Abstract
1. Curcumin is a naturally occurring poly-phenolic compound with a broad range of favorable biological functions including anti-cancer, anti-oxidant and anti-inflammatory activities. The low bioavailability and in vivo stability of curcumin require the development of suitable carrier vehicles to deliver the molecule in a sustained manner at therapeutic levels.
2. We investigated the feasibility and potential of poly(caprolactone) (PCL) nanofibers as a delivery vehicle for curcumin for wound healing applications. By optimizing the electrospinning parameters, bead-free curcumin loaded PCL nanofibers were developed.
3. The fibers showed sustained release of curcumin for 72 h and could be made to deliver a dosage much lower than the reported cytotoxic concentration while remaining bioactive. Human foreskin fibroblast cells (HFF-1) showed more than 70% viability on curcumin loaded nanofibers. The antioxidant activity of curcumin loaded nanofibers was demonstrated using an ORAC assay and by the ability of the fibers to maintain the viability of HFF-1 cells on the fibers under a condition of oxidative stress. The curcumin loaded nanofibers also reduced inflammatory induction as evidenced by low levels of IL-6 release from mouse monocyte-macrophages seeded on the fibers following stimulation by E.coli-derived lipopolysaccharide (LPS). The in vivo wound healing capability of the curcumin loaded PCL nanofibers was demonstrated by an increased rate of wound closure in a streptozotocin (STZ) induced diabetic mice model.
4. These results demonstrate that curcumin loaded PCL nanofiber matrix is bioactive and has potential as a wound dressing with antioxidant and anti-inflammatory properties.
1. Introduction
Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione; Figure 1) is an active ingredient of turmeric isolated from the root of Curcumin Longa L. Turmeric has been used for centuries in South and South East Asia in culinary and traditional medicine. The consumption of curcumin as a dietary spice at doses up to 100 mg/d has been reported and clinical trials reported safety up to about 8g/day [1,2]. Extensive research done over the past three decades has clearly established the biological and pharmacological efficacy of curcumin. Curcumin is known to exhibit strong antioxidant, anti-inflammatory and anti-infective properties which make it a unique molecule for wound healing applications and for treating a variety of inflammatory diseases including atherosclerosis, alzheimer's, diabetes, arthritis and inflammatory bowel disease [3,4].
Figure 1.
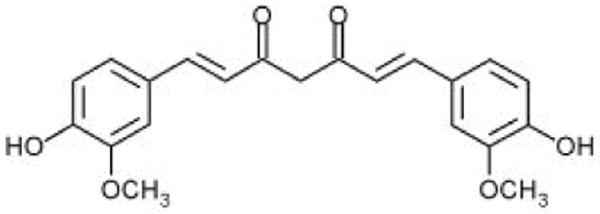
Molecular structure of curcumin.
Several in vitro and in vivo studies demonstrated the ability of curcumin, to decrease the release of inflammatory cytokines from monocytes and macrophages including interleukin-8 (IL-8) and tumor necrosis factor-α (TNF-α), and inhibiting enzymes implicated in inflammation such as cyclooxygenase 2 (COX-2) and lipoxygenase (LOX) [5,6]. The antioxidant property of curcumin is evidenced by its ability to protect fibroblast and keratinocytes against hydrogen peroxide induced damages and to reduce oxidative stress in Alzheimer patients [7,8]. Studies using a rat model attributed the accelerated wound healing of curcumin to its powerful antioxidant property. Curcumin treated cutaneous wound in rats showed decreased levels of lipid peroxides (LPs) and increased levels of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and increased maturation and cross-linking of collagen [9]. Furthermore, the ability of curcumin in assisting wound healing in diabetic mice has also been demonstrated. Curcumin treatment resulted in increased formation of granulation tissue, neovascularization and enhanced biosynthesis of extracellular matrix proteins such as collagen [10].
In spite of these unique biological activities of curcumin, the in vivo stability and bioavailablity of the molecule is very low. Moreover, the biotransformation products of curcumin such as curcumin glucuronide and curcumin sulfate have been shown to have very low activity towards inhibiting COX-2 expression [11]. In a study using 12 patients, daily administration of 450-3600 mg of curcumin showed poor bioavailability following oral administration with only low nanomolar levels of the compound found in the peripheral or portal circulation [12]. Oral administration of curcumin is therefore unlikely to provide pharmacologically beneficial concentrations in the body for many disease conditions. This limitation calls for the development of appropriate carrier vehicles to increase the in vivo stability of the drug and bioavailability. Several carrier vehicles have been investigated. A prolonged release microsphere based curcumin delivery system was developed with high encapsulation efficiency (∼79 and 39%) using bovine serum albumin and chitosan [13]. Another study investigated biodegradable nanosphere formulation by encapsulating curcumin in poly(DL-lactide) nanospheres [14]. A lipid based nanoparticulate carrier vehicle was developed to deliver curcumin to tissue macrophages using intravenous injection [15]. A water dispersible nanoparticle carrier vehicle for curcumin was developed utilizing micellar aggregates of cross-linked polymers of N-isopropyl acrylamide (NIPAM) with N-vinyl-2-pyrrolidone (NVP and poly(ethylene glycol)monoacrylate (PEG-A). The excellent therapeutic efficacy of nanocurcumin has been demonstrated using cell culture studies [16]. Incorporation of curcumin in poly(L-lactic acid) micro fibers was performed to improve the mechanical properties of fibers as well as to reduce the inflammatory responses towards the polyester fibers for cardiovascular stent application [17]. A recent study investigated the feasibility of incorporating curcumin in cellulose acetate nanofibers and release of curcumin in various media [18].
Poly(ε-caprolactone) (PCL) is a biocompatible and biodegradable polymer that has been extensively investigated for tissue regeneration and wound healing applications. Nanofiber matrices are attracting significant attention lately for a variety of biomedical applications as they closely mimic the diameter of collagen fibrils in the natural extracellular matrix [19]. The large surface area of the fiber matrix allow for increased interaction with the tissue, thereby serves as a substrate for the sustained delivery of bioactive molecules as well as to modulate cellular functions during regeneration [20]. The objective of this study was to develop curcumin loaded PCL nanofibers by the process of electrospinning and to evaluate the biological activity of the curcumin loaded fibers using in vitro and in vivo methods. We investigated the feasibility of developing bead free curcumin loaded PCL nanofibers by controlling the elecrospinning parameters. The bioactivity of encapsulated curcumin in the nanofibers was investigated using various in vitro methods and comparisons were made against the corresponding PCL nanofibers. Finally, the in vivo efficacy of the curcumin loaded PCL fibers vs PCL fibers was evaluated using healing impaired diabetic mouse model.
