Abstract
Nitric oxide (NO) is a key neuromodulator of corticostriatal synaptic transmission. We have shown previously that dopamine (DA) D1/5 receptor stimulation facilitates neuronal NO synthase (nNOS) activity in the intact striatum. To study the impact of local manipulations of D1/5 and glutamatergic NMDA receptors on striatal nNOS activity, we combined the techniques of in vivo amperometry and reverse microdialysis. Striatal NO efflux was monitored proximal to the microdialysis probe in urethane anesthetized rats during local infusion of vehicle or drug. NO efflux elicited by systemic administration of SKF-81297 was blocked following intrastriatal infusion of: 1) the D1/5 receptor antagonist SCH-23390, 2) the nNOS inhibitor 7-nitroindazole, 3) the nonspecific ionotropic glutamate receptor antagonist kynurenic acid, and 4) the selective NMDA receptor antagonist 3-phosphonopropyl-piperazine-2-carboxylic acid. Glycine coperfusion did not affect SKF-81297-induced NO efflux. Furthermore, intrastriatal infusion of SKF-81297 potentiated NO efflux evoked during electrical stimulation of the motor cortex. The facilitatory effects of cortical stimulation and SKF-81297 were both blocked by intrastriatal infusion of SCH-23390, indicating that striatal D1/5 receptor activation is necessary for the activation of nNOS by corticostriatal afferents. These studies demonstrate for the first time that reciprocal DA-glutamate interactions play a critical role in stimulating striatal nNOS activity.
Keywords: Nitric oxide, Glutamate, Dopamine, D1/5 receptor, Dorsal striatum, NMDA receptor, Neuronal nitric oxide synthase
INTRODUCTION
Nitric oxide (NO) is a gaseous regulator of numerous physiological and pathophysiological processes in the central nervous system, including immunological defense mechanisms, hemodynamics, and synaptic transmission (Boehning and Snyder 2003; Bredt 2003; Garthwaite 2008). NO is generated by the NO synthase (NOS) family of enzymes which consists of at least three distinct isoforms (Dawson and Dawson 1996; Brenman and Bredt 1997; Alderton et al. 2001). Inducible NOS (iNOS), endothelial NOS (eNOS), and neuronal NOS (nNOS) are present in microglia, endothelial cells, and neurons, respectively (Nathan and Xie 1994). The nNOS isoform is constitutively expressed and activated following transient elevations in intracellular Ca2+ levels mediated via N-methyl-D-aspartate (NMDA)-type glutamate receptor activation (Garthwaite et al. 1988; Garthwaite 2008). This rise in intracellular Ca2+ then allows for calmodulin to associate with the NOS enzyme, thereby inducing a conformation change that simulates enzyme activity (Alderton et al. 2001). Once activated, nNOS synthesizes NO through two successive monooxygenase reactions which require oxygen, NADPH, and the substrate L-arginine for the generation of equimolar concentrations of citrulline and NO (Stuehr et al. 2004).
In the striatum, nNOS is found in a subclass of medium aspiny interneurons which also contain the co-transmitters GABA, neuropeptide Y (NPY), and somatostatin (Kubota et al. 1993; Kharazia et al. 1994; Garthwaite and Boulton 1995; Figueredo-Cardenas et al. 1996; Kawaguchi 1997). Although nNOS-containing interneurons make up only 1–2% of the population of striatal neurons (West et al. 1996; Kawaguchi 1997), they extend highly ramified and far reaching axons which enable these cells to greatly influence the function of striatal circuits (Emson et al. 1993; Kawaguchi 1997).
Striatal NOS-containing interneurons receive asymmetric synaptic inputs from the frontal cortex (Vuillet et al. 1989; Salin et al. 1990). Furthermore, our previous studies using NO microsensor recordings have shown that electrical stimulation of the frontal cortex activates striatal nNOS via a NMDA receptor-dependent mechanism (Sammut et al. 2007b). Neuroanatomical studies have also shown that striatal NOS/somatostatin interneurons express DA D1/5 receptors (Rivera et al. 2002). Consistent with this, electrophysiologically identified nNOS interneurons are potently activated by bath application of DA or D1/5 receptor agonist (Centonze et al. 2002; Centonze et al. 2003). Our recent NO microsensor studies have also shown that electrical and chemical stimulation of the substantia nigra and systemic DA D1/5 receptor agonist administration robustly increases striatal NO efflux (Sammut et al. 2007a; Sammut et al. 2007b). Thus, the DA D1/5 receptor-mediated increase in membrane excitability reported by Centonze and colleagues may also lead to nNOS activation (Calabresi et al. 2008). Given that glutamatergic and DAergic terminals form synapses on NOS interneuron dendrites in close proximity to each other (Hidaka and Totterdell 2001), it is likely that these afferents interact at the presynaptic level or via postsynaptic receptors or signal transduction cascades to regulate striatal NOS activity. This is supported by evidence showing that systemic administration of both NMDA and D1/5 receptor antagonists decrease striatal NOS activity measured in the striatum of intact rats (Morris et al. 1997; Sammut et al. 2006; Sammut et al. 2007b). However, the role of striatal DA and glutamate interactions in regulating NOS activity remains to be characterized using local pharmacological manipulations.
Therefore, the goal of the current study was to determine the impact of striatal DA D1/5 and glutamate receptor manipulations on nNOS activity evoked in vivo following systemic administration of D1/5 receptor agonist or stimulation of cortical afferents. To this end, amperometric microsensors and microdialysis probes were placed in close proximity to each other (~500 μm) in the dorsal striatum to directly measure NO efflux during reverse dialysis of drugs targeting DA, glutamate, and NO transmission.
MATERIALS AND METHODS
Drugs
Urethane, R-(+)-SKF-81297 HBr (SKF), R-(+)-SCH-23390 HCl (SCH), 7-nitroindazole monosodium (7-NI), and kynurenic acid (KYN) were purchased from Sigma (St. Louis, MO, USA). 3-((R)-2-Carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP) and glycine (GLY) were purchased from Tocris (MO, USA). (±)S-Nitroso-N-acetyl-penicillamine (SNAP) used for calibration of NO electrodes was obtained from World Precision Instruments (WPI, Sarasota, FL, USA). All other reagents were of the highest grade commercially available.
Subjects and Surgery
Electrochemical measurements were taken from 42 adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 240–400 g. All animals were housed in groups of two or three per cage in a light- and temperature-controlled room (21–23 °C) maintained at a 12:12h light/dark cycle with food and water provided adlibitum. Daily animal and veterinary care were provided by trained animal care personnel. Rats were allowed at least one week to habituate in the University vivarium. All animal protocols were approved by the University IACUC and conform to the USPHS Guide for the Care and Use of Laboratory Animals. Before surgery, rats were deeply anesthetized with urethane (1.5 g/kg i.p.) and administered analgesic as previously described (Ondracek et al. 2008; Threlfell et al. 2009). Next, an incision (~2–4 cm) was made in the scalp and burr holes (~2–3mm in diameter) were drilled in the skull overlying the frontal cortex (Sammut et al. 2007b). The dura mater was resected and stimulating electrodes and microdialysis probes/NO microsensors were lowered into the motor cortex and dorsal striatum, respectively (see below and (Sammut et al. 2007b; Threlfell et al. 2009)). The level of anesthesia was periodically verified (every 10–20 mins) via testing the hindlimb compression reflex and maintained using supplemental administration of urethane (Sammut et al. 2007b). Core body temperature was monitored via a rectal probe and maintained at 37–38°C using a heating pad (Vl-20F, Fintronics Inc, Orange, CT).
