Abstract
Rationale: Dendritic cells (DCs) have not been well studied in chronic obstructive pulmonary disease (COPD), yet their integral role in activating and differentiating T cells makes them potential participants in COPD pathogenesis.
Objectives: To determine the expression of maturation molecules by individual DC subsets in relationship to COPD stage and to expression of the acute activation marker CD69 by lung CD4+ T cells.
Methods: We nonenzymatically released lung leukocytes from human surgical specimens (n = 42) and used flow cytometry to identify three DC subsets (mDC1, mDC2, and pDC) and to measure their expression of three costimulatory molecules (CD40, CD80 and CD86) and of CD83, the definitive marker of DC maturation. Spearman nonparametric correlation analysis was used to identify significant correlations between expression of DC maturation molecules and COPD severity.
Measurements and Main Results: Expression of CD40 by mDC1 and mDC2 and of CD86 by mDC2 was high regardless of GOLD stage, but CD80 and CD83 on these two DC subsets increased with disease progression. pDC also showed significant increases in expression of CD40 and CD80. Expression of all but one of the DC molecules that increased with COPD severity also correlated with CD69 expression on lung CD4+ T cells from the same patients, with the exception of CD83 on mDC2.
Conclusions: This cross-sectional study implies that COPD progression is associated with significant increases in costimulatory molecule expression by multiple lung DC subsets. Interactions with lung DCs may contribute to the immunophenotype of CD4+ T cells in advanced COPD.
Clinical trial registered with www.clinicaltrials.gov (NCT00281229).
Keywords: human, flow cytometry, B70 costimulatory molecules, CD69 antigen, CD4+, T lymphocytes
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Dendritic cells (DCs) are necessary to activate and induce differentiation of naive and effector T cells in response to antigens. However, the few studies that have addressed the involvement of lung DCs in chronic obstructive pulmonary disease (COPD) have found conflicting answers and have not examined maturational state as a function of disease stage.
What This Study Adds to the Field
DC subsets from human lungs showed increased costimulatory molecule expression that correlated with COPD severity and was not explained by smoking alone. Lung DCs colocalized with CD4+ T cells, and expression of DC activation markers correlated with expression of CD69, an early and transient marker of T-cell activation, on CD4+ T cells from the same patients.
It is crucial to improve understanding of the pathogenesis of chronic obstructive pulmonary disease (COPD), which is the fourth leading cause of death in the United States. COPD is associated with a chronic and augmented inflammatory response, predominantly initiated in susceptible individuals by cigarette smoking. This inflammatory response is characterized by an increased number of macrophages, neutrophils, and lymphocytes in the lungs (1). The increasing infiltration of the small airways by CD4 and CD8 T cells (2) suggests that dendritic cells (DCs), which are necessary for inducing activation and differentiation of naive and effector CD4+ and CD8+ T cells in response to antigens, may also be involved in COPD pathogenesis (3). Indeed, we have previously shown that the expression of T-cell–tropic chemokines by lung DCs increases with worsening COPD severity (4). However, few studies have sought to address the involvement of DCs in COPD, and existing studies have found different answers. Increased numbers of DCs were found in the epithelium and adventitia of small airways in patients with COPD in one study (5), whereas another study found that the number of bronchial mucosal DCs in patients with COPD was similar to or lower than the number of DCs in nonsmoking healthy control subjects (6). In murine model systems, cigarette smoke exposure has been shown to increase numbers of lung Langerhans-type DCs (identified morphologically in tissue section) by 16 weeks (7) and of conventional pulmonary DCs (defined by coexpression of CD11c and class II MHC by flow cytometry) by 24 weeks (8). However, the opposite finding (i.e., decrease in numbers of similarly defined pulmonary DCs after 8–16 weeks of smoke exposure) has also been reported (9).
It is also unclear what functional role DCs play in disease pathogenesis. It is known from experimental systems that immature DCs, which have low costimulatory molecule expression and high antigen-uptake activity, are recruited to the lungs from the circulation to capture antigens for presentation to T cells. After antigen uptake, DCs begin a process of maturation that involves up-regulation of MHC Class II (HLA-DR) and of adhesion and costimulatory molecules (10). DCs isolated from bronchoalveolar lavage fluid from human smokers with virtually normal pulmonary function had evidence of increased DC maturation, as compared with nonsmokers (11). By contrast, DC maturation was suppressed in cigarette smoke–exposed mice, resulting in diminished antigen-specific T-cell proliferation (12). No studies have examined the maturation status of human lung DCs as a function of COPD severity (13).
In the present study, we examined phenotypic markers of maturation of three human pulmonary DC subsets: myeloid DC type 1 (mDC1), myeloid DC type 2 (mDC2), and plasmacytoid DCs (pDCs). We analyzed the cell surface expression of CD40, CD80, CD83, and CD86, all markers of DC maturity. We found that all three DC subsets showed evidence of increased costimulatory molecule expression, which correlates positively with disease severity. Furthermore, expression of the DC activation markers correlated with expression by lung CD4+ T cells from the same patients of the early and transient marker of T-cell activation, CD69 (14). These findings suggest that in later stages of COPD, an increase in the antigen-presenting and immunomodulatory capacity of lung DCs may contribute to an inappropriate and prolonged local immune response.
METHODS
Specimens and Subject Populations
The majority of experiments were performed on specimens obtained from consented patients undergoing clinically indicated resection procedures for pulmonary nodules, lung volume reduction surgery, or lung transplantation at the University of Michigan Healthcare System and the VA Ann Arbor Healthsystem. Studies and consent procedures were approved by Institutional Review Boards. This study was registered with ClinicalTrials.gov as NCT00281229. Only nonneoplastic lung tissue remote from the nodules and lacking postobstructive changes was collected. The study population comprised 42 subjects, all of whom underwent preoperative spirometry and clinical evaluation by a pulmonologist. To categorize subjects based on disease severity, we used the 2008 classification system of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) (15, 16). Consistent with the most recent GOLD criteria, only subjects who had an FEV1/FVC ratio of less than 0.70 were included in GOLD stages 1 through 4. Subjects (n = 11) with a history of smoking, an FEV1/FVC ratio greater than 0.70, normal spirometry, and no clinical diagnosis of COPD represent our smoking control subjects (S). Subjects with no history of smoking and normal spirometry (n = 3) comprised the nonsmoking control group (NS). Table 1 shows the number of subjects, gender ratio, age ranges age, smoking history, spirometry, and emphysema scores for each subject group. Emphysema scores could not be calculated in all subjects. CT scans that were not suitable for quantification were available in the remaining four subjects in the smoking control (S) group. Two subjects showed no emphysema, and there was mild apical emphysema in the other two subjects.
TABLE 1.
