Abstract
Rationale: Sleep studies are laborious, expensive, inaccessible, and inconvenient for diagnosing obstructive sleep apnea (OSA) in children.
Objectives: To examine whether the urinary proteome uncovers specific clusters that are differentially expressed in the urine of children with OSA.
Methods: Two-dimensional differential in-gel electrophoresis (2D-DIGE) and mass spectrometry proteomics followed by validation with western blot of ELISA.
Measurements and Main Results: Morning urine proteins from 60 children with polysomnographically confirmed OSA and from matched children with primary snoring (n = 30) and control subjects (n = 30) were assessed. A total of 16 proteins that are differentially expressed in OSA were identified, and 7 were confirmed by either immunoblots or ELISA. Among the latter, receiver–operator curve analyses of urinary concentrations of uromodulin, urocortin-3, orosomucoid-1, and kallikrein assigned favorable predictive properties to these proteins. Furthermore, combinatorial approaches indicated that the presence of values beyond the calculated cutoff concentrations for three or more of the proteins yielded a sensitivity of 95% and a specificity of 100%.
Conclusions: Proteomic approaches reveal that pediatric OSA is associated with specific and consistent alterations in urinary concentrations of specific protein clusters. Future studies aiming to validate this approach as a screening method of habitually snoring children appears warranted.
Keywords: sleep apnea, biomarkers, children, urine
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Sleep studies are laborious, expensive, relatively inaccessible, and inconvenient for diagnosing obstructive sleep apnea (OSA) in children. Proteomic analyses may uncover specific protein clusters that are differentially expressed in the urine of children with OSA.
What This Study Adds to the Field
Morning urine samples from children fulfilling the polysomnographic and clinical criteria of OSA reveal selective and consistent alterations in specific components of the urinary proteome when compared with children who habitually snore or healthy nonsnoring control children.
Obstructive sleep apnea (OSA) is a frequent condition affecting up to 3% of prepubertal children and has now been recognized as imposing substantial neurocognitive and cardiovascular morbidities (1–3). This condition is characterized by a history of habitual snoring associated with repeated events of partial or complete obstruction of the upper airways during sleep, leading to recurring episodes of hypercapnia, hypoxemia, and arousal throughout the night. Children who snore, but who do not have gas exchange abnormalities or evidence of snore-associated alterations in sleep architecture, are considered to have primary snoring (PS), an extremely frequent condition affecting 10 to 12% of all school-aged children (4–11).
In the clinical setting, history and physical examination have extremely poor predictive value in differentiating between PS and OSA (12–15). Thus, current diagnostic approaches for OSA require an overnight sleep study, which is costly, inconvenient, and labor intensive. Furthermore, because of the relative unavailability of suitable pediatric sleep diagnostic facilities, long waiting periods and unnecessary delays in diagnosis and treatment are frequent. Development of noninvasive biomarkers capable of reliably distinguishing children with PS from those with OSA would greatly facilitate timely screening and diagnosis of OSA in children, and thereby ameliorate the overall outcome of this highly prevalent disorder.
In a previous study, we used two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) to assess protein expression in morning urinary samples obtained from 11 children with OSA and 11 control children (16). Mass spectrometry followed by peptide mass fingerprinting analyses conclusively identified four proteins that were differentially regulated, namely gelsolin, perlecan, albumin, and immunoglobulin (16), and thus suggested that OSA may lead to altered renal glomerular or tubular permeability. Attempts to apply these findings as a diagnostic approach, however, have been limited by the relatively high abundance of these proteins in urine and the relatively underperforming receiver–operator curves (D. Gozal, unpublished observations). In addition, the 2D-PAGE approach is limited in its ability to detect quantitative differences across urinary samples because each of the samples needs to be analyzed separately, and thus the technique is fraught with inherently high inter- and intraindividual variability. Therefore, conventional 2D-PAGE has been more recently combined with protein-labeling strategies using two different fluorescent dyes to allow comparative analysis of different protein samples within a single two-dimensional gel platform. In this technique, termed two-dimensional differential in-gel electrophoresis (2D-DIGE), samples are labeled separately with fluorescent dyes, and then combined and run on the same 2D gel, thereby minimizing experimental variation and greatly facilitating protein spot matching. Images of the gel are acquired using the appropriate incident light wavelengths, and are then superimposed to localize the differentially regulated spots on the 2D gel with highly specialized image analysis software. 2D-DIGE is now recognized as an extremely robust method in comparative proteomics, because the novel fluorescent dyes exhibit unique sensitivity and protein detection in the linear range of concentrations up to five orders of magnitude (17).
In the present study, we aimed to expand on our preliminary findings in defining the unique characteristics of urinary proteins in pediatric OSA, and to further explore, using 2D-DIGE approaches, (1) whether prepubertal children with PS and control subjects exhibit any differences in their urinary proteome; (2) whether the previously identified urinary proteins are indeed confirmed as differentially expressed in a large cohort of children with OSA as compared with either control children or children with polysomnographically defined PS, and (3) whether additional proteins emerge, and thus could provide supplemental biomarker candidates. To this effect, we aimed to validate the presence of differentially expressed proteins by confirming such changes using immunoblotting or ELISA techniques, with the ultimate future goal of developing robust screening methodologies for the diagnosis of OSA in children.