Simultaneous Microdialysis and Amperometry
Concentric microdialysis probes (Bioanalytical Systems, West Lafayette, IN) having 4 mm of exposed membrane (225 μM diameter) were implanted into the striatum (West and Grace 2004; Threlfell et al. 2009). Probes were perfused with aCSF containing (in mM) 145 NaCl, 2.7 KCl, 1.0 MgCl2, 1.2 CaCl2, 2.0 NaH2PO4, and 2.0 Na2HPO4 at a rate of 2μL/min. Striatal NO efflux was determined using an NO selective, amperometric microsensor (AmiNO-100, Innovative Instruments Inc., Tampa, FL) as previously described (Sammut et al. 2006; Sammut et al. 2007a; Sammut et al. 2007b; Ondracek et al. 2008). Calibration curves were constructed in order to determine the sensitivity of the electrode and confirm that the NO oxidation current measured with each individual electrode exhibited a linear response to physiological concentrations of NO (0.6–48 nM). Once calibrated, the NO microsensor was positioned to enter the brain surface 1 mm lateral to the probe and lowered at a 10°angle until the tip of the microsensor was located just ventral to the tip of the probe. Because the active surface of both the microdialysis probes and NO microsensors used in this study was relatively large (~4 mm), it is likely that drug was delivered and NO efflux was sampled within an extensive region of the dorsocentral striatum (Fig 1b). Electrochemical recordings were initiated ~3 hours after implantation of the microdialysis probe and NO microsensor. NO oxidation current (pA) was allowed to stabilize for at least 150 s prior to electrical stimulation or drug administration.
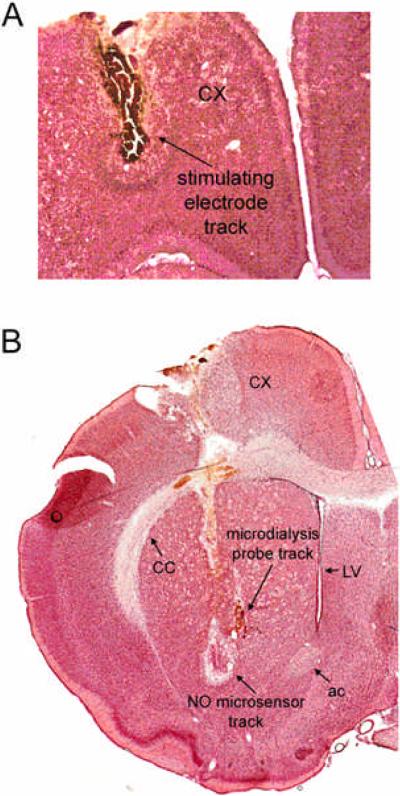
Figure 1. Position of implants in the frontal cortex and dorsal striatum.
A) Stimulating electrodes were implanted into the frontal cortex. B) NO microsensors were implanted into the striatum in close proximity to a microdialysis probe. Abbreviations: ac, anterior commissure; CX, frontal cortex; LV, lateral ventrical; CC, corpus callosum.
Drug Preparation and Administration
Urethane and SKF (Sigma, MO, USA) used for systemic administration were dissolved in physiological saline (0.9%). An effective dose of SKF (0.5 mg/kg i.v.) was derived from the range previously reported in the literature (Ainsworth et al. 1998; Ruskin et al. 1998; Caine et al. 1999). For experiments using reverse microdialysis of drug, all compounds (SKF, SCH, 7-NI, KYN, CPP and GLY) were dissolved in aCSF. The conversion from aCSF to aCSF with drug during the microdialysis procedure was accomplished using a liquid switch as previously described (West and Grace 2004; Threlfell et al. 2009). In the first series of studies (Fig 2), SKF was administered systemically (0.5 mg/kg, i.v.) following intrastriatal infusion (120 min) of either aCSF, SCH (10 μM), 7-NI (300 μM), KYN (100 nM), CPP (100 μM), or GLY (1mM). In the second series of studies (Figs 3, 4), cortically-evoked NO efflux was recorded prior to, and following, intrastriatal infusion of aCSF or SCH (100 μM for 90 min). SKF (5 μM) was then infused for 10 min and cortical stimulation trials were repeated 10–20 and 30–40 min later. Effective doses of each drug were derived from a range previously reported in the literature and/or our previous studies (Ito and Cherubini 1991; West and Galloway 1996; Desvignes et al. 1999; West and Grace 2000; Zackheim and Abercrombie 2001; Flores-Hernandez et al. 2002; West and Grace 2002; Saklayen et al. 2004; Wu et al. 2007).
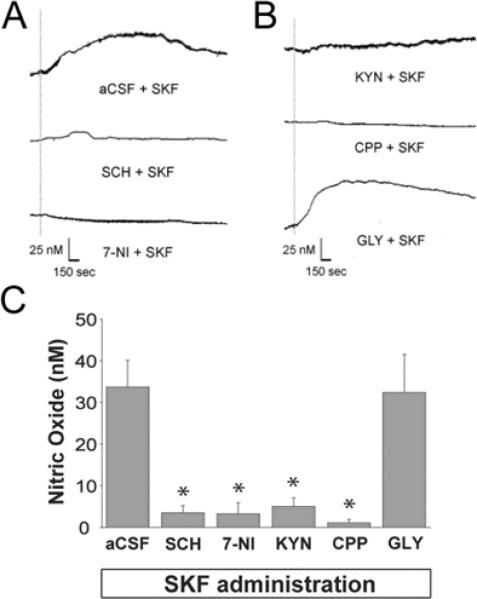
Figure 2. Facilitation of nNOS activity elicited via DA D1/5 receptor stimulation is dependent on NMDA receptor co-activation.
A) Representative recordings showing the effects of systemic administration of the D1/5 receptor agonist SKF (500 μg/kg, i.v.) delivered following intrastriatal infusion (120 min) of either aCSF, SCH (10 μM), or 7-NI (300 μM). Dotted line indicates time of SKF-81297 injection. B) Representative recordings showing the effects of systemic administration of the D1/5 receptor agonist SKF (500 μg/kg, i.v.) delivered following intrastriatal infusion of either KYN (100 nM), CPP (100 μM), or GLY (1 mM). Dotted line indicates time of SKF injection. C) The mean ± S.E.M. increase in NO efflux evoked by DA D1/5 receptor activation was significantly reduced following intrastriatal infusion of SCH, 7-NI, KYN, CPP (*p<0.05 as compared to SKF+aCSF group using ANOVA with Dunnett's post-hoc test, n=5–6 rats/group), but not GLY (p>0.05, n=4 rats).