SUMMARY OF SUBJECT DEMOGRAPHICS, SMOKING HISTORIES, SPIROMETRY, AND EMPHYSEMA SCORES
| Smoking history (pack-years) | Emphysema |
||||||
|---|---|---|---|---|---|---|---|
| Group | Subjects (n)* | Sex ratio (M/F) | Age (yr) | FEV1, % predicted | n† (%) | SD | |
| NS | 3 | 0/3 | 59 (6)‡ | 0 (0) | 91 (5) | 3 (0.6) | 0.3 |
| S | 11 | 8/3 | 68 (11) | 43 (54) | 98 (19) | 7 (5.2) | 3.2 |
| GOLD stage | |||||||
| 1 | 6 | 3/3 | 63 (8) | 33 (130) | 89 (12) | 4 (5.9) | 6.2 |
| 2 | 10 | 8/2 | 61 (5) | 59 (27) | 67 (7) | 7 (6.8) | 12.9 |
| 3 | 5 | 4/1 | 64 (12) | 50 (25) | 44 (4) | 3 (4.3) | 3.8 |
| 4 |
7 |
3/4 |
57 (9) |
38 (22) |
22 (15) |
4 (35.1) |
9.9 |
Definition of abbreviations: NS = nonsmoking control group; S = smoking control group.
Number of subjects for all measures except emphysema score.
Number of subjects for emphysema score.
Numbers in parentheses are SD unless otherwise noted.
Immunohistochemical experiments were performed on frozen lung tissue blocks obtained from the Lung Tissue Research Consortium. This NHLBI-sponsored tissue bank (http://www.ltrcpublic.com) preoperatively collects extensive demographic, physiological, and radiographic information, which is deidentified and available, along with various types of preserved tissue samples, to qualified investigators.
Sample Preparation and Flow Cytometric Analysis
Lung sections weighing approximately 3 g were minced and thoroughly rinsed in RPMI to reduce contamination by blood and homogenized using a Waring blender without enzyme treatments, which we have previously shown efficiently produces single-cell suspensions of high viability (4). Cells were filtered through a 70-μm strainer to remove debris and were resuspended at 10 × 106 cells/ml of staining buffer (2% FBS in PBS). Cells were incubated at 4°C for 10 minutes and added in a volume of 100 μL to each flow tube. We used monoclonal antibodies against the following antigens (clones shown in parentheses): CD45 (HI30), CD3 (UCHT1), CD19 (HIB19), HLA-DR (LN3), CD123 (6H6), CD40 (LOB7/6), CD80 (2D10.4), CD83 (HB15e), CD86 (IT2.2), CD4 (OKT4), CD69 (FN50) (eBioscience, San Diego, CA), BDCA-1 (CD1c; AD5-8E7), BDCA-2 (CD303; AC144), and BDCA-3 (CD141; AD5-14H12) (Miltenyi Biotec). Appropriate isotype-matched controls were used in all experiments. Antibodies were conjugated to fluorescein isothiocyanate, phycoerythrin (PE), phycoerythrin-cyanine 5 (PE-Cy5), phycoerythrin-cyanine 7 (PE-Cy7), allophycocyanin (APC), or biotin, with the biotinylated antibodies developed using streptavidin-allophycocyanin-cyanine 7 (SA-APC-Cy7). Cells were incubated in the dark with primary antibodies and secondary reagents for 25 minutes each at room temperature, with washing between incubations. Cells were analyzed on an LSR II flow cytometer (BD Bioscience, San Jose, CA) equipped with 488-nm blue, 405-nm violet, 633-nm red lasers. Data were collected using FACS Diva software with automatic compensation and were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR). Details of the gating strategy are described in the Results sections. A minimum of 10,000 CD45+ events were collected per sample.
Immunohistochemical Staining
Blocks of frozen tissue were sectioned onto glass slides using a Reichert Jung Cryostat 1800. Slides were brought to room temperature and placed in cold acetone for 10 minutes, followed by immersion in methanol plus hydrogen peroxide for 15 minutes and PBS for 5 minutes. The slides were incubated in normal mouse serum for 20 minutes, which is removed by blotting, and then biotinylated antihuman BDCA-1, BDCA-2, BDCA-3 (Miltenyi Biotec) or appropriate isotype control antibodies were added for 1 hour. After a 5-minutes wash in PBS, slides were incubated with Universal ABC peroxidase complex (Vector Laboratories, Inc., Burlingame, CA) for 30 minutes and washed in PBS for 5 minutes, followed by 3-amino-9-ethylcarbazole peroxidase substrate for 10 minutes. For double-stained slides, this protocol was followed by a second incubation in normal mouse serum for 20 minutes, then in biotinylated antihuman CD4 (BD Pharmingen) or isotype control for 1 hours. After washing in PBS, alkaline phosphatase complex (Vector Laboratories) was added for 30 minutes, followed by alkaline phosphatase substrate for 10 minutes. Single- and double-stained slides were counterstained with hematoxylin for 3 minutes, rinsed in tap water, and mounted with a coverslip using aqueous mounting medium (Gel/Mount; Biømeda, Foster City, CA). Photomicrographs were obtained using an Olympus BX51 digital camera system.
Analysis of Radiographic Images
Noncontrast enhanced CT images of the chest were analyzed for percent emphysema using 3D Slicer software (www.airwayinspector.org) and using a threshold of less than −950 Hounsfield units.
Statistics
Initial statistical analysis was performed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA). One-way analysis of variance, with Dunn's post-hoc testing, was used to determine statistical differences between frequencies of individual DC subsets in the entire group of subjects. Nonparametric (Spearman) correlation analysis was used to determine the correlation coefficient. A two-tailed P value less than 0.05 was considered to indicate significance. Nonparametric Kruskal-Wallis and Mann-Whitney U tests with Bonferroni corrections were performed using SAS 9.1 statistical software (Cary, NC) to look for significant differences between subject groups. A similar approach was used to examine differences between smoking status groups (never-smoker, ex-smoker, active smoker). Regression analysis was used to examine the relationship of smoking status and smoking exposure (pack-years, time since quitting) to the relationship between FEV1 % predicted (continuous or categorically defined) and expression of markers by DCs or CD4+ T cells.
RESULTS
mDC2s Are the Predominant DC Subset in Human Lung Tissue Regardless of COPD Severity
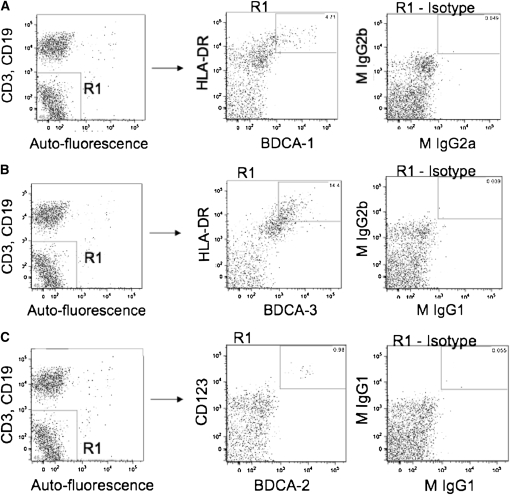
To assess accurately the surface molecule expression of individual subsets of pulmonary DCs, we homogenized lung tissue from consented patients, and, without additional purification steps, used immunofluorescence staining and multiparameter flow cytometry on the entire lung leukocyte population. To identify mDC1, mDC2, and pDC subsets among this mixture of lung leukocytes, we used blood dendritic cell antigen (BDCA) markers as demonstrated by Demedts and colleagues (17). Although we had previously used CD1a to identify DCs in human lung tissue (4), we chose not to in this study because there is concern that CD1a does not identify a unique DC subset (17). Using a procedure similar to the “third gating strategy” of Demedts and colleagues (17), we gated on CD45+ cells and then excluded CD3+, CD19+, and high auto-fluorescent cells. This step eliminated T cells, B cells, and alveolar macrophages. To identify the mDC1 population, we selected cells that were double positive for HLA-DR and BDCA-1 (CD1c), whereas mDC2 cells were identified as being double positive for HLA-DR and BDCA-3 (CD141). To identify pDC, we selected cells that were CD123+ and BDCA-2 (CD303)+. Hence, each DC subset was defined by two receptors, including class II major histocompatibility antigens for both mDC subsets; this procedure has been shown to exclude monocytes and immature DCs (17).
