table 3). Recently, Suh et al. (2005) reported that the inhibition of GM-CSF reduces microglial activation and proliferation in cell cultures. This observation further supports our real-time RT-PCR findings of cytokines (Table 3) and the view that GM-CSF is critical for microglial activation.
Further, our quantitative immunohistochemistry analysis of CD40 in C57BL6 mice injected with recombinant mouse GM-CSF compared to C57BL6 mice injected with PBS (control mice) revealed that CD40 immunoreactivity in the brains of C57BL6 mice injected with GM-CSF was significantly increased (P<0.003), suggesting that recombinant GM-CSF is a potent inducer of microglia in C57BL6 mice.
GM-CSF antibody suppresses microglia
We found decreased mRNA expression of gp91, CD11b, and MHC II in the cerebral cortices of the C57BL6 mice that we had injected with the GM-CSF antibody, compared to the C57BL6 mice injected with PBS. Further, we also found decreased mRNA expression of TNFα, gp91, CD11c, MHCII in C57BL6 mice injected with the recombinant GM-CSF plus the GM-CSF antibody, compared to C57BL6 mice injected with PBS vehicle. These findings suggest that the GM-CSF antibody suppresses the microglial activation in brains of C57BL6 mice. These observations are supported by a recent study of Manczak et al. 2009. Manczak and colleagues found decreased levels of microglial mRNA expression and Aβ deposits in 10-month-old AD transgenic mice (Tg2576 mouse line) injected with the anti-GM-CSF anti-mouse antibody, compared to PBS vehicle-injected Tg2576 control mice, suggesting that the anti-GM-CSF antibody suppresses excessive microglial activity. That anti-GM-CSF antibody may be a potent suppressor of activated microglia in the CNS is suggested by our findings that anti-GM-CSF antibody suppresses excessive microglial activity in the CNS. The present study findings together with our previous observations suggest that anti-GM-CSF antibody may have some therapeutic implications as an anti-inflammatory in AD patients (Manczak et al. 2009).
GM-CSF induction and CD40 and TNFα expression in CNS of C57BL6 mice
We found increased expression of CD40 in 8-month-old C57BL6 mice induced by mouse recombinant GM-CSF. Our double-labeling analysis of CD40 and GFAP revealed CD40 expressed in astrocytes (Figs. 6 and 7), suggesting that GM-CSF induction is activated by astrocytes. We also found CD40 expression in hippocampal pyramidal neurons indicating that GM-CSF induction activates CD40 expression in neurons as well. Overall, our present study findings suggest that GM-CSF induction increases CD40 expression in astrocytes, microglia and also in neurons. However, as discussed above, the GM-CSF antibody is capable of suppressing GM-CSF associated with microglial and astrocytic expressions in the CNS of C57BL6 mice.
TNFα is pro-inflammatory cytokine that is expressed in microglia, in astrocytes, and to some extent, in neurons (Ignatowski et al. 1996; Takei and Luskey 2008). We found increased expression of TNFα in 8-month-old C57BL6 mice induced with mouse recombinant GM-CSF (Table 3). Our double-labeling analysis of TNFα and GFAP revealed TNFα co-expressed in astrocytes (Fig. 8), suggesting that GM-CSF induction is activated by TNFα marked by microglia and astrocytes.
GM-CSF activation and neuronal change
Our present study findings suggest that GM-CSF induction increases the production of microglia (marked by TNFα, CD40) and astrocytes. These increased productions of microglia and astrocytes develop chronic inflammation in CNS. In CNS diseases, such as AD, increased production of microglia and astrocytes were reported to increase inflammation, neuronal damage, and ultimate neuronal loss (Wyss Coray 2006).
On the contrary, GM-CSF activation is involved in several cellular functions and beneficial effects, including axonal regeneration, removing myelin debris (after CNS injury), actions of neurotrophic factors and anti-apoptosis (Kannan et al. 2000). Giulian et al. 1994; Franzen et al. 2004). However, hyperactivation of GM-CSF is harmful to the neurons.