2. Materials and Methods
2.1 Materials
Poly(ε-caprolactone) [PCL; Mwt: 65,000] was procured from Aldrich Chemical Company, Inc (Milwaukee WI, USA). Curcumin, lipopolysaccharide (LPS), streptozotocin (STZ), xylazin, and ketamine were procured from Sigma-Aldrich (St. Louis, USA). MTS biochemical assay kit was purchased from Promega (Madison, WI, USA). Sodium Fluorescein (FL) was purchased from Invitrogen (Carlsbad, CA). 2,2′-Azobis(2-amidino-propane)dihydrochloride (AAPH) was procured from Wako Chemicals (Richmond, VA). Human foreskin fibroblast cells (HFF-1) and Mouse monocyte-macrophages (J774A1) were procured from ATCC (Manassas, VA, USA). All other reagents were of analytical grade and used as received.
2.2. Fabrication of PCL and Curcumin loaded PCL Nanofibers
A weighed amount of PCL was dissolved in 3:1 v/v chloroform/methanol solvent mixture to prepare the solution for electrospinning PCL nanofibers. For curcumin loaded nanofiber preparation, a weighed amount of PCL was dissolved in chloroform /methanol mixture (3:1) and appropriate amounts of curcumin were added and allowed to dissolve by vortexing the solution for an hour before electrospinning. Different concentrations of PCL solutions (7-18% wt/v) were prepared to fabricate bead free nanofibers. Two different concentrations of curcumin (3 & 17% wt/wt) were used for fabricating curcumin loaded nanofibers. The electrospinning apparatus consists of a 5mL syringe fitted with a blunt end needle (14 or 18 gauge) and a grounded electrode. The grounded electrode consists of a copper plate covered with aluminum foil placed at a predetermined distance from the needle tip (10, 20 or 30 cm). The syringe was fitted perpendicular to the collection screen and the polymer solution was injected at a flow rate of 2.0 mL/h using a syringe pump. A Gamma high voltage supply (25 kV) was used as the power source. The electrospinning was carried out at ambient temperature and pressure. The spun nanofibers were dried under vacuum at room temperature for 48 hours. The optimized parameters used for fabricating PCL and curcumin loaded PCL nanofibers are summarized as follows: PCL concentration of 15%w/v in 3:1 chloroform/methanol solvent mixture, curcumin concentration of 3 and 17%wt/wt, an applied voltage of 25kV, tip–to-target distance of 10 cm, solution flow rate of 2mL/hr and a 14 gauge blunt tip needle.
2.3 Scanning Electron Microscopy (SEM)
The morphology and diameter distribution of the fibers were determined using scanning electron microscopy (JEOL, 6400). The SEMs of the fibers were obtained after coating the fibers with gold for 200sec at 60mA under 0.5mbar pressure using a Bal-Tec SCD 005 sputtering system. The samples were viewed at an accelerating voltage of 15kV. Average fiber diameters were determined by measuring 100 fibers selected randomly from each electrospun mat using Image J software (National Institute of Health, USA).
2.4 In vitro Curcumin Release
PCL and Curcumin loaded PCL nanofiber matrices (1cm2) were incubated in 1 mL of cell culture media (Dubelcco's modified Eagles Media supplemented with 10% FBS, 1% penicillin/streptomycin) for 72 hours at 37°C. At predetermined intervals, the extraction media was removed and placed in a 96 well plate (BD falcon) and same amount of fresh media was added to the extraction media. The media was allowed to evaporate and the dried samples were reconstituted in 1mL of 50% ethanol solution. The amount of curcumin released was quantified using a fluorometer (excitation 430, nm emission 535 nm) and the results were correlated to a curcumin standard curve in 50% ethanol.
2.5 In vitro antioxidant Study
An Oxygen Radical Absorbance Capacity Assay (ORAC) was used to determine the antioxidant activity of curcumin loaded PCL nanofibers. The ORAC assay has been used for determining the anti-oxidant potential of many molecules [21, 22]. PCL and Curcumin loaded PCL nanofiber matrices (0.5cm2) were placed in 48 well tissue culture plates. Wells bordering the outermost edge of the plate were not used as they have been found to sometimes distort fluorescence measurements as well as introduce temperature variance in the assay [23]. A water soluble azo compound 2,2′-Azobis(2-amidino-propane)dihydrochloride (AAPH) was used as a radical initiator to generate free radicals at a constant rate. Fluorescein (FL) was used as the probe to assess the antioxidant activity. A positive control (FL solution containing AAPH free radical generator), a negative control (FL solution containing no AAPH) and blanks (curcumin loaded PCL nanofibers and PCL nanofibers in PBS) were run simultaneously. Briefly, 170μL of FL solution was added to each well (PCL and curcumin loaded PCL nanofibers and blank wells) to initiate the assay. 30μL of AAPH solution was then added to all wells except for the negative control which received 30μL of phosphate buffer solution. A timer was started upon introduction of the free radical generator and the plate was stored in the dark at room temperature. At each time points the solution fluorescence was measured after taking out the nanofiber mats from the wells (excitation 492, emission 535 nm) using a TECAN Spectrafluor Plus™ and plotted as a function of time.
2.6. In vitro Cytotoxicity Evaluation
PCL and curcumin loaded PCL nanofibers (1 cm2) were treated with 10% ethanol solution to minimize bacterial contamination. HFF-1 cells were plated in a monolayer in tissue culture flasks (75 cm2) and cultured to confluence in DMEM supplemented with 10% fetal bovine serum and 1% penicillin and streptomycin. The media was replaced every other day and the culture was maintained in a tissue culture incubator at 37°C and 5% carbon dioxide. Once the cells reached 80% confluency, they were trypsinized and seeded onto the surface of the nanofiber matrices at a seeding density of 100,000 cells/well and cultured for 48 h. The viability of the cells adhered on the surface of nanofibers was evaluated using the MTS assay [25]. Appropriate blank samples (PCL and curcumin loaded PCL nanofibers without cells) were run simultaneously.
2.7. In vitro Cytoprotective Evaluation
An assay was performed to determine the cytoprotective potential of curcumin loaded PCL nanofibers compared to PCL fibers in a situation of oxidative stress. HFF-1 cells were seeded onto the nanofiber matrices as described above and cultured for 48 hours. Half of the samples were then exposed to 1% hydrogen peroxide for 4 hours and incubated at 37°C. At the end of the incubation time, the viability of cells on the matrices was evaluated using the MTS assay. Appropriate blank samples (PCL and curcumin loaded PCL nanofibers without cells and hydrogen perioxide) were run simultaneously.