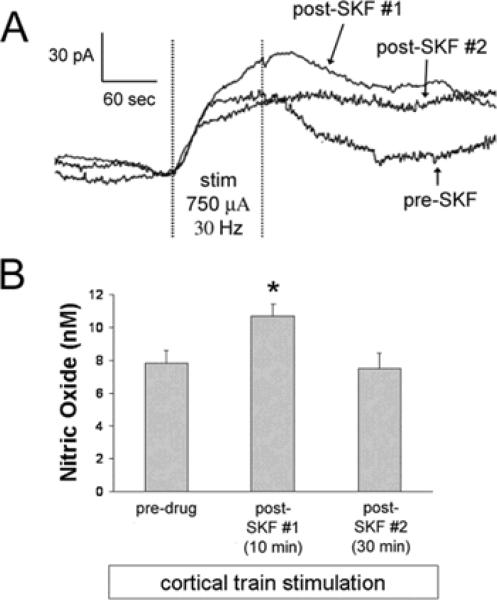
Figure 3. Intrastriatal infusion of D1/5 agonist increases NO efflux evoked via train stimulation of the frontal cortex.
A) Representative traces of cortically-evoked NO efflux recorded prior to, and 10–20 (#1) or 30–40 (#2) min following intrastriatal SKF (5 μM for 10 min) infusion. Electrical stimuli were delivered for 100 sec as trains (30 Hz, 750 μA, 800 ms train duration, 2 sec inter-train interval) and all stimulations were separated by >15 min. B) The mean ± S.E.M. increase in NO efflux evoked by cortical train stimulation was transiently potentiated by intrastriatal infusion of SKF (*p<0.05 as compared to pre-SKF group using one-way ANOVA with Bonferroni post-hoc test, n=6 rats).
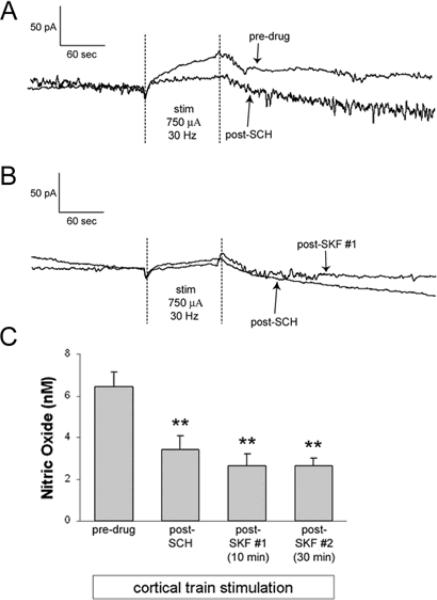
Figure 4. Intrastriatal infusion of D1/5 antagonist attenuates NO efflux evoked via train stimulation of the frontal cortex and blocks the facilitatory effects of D1/5 agonism.
A) Recordings of NO efflux evoked prior to, and following, intrastriatal SCH infusion. Electrical stimuli were delivered as described above. B) Representative traces of NO efflux evoked approximately 90 mins following intrastriatal SCH (100 μM) infusion and 10–20 and 30–40 min following intrastriatal SKF infusion (5 μM for 10 min). Electrical stimuli were delivered as described above. C) The mean ± S.E.M. increase in NO efflux evoked by cortical train stimulation was significantly attenuated following SCH infusion as compared to pre-drug conditions (**p<0.001 as compared to pre-SKF group using one-way ANOVA with Bonferroni post-hoc test, n=5 rats). The mean ± S.E.M. increase in NO efflux evoked by cortical train stimulation applied during intrastriatal SKF infusion was significantly attenuated at both time points as compared to pre-drug conditions (**p<0.001 as compared to pre-SKF group using one-way ANOVA with Bonferroni post-hoc test, n=5 rats). No significant differences in measures of NO efflux were observed during SCH and SCH+SKF infusion periods.
Electrical Stimulation
Stimulus pulses having a duration of 500 μs and an intensity of 0.75 mA were generated using a stimulator and photoelectric constant current/stimulus isolation unit (S88 stimulator with PSIU6F stimulus isolation unit, Grass Instruments, Quincy, MA) and delivered in stimulus trains (30 Hz, 800 ms train duration, 1.2 sec inter-train interval (ITI)) for a duration of 100 seconds (50 trains) as previously reported (Sammut et al. 2007b; Ondracek et al. 2008). The response to cortical stimulation was tested multiple times prior to vehicle or vehicle/drug administration and stimulation trials were performed every 15–20 minutes (Sammut et al. 2007b; Ondracek et al. 2008). The average of these measures was considered the control response to which responses following drug administration were compared.
Histology
Animals were deeply anesthesized with urethane following the completion of each experiment and subsequently perfused transcardially with ice-cold saline and 10 % formalin in buffered phosphate (PB) (EMS, Hatfield, PA). Brains were removed and postfixed in 10 % formalin/PB for at least 3 days and then saturated in PBS/sucrose solution (30%) and stored at 4–6 °C. Brains were then coronally sectioned into 50 μm slices, mounted, and stained with Neutral red/Cresyl Violet (10:1) solution to enable the histological assessment of NO microsensor and microdialysis probe placements (Fig 1).
Data Analyses
Drug- and stimulation-induced changes in NO oxidation current were measured as previously described (Sammut et al. 2007a; Sammut et al. 2007b). Briefly, the latency of the peak SKF-induced change in NO oxidation current was determined for each animal in the control group (aCSF + SKF) and averaged to generate a mean group value (Sammut et al. 2006). The mean NO oxidation current was then measured across a 30 sec epoch which enveloped this group value using Apollo 4000 (WPI) software applications (15 sec prior to and after peak NO oxidation current). In all cases, the mean NO oxidation current recorded during the last 30 s of the pre-SKF injection period was subtracted from the above measures to yield the net SKF-induced change in NO oxidation current. In experiments using electrical stimulation of the frontal cortex, the NO oxidation current recorded during the last 30 s of the stimulation period was averaged as described above and subtracted from the NO oxidation current recorded during the last 30 sec before the stimulation period (Sammut et al. 2007b). Cumulative data are expressed as nM ± SEM NO as determined from in vitro calibration curves (Sammut et al. 2006; Sammut et al. 2007a; Sammut et al. 2007b). The statistical significance of drug-induced changes in NO oxidation current was assessed across time using one-way analysis of variance (ANOVA) with repeated measures (RM, Sigma Stat, Jandel, Scientific, San Rafael, CA). Dunnett's or Bonferroni post-hoc tests were used as indicated to determine which group(s) contributed to overall differences seen with ANOVA.