GM-CSF and implications to Alzheimer's disease and Multiple Sclerosis
The present study findings suggest that GM-CSF activate microglia and astrocytes that ultimately leads to the development of chronic inflammation in CNS. Further, increasing evidence suggests that GM-CSF activation is involved in AD pathogenesis (Tarkowski et al. 2001, 2005; Manczak et al. 2009). In addition, present study findings also suggest that anti-GM-CSF antibodies suppress microglial and astrocytic activation in CNS. In support of this observation, in a recent study of the anti-GM-CSF antibody using AD transgenic mice (Tg2576 line), we found decreased levels of Aβ deposits and microglial mRNA expression in 10-month-old Tg2576 mice injected with the anti-GM-CSF antibody, compared to the PBS vehicle-injected Tg2576 control mice (Manczak et al. 2009), suggesting that the anti-GM-CSF antibody suppresses excessive microglial activity, Aβ deposits associated with GM-CSF, and other pro-inflammatory cytokines. (Manczak et al. 2009). Overall, findings of this present study together with our earlier study (by Manczak et al. 2009) suggest that GM-CSF is involved in inflammation in CNS, and suppression of excessive microglial activation may have therapeutic implications to AD.
It has been reported that GM-CSF-deficient mice are resistant to experimental autoimmune encephalomyelitis (EAE) following immunization with myelin self-Ag (Stanley et al. 1995). Further, recently, Ponomarev et al. (2007) investigated the cellular source of the GM-CSF and found that GM-CSF production by encephalitogenic T cells (but not by other peripheral cells) is required for EAE induction. They also found that microglial cell activation is a GM-CSF-dependent process. Activation of microglial cells through the injection of lipopolysaccharid abrogated the GM-CSF requirement for EAE induction, suggesting that microglial cell activation is required for EAE onset. In our ongoing study of GM-CSF knockout mice and MOG induction, we found GM-CSF knockout mice are resistant to develop EAE, indicating that GM-CSF is critical for the development of EAE (unpublished observations, Reddy et al) Overall, these observations, suggest that suppression of excessive microglial activation may become a therapeutic strategy to neurodegenerative diseases, particularly in EAE mice or persons with MS.
Conclusions
The objective of our study was to determine GM-CSF activity in the brain following GM-CSF induction. Our real-time RT-PCR analysis revealed increased mRNA expression of CD40, TNFα, CD45, GFAP, and CD11c in the mice induced with recombinant mouse GM-CSF. Our immunoblotting and immunohistochemistry/immunofluorescence analyses findings concurred with our real-time RT-PCR findings. Our extensive double-labeling analysis of CD40 and GFAP, and TNFα and GFAP revealed that CD40 and TNFα are co-localized with GFAP in the hippocampus and the cerebral cortical regions of the C57BL5 mouse brain that we injected with recombinant GM-CSF. These findings suggest that GM-CSF associated cytokines are also expressed in GFAP. Overall, these findings suggest that GM-CSF is critical for microglial activation. Further, the anti-GM-CSF antibody suppressed both endogenous and GM-CSF-induced microglial activity in the C57BL6 mice. Overall, these findings may have implications in developing anti-inflammatory therapies for AD and MS patients.
Supplementary Material
Double labeling immunofluorescence analysis of CD40 and GFAP in the cerebral cortex and hippocampal regions of the brain of a representative 8-month-old C57BL mouse injected with recombinant GM-CSF. (A) represents immunoreactivity of CD40 in the hippocampus and cerebral cortex (in green), (B) GFAP (in red) of the same section and (C) Overlay of CD40 and GFAP. Sections were photographed at 5× the original magnification.
Double labeling immunofluorescence analysis of CD40 and GFAP in the midbrain regions of a representative 8-month-old C57BL mouse injected with recombinant GM-CSF. (A) represents immunoreactivity of CD40 in the midbrain regions including striatum and thalamus (in green), (B) GFAP (in red) of the same section and (C) Overlay of CD40 and GFAP. Sections were photographed at 5× the original magnification.
Double labeling immunofluorescence analysis of TNFα and GFAP in the cerebral cortex and hippocampal regions of brain in a representative 8-month-old C57BL mouse injected with recombinant GM-CSF. (A) represents immunoreactivity of TNFα in the hippocampus and cerebral cortex (in red), (B) GFAP (in green) of the same section and (C) Overlay of TNFα and GFAP. Sections were photographed at 5× the original magnification.
Acknowledgments
The research presented in this paper was supported by KaloBios Pharmaceuticals via OHSU-Technology Research Collaborations Research Agreement and NIH grants (AG028072, AG026051, and RR00163). We thank Dr. Shaun Morrison for allowing us to use the stereotaxic apparatus for GM-CSF experiments conducted in this study.