2.8 In Vitro Anti-Inflammatory Property
PCL and curcumin loaded PCL nanofibers (1 cm2) were seeded with J774A1 cells at a seeding density of 700,000 cells/matrix and cultured in DMEM for 6 h. The culture media was removed from all the wells and half of the samples (PCL and curcumin loaded PCL nanofibers with cells) were treated with 2mL DMEM containing 1μg/ml lipopolysaccharide (LPS) to elicite the inflammatory response. The cells were then cultured for 14 hours after which the release of IL-6 was quantified using an ELISA (Sandwich ELISA™)
2.9 In Vivo Diabetic Mouse Wound Closure
Male C57/B6 mice aged 8-12 weeks were purchased from Jackson Laboratories, Bar Harbor, Maine. The study was approved by the animal research committees of the Michigan State University. The mice were injected intraperitoneally with streptozotocin (STZ) dissolved in sterile citrate buffer (0.05 mol/L sodium citrate, pH 4.5, 4.5 mg/kg). Mice were treated with five consecutive daily injections of STZ during the first week of the study. Non-fasting blood glucose levels were measured once a week, following administration of STZ by a blood glucose analyzer (2300 Stat Plus analyzer, YSI Incorporated) by drawing blood from the dorsal vein of the mouse hind foot with the use of Microvette blood collection tubes (Microvette, CB-300 LH, Sarstedt). Mice with blood glucose level ≥280 mg/dL were considered diabetic and used for the wound healing experiment. [26]
Ten weeks after streptozotocin injection, mice were anesthetized with ketamine (100 mg/kG IP) and xylazine (10 mg/kg IP) and the dorsum was clipped free of hair. Two, six mm circular wounds were created with biopsy punch. In half of the mice the wounds were dressed using 17% curcumin loaded PCL nanofibers and the other half dressed with PCL nanofibers. The wound closure rates were measured by tracing the wound area onto a Bioclusive transparent dressing. The tracings were digitized, and the areas were calculated in blinded fashion with the use of a computerized algorithm (Sigma, Scan; Jandel Scientific).
2.10 Statistical Analysis
Data was analyzed using the software program Origin Pro 7.5. Statistical comparison between curcumin loaded PCL nanofibers and PCL nanofibers was determined by using one-way ANOVA with a Tukey test (p ≤ 0.05)
Results
3.1. SEM Analysis
Figure 2 shows the morphologies of PCL nanofibers electrospun using various polymer concentrations. In the present study, it was found that the concentration of PCL solution ≤ 14% (w/w) resulted in the formation of beads along the fibers (Figure 2 A & B). Electrospinning of 15% (w/v) PCL in chloroform/methanol gave rise to bead-free nanofibrous matrices with median diameter in the range of 300-400 nm (Figure 2C). Figure 3A shows the morphology of curcumin loaded (3% w/w) PCL nanofibers under the optimized electrospinning conditions as described above. Similar to the PCL nanofibers (Figure 2C) curcumin loaded (3% w/w) PCL nanofibers showed bead free morphology. However, the loading of curcumin significantly changed the diameter distribution of fibers. Fibers with broad diameter distribution (200-800 nm) were observed upon the incorporation of curcumin. Increasing curcumin concentration to 17%w/w did not significantly change the morphology and frequency of fiber distribution compared to fibers loaded with 3% (wt/wt) curcumin (Figure 3B). No aggregates of curcumin were found on the surface of the fibers. The maximum concentration of curcumin that can be loaded in the PCL nanofibers under the optimized condition was found to be 17% (w/w). Further increase in curcumin concentration resulted in the precipitation of curcumin from the polymer solution.
Figure 2.
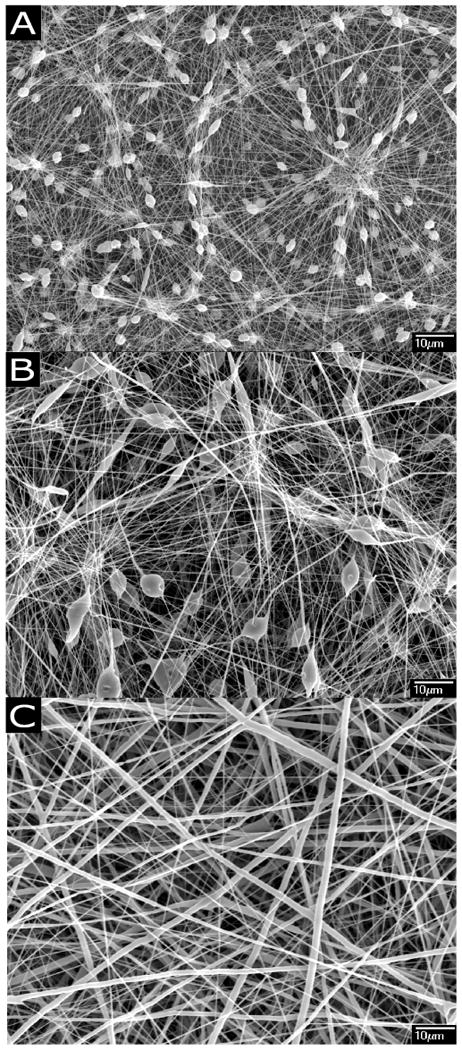
Morphology of Poly(ε-caprolactone) (PCL) nanofibers A: 7.5% (w/v) PCL in chloroform/methanol; B: 13% (w/v) PCL in chloroform/methanol; C: 15% (w/v) PCL in chloroform/methanol showing bead free fiber morphology.
Figure 3.