RESULTS
Stimulating electrode, NO microsensor, and microdialysis probe placements
All tracks resulting from implantation of stimulating electrodes were confirmed to terminate in the frontal cortex between 3.2 and 4.7 mm anterior to bregma, 1.0 and 3.0 mm lateral to the midline, and 1.5 and 4.8 mm ventral to the surface of the skull (Paxinos and Watson 1986) (Fig 1 a). All identified tracks resulting from implantation of NO microsensors and microdialysis probes into the striatum (Fig. 1b) were verified to terminate between 0.3 mm posterior and 1.7 mm anterior to bregma, 2.0 and 3.8 mm lateral to midline, and 4.0 and 8.0 mm ventral to the dural surface (Paxinos and Watson 1986).
Impact of intrastriatal drug manipulations on NO efflux elicited by systemic D1/5 agonist administration
Our previous NO microsensor recording studies have shown that electrical and chemical stimulation of the substantia nigra and systemic D1/5 receptor agonist administration both robustly increase striatal NO efflux via nNOS and D1/5 receptor-dependent mechanisms (Sammut et al. 2006; Sammut et al. 2007a). In the current studies, NO microsensor recordings and reverse microdialysis were used together for the first time to enable local delivery of glutamatergic and nitrergic drugs (Fig 2). Local and systemic administration of vehicle did not affect basal NO efflux (data not shown). Consistent with our previous work using systemic drug administration (Sammut et al. 2006), intrastriatal infusion of both 7-NI and SCH blocked the increase in NO efflux evoked by SKF (Fig 2a,c; F(5,23)=10.799, p<0.001 as compared to aCSF control group using ANOVA with Dunnett's post-hoc test, p<0.05). Intrastriatal infusion of the nonspecific ionotropic glutamate receptor antagonist KYN (100 nM) or the specific NMDA receptor antagonist CPP (100 μM) also induced a significant attenuation in SKF-induced NO efflux (Fig 2b,c; F(5,23)=10.799, p<0.001 as compared to aCSF control group using ANOVA with Dunnett's post-hoc test, p<0.05). No significant changes in striatal NO efflux were observed following GLY (1mM) co-administration (Fig 2c; p>0.05).
Impact of intrastriatal SKF infusion on NO efflux evoked by electrical stimulation of cortical afferents
In a subset of studies the ipsilateral frontal cortex was activated using a well characterized electrical stimulation protocol in which trains of stimuli (30 Hz, 800 ms train duration, 2 sec inter-train interval) are delivered for 100 sec (Sammut et al. 2007a; Ondracek et al. 2008). Similar to our previous studies, this stimulation paradigm robustly increased striatal NO efflux in a manner which was highly reproducible across time (Sammut et al. 2007a; Ondracek et al. 2008). Moreover, intrastriatal SKF (5μM, 10 min) infusion transiently increased cortically-evoked NO efflux significantly over levels observed during local aCSF infusion (Fig 3a,b; F(2,10)=6.127, p<0.005 as compared to pre-SKF-81297 group using one-way ANOVA with Bonferroni post-hoc test, p<0.05). Cortically-evoked NO efflux returned to pre-SKF levels approximately 30 min after reperfusion with aCSF (Fig 3a,b; p>0.05).
Impact of intrastriatal SCH infusion on NO efflux evoked by electrical stimulation of cortical afferents and local SKF co-perfusion
The role of striatal D1/5 receptor activation in the facilitation of cortically-evoked NO-efflux observed following intrastriatal infusion of SKF was examined further in studies using co-perfusion of the D1/5 receptor antagonist SCH. The electrical stimulation paradigm and the reverse dialysis approach used in these studies were identical to that described above. The response to cortical stimulation was tested multiple times following SCH (100 μM, 90 mins) infusion and again 10 and 30 min after SKF (5μM, 10 min) infusion. Intrastriatal SCH infusion significantly attenuated cortically-evoked NO efflux (Fig 4a,c; F(3,12)=45.450, p<0.001 as compared to pre-SKF-81297 group using one-way ANOVA with Bonferroni post-hoc test, p<0.001) and blocked the facilitatory effects of SKF (Fig 4b,c; F(3,12)=45.450, p<0.001 as compared to pre-SKF group using one-way ANOVA with Bonferroni post-hoc test, p<0.001).
DISCUSSION
This study investigated the impact of striatal DA and glutamate interactions on nNOS activity using the combined techniques of in vivo amperometry and reverse microdialysis. Our data demonstrate for the first time that striatal NMDA receptor activation plays a critical role in the facilitatory effects of D1/5 receptor activation on NO efflux. Local striatal D1/5 receptor activation also facilitated NO-efflux evoked via electrical stimulation of the frontal cortex, whereas local D1/5 receptor antagonism blocked NO efflux evoked by cortical stimulation and local SKF infusion. Given these findings, it is likely that reciprocal DA and glutamate interactions are crucial for the regulation of striatal nNOS and activation of NO transmission.
Technical Considerations
We have developed a novel approach for administering drugs locally into the striatum of intact rats in the vicinity of a NO microsensor using reverse microdialysis. Experiments were performed in intact urethane-anesthetized rats, and with the exception of SKF, all drugs were administered locally via a microdialysis probe. Similar to our previous studies using systemic drug administration (Sammut et al. 2007b; Ondracek et al. 2008), NO efflux recorded proximal to the probe under basal conditions and during electrical stimulation of the cortex was stable over time and across consecutive stimulation intervals. Given this, it is likely that changes in NO efflux observed following local drug infusions were not due to time or vehicle effects, but a result of pharmacological manipulations of glutamate and DA receptors.
Consistent with our previous work (Sammut et al. 2006; Sammut et al. 2007a), the increase in NO efflux evoked by systemic administration of the D1/5 receptor agonist SKF was attenuated following local antagonism/inhibition of D1/5 receptors and nNOS, respectively. Compared to outcomes from the current study, however, previous studies using systemic administration of the nNOS inhibitor 7-NI observed very modest maximal decreases in nNOS activity (~33–60%), even when substantial doses (50–80 mg/kg, i.p.) of drug were tested (Kalisch et al. 1996; Sammut et al. 2006; Sammut et al. 2007b; Ondracek et al. 2008). Similar findings were observed with other selective and non-selective nNOS inhibitors (Kalisch et al. 1996; Sammut et al. 2006; Sammut et al. 2007b; Ondracek et al. 2008), raising the possibility that currently available drugs are not very effective when given systemically, or that a drug-resistant isoform of striatal NOS may contribute to outcomes measured in these studies. Our observations that local infusion of 7-NI effectively blocked SKF-induced NO efflux indicate that nNOS is the primary mediator of D1/5 receptor-dependent activation of striatal NO synthesis. Therefore, the modest efficacy observed following systemic drug administration in the above studies is likely to result from poor brain penetration, rather than a novel, drug-resistant isoform of NOS.
Given that the facilitatory effects of SKF on NO efflux were observed following both intravenous and intrastriatal administration, it is likely that D1/5 receptor activation under these conditions is occurring in the striatum at the level of the nNOS interneuron. In support of this, the membrane excitability of electrophysiologically-identified striatal nNOS interneurons was shown to increase robustly following bath application of either DA or the D1/5 receptor agonist (Centonze et al. 2002; Centonze et al. 2003). Furthermore, a robust D1/5 receptor-dependent depolarization of the membrane potential of nNOS interneurons was observed in striatal slices pretreated with tetrodotoxin (Centonze et al. 2002; Centonze et al. 2003), indicating a direct effect of D1/5 receptor agonism on these cells.