References
- Abbas N, Bednar I, Mix E, Marie S, Paterson D, Ljungberg A, Morris C, Winblad B, Nordberg A, Zhu J. Up-regulation of the inflammatory cytokines IFN-gamma and IL-12 and down-regulation of IL-4 in cerebral cortex regions of APP(SWE) transgenic mice. J Neuroimmunol. 2002;126:50–57. doi: 10.1016/s0165-5728(02)00050-4. [DOI] [PubMed] [Google Scholar]
- Apelt J, Schliebs R. Beta-amyloid-induced glial expression of both pro- and anti-inflammatory cytokines in cerebral cortex of aged transgenic Tg2576 mice with Alzheimer plaque pathology. Brain Res. 2001;891:21–30. doi: 10.1016/s0006-8993(00)03176-0. [DOI] [PubMed] [Google Scholar]
- Banati RB, Beyreuther K. Alzheimer's Disease. In: Kettenma H, Ransom BR, editors. Neuroglia. New Yourk, Oxford: Oxford University Press; 1995. pp. 1027–1043. [Google Scholar]
- Brook GA, Plate D, Franzen R, Martin D, Moonen G, Schoenen J, Schmitt AB, Noth J, Nacimiento W. Spontaneous longitudinally orientated axonal regeneration is associated with the Schwann cell framework within the lesion site following spinal cord compression injury of the rat. J Neurosci Res. 1998;53:51–65. doi: 10.1002/(SICI)1097-4547(19980701)53:1<51::AID-JNR6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- Cosenza MA, Zhao ML, Si Q, Lee SC. Human brain parenchymal microglia express CD14 and CD45 and are productively infected by HIV in HIV-1 encephalitis. Brain Pathol. 2002;12:442. doi: 10.1111/j.1750-3639.2002.tb00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derigs HG, Reifel-Miller A, Kaushansky K, Hromas RA, Boswell HS. Granulocyte-macrophage colony-stimulating factor expression is regulated at transcriptional and posttranscriptional levels in a murine bone marrow stromal cell line. Exp Hematol. 1994;22:924–933. [PubMed] [Google Scholar]
- Fleetwood AJ, Cook AD, Hamilton JA. Functions of granulocyte colony stimulating factor. Crit Rev Immunol. 2005;25:405–428. doi: 10.1615/critrevimmunol.v25.i5.50. [DOI] [PubMed] [Google Scholar]
- Franzen R, Bouhy D, Schoenen J. Nervous system: focus on the inflammatory cytokine granulocyte-macrophage colony stimulating factor. Neurosci Lett. 2004;361:76–78. doi: 10.1016/j.neulet.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Frautschy SA, Yang F, Irrizarry M, Hyman B, Saido TC, Hsiao K, Cole GM. Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pathol. 1998;152:307–317. [PMC free article] [PubMed] [Google Scholar]
- Sawada M, Itoh Y, Suzumura A, Marunouchi T. Expression of cytokine receptors in cultured neuronal and glial cells. Neurosci Lett. 1993;160:131–134. doi: 10.1016/0304-3940(93)90396-3. [DOI] [PubMed] [Google Scholar]
- Giulian D, Ingeman JE. Colony-stimulating factors as promoters of ameboid microglia. J Neurosci. 1988;8:4707–4717. doi: 10.1523/JNEUROSCI.08-12-04707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutala RV, Reddy PH. The use of real-time RT-PCR analysis in a gene expression study of Alzheimer's disease postmortem brains. J Neurosci Methods. 2004;132:101–107. doi: 10.1016/j.jneumeth.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Hamilton JA. GM-CSF in inflammation and autoimmunity. Trends Immunol. 2002;23:403–408. doi: 10.1016/s1471-4906(02)02260-3. [DOI] [PubMed] [Google Scholar]
- Hamilton JA. Colony stimutation groewth factors. Nat Rev Immunol. 2008;8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- Hornell TM, Beresford GW, Bushy A, Boss JM, Mellins ED. Regulation of the class II MHC pathway in primary human monocytes by granulocyte-macrophage colony-stimulating factor. J Immunol. 2003;171:2374–2383. doi: 10.4049/jimmunol.171.5.2374. [DOI] [PubMed] [Google Scholar]
- Ignatowski TA, Chou RC, Spengler RN. Changes in noradrenergic sensitivity to tumor necrosis factor-alpha in brains of rats administered clonidine. J Neuroimmunol. 1996;70:55–63. doi: 10.1016/s0165-5728(96)00098-7. [DOI] [PubMed] [Google Scholar]