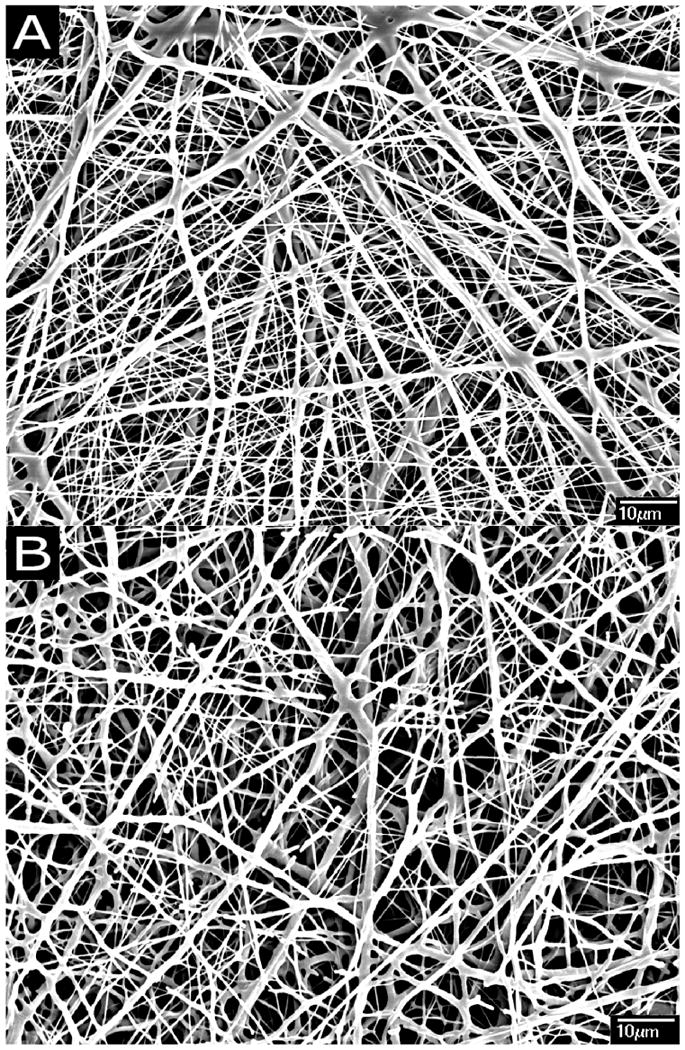
Morphology of curcumin loaded PCL nanofibers A: PCL nanofibers having 3% (w/w) of curcumin; B: PCL nanofibers having 17% (w/w) of curcumin
3.2 In Vitro Curcumin Release from the Nanofibers
Figure 4 shows the in vitro release of curcumin into cell culture media for a period of 3 days. No significant difference in curcumin release was observed between fibers containing 3 and 17% curcumin in the first 12 hours. After 12 h the amount of curcumin released from fibers loaded with 17%(w/w) of curcumin was found to be significantly higher than fibers containing 3%(w/w) curcumin. By day 3, ∼35μg of curcumin was released from 17% loaded fibers and ∼20μg of curcumin was released from 3% loaded fibers.
Figure 4.
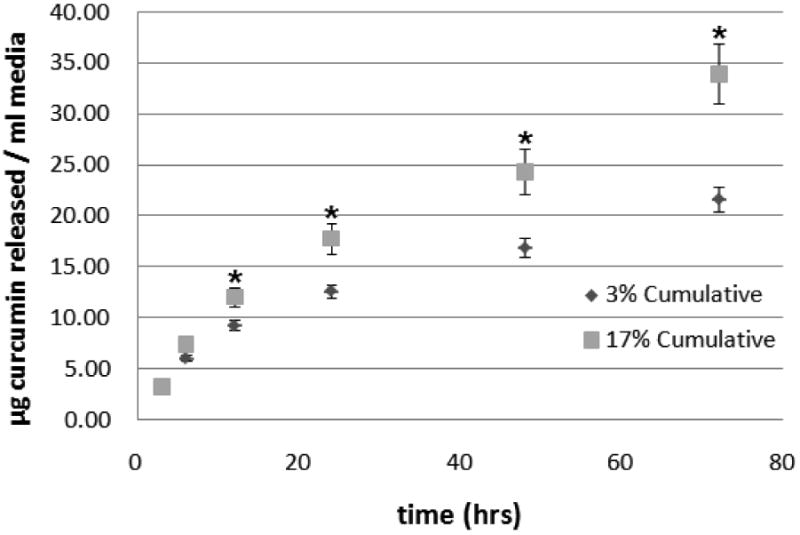
Cumulative release of curcumin from curcumin loaded nanofibers as a function of time. ■ Curcumin released from PCL nanofibers loaded with 17%(w/w) of curcumin; ◆ Curcumin released from PCL nanofibers loaded with 3%(w/w) of curcumin. Significantly more curcumin (* p ≤ 0.05) was released from fibers having higher concentration of curcumin after 12 hours.
3.3 In Vitro Antioxidant Activity of Curcumin Loaded PCL Nanofibers
The antioxidant activity of curcumin loaded fibers relates to the ability of curcumin to inhibit the free radical damage of a fluorescent probe, fluorescein (FL). This inhibition is observed as a preservation of the fluorescent signal. Figure 5 shows the fluorescence of solutions up to 4 h of incubation at room temperature. The assay did not run to completion (the fluorescence was preserved) even after 4 h of incubation at room temperature for curcumin loaded PCL nanofibers suggesting the high antioxidant potential of the curcumin loaded nanofibers. The PCL nanofibers showed some inhibition at early time point but it showed almost complete quenching by 4 h. The area under the curve has not been determined in this case since the activity did not decay to zero even after 4h of incubation for curcumin loaded PCL nanofibers.
Figure 5.
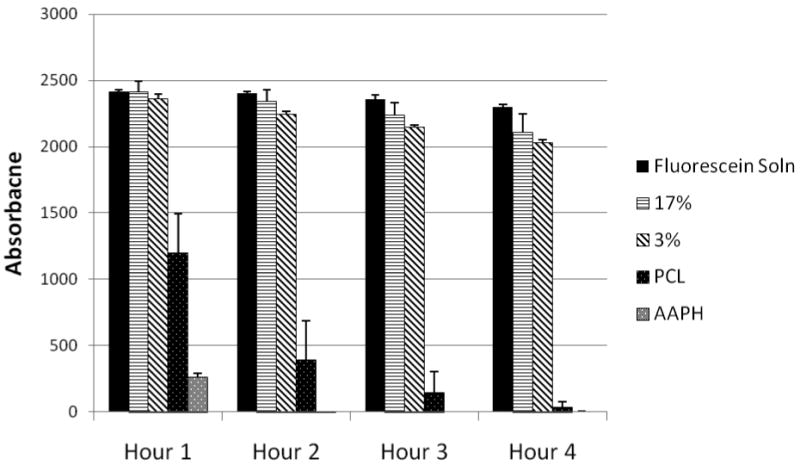
Oxygen Radical Absorbance Capacity (ORAC) assay. Fluorescence quenching plotted as a function of time. Curcumin loaded fiber matrices absorb free radicals and preserve fluorescence better than curcumin free fibers and control with no fibers.