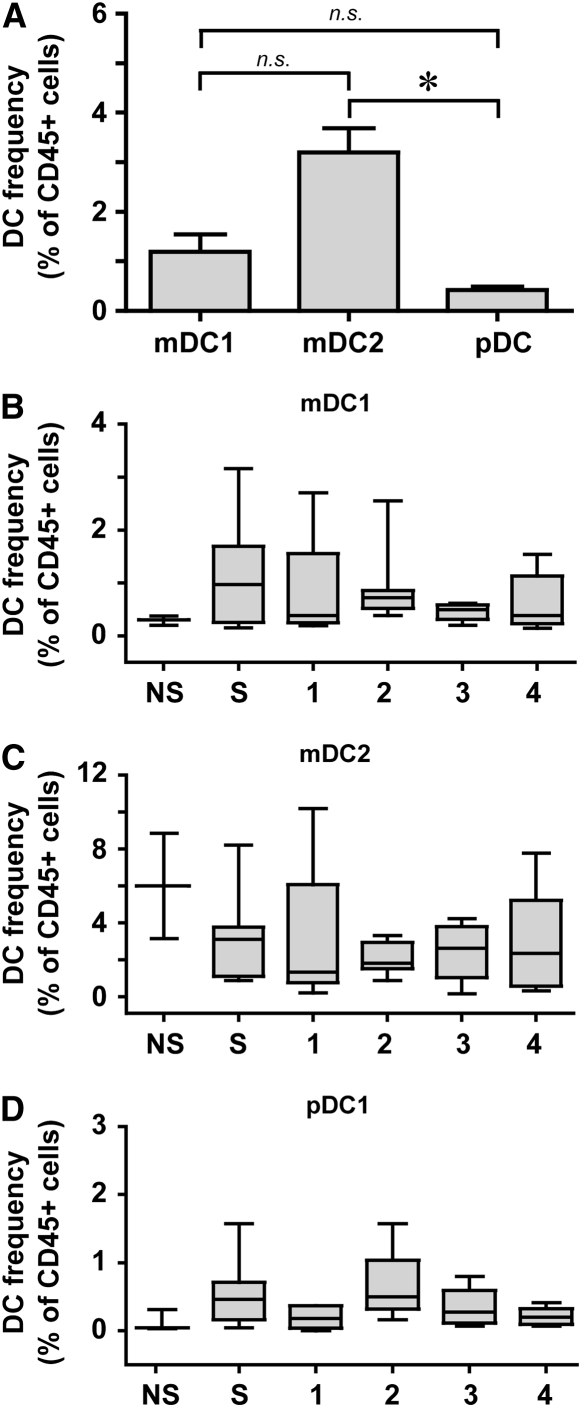
By means of this gating strategy (Figure 1), we successfully identified all three DC subsets in the resected lung tissue of all of our subjects (n = 42). The most abundant DC subset was mDC2 cells, at approximately 3.2% of all lung leukocytes (Figure 2A). The other DC subsets, mDC1 cells at approximately 1.1% and pDC at less than 0.5%, were also found in all subjects. When we examined the relationships between the frequency of DC subsets in individual subjects, there were highly significant correlations between mDC1 and pDC (r = 0.676; P < 0.0001) and between mDC2 and pDC (r = 0.357; P = 0.022) but not between the two mDC subsets (r = 0.0999; P = 0.5344). No statistically significant differences in the frequency of any lung DC subset were seen between individual GOLD stages, smokers without COPD, or never-smokers (Figures 2B−2D). Additional exploratory analyses showed no significant correlations of DC frequency among lung leukocytes with total smoking exposure or duration since cessation of smoking.
Figure 1.
Gating strategy used to identify dendritic cell (DC) subsets among human lung leukocytes. Lung tissue was dispersed nonenzymatically and was stained with monoclonal antibodies to identify DC subsets. After gating on CD45+ cells (left column), cells that had low autofluorescence and that were CD3− and CD19− were selected (gate R1). From within gate R1, we then selected cells (middle column) that were (A) BDCA-1+ and HLA-DR+ (mDC1), (B) BDCA-3+ and HLA-DR+ (mDC2), or (C) BDCA-2+ and CD123+ (pDC). Isotype controls are shown in the right column. Representative data are from individual subjects.
Figure 2.
Myeloid DCs are more prevalent in human lungs than plasmacytoid DCs. (A) Frequency of DC subsets in entire group (n = 42), determined as a percent of CD45+ cells. Data represent the mean ± SEM. * P < 0.001 (one-way analysis of variance, with Dunn's post hoc testing). mDC1 = myeloid dendritic cell type 1; mDC2 = myeloid dendritic cell type 2; n.s.,= nonsignificant; pDC = plasmacytoid dendritic cell. (B–D) Frequency of DC subsets stratified by group. (B) mDC1. (C) mDC2. (D) pDC. NS = neversmokers; S = smokers without COPD; 1–4, GOLD stages. Box and whisker plots show the median (central bar), interquartiles (boxes), and range (whiskers) of the data; note differences in scales. There were no significant differences between groups (nonparametric Kruskal-Wallis and Mann-Whitney U tests).
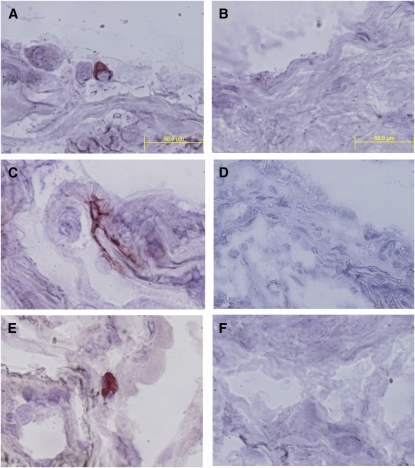
Because these small lung samples, predominantly from distal lung parenchyma, could not be perfused during sample preparation, it was possible that some of the DCs in these samples might reside within pulmonary vasculature. To verify the presence of lung DCs within the lung interstitium, in separate experiments we used frozen samples of lung tissue from a different source to perform immunohistochemical staining for all three DC subsets, using the same clones of anti-BCDA monoclonal antibodies (Figure 3). Results confirmed the presence of mDC2 with typical stellate morphology (Figure 3A), whereas mDC1 and pDC were ovoid cells without elaborate processes (Figures 3B−3C). These results, and the absence of DCs within pulmonary vessels in any section examined (not shown), implied that the majority of DCs were within the lung interstitium and airways.
