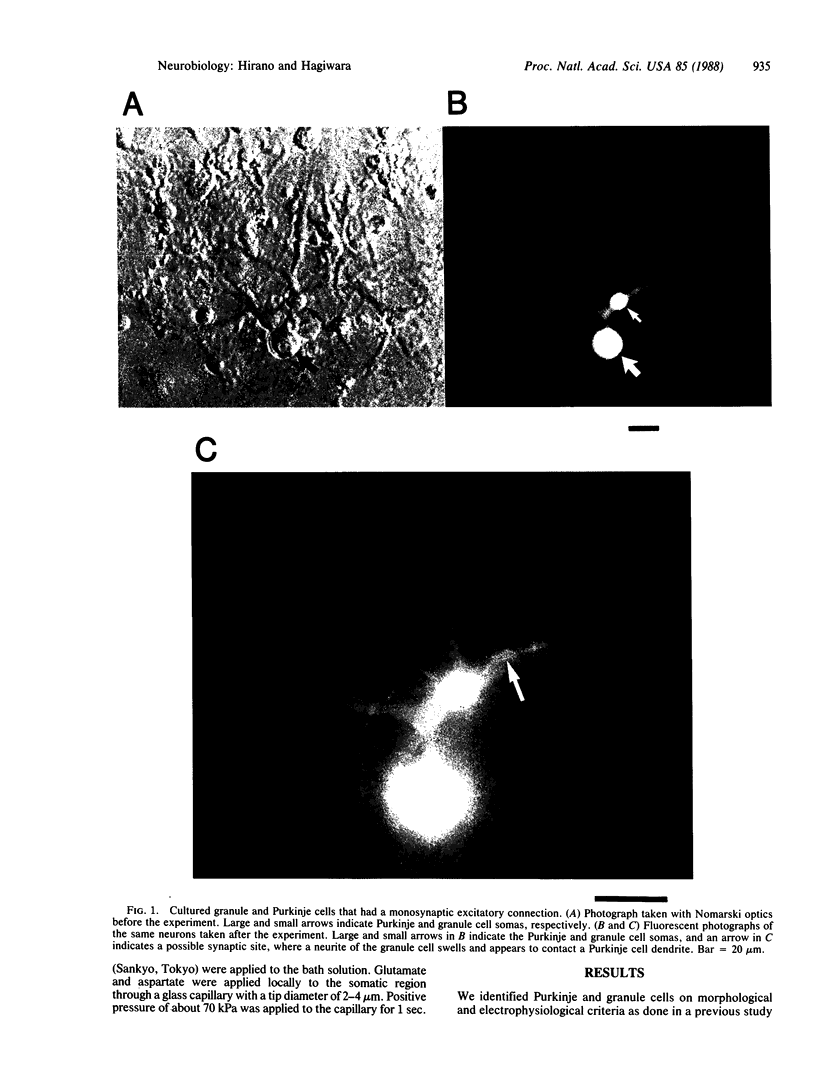
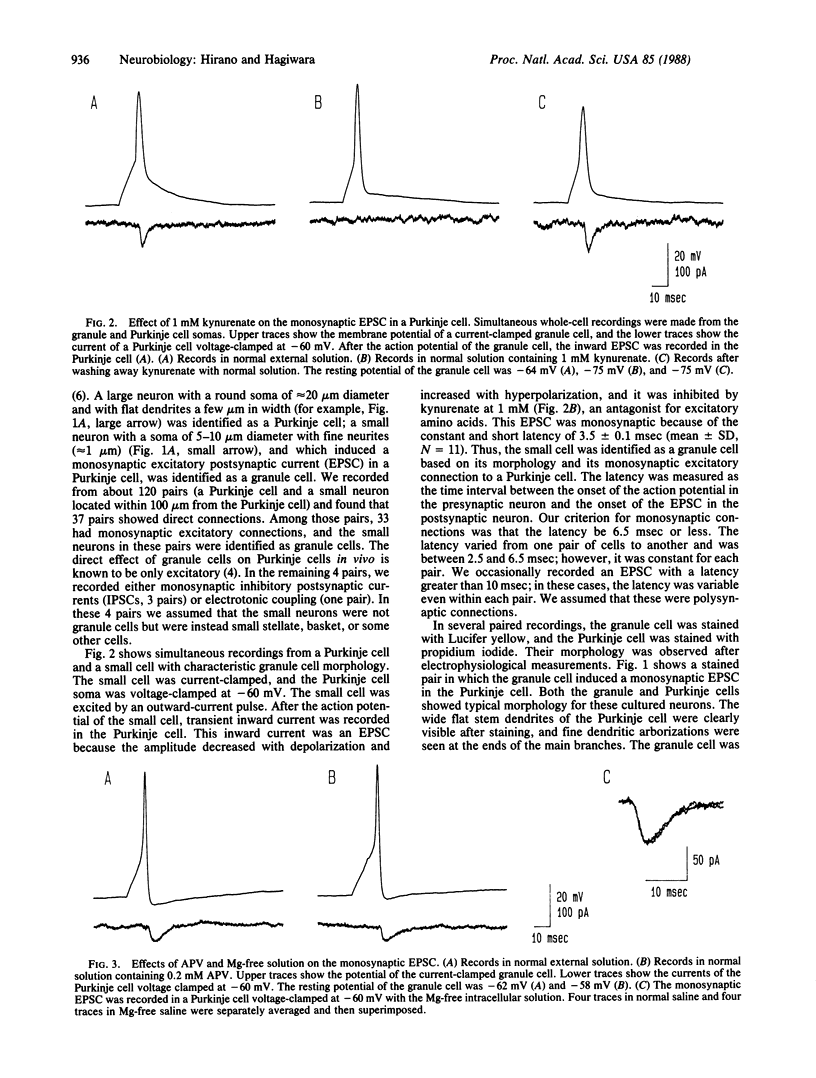
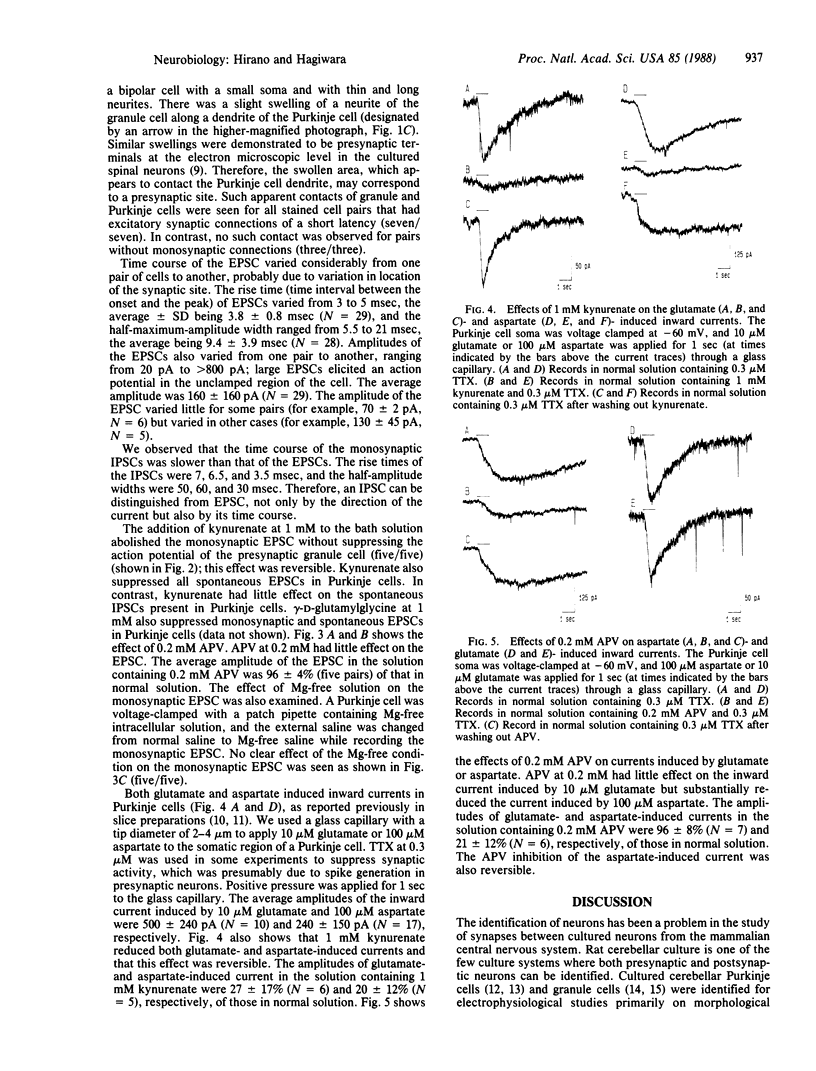
Abstract
Monosynaptic excitatory connections between cerebellar granule and Purkinje cells were studied in dissociated cell cultures, and identification of the transmitter and the postsynaptic receptor at this synapse was pharmacologically investigated. The presynaptic granule cell and the postsynaptic Purkinje cell were voltage- or current-clamped simultaneously, and the excitatory postsynaptic current induced by the granule cell was examined. The neurons and monosynaptic excitatory connections were identified as in our earlier study. Several pairs of granule and Purkinje cells were stained with Lucifer yellow and propidium iodide, respectively, and their morphology was examined after electrophysiological recording. The monosynaptic excitatory postsynaptic current was suppressed by 1 mM kynurenate, an antagonist for excitatory-amino acid receptors, but was little affected by 0.2 mM DL-2-amino-5-phosphonovalerate, a selective antagonist of N-methyl-D-aspartate receptors. Glutamate and aspartate induced inward current in the Purkinje cells. These currents were suppressed by kynurenate at 1 mM. DL-2-Amino-5-phosphonovalerate at 0.2 mM suppressed the inward current induced by 100 microM aspartate but did not affect the inward current induced by 10 microM glutamate. These results are consistent with the idea that glutamate, or a glutamate-like substance, but not aspartate is the transmitter released at the synapse between granule and Purkinje cells and that non-N-methyl-D-aspartate receptor channels are functioning in the postsynaptic membrane.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crepel F., Dhanjal S. S., Sears T. A. Effect of glutamate, aspartate and related derivatives on cerebellar purkinje cell dendrites in the rat: an in vitro study. J Physiol. 1982 Aug;329:297–317. doi: 10.1113/jphysiol.1982.sp014304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N., Roberts A. Dual-component amino-acid-mediated synaptic potentials: excitatory drive for swimming in Xenopus embryos. J Physiol. 1985 Jun;363:35–59. doi: 10.1113/jphysiol.1985.sp015694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Francis A. A., Jones A. W., Watkins J. C. 2-Amino-5-phosphonovalerate (2APV), a potent and selective antagonist of amino acid-induced and synaptic excitation. Neurosci Lett. 1981 Jan 1;21(1):77–81. doi: 10.1016/0304-3940(81)90061-6. [DOI] [PubMed] [Google Scholar]