- Kannan Y, Moriyama M, Sugano T, Yamate J, Kuwamura M, Kagaya A, Kiso Y. Neurotrophic action of interleukin 3 and granulocyte-macrophage colony-stimulating factor on murine sympathetic neurons. Neuroimmunomodulation. 2000;8:132–141. doi: 10.1159/000054273. [DOI] [PubMed] [Google Scholar]
- Manczak M, Mao P, Nakamura K, Bebbington C, Park B, Reddy PH. Granulocyte Macrophage Colony Stimulating Factor Immunization decreases Amyloid beta 1-42 and suppresses microglial activity in a Transgenic Mouse Model of Alzheimer's Disease. Hum Mol Genet. 2009;18:3876–3893. doi: 10.1093/hmg/ddp331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manczak M, Park BS, Jung Y, Reddy PH. Differential expression of oxidative phosphorylation genes in patients with Alzheimer's disease: implications for early mitochondrial dysfunction and oxidative damage. Neuromolecular Med. 2004;5:147–162. doi: 10.1385/NMM:5:2:147. [DOI] [PubMed] [Google Scholar]
- Maier M, Peng Y, Jiang L, Seabrook TJ, Carroll MC, Lemere CA. Complement C3 deficiency leads to accelerated amyloid beta plaque deposition and neurodegeneration and modulation of the microglia/macrophage phenotype in amyloid precursor protein transgenic mice. J Neurosci. 2008;28:6333–6341. doi: 10.1523/JNEUROSCI.0829-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malipiero VV, Frei K, Fontana A. Production of hemopoietic colony-stimulating factors by astrocytes. J Immunol. 1990;144:3816–3821. [PubMed] [Google Scholar]
- Mao P, Reddy PH. Is multiple sclerosis a mitochondrial disease. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbadis.2009.07.002. 2009 Jul 13. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Hansen L, Alford M, Albright T, Terry R, Shapiro P, Sundsmo M, Saitoh T. Immunoreactivity of CD45, a protein phosphotyrosine phosphatase, in Alzheimer's disease. Acta Neuropathol (Berl) 1991;83:12–20. doi: 10.1007/BF00294425. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Picciano M, La Francois J, Duff K. Fibrillar beta-amyloid evokes oxidative damage in a transgenic mouse model of Alzheimer's disease. Neuroscience. 2001;104:609–613. doi: 10.1016/s0306-4522(01)00115-4. [DOI] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL. Inflammatory processes in Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:741–749. doi: 10.1016/S0278-5846(03)00124-6. [DOI] [PubMed] [Google Scholar]
- McLay RN, Banks WA, Kastin AJ. Granulocyte macrophage colony stimulating factor crosses the blood testis barrier in mice. Biol Reprod. 1997;57:822–826. doi: 10.1095/biolreprod57.4.822. [DOI] [PubMed] [Google Scholar]
- McLay RN, Kimura M, Banks WA, Kastin AJ. Granulocyte macrophage colony stimulating factor crosses the blood brain and blood spinal cord barriers. Brain. 1997;120:2083–2091. doi: 10.1093/brain/120.11.2083. [DOI] [PubMed] [Google Scholar]
- Mehlhorn G, Hollborn M, Schliebs R. Induction of cytokines in glial cells surrounding cortical beta-amyloid plaques in transgenic Tg2576 mice with Alzheimer pathology. Int J Dev Neurosci. 2000;18:423–431. doi: 10.1016/s0736-5748(00)00012-5. [DOI] [PubMed] [Google Scholar]
- Ponomarev ED, Shriver LP, Maresz K, Pedras-Vasconcelos J, Verthelyi D, Dittel BN. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:39–48. doi: 10.4049/jimmunol.178.1.39. [DOI] [PubMed] [Google Scholar]
- Raivich G, Gehrmann J, Kreutzberg GW. Increase of macrophage colony-stimulating factor and granulocyte-macrophage colony-stimulating factor receptors in the regenerating rat facial nucleus. J Neurosci Res. 1991;30:682–686. doi: 10.1002/jnr.490300412. [DOI] [PubMed] [Google Scholar]
- Schäbitz WR, Krüger C, Pitzer C, Weber D, Laage R, Gassler N, Aronowski J, Mier W, Kirsch F, Dittgen T, Bach A, Sommer C, Schneider A. A neuroprotective function for the hematopoietic protein granulocyte-macrophage colony stimulating factor (GM-CSF) J Cereb Blood Flow Metab. 2008;28:29–43. doi: 10.1038/sj.jcbfm.9600496. [DOI] [PubMed] [Google Scholar]