Figure 3.
All three DC subsets can be identified in lung interstitium in chronic obstructive pulmonary disease (COPD). Frozen tissue sections were stained with antibody against (A) BDCA1 (mDC1), (C) BDCA-3 (mDC2), (E) BDCA-2 (pDC), or (B, D, F) the respective isotype control antibodies. The immunoperoxidase reaction was visualized using 3-amino-9-ethylcarbazole substrate (red) with hematoxylin counterstaining. Magnification: 1,000×, scale = 50 μm.
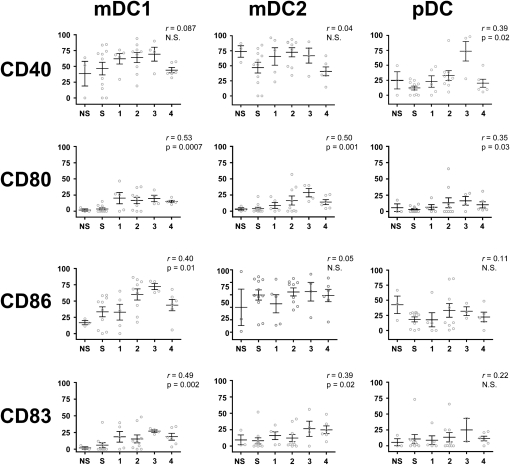
Percentage of Activated DCs Increases with COPD Severity
Although our study used a small number of cells, our flow cytometric staining strategy was sufficiently sensitive to ascertain the percentage of DCs expressing CD40, CD80, CD83, or CD86 in all subjects (Figure 4). CD40, a member of the tumor necrosis factor receptor (TNFR) superfamily (also known as TNFR5), is frequently used to verify DC activation because it is one of the most rapidly up-regulated costimulatory molecules (18). Approximately 50% of mDC1 and mDC2 cells from never smokers, smokers without COPD, and patients from all GOLD stages expressed CD40, without significant differences between groups (Figure 4, first row). This relationship was not influenced by smoking status. However, expression of CD40 on pDC, which was low at early GOLD stages, rose markedly in GOLD stage 3 subjects before falling in GOLD 4. This correlation was significant (P < 0.02; r = 0.39) and persisted after adjustment for age and gender.
Figure 4.
Activation markers on DC subsets increase in correlation with disease severity. Using flow cytometry, expression of CD40, CD80, CD86, and CD83 on mDC1 (left column), mDC2 (middle column), and pDC (right column) subsets was determined and correlated to GOLD stage. The frequency (as percentage of positive cells) of a given marker is shown on the vertical axis, and subject group is shown on the horizontal axis, NS = never-smokers; S = smokers without COPD. Spearman nonparametric correlation analysis generated an r value that was used to determine significance. Open circles represent individual patients. Bars represent the mean ± SEM.
CD80 and CD86, also known as B7-1 and B7-2, respectively, are costimulatory ligands expressed on the surface of DCs. Binding of these ligands to T-cell receptors such as CD28 is essential for T-cell activation. In all three lung DC subsets, CD80 was expressed by only a small percentage of DCs from never-smokers and smokers without COPD but increased to approximately 20% of DCs by GOLD stage 4 (Figure 4, second row). Thus, there was a significant correlation of CD80 expression with increasing COPD severity, with r values of 0.53 for mDC1, 0.50 for mDC2, and 0.35 for pDC. Where appropriate to test, the direction of the correlation did not vary when adjusted for age and gender. By contrast, CD86 expression (Figure 4, third row) was higher at baseline, at over a quarter of subjects for mDC1 and pDC, and especially on mDC2, where the majority of subjects showed CD86 expression. A significant change in CD86 expression with COPD severity was seen only on mDC1 cells (r = 0.40). The strength of this relationship persisted after adjustment for age and pack-years.
We also examined expression of CD83, a molecule of unknown function that appears to be a specific marker for mature human DCs (19). Similar to CD80, never-smokers and smokers without COPD tended to express little to no CD83 on any of the DC subsets (Figure 4, fourth row). However, CD83 expression showed a slight increase, which correlated with COPD severity on mDC1 and mDC2 subsets (r = 0.49 and 0.39, respectively), but showed no significant change on pDC. The strength of this relationship persisted after adjustment for age.
Kruskal-Wallis nonparametric analyses confirmed that four of the activation markers exhibited significant differences between COPD severity groups; these were CD80 (P = 0.022) and CD86 (P = 0.010) expression on mDC1, CD80 (P = 0.035) on mDC2, and CD40 (P = 0.0008) on pDC. Further analyses confirmed significant differences between subject groups. CD80+ mDC1 showed significant differences between never-smokers and GOLD stage 3 (P = 0.034) and GOLD stage 4 (P = 0.001) and between smokers without COPD and all GOLD stages (P = 0.016 for GOLD 1; P = 0.014 for GOLD 2; P = 0.001 for GOLD 3; and P = 0.002 for GOLD 4). CD86+ mDC1 showed significant differences between never-smokers and GOLD stage 2 (P = 0.02) and GOLD stage 3 (P = 0.0003). Similarly, smokers without COPD showed significant differences in expression of CD86 on mDC1 with GOLD stage 2 (P = 0.03) and GOLD stage 3 (P = 0.01). CD80 expression on mDC2 was significantly different between never-smokers and GOLD stage 3 (P = 0.02) and between smokers without COPD and GOLD stage 3 (P = 0.0003) and GOLD stage 4 (P = 0.02). CD40 expression on pDC was only significantly different between smokers without COPD and GOLD stage 2 (P = 0.02) and GOLD stage 3 (P = 0.0001).
We further analyzed whether the levels of expression of CD40, CD80, CD83, and CD86 within a given DC subset would correlate with each other on cells from individual patients, using pair-wise comparisons between individual markers. With the exception of the relationship between CD40 and CD83 on the mDC1 (r = 0.301; P = 0.067), we found that all the markers showed highly significant correlations (mean r = 0.533), with most comparisons significant at the P < 0.001 level. This concordance favors the interpretation that the maturation markers are being regulated in a parallel fashion on individual lung DCs, although the eight-parameter flow cytometry used in this study did not directly compare the simultaneous expression of all four maturation markers on single DCs.
We also sought possible correlations between the levels of maturation marker expression and total smoking exposure or duration since cessation of smoking. The only significant correlation was between CD86 expression on mDC1 and pack-years (r = 0.39; P = 0.02). Collectively, these data showing increased expression of multiple activation markers imply that DCs in the distal lungs have a more mature phenotype in later stages of COPD and that these change do not reflect the effect of smoking alone.