- Foster A. C., Fagg G. E. Acidic amino acid binding sites in mammalian neuronal membranes: their characteristics and relationship to synaptic receptors. Brain Res. 1984 May;319(2):103–164. doi: 10.1016/0165-0173(84)90020-1. [DOI] [PubMed] [Google Scholar]
- Ganong A. H., Lanthorn T. H., Cotman C. W. Kynurenic acid inhibits synaptic and acidic amino acid-induced responses in the rat hippocampus and spinal cord. Brain Res. 1983 Aug 22;273(1):170–174. doi: 10.1016/0006-8993(83)91108-3. [DOI] [PubMed] [Google Scholar]
- Gruol D. L. Cultured cerebellar neurons: endogenous and exogenous components of Purkinje cell activity and membrane response to putative transmitters. Brain Res. 1983 Mar 21;263(2):223–241. doi: 10.1016/0006-8993(83)90315-3. [DOI] [PubMed] [Google Scholar]
- Gruol D. L., Franklin C. L. Morphological and physiological differentiation of Purkinje neurons in cultures of rat cerebellum. J Neurosci. 1987 May;7(5):1271–1293. doi: 10.1523/JNEUROSCI.07-05-01271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Ohmori H. Studies of calcium channels in rat clonal pituitary cells with patch electrode voltage clamp. J Physiol. 1982 Oct;331:231–252. doi: 10.1113/jphysiol.1982.sp014371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Kubo Y., Wu M. M. Cerebellar granule cells in culture: monosynaptic connections with Purkinje cells and ionic currents. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4957–4961. doi: 10.1073/pnas.83.13.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Ohmori H. Voltage-gated and synaptic currents in rat Purkinje cells in dissociated cell cultures. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1945–1949. doi: 10.1073/pnas.83.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockberger P. E., Tseng H. Y., Connor J. A. Immunocytochemical and electrophysiological differentiation of rat cerebellar granule cells in explant cultures. J Neurosci. 