Regulation of NOS activity by glutamate-dopamine interactions
NOS-containing interneurons receive synaptic inputs from glutamatergic, GABAergic, and DAergic neurons (Vuillet et al. 1989; Salin et al. 1990; Vuillet et al. 1992; Fujiyama and Masuko 1996; Morello et al. 1997; Bevan et al. 1998; Hidaka and Totterdell 2001; French et al. 2005). Our previous work performed in intact rats has shown that stimulation of the frontal cortex facilitated striatal nNOS activity in a stimulus frequency and intensity-dependent manner (Sammut et al. 2007b). This effect was also significantly attenuated following NMDA receptor antagonist and nNOS inhibitor administration (Sammut et al. 2007b). Additionally, phasic stimulation of nigrostriatal DA cells or pharmacological activation of the D1/5 receptor was shown to activate striatal nNOS activity (Sammut et al. 2007a). These findings are supported by histochemical evidence showing that both NMDA and D1/5 receptor antagonists decrease NOS activity measured ex vivo (Morris et al. 1997).
Taken together, the above literature suggests that reciprocal D1/5-NMDA receptor interactions may play a critical role in regulating striatal nNOS activity. In support of this, the current studies demonstrated that the increase in striatal NO efflux elicited by systemic administration of D1/5 agonist is blocked by intrastriatal infusion of the non-selective ionotropic glutamate receptor antagonist KYN and the selective NMDA receptor antagonist CPP. Thus, D1/5 receptor-mediated NO efflux is dependent on concurrent, tonic NMDA receptor activation.
Interestingly, intrastriatal infusion of the NMDA receptor co-agonist GLY did not affect SKF-induced NO efflux. This finding was unexpected given previous work showing that local GLY infusion enhances striatal NO efflux induced via local infusion of NMDA (Crespi and Rossetti 2004). The lack of effect of GLY on SKF-mediated NO efflux observed in the current study may be due to saturation of GLY binding sites under the current experimental conditions or a ceiling effect with regard to nNOS activation. Furthermore, previous electrophysiological studies of neurons recorded in the ventromedial versus dorsolateral striatum have reported regional differences in the sensitivity of NMDA receptor-mediated excitatory postsynaptic currents to GLY and D-serine (Chapman et al. 2003). This work indicates that the nature of GLY-mediated effects may depend on regional variations in NMDA receptor subunit expression and the pharmacological and biophysical properties of these receptor subunits (Chapman et al. 2003). In the current study, NO microsensor recordings were primarily performed in the dorsocentral striatum. Therefore, additional studies are needed to determine whether outcomes observed herein translate to other striatal subregions. In any event, the current outcomes observed with KYN and CPP demonstrate for the first time that tonic striatal NMDA receptor activation plays a critical role in the facilitatory effects of D1/5 receptor activation on striatal nNOS activity.
Modulation of cortically-evoked NO efflux by local D1/5 receptor manipulations
Using a previously characterized electrical stimulation paradigm (Sammut et al. 2007b; Ondracek et al. 2008) and the reverse dialysis approach described above, we found that local striatal D1/5 receptor activation facilitates NO-efflux evoked by stimulation of cortical afferents. In addition, cortically-evoked NO efflux was strongly attenuated following intrastriatal administration of the D1/5 receptor antagonist SCH, indicating that D1/5 receptor activation is crucial for the glutamatergic facilitation of NO synthesis. The D1/5 receptor agonist-induced facilitation of cortically-evoked NO efflux was also abolished following pretreatment with the D1/5 receptor antagonist, indicating that the observed effects of drug manipulations were receptor mediated. We have shown previously that systemic administration of the NMDA receptor antagonist MK-801 strongly attenuated NO efflux evoked by the same stimulation protocol used in the current study (Sammut et al. 2007b). Together with the current observations using local SCH infusion, these results indicate that reciprocal D1/5 and NMDA receptor interactions are critical for the activation of striatal nNOS by cortical afferents.
Consistent with the above conclusions, many studies have shown that NMDA and D1/5 receptors have reciprocal interactions which increase the membrane excitability of striatal projection neurons (for review see (Cepeda and Levine 2006)). Interestingly, a previous study observed that treatment of cultured striatal neurons with NMDA increased the recruitment of D1/5 receptors from the cytosol to the plasma membrane, an effect that was abolished following pretreatment with an NMDA receptor antagonist (Scott et al. 2002). D1/5 receptors were also shown to form an oligomer with the NR1 subunit of the NMDA receptor that regulates D1/5 receptor trafficking to the plasma membrane (Fiorentini et al. 2003). Furthermore, exposure to NMDA induced an increase in D1/5 receptor-positive spines and a reduction in lateral movement of D1/5 receptors within the membrane of striatal neurons (Scott et al. 2006). These findings suggest that NMDA receptor activation induces the formation of spines that trap D1/5 receptors, leading to the generation of D1/5-NMDA heteroreceptor complexes (Cepeda and Levine 2006). Additional studies have shown that application of D1/5 receptor agonist to the synaptosomal membrane compartment of striatal cells induced a rapid increase in the expression of the NMDA receptor subunits NR1, NR2A, and NR2B (Dunah and Standaert 2001).
Given the above observations, it is plausible that the expression of functional NMDA receptor subunits within glutamatergic synapses on striatal nNOS cells requires tonic D1/5 receptor activation. Although speculative, the sustained pharmacological blockade of striatal D1/5 receptors (produced in the current study following intrastriatal infusion of SCH) could conceivably disrupt this process and lead to blockade of NMDA receptor-dependent activation of nNOS and cortically-evoked NO efflux. It is also possible that the observed effects of striatal D1/5 receptor antagonism on cortically-evoked NO efflux were mediated partially via circuit-level changes in brain function. In support of this, studies examining the impact of striatal D1 receptor activation on gene expression in the basal ganglia and cortex have shown that unilateral intrastriatal SCH infusion blocks the widespread activation of the frontal cortex observed following systemic administration of the DA D1/D2 receptor agonist apomorphine (Steiner and Kitai 2000). Thus, some of the dampening effects of intrastriatal SCH infusion on cortically-evoked NO efflux observed in the current study may have arisen as a result of indirect circuit level effects on cortical excitability. Additional studies using reduced preparations are needed to determine whether these DA-glutamate receptor interactions occur at the level of striatal NO producing interneurons and if they play a critical role in stimulating nNOS activity in these cells.