Lung DC Activation Markers Correlate with Expression of CD69+ by Lung CD4+ T Cells
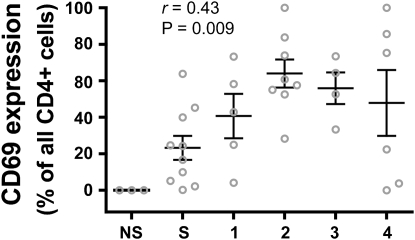
To ascertain whether the increased costimulatory molecule expression by lung DCs was associated with a similar increase in lung T-cell activation, we examined the expression of CD69 on CD4+ T cells from lungs of the same subjects. CD69 is an acute marker of activation that appears to be the earliest cell surface molecule induced during lymphocyte activation. The percentage of lung CD4+ T cells expressing CD69 correlated with COPD severity (r = 0.43) (Figure 5), a novel finding in this disease. Analysis of individual groups revealed statistically significant differences between never-smokers and GOLD stages 1 (P = 0.046), 2 (P = 0.0008), and 3 (P = 0.003) and between smokers without COPD and GOLD stages 2 (P = 0.001) and 3 (P = 0.017). Additional analyses showed no significant correlation between CD4+ CD69+ cells and age, gender, or duration since cessation of smoking. However, there was a correlation between CD69 expression on CD4+ T cells and pack-years (r = 0.48; P = 0.004). Furthermore, smoking status appeared to have a significant effect on CD69 expression by lung CD4+ T cells, as determined by Kruskal-Wallis testing (P = 0.015). Mean CD69 expression was 0% for never-smokers (n = 3), 39.3% for ex-smokers (n = 25), and 65.5% for active smokers (n = 4). CT scans of the chest were available and suitable for analysis on 27 subjects. There was no correlation of % emphysema and expression of CD69 by lung CD4+ T cells or with the frequency of individual DC subsets or their activation markers (data not shown).
Figure 5.
CD69 expression on lung CD4+ T cells correlates with COPD severity. Surface expression of CD69 on lung CD4+ T cells was determined by flow cytometry and is expressed as the percentage of all lung CD4+ T cells on the vertical axis; subject groups are shown on the horizontal axis. NS = never-smokers; S = smokers without COPD. Spearman nonparametric correlation analysis is shown. Open circles represent individual patients. Bars represent the mean ± SEM.
When we examined the correlation among individual patients between the percentage of lung CD4+ T cells expressing CD69 and lung DC expression of activation markers (Table 2), we found that almost all the same activation markers that had shown significant correlations with disease severity (Figure 4) correlated significantly with CD69+ expression by lung CD4+ T cells. The single exception was CD83 on the mDC2 subset, which had shown a weak (r = 0.39) but significant (P = 0.02) correlation with GOLD stage but which did not correlate with lung CD4+ T-cell expression of CD69. Collectively, these results show a strong association in patients with advanced COPD between markers of pulmonary DC maturity and evidence of acute activation of lung CD4+ T cells.
TABLE 2.
SUMMARY OF CORRELATION BETWEEN PERCENTAGE OF LUNG CD4+ T CELLS EXPRESSING CD69 AND LUNG DENDRITIC CELL EXPRESSION OF ACTIVATION MARKERS
| DC Subset | Activation Marker | r Value | P Value |
|---|---|---|---|
| mDC1 | CD40 | 0.25 | ns |
| CD80 | 0.65 | <0.0005 | |
| CD86 | 0.55 | <0.0005 | |
| CD83 | 0.62 | <0.0005 | |
| mDC2 | CD40 | 0.25 | ns |
| CD80 | 0.57 | <0.0005 | |
| CD86 | 0.27 | ns | |
| CD83 | 0.21 | ns | |
| pDC | CD40 | 0.51 | <0.005 |
| CD80 | 0.47 | <0.005 | |
| CD86 | 0.28 | ns | |
| CD83 |
0.31 |
ns |
Definition of abbreviations: DC = dendritic cell; mDC1 = myeloid dendritic cell type 1; mDC2 = myeloid dendritic cell type 2; ns = not significant; pDC = plasmacytoid dendritic cell.
Immunohistochemical Staining of Lung Tissue Reveals Interactions between DCs and CD4+ T Cells
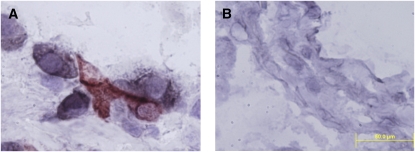
To investigate the interaction of CD4+ T cells with mDC2 cells, the most numerous lung DC subset in all COPD stages (see Figure 2), we performed double-staining experiments on frozen lung tissue (Figure 6). Results demonstrated direct contact between the two cell types, thereby strengthening our conclusion that lung DCs may contribute to the phenotype of CD4+ T cells in COPD.
Figure 6.
Lung mDC2 interact physically with lung CD4+ T cells. Frozen lung tissue sections were stained with antibodies against (A) BDCA-3 (immunoperoxidase/3-amino-9-ethylcarbazole, red) and CD4 (alkaline phosphatases, black) or (B) isotype control. Magnification: 1,000×; scale = 50 μm.
DISCUSSION
This is the first study to examine systematically maturation molecules on pulmonary DCs from patients with COPD. Results reveal that on all three lung DC subsets, CD80 expression increased with worsening COPD severity, as assessed by GOLD stage. In addition, expression of CD83 and CD86 on mDC1 cells, of CD83 on mDC2 cells, and of CD40 on pDC increased significantly with disease severity. Several of the exceptions to this trend toward increased DC maturation molecule expression appear to be explained in part by relatively high expression in all GOLD stages (e.g., CD40 on mDC1 and mDC2 and CD86 on mDC2). Other exceptions may relate in part to differences in the kinetics of costimulatory molecule expression. CD86 is rapidly up-regulated and maximally expressed immediately after interaction with CD28 (20), whereas CD80 is slowly induced and stable for a longer period (21–23). This difference in kinetics might explain why later stages of COPD were associated with an increase in CD80 expression for all three DC subsets but with an increase in CD86 expression only on the mDC1 subset. Furthermore, we demonstrated that the identical DC maturation molecules showing disease stage–specific increases correlated with expression of the acute activation marker CD69 on lung CD4+ T cells from individual patients. Our results complement and extend previous studies of human lung DCs (5, 6, 11, 17, 24–26) due to the size of our patient group (n = 42) and our analysis of patients with a range of COPD severities, including never-smokers and smokers without COPD. Although we have a small sample size of never-smokers, this is a critical population for which little data exist. Based on the accumulation of follicle-forming T and B lymphocytes in the lung parenchyma in severe COPD (2), the disease has come to be viewed as an inflammatory condition that progresses from initial involvement of the innate immune system to later participation of adaptive immunity (27). Additionally, recent evidence that antielastin and antiendothelial cell antibodies might contribute to COPD pathology (28, 29) support the hypothesis of an autoimmune component to at least some cases of advanced COPD (30). Thus, studying lung DCs, key immunoregulatory cell types, and their interactions with other lung leukocytes is a promising direction to better understand COPD pathogenesis.