3.4 In Vitro Cytotoxicity Assay
Figure 6 shows the viability of HFF-1 cells cultured directly on curcumin loaded PCL nanofibers and cells cultured on PCL nanofibers. After 48 h of culture on 3 and 17% (wt/wt) curcumin loaded PCL fibers was found to be significantly lower than the PCL fibers. However, more than 70% of the cells were viable on the both curcumin loaded nanofiber matrices indicating their low cytotoxicity.
Figure 6.
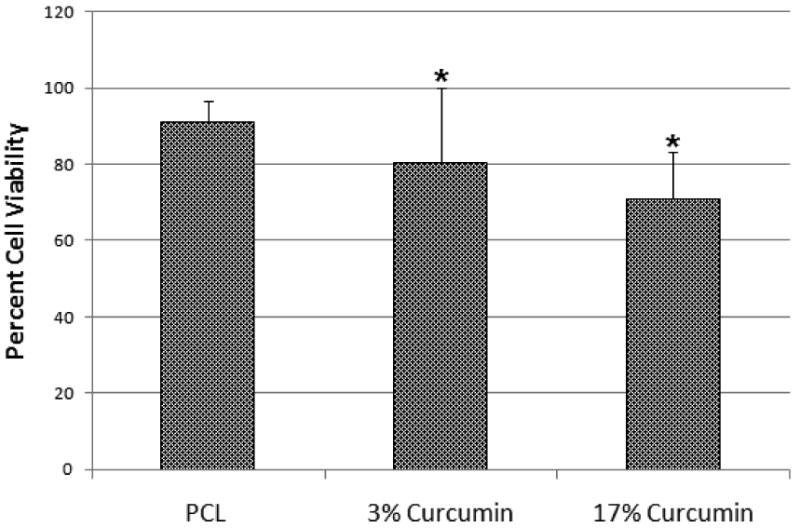
Viability of Human Foreskin Fibroblast (HFF-1) cells cultured on PCL and (3 and 17% (wt/wt)) curcumin loaded PCL nanofibers after 48 h. Viability was significantly reduced (* p ≤ 0.05) though more than 70% of cells were viable on both scaffolds suggesting the low cytotoxicity of curcumin loaded fibers.
3.5 In Vitro Cytoprotective Effect
Curcumin is known to have cytoprotective effects under a variety of conditions [28]. Figure 7 shows the viability of HFF-1cells cultured on tissue culture polystyrene (TCPS), PCL nanofibers, 3%(wt/wt) curcumin loaded PCL nanofibers and 17%(wt/wt) curcumin loaded PCL nanofibers. Cells seeded on TCPS and PCL nanofiber showed a significant decrease in cell viability whereas cells seeded on curcumin loaded nanofibers did not show any significant difference in cell viability after exposing the cells to hydrogen peroxide.
Figure 7.
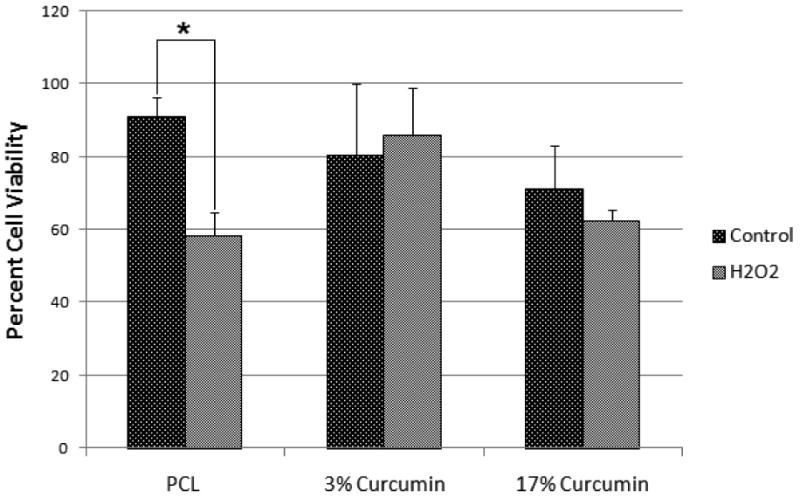
Viability of HFF-1 cells cultured in the presence and absence of hydrogen peroxide on PCL nanofibers, 3 and 17% (wt/wt) curcumin loaded nanofibers. PCL nanofibers lacking curcumin, were significantly less capable (* p ≤ 0.05) of preventing cell death due to oxidative stress.
3.6 In Vitro Anti-Inflammatory Effect
We examined the effect of curcumin loaded PCL nanofibers compared to PCL nanofibers after stimulating mouse peritoneal macrophages with LPS. Figure 8 shows IL-6 expression of the macrophages seeded on PCL nanofibers and curcumin loaded PCL nanofibers. Mouse peritoneal macrophages showed a significantly reduced pro-inflammatory response when seeded on curcumin loaded nanofibers compared to cells on PCL nanofibers. Even without LPS stimulation, cells seeded on curcumin loaded PCL nanofiber matrices exhibited a significantly lower IL-6 release than cells seeded on PCL nanofiber matrices (Figure 8). The amount of curcumin loaded in the fibers also affected the extent of IL-6 release. The 17% (wt/wt) curcumin loaded fibers showed significantly lower IL-6 release compared with 3% (wt/wt) curcumin loaded fibers.
Figure 8.
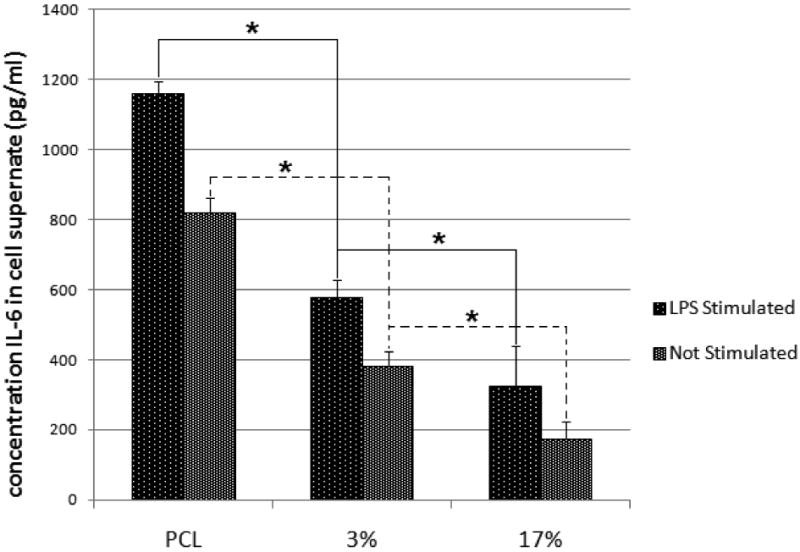
Concentration of interleukin-6 (IL-6) released from lipopolysaccharide (LPS) stimulated macrophage cells seeded on PCL nanofibers, 3%, and 17% (wt/wt) curcumin loaded nanofibers. IL-6 release was significantly reduced (* p ≤ 0.05) due to the presence of curcumin in fibers.