Functional Implications
This is the first study to demonstrate that striatal NMDA receptor co-activation is necessary for D1/5 receptor stimulation of nNOS activity. In addition, striatal D1/5 receptor activation may be critical for the NMDA receptor-dependent activation of nNOS. These findings add to the growing body of literature indicating that interactions between striatal DAergic, glutamatergic, and nitrergic systems play a significant role in the regulation of striatal function (West et al. 2002; Del Bel et al. 2005). In support of this, previous studies have reported abnormal locomotor activity in rats and mice following systemic administration of nNOS inhibitors (Starr and Starr 1995; Dzoljic et al. 1997; Araki et al. 2001; Dall'Igna et al. 2001; Uzbay 2001; Del Bel et al. 2002) and genetic deletion of the nNOS gene (Kriegsfeld et al. 1999) In addition, locomotor activity stimulated by NMDA receptor antagonism (Starr and Starr 1995) and D1/5 receptor agonism (Deutsch et al. 1996) was attenuated following pretreatment with NOS inhibitors. Thus, a better understanding of the complexities of NMDA-D1/5 receptor interactions as they pertain to NO synthesis holds considerable promise for the development of novel therapeutic strategies for reversing motor dysfunction.
ACKNOWLEDGEMENTS
The authors thank Dr. Stephen Sammut for his valuable assistance with the techniques associated with this study. We are also grateful to Michael Park for his assistance with histology. This work was supported by the Chicago Medical School and United States Public Health grant NS 047452 (ARW).
Abbreviations
- NO
nitric oxide
- DA
dopamine
- NOS
nNOS
- neuronal nitric oxide synthase
- CPP
3-phosphonopropyl-piperazine-2-carboxylic acid
- NOS
nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- eNOS
endothelial nitric oxide synthase
- NMDA
N-methyl-D-aspartate
- NPY
neuropeptide Y
- SKF
R-(+)-SKF-81297 HBr
- SCH
R-(+)-SCH-23390 HCl or SCH-23390
- GLY
glycine
- 7-NI
7-nitroindazole monosodium
- KYN
kynurenic acid
- SNAP
(±)S-Nitroso-N-acetyl-penicillamine
- PB
phosphate buffer
- Hz
hertz
Reference List
- Ainsworth K, Smith SE, Sharp T. Repeated administration of fluoxetine, desipramine and tranylcypromine increases dopamine D2-like but not D1-like receptor function in the rat. J. Psychopharmacol. 1998;12:252–257. doi: 10.1177/026988119801200304. [DOI] [PubMed] [Google Scholar]
- Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem. J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, Mizutani H, Matsubara M, Imai Y, Mizugaki M, Itoyama Y. Nitric oxide synthase inhibitors cause motor deficits in mice. Eur. Neuropsychopharmacol. 2001;11:125–133. doi: 10.1016/s0924-977x(01)00077-3. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Booth PA, Eaton SA, Bolam JP. Selective innervation of neostriatal interneurons by a subclass of neuron in the globus pallidus of the rat. J. Neurosci. 1998;18:9438–9452. doi: 10.1523/JNEUROSCI.18-22-09438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehning D, Snyder SH. Novel neural modulators. Annu. Rev. Neurosci. 2003;26:105–131. doi: 10.1146/annurev.neuro.26.041002.131047. [DOI] [PubMed] [Google Scholar]
- Bredt DS. Nitric oxide signaling in brain: potentiating the gain with YC-1. Mol. Pharmacol. 2003;63:1206–1208. doi: 10.1124/mol.63.6.1206. [DOI] [PubMed] [Google Scholar]
- Brenman JE, Bredt DS. Synaptic signaling by nitric oxide. Curr. Opin. Neurobiol. 1997;7:374–378. doi: 10.1016/s0959-4388(97)80065-7. [DOI] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK, Bergman J. Effects of dopamine D(1-like) and D(2-like) agonists in rats that self-administer cocaine. J. Pharmacol. Exp. Ther. 1999;291:353–360. [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Di FM. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Centonze D, Bracci E, Pisani A, Gubellini P, Bernardi G, Calabresi P. Activation of dopamine D1-like receptors excites LTS interneurons of the striatum. Eur. J. Neurosci. 2002;15:2049–2052. doi: 10.1046/j.1460-9568.2002.02052.x. [DOI] [PubMed] [Google Scholar]
- Centonze D, Grande C, Saulle E, Martin AB, Gubellini P, Pavon N, Pisani A, Bernardi G, Moratalla R, Calabresi P. Distinct roles of D1 and D5 dopamine receptors in motor activity and striatal synaptic plasticity. J. Neurosci. 2003;23:8506–8512. doi: 10.1523/JNEUROSCI.23-24-08506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Levine MS. Where do you think you are going? The NMDA-D1 receptor trap. Sci. STKE. 2006;2006:e20. doi: 10.1126/stke.3332006pe20. [DOI] [PubMed] [Google Scholar]
- Chapman DE, Keefe KA, Wilcox KS. Evidence for functionally distinct synaptic NMDA receptors in ventromedial versus dorsolateral striatum. J. Neurophysiol. 2003;89:69–80. doi: 10.1152/jn.00342.2002. [DOI] [PubMed] [Google Scholar]
- Crespi F, Rossetti ZL. Pulse of Nitric Oxide Release in Response to Activation of N-Methyl-D-aspartate Receptors in the Rat Striatum: Rapid Desensitization, Inhibition of Receptor Antagonists, and Potentiation by Glycine. JPET. 2004;309:462–468. doi: 10.1124/jpet.103.061069. [DOI] [PubMed] [Google Scholar]
- Dall'Igna OP, Dietrich MO, Hoffmann A, Neto W, Vendite D, Souza DO, Lara DR. Catalepsy and hypolocomotion induced by a nitric oxide donor: attenuation by theophylline. Eur. J. Pharmacol. 2001;432:29–33. doi: 10.1016/s0014-2999(01)01457-1. [DOI] [PubMed] [Google Scholar]
- Dawson VL, Dawson TM. Nitric oxide actions in neurochemistry. Neurochem. Int. 1996;29:97–110. doi: 10.1016/0197-0186(95)00149-2. [DOI] [PubMed] [Google Scholar]
- Del Bel EA, Guimaraes FS, Bermudez-Echeverry M, Gomes MZ, Schiaveto-desouza A, Padovan-Neto FE, Tumas V, Barion-Cavalcanti AP, Lazzarini M, Nucci-da-Silva LP, de Paula-Souza D. Role of nitric oxide on motor behavior. Cell Mol. Neurobiol. 2005;25:371–392. doi: 10.1007/s10571-005-3065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bel EA, Souza AS, Guimaraes FS, da-Silva CA, Nucci-da-Silva LP. Motor effects of acute and chronic inhibition of nitric oxide synthesis in mice. Psychopharmacology (Berl) 2002;161:32–37. doi: 10.1007/s00213-002-1009-2. [DOI] [PubMed] [Google Scholar]