DCs comprise a highly heterogeneous population of ultimately hematopoietically derived cells that inhabit lymphoid and nonlymphoid tissues (31). The terminologies applied to DC lineages differ somewhat between the human and mice. In humans, the major DC population found in nonlymphoid tissues such as lung (17, 19, 32) continues to be called myeloid (mDC), but in mice, the preferred term is increasingly conventional DC because it was recognized that this cell type derive from both myeloid and lymphoid precursors (33). In both species, an independent plasmacytoid lineage (pDC) specialized for type-1 IFN production is recognized (34). The balance between total numbers of conventional DCs and pDCs has been suggested to be crucial for maintaining tolerance to inhaled antigens (35).
Existing at the interface between innate and adaptive immunity, DCs are essential for immune function not only as the most effective activators of naive CD4+ T cells but also because their ability to sense environmental cues may allow them to dictate to the T cell the type of response necessary to counteract a particular antigen. Mainstream views of DC biology have highlighted the CCR7-dependent migration of immature DCs after exposure to antigen in combination with appropriate danger signals (i.e., inflammatory cytokines or pathogen-associated molecular patterns) to secondary lymphoid tissues (36, 37). There, the newly mature DCs present processed antigen along with costimulatory molecules, inducing the proliferation of antigen-specific CD4+ T cells. Furthermore, based on the environment in which they were initially activated, DCs are able to direct the fate of CD4+ T cells (38). DC-derived cytokines, such as IL-12, IL-18, and IFN-α, can bias CD4+ T-cell priming toward a proinflammatory T helper 1 cell fate, whereas selective expression by DCs of members of the Jagged family of Notch ligands is thought to promote T helper 2 cell responses (39). Thus, one uncontested role of DCs is ultimately mediated in the lymph nodes. This role appears to be important for surveillance of the airways (40, 41). However, it has become evident that some experimental pulmonary immune responses and infections are associated with recruitment from the bloodstream and accumulation within lung parenchyma of large numbers of DCs that do not appear to migrate to lymph nodes (42–45). Such findings imply that potential roles of DCs within lung parenchyma and independent of migration to the regional nodes (39) also need to be considered in COPD.
The markers we used to identify DCs (BDCA-1, BDCA-2, and BDCA-3) were first used to stain peripheral blood DCs but were later shown to be present on DCs in human lungs (17). Our data on the relative frequency of specific DC subsets agrees closely with the findings by Demedts and colleagues, but our results for expression of maturation molecules on pDCs do not. They reported that lung pDCs have almost no expression of maturation markers, whereas, in our study, pDCs showed expression of maturation markers, albeit at lower levels than mDCs. This disparity could be due to the different methods used to generate single-cell suspensions from the lung tissue because DC activation molecules are reportedly sensitive to enzymatic digestion, gradient centrifugation, and overnight incubation (19). Because we used only mechanical means to disaggregate the tissue, and stained DC immediately, we are confident that DC surface markers were not affected by our procedure. Our results also contradict recent data of Tsoumakidou and colleagues (46), who found using immunohistochemical analysis that the volume of DCs expressing CD83 as a percentage the surface area of small airway (by point-counting) and alveolar walls (semiquantitatively) was lower in subjects with COPD than in subjects without COPD. This disparity in results likely relates to the considerable difference in the amount of lung tissue sampled and possibly by relative sensitivities of the two techniques. We also considered the possibility (suggested by Marsten and colleagues [25]) that our staining strategy underestimated lung pDCs because BDCA-2, which is highly specific for pDCs in human peripheral blood (47), is down-regulated as pDCs mature. Because the percentage of BDCA-2+ cells remained the same at all GOLD stages, despite increases at later stages in expression of CD40 and CD80 that imply greater pDC maturity, we suspect that such is not the case but cannot exclude that possibility. We have begun experiments simultaneously using greater numbers of antibodies that will eventually address possible underestimation of lung pDCs.
One advantage of our flow cytometric methodology was that we were able to gather accurate phenotypic data on all three DC populations without the losses and error inherent in purification techniques such as used in our previous study of lung DCs in COPD (4). However, the disadvantage of such a direct staining approach is that, unless supplemented by expensive cell sorting, it yields no purified DCs for functional or PCR assays. Such investigations will be our focus in future studies. The methods we used to quantify lung DCs as a fraction of total lung leukocytes complement the more recent results of Demedts and colleagues (5). Using immunohistochemistry, they found an increase in absolute numbers of DCs in the epithelium and adventitia in the small airways of patients with COPD. Although our flow cytometric method did not detect any correlations between COPD severity and the frequency of individual DC subsets, expressed as the percent of total leukocytes (see Figure 2), we believe our data imply the same increase in absolute numbers of lung DCs with worsening COPD severity shown in their study. We base this inference on the established finding that numbers of total inflammatory cells in the lungs increases with GOLD stage. Thus, if absolute numbers of parenchymal DCs did not increase as well, they should have fallen as a fraction of total leukocytes, which was not the case. We have not succeeded in devising a means of determining an accurate total of cell numbers in surgical specimens (e.g., relative to lung weight) because the lungs are variably bloody or already immersed in culture medium. Moreover, because it is not feasible to perfuse the small samples we receive after processing by the pathologist, it is not possible to exclude some contamination by DCs within the pulmonary vasculature. Despite these limitations, flow cytometry has distinct advantages, notably the ability to examine simultaneously and quantitatively multiple receptors on large numbers of individual cells.
Our data differ from those of Rogers and colleagues (6). Using transmission electron microscopy to identify DC in endobronchial biopsies, they found decreased numbers of DCs in the bronchial mucosal of current smokers with COPD, as compared with healthy control subjects. However, in that study, DC numbers in patients who had quit smoking were similar to healthy control subjects. The disparity in findings likely relates to the difference in anatomic sites studied, in particular the possible greater effect of active smoking on airway DCs in the study of Rogers and colleagues versus the disease stage–related changes found in this study and by Demedts and colleagues (5), both of which examined distal lung parenchyma, the site of the predominant pathology in COPD. DCs are present in multiple anatomic compartments, including epithelium and submucosa of large and small airway, in lung interstitium, and within the alveoli, and may mediate different effects in each site. Therefore, to gain a fuller understanding of the contribution of DCs to lung host defense and pathology, studies such as ours on lung digests must be complemented in the future by immunolocatization studies.
Increased DC expression of costimulatory molecules does not necessarily equate with greater ability to induce cytokine elaboration or other effector functions in lung T cells because such “mature” DCs may also induce tolerance (39). One reason for this apparent paradox is that the signal delivered to the DCs by CD80 and CD86 ligation depends on the T-cell receptors with which they interact. When CD80 and CD86 are bound by CD28, DCs increase their IL-6 production, which stimulates T-cell activation (48). However, binding of CD80 and CD86 by CTLA-4 induces a DC phenotype that suppresses T-cell proliferation via indoleamine 2,3-dioxygenase-dependent tryptophan degradation (49). Together with the consideration that DCs at sites of inflammation may have purely local actions not dependent on migration to the lymph nodes, these factors imply that caution is warranted in extrapolating the conventional DC maturation paradigm to specific disease states, including COPD.