3.7. In Vivo Wound Closure in Diabetic Mice
To evaluate if curcumin loaded nanofibers could enhance the wound closure rate, diabetic mice having impaired wound healing were created by the injection of low doses of streptozotocin. Figure 9 shows the photograph of mouse treated with curcumin loaded PCL nanofibers. The bright yellow color of the dressing is due to the incorporated curcumin. Figure 10 shows the percentage of wound closure represented as a function of time. The mice treated with curcumin loaded PCL nanofibers showed a significant increase in the rate of wound closure compared to those treated with PCL nanofibers. By day 10, mice treated with curcumin loaded PCL nanofibers showed almost 80% wound closure, however, only ∼60% wound closure was achieved in mice treated with PCL nanofibers.
Figure 9.
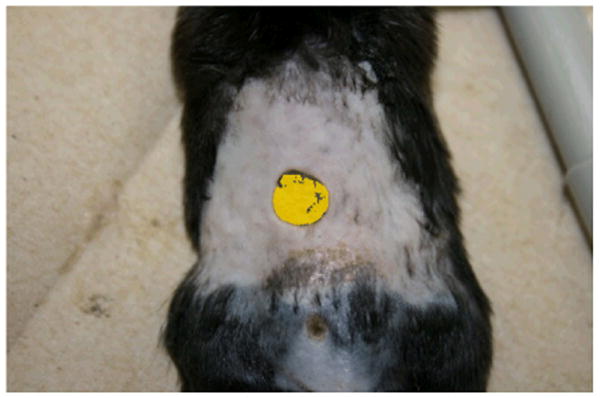
Mouse back punch biopsy model shown with curcumin dressing.
Figure 10.
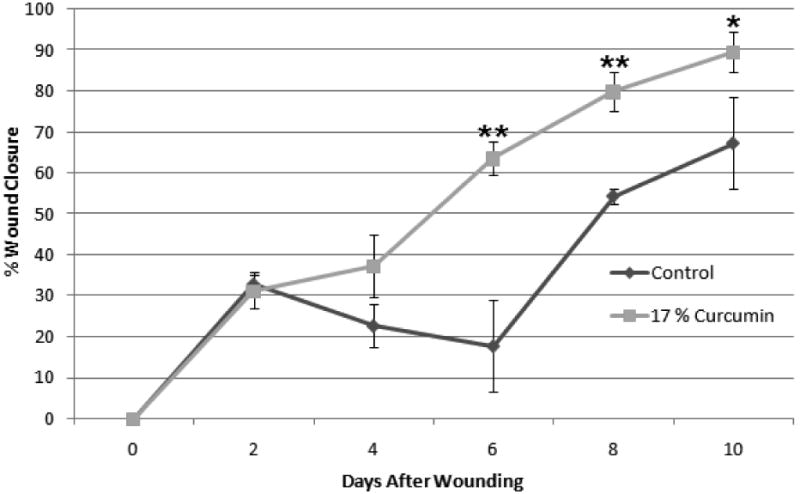
Percent wound closure of diabetic wound as a function of time. Wound closure was significantly higher in curcumin dressing after 6 days (* p ≤ 0.05, ** p ≤ 0.01).
4. Discussion
The aim of this study was to demonstrate the feasibility of developing curcumin loaded PCL nanofibers and evaluate their wound healing activity in vitro and in vivo. Several groups have reported the feasibility of electrospinning PCL from various solvents [27]. In the present study we have used a mixture of chloroform and methanol as the spinning solution, as curcumin is highly soluble in methanol. Many studies have investigated how various electrospinning parameters affect fiber diameter and morphology [19]. We have varied different electrospinning parameters such as polymer concentration, voltage, distance and needle diameter to fabricate bead free nanofibers. Our study showed that high concentration of PCL (∼15% wt/v) is required to fabricate bead free nanofibers under the electrospinning conditions described (an applied voltage of 25kV, tip–to-target distance of 10 cm and a 14 gauge blunt tip needle). A multimodal distribution of fiber diameter was observed in the case of PCL nanofibers with most of the fibers exhibiting diameters in the range of 300-400 nm. The incorporation of curcumin did not significantly change the morphology of the PCL fibers however, fibers with a broader diameter distribution were observed upon curcumin loading. Our previous study using human skin fibroblast cells demonstrated the dependency of fibroblast proliferation and gene expression on fiber diameter. Unlike for osteoblast cells, fibers having diameters in the range of 350-1100 nm were found to be optimal for fibroblast proliferation and matrix deposition [20].