- Desvignes C, Bert L, Vinet L, Denoroy L, Renaud B, Lambas-Senas L. Evidence that the neuronal nitric oxide synthase inhibitor 7-nitroindazole inhibits monoamine oxidase in the rat: in vivo effects on extracellular striatal dopamine and 3,4-dihydroxyphenylacetic acid. Neurosci. Lett. 1999;264:5–8. doi: 10.1016/s0304-3940(99)00139-1. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Rosse RB, Paul SM, Tomasino V, Koetzner L, Morn CB, Mastropaolo J. 7-Nitroindazole and methylene blue, inhibitors of neuronal nitric oxide synthase and NO-stimulated guanylate cyclase, block MK-801-elicited behaviors in mice. Neuropsychopharmacology. 1996;15:37–43. doi: 10.1016/0893-133X(95)00153-5. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Standaert DG. Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane. J. Neurosci. 2001;21:5546–5558. doi: 10.1523/JNEUROSCI.21-15-05546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzoljic E, De VR, Dzoljic MR. New and potent inhibitors of nitric oxide synthase reduce motor activity in mice. Behav. Brain Res. 1997;87:209–212. doi: 10.1016/s0166-4328(97)02281-x. [DOI] [PubMed] [Google Scholar]
- Emson PC, Augood SJ, Senaris R, Guerara GR, Kishimoto J, Kadowaki K, Norris PJ, Kendrick KM. Chemical signalling and striatal interneurones. Prog. Brain Res. 1993;99:155–165. doi: 10.1016/s0079-6123(08)61344-8. [DOI] [PubMed] [Google Scholar]
- Figueredo-Cardenas G, Morello M, Sancesario G, Bernardi G, Reiner A. Colocalization of somatostatin, neuropeptide Y, neuronal nitric oxide synthase and NADPH-diaphorase in striatal interneurons in rats. Brain Res. 1996;735:317–324. doi: 10.1016/0006-8993(96)00801-3. [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Gardoni F, Spano P, Di LM, Missale C. Regulation of dopamine D1 receptor trafficking and desensitization by oligomerization with glutamate N-methyl-D-aspartate receptors. J. Biol. Chem. 2003;278:20196–20202. doi: 10.1074/jbc.M213140200. [DOI] [PubMed] [Google Scholar]
- Flores-Hernandez J, Cepeda C, Hernandez-Echeagaray E, Calvert CR, Jokel ES, Fienberg AA, Greengard P, Levine MS. Dopamine enhancement of NMDA currents in dissociated medium-sized striatal neurons: role of D1 receptors and DARPP-32. J. Neurophysiol. 2002;88:3010–3020. doi: 10.1152/jn.00361.2002. [DOI] [PubMed] [Google Scholar]
- French SJ, Ritson GP, Hidaka S, Totterdell S. Nucleus accumbens nitric oxide immunoreactive interneurons receive nitric oxide and ventral subicular afferents in rats. Neuroscience. 2005;135:121–131. doi: 10.1016/j.neuroscience.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Fujiyama F, Masuko S. Association of dopaminergic terminals and neurons releasing nitric oxide in the rat striatum: an electron microscopic study using NADPH-diaphorase histochemistry and tyrosine hydroxylase immunohistochemistry. Brain Res. Bull. 1996;40:121–127. doi: 10.1016/0361-9230(96)00035-4. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Concepts of neural nitric oxide-mediated transmission. Eur. J. Neurosci. 2008;27:2783–2802. doi: 10.1111/j.1460-9568.2008.06285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J, Boulton CL. Nitric oxide signaling in the central nervous system. Annu. Rev. Physiol. 1995;57:683–706. doi: 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Charles SL, Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988;336:385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Hidaka S, Totterdell S. Ultrastructural features of the nitric oxide synthase-containing interneurons in the nucleus accumbens and their relationship with tyrosine hydroxylase-containing terminals. J. Comp Neurol. 2001;431:139–154. doi: 10.1002/1096-9861(20010305)431:2<139::aid-cne1061>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Ito S, Cherubini E. Strychnine-sensitive glycine responses of neonatal rat hippocampal neurones. J. Physiol. 1991;440:67–83. doi: 10.1113/jphysiol.1991.sp018696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch BE, Connop BP, Jhamandas K, Beninger RJ, Boegman RJ. Differential action of 7-nitro indazole on rat brain nitric oxide synthase. Neurosci. Lett. 1996;219:75–78. doi: 10.1016/s0304-3940(96)13194-3. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Neostriatal cell subtypes and their functional roles. Neurosci. Res. 1997;27:1–8. doi: 10.1016/s0168-0102(96)01134-0. [DOI] [PubMed] [Google Scholar]
- Kharazia VN, Schmidt HH, Weinberg RJ. Type I nitric oxide synthase fully accounts for NADPH-diaphorase in rat striatum, but not cortex. Neuroscience. 1994;62:983–987. doi: 10.1016/0306-4522(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Eliasson MJ, Demas GE, Blackshaw S, Dawson TM, Nelson RJ, Snyder SH. Nocturnal motor coordination deficits in neuronal nitric oxide synthase knock-out mice. Neuroscience. 1999;89:311–315. doi: 10.1016/s0306-4522(98)00614-9. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Mikawa S, Kawaguchi Y. Neostriatal GABAergic interneurones contain NOS, calretinin or parvalbumin. Neuroreport. 1993;5:205–208. doi: 10.1097/00001756-199312000-00004. [DOI] [PubMed] [Google Scholar]
- Morello M, Reiner A, Sancesario G, Karle EJ, Bernardi G. Ultrastructural study of nitric oxide synthase-containing striatal neurons and their relationship with parvalbumin-containing neurons in rats. Brain Res. 1997;776:30–39. doi: 10.1016/s0006-8993(97)00997-9. [DOI] [PubMed] [Google Scholar]
- Morris BJ, Simpson CS, Mundell S, Maceachern K, Johnston HM, Nolan AM. Dynamic changes in NADPH-diaphorase staining reflect activity of nitric oxide synthase: evidence for a dopaminergic regulation of striatal nitric oxide release. Neuropharmacology. 1997;36:1589–1599. doi: 10.1016/s0028-3908(97)00159-7. [DOI] [PubMed] [Google Scholar]
- Nathan C, Xie QW. Regulation of biosynthesis of nitric oxide. J. Biol. Chem. 1994;269:13725–13728. [PubMed] [Google Scholar]
- Ondracek JM, Dec A, Hoque KE, Lim SA, Rasouli G, Indorkar RP, Linardakis J, Klika B, Mukherji SJ, Burnazi M, Threlfell S, Sammut S, West AR. Feed-forward excitation of striatal neuron activity by frontal cortical activation of nitric oxide signaling in vivo. Eur. J. Neurosci. 2008;27:1739–1754. doi: 10.1111/j.1460-9568.2008.06157.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1986. [Google Scholar]
- Rivera A, Alberti I, Martin AB, Narvaez JA, de la Calle A, Moratalla R. Molecular phenotype of rat striatal neurons expressing the dopamine D5 receptor subtype. Eur. J. Neurosci. 2002;16:2049–2058. doi: 10.1046/j.1460-9568.2002.02280.x. [DOI] [PubMed] [Google Scholar]