- Stalder M, Phinney A, Probst A, Sommer B, Staufenbiel M, Jucker M. Association of microglia with amyloid plaques in brains of APP23 transgenic mice. Am J Pathol. 1999;154:1673–1684. doi: 10.1016/S0002-9440(10)65423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh HS, Kim MO, Lee SC. Inhibition of granulocyte-macrophage colony-stimulating factor signaling and microglial proliferation by anti-CD45RO: role of Hck tyrosine kinase and phosphatidylinositol 3-kinase/Akt. J Immunol. 2005;174:2712–9. doi: 10.4049/jimmunol.174.5.2712. [DOI] [PubMed] [Google Scholar]
- Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JA, Maher DW, Cebon J, Sinickas V, Dunn AR. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci U S A. 1994;91:5592–5596. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei Y, Laskey R. Interpreting crosstalk between TNF-alpha and NGF: potential implications for disease. Trends Mol Med. 2008;14:381–388. doi: 10.1016/j.molmed.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Tarkowski E, Wallin A, Regland B, Blennow K, Tarkowski A. Local and systemic GM-CSF increase in Alzheimer's disease and vascular dementia. Acta Neurol Scand. 2001;103:166–174. doi: 10.1034/j.1600-0404.2001.103003166.x. [DOI] [PubMed] [Google Scholar]
- Tarkowski E, Andreasen N, Tarkowski A, Blennow K. Intrathecal inflammation precedes development of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2003;74:1200–1205. doi: 10.1136/jnnp.74.9.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel J, Wang KC, Imaki H, Rubenstein R, Wronska A, Osuchowski M, Lipinski WJ, Walker LC, LeVine H. The role of microglial cells and astrocytes in fibrillar plaque evolution in transgenic APP(SW) mice. Neurobiol Aging. 2001;22:49–61. doi: 10.1016/s0197-4580(00)00181-0. [DOI] [PubMed] [Google Scholar]
- Wirths O, Breyhan H, Marcello A, Cotel MC, Brück W, Bayer TA. Inflammatory changes are tightly associated with neurodegeneration in the brain and spinal cord of the APP/PS1KI mouse model of Alzheimer's disease. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.06.011. in press. [DOI] [PubMed] [Google Scholar]
- Whetton AD, Dexter TM. Myeloid haemopoietic growth factors. Biochim Biophys Acta. 1989;989:111–132. doi: 10.1016/0304-419x(89)90038-3. [DOI] [PubMed] [Google Scholar]
- Wilcock DM, Gordon MN, Morgan D. Quantification of cerebral amyloid angiopathy and parenchymal amyloid plaques with Congo red histochemical stain. Nat Protoc. 2006;1:1591–155. doi: 10.1038/nprot.2006.277. [DOI] [PubMed] [Google Scholar]
- Yamanishi T, Yamamoto Y, Hatakeyama T, Yamaguchi K, Oda T. CEL-I, an invertebrate N-acetylgalactosamine-specific C-type lectin, induces TNF-alpha and G-CSF production by mouse macrophage cell line RAW264.7 cells. J Biochem. 2007;142:587–595. doi: 10.1093/jb/mvm164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Double labeling immunofluorescence analysis of CD40 and GFAP in the cerebral cortex and hippocampal regions of the brain of a representative 8-month-old C57BL mouse injected with recombinant GM-CSF. (A) represents immunoreactivity of CD40 in the hippocampus and cerebral cortex (in green), (B) GFAP (in red) of the same section and (C) Overlay of CD40 and GFAP. Sections were photographed at 5× the original magnification.
Double labeling immunofluorescence analysis of CD40 and GFAP in the midbrain regions of a representative 8-month-old C57BL mouse injected with recombinant GM-CSF. (A) represents immunoreactivity of CD40 in the midbrain regions including striatum and thalamus (in green), (B) GFAP (in red) of the same section and (C) Overlay of CD40 and GFAP. Sections were photographed at 5× the original magnification.
Double labeling immunofluorescence analysis of TNFα and GFAP in the cerebral cortex and hippocampal regions of brain in a representative 8-month-old C57BL mouse injected with recombinant GM-CSF. (A) represents immunoreactivity of TNFα in the hippocampus and cerebral cortex (in red), (B) GFAP (in green) of the same section and (C) Overlay of TNFα and GFAP. Sections were photographed at 5× the original magnification.