The associations shown in Table 2 have several possible explanations. It is intriguing to speculate that lung DCs are activated by recognition of danger signals, such as from respiratory pathogens or damaged tissues, and then activate T cells within the lung parenchyma. The higher expression of CD40, relative to the other DC molecules examined in this study, could relate in part to either type of danger signal because CD40 has been shown to bind both microbial and mammalian heat-shock protein 70 (50). However, it is equally possible that the DC maturation molecules were up-regulated in response to interactions with activated CD4+ T cells. The experiments necessary to demonstrate thoroughly that lung DCs are able to induce T-cell activation are beyond the scope of the current study. It is also possible that these lung DCs contribute directly to lung destruction in COPD. Lung DCs exposed to cigarette smoke were shown to produce increased levels of matrix metalloproteinase-12, which could contribute to tissue damage (51).
In summary, we found that all three subsets of DCs harvested from distal pulmonary parenchyma showed evidence of increased maturity and activation that correlates positively with worsening COPD severity and that appears not to result fom recent smoking or cumulative smoke exposure. Furthermore, expression of the lung DC activation markers correlated positively with expression of CD69 on lung CD4+ T cells from the same patients, and we show evidence of physical contact between lung DCs and lung CD4+ T cells. Our findings suggest that as COPD progresses, the lung is infiltrated by mature DCs that may contribute to an inappropriate, sustained, and destructive immune response. Determining the functional capacities of individual lung DC subsets, and the degree to which their activation states relates to stimulation by lung microbiota or altered self-antigens, are important goals for future studies.
Acknowledgments
The authors thank Dr. George R. Washko, Jr. for assistance with 3D Slicer software; the reviewers for prompting profitable additional experiments; all the members of the Ann Arbor Veteran's Affairs Research Enhancement Award Program for helpful suggestions and discussion; Dr. Galen B. Toews for reviewing the manuscript; Liujian Zhao for assistance in tissue processing; Mary Christensen, Charlotte Jett, and Dr. Deborah Thompson for assistance in patient recruitment and regulatory activities; and Mary Freer, Joyce O'Brien,and Rebecca Weeks for administrative support.
Supported by grants R01 HL082480, T32 HL07749, KL2 RR024987, and K24 HL04212 from the USPHS; by Career Development Award (C.M.F.) and a Research Enhancement Award Program from the Biomedical Laboratory Research and Development Service, Department of Veterans Affairs; by the Tissue Procurement Core of the University of Michigan Comprehensive Cancer Center, grant P30 CA46952; and by the Lung Tissue Research Consortium (Clinical Centers), grant N01 HR046162.
Originally Published in Press as DOI: 10.1164/rccm.200904-0552OC on September 3, 2009
Conflict of Interest Statement: C.M.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. F.J.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.K.H. received up to $1,000 from Novartis in consultancy fees, $1,001–$5,000 for serving on an advisory board for CSL Behring, $5,001–$10,000 in lecture fees from GlaxoSmithKline, and up to $1,000 in royalties from UpToDate. T.M.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.W.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.C.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.A.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.A.M . does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.L.C. received $10,001–$50,001 in capitation for a clinical trial from Boehringer Ingelheim.
References
- 1.O'Donnell R, Breen D, Wilson S, Djukanovic R. Inflammatory cells in the airways in COPD. Thorax 2006;61:448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645–2653. [DOI] [PubMed] [Google Scholar]
- 3.Lambrecht BN, Hammad H. Taking our breath away: dendritic cells in the pathogenesis of asthma. Nat Rev Immunol 2003;3:994–1003. [DOI] [PubMed] [Google Scholar]
- 4.Freeman CM, Curtis JL, Chensue SW. CC chemokine receptor 5 and CXC chemokine receptor 6 expression by lung CD8+ cells correlates with chronic obstructive pulmonary disease severity. Am J Pathol 2007;171:767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demedts IK, Bracke KR, Van Pottelberge G, Testelmans D, Verleden GM, Vermassen FE, Joos GF, Brusselle GG. Accumulation of dendritic cells and increased CCL20 levels in the airways of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;175:998–1005. [DOI] [PubMed] [Google Scholar]
- 6.Rogers AV, Adelroth E, Hattotuwa K, Dewar A, Jeffery PK. Bronchial mucosal dendritic cells in smokers and ex-smokers with COPD: an electron microscopic study. Thorax 2008;63:108–114. [DOI] [PubMed] [Google Scholar]
- 7.Zeid NA, Muller HK. Tobacco smoke induced lung granulomas and tumors: association with pulmonary Langerhans cells. Pathology 1995;27:247–254. [DOI] [PubMed] [Google Scholar]
- 8.D'Hulst AI, Maes T, Bracke KR, Demedts, IK, Tournoy KG, Joos GF, Brusselle GG. Cigarette smoke-induced pulmonary emphysema in scid-mice. Is the acquired immune system required? Respir Res 2005;6:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robbins CS, Dawe DE, Goncharova SI, Pouladi MA, Drannik AG, Swirski FK, Cox G, Stampfli MR. Cigarette smoke decreases pulmonary dendritic cells and impacts antiviral immune responsiveness. Am J Respir Cell Mol Biol 2004;30:202–211. [DOI] [PubMed] [Google Scholar]
- 10.Hart DN. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood 1997;90:3245–3287. [PubMed] [Google Scholar]
- 11.Bratke K, Klug M, Bier A, Julius P, Kuepper M, Virchow JC, Lommatzsch M. Function-associated surface molecules on airway dendritic cells in cigarette smokers. Am J Respir Cell Mol Biol 2008;38:655–660. [DOI] [PubMed] [Google Scholar]
- 12.Robbins CS, Franco F, Mouded M, Cernadas M, Shapiro SD. Cigarette smoke exposure impairs dendritic cell maturation and T cell proliferation in thoracic lymph nodes of mice. J Immunol 2008;180:6623–6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsoumakidou M, Demedts IK, Brusselle GG, Jeffery PK. Dendritic cells in chronic obstructive pulmonary disease: new players in an old game. Am J Respir Crit Care Med 2008;177:1180–1186. [DOI] [PubMed] [Google Scholar]
- 14.Ziegler SF, Ramsdell F, Alderson MR. The activation antigen CD69. Stem Cells 1994;12:456–465. [DOI] [PubMed] [Google Scholar]
- 15.GOLD Executive Committee. Global strategy for the diagnosis, management, and prevention of COPD [updated 2008]. Available from http://www.goldcopd.com/GuidelinesResources.asp?l1=2&l2=0 (accessed 2009 Mar 29).