Curcumin is a highly hydrophobic molecule with very low solubility in water. In order to correlate the results of in vitro cell culture studies, we performed in vitro release study of curcumin from curcumin loaded nanofibers in cell culture media. The results were expressed as cumulative micrograms of curcumin released as a function of time up to 72 hours. Curcumin is known to exhibit a wide range of pharmacological effects including anti-inflammatory, antioxidant and anti-carcinogenic activities which is known to be concentration dependent. In a dose dependent manner curcumin can induce apoptosis in a wide variety of cells, down-regulate transcription factors such as nuclear factor-κB [29]. Studies indicate that the cytotoxic effects of curcumin (less than 50% cell survival) are exhibited at only high curcumin concentrations (∼25 μM) [30]. Even in cell culture media supplemented with FBS, a slow release of curcumin was observed from the nanofibers (Figure 4). The good cytocompatibilty of curcumin loaded nanofibers (Figure 6) can be attributed to this very slow release of curcumin from the nanofiber matrix. However, increasing the concentration of curcumin in the fibers showed further decrease in cell viability (17% <3%). Moreover, the cells on 17% (wt/wt) curcumin loaded fibers showed less spreading compared to PCL and 3% (wt/wt) loaded curcumin nanofibers (figure not shown) even though the amount of curcumin released from the fibers is much lower than the cytotoxic concentration. This can be attributed to the increase in concentration of curcumin on the surface of the fibers with increase in curcumin loading. The aqueous stability of curcumin has been studied and it varies with the pH of the solution [31]. It has been reported that the presence of fetal calf serum or human blood can significantly increase the stability of curcumin [32]. The stability of the curcumin in PCL nanofibers after incubation in cell culture media containing FBS needs to be further investigated. The maintenance of chemical integrity of chitosan under the high electrical potential of the electrospinning process was demonstrated earlier [18]. One of the important reasons for the better wound healing property of curcumin is its antioxidant property. Curcumin has been reported to be at least ten times more active as an antioxidant than Vitamin E [36]. The phenolic, and the methoxy group on the phenyl ring and the 1,3 diketone system in curcumin has shown to contribute towards its antioxidant activity [37]. Since the water solubility of curcumin is very low, we qualitatively investigated the antioxidant potential of PCL nanofibers loaded with curcumin using a standard ORAC assay (Figure 5). Even after 4 h of incubation, the FITC solution containing PCL nanofibers loaded with curcumin (3 and 17% w/w) showed very high fluorescence compared to PCL or the positive control. The study indicated that the curcumin loaded nanofibers were effective in quenching the radicals that would have inhibited the fluorescence of FITC. The radical quenching ability of curcumin loaded nanofibers was further demonstrated by evaluating the inhibition of cell death due to oxidative stress induced by hydrogen peroxide (Figure 7) [28]. Another biological activity of curcumin that has been extensively investigated is its ability to inhibit the induction of inflammation. It has been shown that curcumin is able to inhibit the production of proinflammatory cytokines TNF-α and IL-6 in macrophages and also other inflammatory mediators such as COX-2 and iNOS [33-35]. As shown in Figure 8, curcumin loaded PCL nanofibers significantly decreased the IL-6 production by LPS stimulated macrophages indicating its potential to reduce inflammation. This could have beneficial effects while using curcumin loaded nanofibers as wound dressing for treating diabetic wounds which are characterized by persistant inflammation. Chronic inflammation significantly inhibits re-epithelializatoin and wound closure. Curcumin on the other hand has shown to accelerate the re-epithelializatoin of epidermis, stimulate increased migration of various cells including myofibroblasts, fibroblasts and macrophages in the wound bed, and enhance neovascularization and collagen deposition at lower concentration. However, at high concentrations (>25 μM) it has been reported that curcumin will inhibit wound contraction [30]. Our preliminary study has demonstrated that the low concentration of curcumin released from curcumin loaded PCL nanofibers maintains the biological activity of curcumin and is efficient in accelerating the punch wound closure rate in STZ induced diabetic mice compared to PCL nanofibers (Figure 9 and 10).
5. Conclusions
In summary, we have demonstrated the feasibility of developing bead free PCL nanofibers loaded with different concentrations of curcumin by optimizing the electrospinning parameters. The electrospinning of PCL-curcumin solution resulted in the formation of yellow fibers with no visible aggregation of curcumin on the surface. The curcumin loaded nanofibers showed a broader diameter distribution with fibers having diameters in the range of 200-1000 nm. The in vitro release study demonstrated a slow release of curcumin from the curcumin loaded nanofibers. The curcumin loaded fibers exhibited antioxidant property as evident from the ORAC assay. The fibers were found to be cytocompatible and exhibited cytoprotective effect towards human fibroblast cells under a condition of oxidative stress. The anti-inflammatory activity of curcumin loaded nanofibers is evidenced by the lower IL-6 production of macrophages seeded on the fibers compared to PCL fibers. Our result also demonstrated the ability of curcumin loaded nanofibers in enhancing the rate of wound closure in vivo in a diabetic mouse model. Since the biological activity of curcumin is highly concentration dependent, the long term release of curcumin from the fibers and its subsequent biological activity need to be further investigated.
Acknowledgments
This work was supported in part by Coulter Foundation (to Dr. LS Nair) and NIH R01 GM077352, American Heart Association Grant-in-Aid 0855601G, and American Diabetes Association Research Award 7-08-RA-23 (to Dr. AF Chen).
References
- 1.Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- 2.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 3.Liang G, Li X, Chen L, Yang S, Wu X, Studer E, Gurley E, Hylemon PB, Ye F, Li Y, Zhou H. Synthesis and anti-inflammatory activities of mono-carbonyl analogues of curcumin. Bioorganic and Medicinal Chemistry Letters. 2008;18:1525–1529. doi: 10.1016/j.bmcl.2007.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams MD, Nadler JL. Inflammatory mechanisms of diabetic complications. Curr Diab Rep. 2007;7:242. doi: 10.1007/s11892-007-0038-y. [DOI] [PubMed] [Google Scholar]
- 5.Abe Y, Hashimoto S, Horie T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol Res. 1999;39:41–47. doi: 10.1006/phrs.1998.0404. [DOI] [PubMed] [Google Scholar]
- 6.Huang MT, Lysz T, Ferraro T, Abidi TF, Laskin JD, Conney AH. Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer Res. 1991;51:813–819. [PubMed] [Google Scholar]