- Ruskin DN, Rawji SS, Walters JR. Effects of full D1 dopamine receptor agonists on firing rates in the globus pallidus and substantia nigra pars compacta in vivo: tests for D1 receptor selectivity and comparisons to the partial agonist SKF 38393. J. Pharmacol. Exp. Ther. 1998;286:272–281. [PubMed] [Google Scholar]
- Saklayen SS, Mabrouk OS, Pehek EA. Negative feedback regulation of nigrostriatal dopamine release: mediation by striatal D1 receptors. J. Pharmacol. Exp. Ther. 2004;311:342–348. doi: 10.1124/jpet.104.067991. [DOI] [PubMed] [Google Scholar]
- Salin P, Kerkerian-Le GL, Heidet V, Epelbaum J, Nieoullon A. Somatostatin-immunoreactive neurons in the rat striatum: effects of corticostriatal and nigrostriatal dopaminergic lesions. Brain Res. 1990;521:23–32. doi: 10.1016/0006-8993(90)91520-q. [DOI] [PubMed] [Google Scholar]
- Sammut S, Bray KE, West AR. Dopamine D2 receptor-dependent modulation of striatal NO synthase activity. Psychopharmacology (Berl) 2007a;191:793–803. doi: 10.1007/s00213-006-0681-z. [DOI] [PubMed] [Google Scholar]
- Sammut S, Dec A, Mitchell D, Linardakis J, Ortiguela M, West AR. Phasic dopaminergic transmission increases NO efflux in the rat dorsal striatum via a neuronal NOS and a dopamine D(1/5) receptor-dependent mechanism. Neuropsychopharmacology. 2006;31:493–505. doi: 10.1038/sj.npp.1300826. [DOI] [PubMed] [Google Scholar]
- Sammut S, Park DJ, West AR. Frontal cortical afferents facilitate striatal nitric oxide transmission in vivo via a NMDA receptor and neuronal NOS-dependent mechanism. J. Neurochem. 2007b;103:1145–1156. doi: 10.1111/j.1471-4159.2007.04811.x. [DOI] [PubMed] [Google Scholar]
- Scott L, Kruse MS, Forssberg H, Brismar H, Greengard P, Aperia A. Selective up-regulation of dopamine D1 receptors in dendritic spines by NMDA receptor activation. Proc. Natl. Acad. Sci. U. S. A. 2002;99:1661–1664. doi: 10.1073/pnas.032654599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott L, Zelenin S, Malmersjo S, Kowalewski JM, Markus EZ, Nairn AC, Greengard P, Brismar H, Aperia A. Allosteric changes of the NMDA receptor trap diffusible dopamine 1 receptors in spines. Proc. Natl. Acad. Sci. U. S. A. 2006;103:762–767. doi: 10.1073/pnas.0505557103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr MS, Starr BS. Do NMDA receptor-mediated changes in motor behaviour involve nitric oxide? Eur. J. Pharmacol. 1995;272:211–217. doi: 10.1016/0014-2999(94)00644-m. [DOI] [PubMed] [Google Scholar]
- Steiner H, Kitai ST. Regulation of Rat Cortex Function by D1 Dopamine Receptors in the Striatum. J. Neurosci. 2000;20:5449–5460. doi: 10.1523/JNEUROSCI.20-14-05449.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr DJ, Santolini J, Wang ZQ, Wei CC, Adak S. Update on mechanism and catalytic regulation in the NO synthases. J Biol. Chem. 2004;279:36167–36170. doi: 10.1074/jbc.R400017200. [DOI] [PubMed] [Google Scholar]
- Threlfell S, Sammut S, Menniti FS, Schmidt CJ, West AR. Inhibition of Phosphodiesterase 10A Increases the Responsiveness of Striatal Projection Neurons to Cortical Stimulation. J. Pharmacol. Exp. Ther. 2009;328:785–795. doi: 10.1124/jpet.108.146332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzbay IT. L-NAME precipitates catatonia during ethanol withdrawal in rats. Behav. Brain Res. 2001;119:71–76. doi: 10.1016/s0166-4328(00)00332-6. [DOI] [PubMed] [Google Scholar]
- Vuillet J, Dimova R, Nieoullon A, Kerkerian-Le GL. Ultrastructural relationships between choline acetyltransferase- and neuropeptide y-containing neurons in the rat striatum. Neuroscience. 1992;46:351–360. doi: 10.1016/0306-4522(92)90057-9. [DOI] [PubMed] [Google Scholar]
- Vuillet J, Kerkerian L, Kachidian P, Bosler O, Nieoullon A. Ultrastructural correlates of functional relationships between nigral dopaminergic or cortical afferent fibers and neuropeptide Y-containing neurons in the rat striatum. Neurosci. Lett. 1989;100:99–104. doi: 10.1016/0304-3940(89)90667-8. [DOI] [PubMed] [Google Scholar]
- West AR, Galloway MP. Intrastriatal infusion of (+/−)-S-nitroso-N-acetylpenicillamine releases vesicular dopamine via an ionotropic glutamate receptor-mediated mechanism: an in vivo microdialysis study in chloral hydrate-anesthetized rats. J. Neurochem. 1996;66:1971–1980. doi: 10.1046/j.1471-4159.1996.66051971.x. [DOI] [PubMed] [Google Scholar]
- West AR, Galloway MP, Grace AA. Regulation of striatal dopamine neurotransmission by nitric oxide: effector pathways and signaling mechanisms. Synapse. 2002;44:227–245. doi: 10.1002/syn.10076. [DOI] [PubMed] [Google Scholar]
- West AR, Grace AA. Striatal nitric oxide signaling regulates the neuronal activity of midbrain dopamine neurons in vivo. J. Neurophysiol. 2000;83:1796–1808. doi: 10.1152/jn.2000.83.4.1796. [DOI] [PubMed] [Google Scholar]
- West AR, Grace AA. Opposite influences of endogenous dopamine D1 and D2 receptor activation on activity states and electrophysiological properties of striatal neurons: studies combining in vivo intracellular recordings and reverse microdialysis. J. Neurosci. 2002;22:294–304. doi: 10.1523/JNEUROSCI.22-01-00294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AR, Grace AA. The nitric oxide-guanylyl cyclase signaling pathway modulates membrane activity States and electrophysiological properties of striatal medium spiny neurons recorded in vivo. J. Neurosci. 2004;24:1924–1935. doi: 10.1523/JNEUROSCI.4470-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Ostergaard K, Andreassen OA, Finsen B. Estimation of the number of somatostatin neurons in the striatum: an in situ hybridization study using the optical fractionator method. J. Comp Neurol. 1996;370:11–22. doi: 10.1002/(SICI)1096-9861(19960617)370:1<11::AID-CNE2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Wu HQ, Rassoulpour A, Schwarcz R. Kynurenic acid leads, dopamine follows: a new case of volume transmission in the brain? J. Neural Transm. 2007;114:33–41. doi: 10.1007/s00702-006-0562-y. [DOI] [PubMed] [Google Scholar]
- Zackheim JA, Abercrombie ED. Decreased striatal dopamine efflux after intrastriatal application of benzazepine-class D1 agonists is not mediated via dopamine receptors. Brain Res. Bull. 2001;54:603–607. doi: 10.1016/s0361-9230(01)00462-2. [DOI] [PubMed] [Google Scholar]