- 16.Fabbri L, Pauwels RA, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary updated 2003. COPD 2004;1:105–141. [DOI] [PubMed] [Google Scholar]
- 17.Demedts IK, Brusselle GG, Vermaelen KY, Pauwels RA. Identification and characterization of human pulmonary dendritic cells. Am J Respir Cell Mol Biol 2005;32:177–184. [DOI] [PubMed] [Google Scholar]
- 18.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol 2004;22:307–328. [DOI] [PubMed] [Google Scholar]
- 19.Vermaelen K, Pauwels R. Pulmonary dendritic cells. Am J Respir Crit Care Med 2005;172:530–551. [DOI] [PubMed] [Google Scholar]
- 20.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol 2005;23:515–548. [DOI] [PubMed] [Google Scholar]
- 21.Hathcock KS, Laszlo G, Pucillo C, Linsley P, Hodes RJ. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J Exp Med 1994;180:631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inaba K, Witmer-Pack M, Inaba M, Hathcock KS, Sakuta H, Azuma M, Yagita H, Okumura K, Linsley PS, Ikehara S, et al. The tissue distribution of the B7-2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro. J Exp Med 1994;180:1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenschow DJ, Su GH, Zuckerman LA, Nabavi N, Jellis CL, Gray GS, Miller J, Bluestone JA. Expression and functional significance of an additional ligand for CTLA-4. Proc Natl Acad Sci USA 1993;90:11054–11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsoumakidou M, Tzanakis N, Papadaki HA, Koutala H, Siafakas NM. Isolation of myeloid and plasmacytoid dendritic cells from human bronchoalveolar lavage fluid. Immunol Cell Biol 2006;84:267–273. [DOI] [PubMed] [Google Scholar]
- 25.Masten BJ, Olson GK, Tarleton CA, Rund C, Schuyler M, Mehran R, Archibeque T, Lipscomb MF. Characterization of myeloid and plasmacytoid dendritic cells in human lung. J Immunol 2006;177:7784–7793. [DOI] [PubMed] [Google Scholar]
- 26.Tsoumakidou M, Zhu J, Wang Z, Thorley A, Kemp S, Tetley T, Jeffery PK. Immunohistochemical detection of dendritic cells in human lung tissue. Histopathology 2007;51:565–568. [DOI] [PubMed] [Google Scholar]
- 27.Curtis JL, Freeman CM, Hogg JC. The immunopathogenesis of chronic obstructive pulmonary disease: insights from recent research. Proc Am Thorac Soc 2007;4:512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, Green L, Hacken-Bitar J, Huh J, Bakaeen F, et al. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med 2007;13:567–569. [DOI] [PubMed] [Google Scholar]
- 29.Taraseviciene-Stewart L, Burns N, Kraskauskas D, Nicolls MR, Tuder RM, Voelkel NF. Mechanisms of autoimmune emphysema. Proc Am Thorac Soc 2006;3:486–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agusti A, MacNee W, Donaldson K, Cosio M. Hypothesis: does COPD have an autoimmune component? Thorax 2003;58:832–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol 2007;7:19–30. [DOI] [PubMed] [Google Scholar]
- 32.Sertl K, Takemura T, Tshachler E, Ferrans VJ, Kaliner MA, Shevach EM. Dendritic cells with antigen-presenting capability reside in airway epithelium, lung parenchyma and visceral pleura. J Exp Med 1986;163:436–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu L, Liu YJ. Development of dendritic-cell lineages. Immunity 2007;26:741–750. [DOI] [PubMed] [Google Scholar]
- 34.Liu YJ. IPC: professional Type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol 2004;23:275–306. [DOI] [PubMed] [Google Scholar]
- 35.De Heer HJ, Hammad H, Soullie T, Hijdra D, Vos N, Willart MA, Hoogsteden HC, Lambrecht BN. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med 2004;200:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cook DN, Bottomly K. Innate immune control of pulmonary dendritic cell trafficking. Proc Am Thorac Soc 2007;4:234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alvarez D, Vollmann EH, von Andrian UH. Mechanisms and consequences of dendritic cell migration. Immunity 2008;29:325–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diebold SS. Determination of T-cell fate by dendritic cells. Immunol Cell Biol 2008;86:389–397. [DOI] [PubMed] [Google Scholar]
- 39.Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol 2006;6:476–483. [DOI] [PubMed] [Google Scholar]
- 40.Holt PG, Haining S, Nelson DJ, Sedgwick JD. Origin and steady-state turnover of class II MHC-bearing dendritic cells in the epithelium of the conducting airways. J Immunol 1994;153:256–261. [PubMed] [Google Scholar]
- 41.von Garnier C, Filgueira L, Wikstrom M, Smith M, Thomas JA, Strickland DH, Holt PG, Stumbles PA. Anatomical location determines the distribution and function of dendritic cells and other APCs in the respiratory tract. J Immunol 2005;175:1609–1618. [DOI] [PubMed] [Google Scholar]
- 42.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol 2006;7:311–317. [DOI] [PubMed] [Google Scholar]
- 43.Robays LJ, Maes T, Lebecque S, Lira SA, Kuziel WA, Brusselle GG, Joos GF, Vermaelen KV. Chemokine receptor CCR2 but not CCR5 or CCR6 mediates the increase in pulmonary dendritic cells during allergic airway inflammation. J Immunol 2007;178:5305–5311. [DOI] [PubMed] [Google Scholar]
- 44.Osterholzer JJ, Curtis JL, Polak T, Ames T, Chen GH, McDonald R, Huffnagle GB, Toews GB. CCR2 mediates conventional dendritic cell recruitment and the formation of bronchovascular mononuclear cell infiltrates in the lungs of mice infected with Cryptococcus neoformans. J Immunol 2008;181:610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGill J, Van Rooijen N, Legge KL. Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. J Exp Med 2008;205:1635–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsoumakidou M, Koutsopoulos AV, Tzanakis N, Dambaki K, Tzortzaki E, Zakynthinos S, Jeffery PK, Siafakas NM. Decreased small airway and alveolar CD83+ dendritic cells in COPD. Chest 2009;136:726–733. [DOI] [PubMed] [Google Scholar]
- 47.Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol 2000;165:6037–6046. [DOI] [PubMed] [Google Scholar]
- 48.Orabona C, Grohmann U, Belladonna ML, Fallarino F, Vacca C, Bianchi R, Bozza S, Volpi C, Salomon BL, Fioretti MC, et al. CD28 induces immunostimulatory signals in dendritic cells via CD80 and CD86. Nat Immunol 2004;5:1134–1142. [DOI] [PubMed] [Google Scholar]
- 49.Munn DH, Sharma MD, Mellor AL. Ligation of B7–1/B7–2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol 2004;172:4100–4110. [DOI] [PubMed] [Google Scholar]
- 50.Calderwood SK, Mambula SS, Gray PJ Jr, Theriault JR. Extracellular heat shock proteins in cell signaling. FEBS Lett 2007;581:3689–3694. [DOI] [PubMed] [Google Scholar]
- 51.Bracke K, Cataldo D, Maes T, Gueders M, Noel A, Foidart JM, Brusselle G, Pauwels RA. Matrix metalloproteinase-12 and cathepsin D expression in pulmonary macrophages and dendritic cells of cigarette smoke-exposed mice. Int Arch Allergy Immunol 2005;138:169–179. [DOI] [PubMed] [Google Scholar]