- 7.Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgene mouse. J Neurosci. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duvoix A, Blasius R, Delhalle S, Schnekenburger M, Morceau F, Henry E, Dicato M, Diederich M. Chemopreventive and therapeutic effects of curcumin. Cancer Letter. 2005;223:181–190. doi: 10.1016/j.canlet.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 9.Panchatcharam M, Miriyala S, Gayathri VS, Suguna L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol Cell Biochem. 2006;290:87–96. doi: 10.1007/s11010-006-9170-2. [DOI] [PubMed] [Google Scholar]
- 10.Sidhu GS, Mani H, Gaddipati JP, Singh AK, Seth P, Banaudha KK, Patnair GK, Maheshwari RK. Curcumin enhances wound healing in streptozotocin induced diabetic rats and genetically diabetic mice. Wound Repair and Regeneration. 1999;7:362–374. doi: 10.1046/j.1524-475x.1999.00362.x. [DOI] [PubMed] [Google Scholar]
- 11.Ireson CR, Jones DJ, Orr S, Coughtrie MW, Boocock DJ, Williams ML, Farmer PB, Steward WP, Gescher AJ. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestive. Cancer Epidemiol Biomarkers Prev. 2002;11:105–111. [PubMed] [Google Scholar]
- 12.Garcea G, Berry DP, Jones DJ, Singh R, dennison AR, Farmer PB, Sharma RA, Steward WP, Gescher AJ. Consumption of the purative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidermiol Biomarkers Prev. 2005;14:120–125. [PubMed] [Google Scholar]
- 13.Kumar V, Lewis SA, Mutalik S, Shenoy DB, Venkatesh Udupa N. Biodegradable microspheres of curcumin for treatment of inflammation. Indian J Physiol Pharmacol. 2002;46:209–217. [PubMed] [Google Scholar]
- 14.Zhang SM, Cao R, Liu J, Liu L, Lu P, Zhou W, Cheng L, Chen P, Luo QM. Fabrication and characterization of biodegradable nanospheres using curcumin. Solid State Phenomena. 2007:121–23. 767–770. [Google Scholar]
- 15.Sou K, Inenaga S, Takeoka S, Tsuchida E. Loading of curcumin in macrophages using lipid based nanoparticles. Int J Pharmaceutics. 2008;352:287–293. doi: 10.1016/j.ijpharm.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 16.Bisht S, Feldmann G, Soni S, Maitra A, Maitra A. Polymeric nanoparticle-encapsulated curcumin (nanocurcumin): a novel strategy for human cancer therapy. J Nano Biotechnology. 2007;5:3. doi: 10.1186/1477-3155-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su SH, Nguyen KT, Satasiya P, Greilich PE, Tang L, Eberhart RC. Curcumin impregnation improves the mechanical properties and reduces the inflammatory response associated with poly(L-lactic acid) fiber. J Biomater Sci, Polym Ed. 2005;16:353–370. doi: 10.1163/1568562053654077. [DOI] [PubMed] [Google Scholar]
- 18.Suwantong O, Opanasopit P, Ruktanonchai U, Supaphol P. Electrospun cellulose acetate fiber mats containing curcumin and release characteristic of the herbal substance. Polymer. 2007;48:7546–7557. [Google Scholar]
- 19.Laurencin CT, Nair LS, editors. Nanotechnology and Tissue Engineering: The Scaffold. CRC Press, Taylor and Francis; Boco-Raton, FL: 2008. [Google Scholar]
- 20.Kumbar SG, Nukavarapu S, James R, Nair LS, Laurencin CT. Electrospun poly(lactic acid-glycolic acid) scaffolds for skin tissue engineering. Biomaterials. 2008;29:4100–4107. doi: 10.1016/j.biomaterials.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pino Eduardo, Campos Ana M, Lopez-Alarcon Camilo, Aspee Alexis, Lissi Eduarde. Free radical scavenging capacity of hydroxycinnamic acids and related compounds. 2006;19:759–764. [Google Scholar]
- 22.Devaraj Sridevi, Vega-Lopez Sonia, Kaul Nalini, Schonlau Frank, Rohdewald Peter, Jialal Ishwarlal. Supplementation with a pine bark extract rich in polyphenols increases plasma antioxidant capacity and alters the plasma lipoprotein profile. 2002;37:931–934. doi: 10.1007/s11745-006-0982-3. [DOI] [PubMed] [Google Scholar]
- 23.Paul Held. Performing Oxygen Radical Absorbance Capacity (ORAC) Assays with Synergy™ HT Multi-Detection Microplate Reader. 2005 [Google Scholar]
- 24.Cao G, Prior RL. Measurement of oxygen radical absorbance capacity in biological samples. Methods Enzymol. 1999;299:50–62. doi: 10.1016/s0076-6879(99)99008-0. [DOI] [PubMed] [Google Scholar]
- 25.Nair LS, Lee D, Bender JD, Barrett EW, Greish YE, Brown PW, Allcock HR, Laurencin CT. Synthesis, Characterization and Osteocompatibility Evaluations of Novel Alanine Based Polyphosphazenes. J Biomed Mater Res. 2006;76A:206–213. doi: 10.1002/jbm.a.30532. [DOI] [PubMed] [Google Scholar]
- 26.Luo JD, Wang YY, Fu W, Wu J, Chen AF. Gene therapy of eNOS and MnSOD restores delayed wound healing in type 1 diabetic mice. Circulation. 2004;110:2484–93. doi: 10.1161/01.CIR.0000137969.87365.05. [DOI] [PubMed] [Google Scholar]
- 27.Fujihara K, Kotaki M, Ramakrishna S. Guided bone regeneration membrane made of polycaprolactone/calcium carbonate composite nanofibers. Biomaterials. 2005;26:4139–4147. doi: 10.1016/j.biomaterials.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 28.Mahakunakorn P, Tohda M, Murakami V, Matsumoto K, Watanabe H, Vajaragupta O. Cytoprotective and cytotoxic effects of curcumin: dual action on hydrogen peroxide induced oxidative cell damage in NG108-15 cells. Biol Pharm Bull. 2003;26:725–8. doi: 10.1248/bpb.26.725. [DOI] [PubMed] [Google Scholar]
- 29.Notoya M, Nishimura H, Woo JT, Nagai K, Ishihara Y, Hagiwara H. Curcumin inhibits the proliferation and mineralization of cultured osteoblasts. Eur J Pharm. 2008;534:55–62. doi: 10.1016/j.ejphar.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 30.Scharstuhl A, Mutsaers HA, Pennings SW, Szarek WA, Russel FG, Wagener FA. Curcumin induced fibroblast apoptosis and in vitro wound contraction are regulated by antioxidants and heme oxygenase” Implications for scar formation. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00339.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernabe-Pineda M, Ramirez-Silva MT, Romero-Romo M, Gonzalez-Vergara E, Rojas-Hernandez A. Determination of acidity constants of curcumin in aqueous solution and apparent rate constant its decomposition. Spectrochim Acta A Mol Biomol Spectrosc. 2004;60:1091–1097. doi: 10.1016/S1386-1425(03)00342-1. [DOI] [PubMed] [Google Scholar]
- 32.Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, Lin JK. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal. 1997;15:1867–1876. doi: 10.1016/s0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- 33.Cho JW, Lee KS, Kim CW. Curcumin attenuates the expression of IL-1 beta and TNF-alpha as well as cyclin E in TNF-alpha treated HaCaT cells; NF-kappaB and MAPKs as potential upstream targets. Int J Mol Med. 2007;19:469. [PubMed] [Google Scholar]
- 34.Chan MM. Inhibition of tumor necrosis factor by curcumin. A phytochemical Pharmacology. 1995;49:1551. doi: 10.1016/0006-2952(95)00171-u. [DOI] [PubMed] [Google Scholar]
- 35.Rao CV. Regulation of COX and LOX by curcumin. Adv Exp Med Biol. 2007;595:213. doi: 10.1007/978-0-387-46401-5_9. [DOI] [PubMed] [Google Scholar]
- 36.Kowluru RA, Kanwar M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr Metab (Lond) 2007;4:8. doi: 10.1186/1743-7075-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jayaprakash GK, Rao LJM, Sakariah KK. Chemistry and biological activities of C. Longa. Trends in Food Sci Tech. 2005;16:533. [Google Scholar]