1987 May;7(5):1370–1383. doi: 10.1523/JNEUROSCI.07-05-01370.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Jessell T. M. Synaptic transmission between dorsal root ganglion and dorsal horn neurons in culture: antagonism of monosynaptic excitatory postsynaptic potentials and glutamate excitation by kynurenate. J Neurosci. 1985 Aug;5(8):2281–2289. doi: 10.1523/JNEUROSCI.05-08-02281.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M., Kato M. Quisqualate receptors are specifically involved in cerebellar synaptic plasticity. Nature. 1987 Jan 15;325(6101):276–279. doi: 10.1038/325276a0. [DOI] [PubMed] [Google Scholar]
- Kimura H., Okamoto K., Sakai Y. Pharmacological evidence for L-aspartate as the neurotransmitter of cerebellar climbing fibres in the guinea-pig. J Physiol. 1985 Aug;365:103–119. doi: 10.1113/jphysiol.1985.sp015761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Moonen G., Neale E. A., Macdonald R. L., Gibbs W., Nelson P. G. Cerebellar macroneurons in microexplant cell culture. Methodology, basic electrophysiology, and morphology after horseradish peroxidase injection. Brain Res. 1982 Sep;281(1):59–73. doi: 10.1016/0165-3806(82)90113-4. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- O'Brien R. J., Fischbach G. D. Characterization of excitatory amino acid receptors expressed by embryonic chick motoneurons in vitro. J Neurosci. 1986 Nov;6(11):3275–3283. doi: 10.1523/JNEUROSCI.06-11-03275.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun R. Y., Neale E. A., Guthrie P. B., Nelson P. G. Active and inactive central synapses in cell culture. J Neurophysiol. 1986 Nov;56(5):1242–1256. doi: 10.1152/jn.1986.56.5.1242. [DOI] [PubMed] [Google Scholar]
- Vicini S., Wroblewski J. T., Costa E. Pharmacological modulation of GABAergic transmission in cultured cerebellar neurons. Neuropharmacology. 1986 Feb;25(2):207–211. doi: 10.1016/0028-3908(86)90043-2. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- Weber A., Schachner M. Maintenance of immunocytologically identified Purkinje cells from mouse cerebellum in monolayer culture. Brain Res. 1984 Oct 8;311(1):119–130. doi: 10.1016/0006-8993(84)91404-5. [DOI] [PubMed] [Google Scholar]