METHODS
Children who were referred for evaluation of habitual snoring and suspected sleep-disordered breathing to the Pediatric Sleep Medicine Center (Louisville, KY) were recruited. As control subjects, we invited children from the community who had no history of any chronic or acute disorder and who did not snore. Exclusion criteria for all subjects included the presence of genetic or systemic disorders, significant neuromuscular diseases, renal disease, or any acute infectious processes. All parents completed a detailed intake clinical questionnaire. Height and weight and vital signs were recorded for each child, and body mass index (BMI) z-score was calculated on the basis of CDC 2000 growth standards (www.cdc.gov/growthcharts) and using online software (www.cdc.gov/epiinfo). A BMI z-score exceeding 1.65 (>95th percentile) was considered as fulfilling criteria for obesity. The study was approved by the institutional review boards at the University of Louisville (Louisville, KY); informed consent and, when appropriate, assent for minors, were obtained.
Overnight Polysomnography
All subjects underwent overnight polysomnography, using standard techniques (18). Children were studied for up to 12 hours in a quiet, darkened room maintained at an ambient temperature of 24°C. No medication was used to induce sleep. The following parameters were measured: chest and abdominal wall movement by inductance plethysmography, and heart rate by electrocardiography; air flow was triply monitored with a side-stream end-tidal capnograph that also provides breath-by-breath assessment of end-tidal carbon dioxide levels (BCI SC-300; BCI, Menomonee Falls, WI), a nasal pressure cannula, and an oronasal thermistor. Arterial pulse oxygen saturation (SpO2) was assessed by pulse oximetry (Nellcor N 100; Nellcor Inc, Hayward, CA), with simultaneous recording of the pulse waveform. The bilateral electro-oculogram, eight channels of electroencephalogram (two frontal, two occipital, two temporal, and two central leads), chin and anterior tibial electromyograms, and analog output from a body position sensor were also monitored. All measures were digitized with a commercially available system (Sandman [Nellcor Puritan Bennett, Kanata, ON, Canada]; or Stellate Instruments [Montreal, QC, Canada]). Tracheal sound was monitored with a microphone sensor, and a digital time-synchronized video recording was performed. The sleep technician monitored patient behavior and confirmed sleep position by means of the infrared camera inside the room. All the studies were initially scored by a certified technician and were then reviewed by a physician who was experienced in pediatric polysomnography and underwent training in an accredited fellowship program.
Sleep architecture was assessed by standard techniques (19). Central, obstructive, and mixed apneic events were counted. Obstructive apnea was defined as the absence of airflow with continued chest wall and abdominal movement for a duration of at least two breaths (18). Hypopneas were defined as a decrease in oronasal flow of at least 50% with a corresponding decrease in SpO2 of 3% or more and/or an arousal (18). The obstructive apnea–hypopnea index was defined as the number of obstructive apneas and hypopneas per hour of total sleep time (TST). Arousals were identified as defined by the American Sleep Disorders Association Task Force report (20).
Subjects were then divided into three groups according to whether they had habitual snoring or not and their sleep study findings. Criteria for OSA included an obstructive apnea index greater than 1/hour TST, and/or an obstructive apnea–hypopnea index greater than 2/hour TST with a nadir oxygen saturation value less than 92%. children with PS were defined as children with habitual snoring and an apnea–hypopnea index not exceeding 2/hour TST, with no evidence of any alterations in arousal indices and normal gas exchange during sleep. Control children were nonsnoring children with sleep findings similar to those of children with PS.
Urine Collection
The first morning void was collected on the day after the sleep study. Urine samples (20 ml) were collected in 1 ml of protease inhibitor cocktail (leupeptin [0.1 mg/ml], phenylmethylsulfonyl fluoride [0.1 mg/ml], and 1 mM sodium azide in 1 M TRIS, pH 6.8) and were stored at −80°C until analysis.
Urine Sample Preparation and 2D-DIGE
Samples were centrifuged at 1,000 × g for 5 minutes and passed through 0.34-mm pore size Whatman chromatography paper to remove cell debris and nuclei. Supernatants were then subjected to centrifugation (12,000 rpm for 5 min) and pellets were resuspended in 200 μl of 2D cell lysis buffer of the following composition: TRIS-HCl (30 mmol/L, pH 8.8) containing urea (7 mol/L), thiourea (2 mol/L), and 4% 3-[(3-cholamidopropyl)dimethylammonio]propanesulfonate (CHAPS). These procedures were performed at 4°C. The protein concentrations were measured with a protein assay kit (Bio-Rad, Hercules, CA). To label proteins (analytical gel), 30 μg of each protein sample was incubated with 0.7 μl of diluted CyDye (diluted 1:5 in dimethylformamide from a 1 nmol/μl stock; GE Healthcare, Piscataway, NJ) at 4°C for 30 minutes. The labeling was stopped by adding 0.7 μl of l-lysine (10 mmol/L) and incubating at 4°C for 15 minutes. The labeled samples were mixed together, and an equal volume of 2× 2D sample buffer (urea at 8 mol/L, 4% CHAPS, dithiothreitol [DTT] at 20 mg/ml, 2% Pharmalytes, and a trace amount of bromophenol blue) and 100 μl of destreak solution (GE Healthcare) were added. The total sample volume was adjusted to 260 μl by adding rehydration buffer (urea at 7 mol/L, thiourea at 2 mol/L, 4% CHAPS, DTT at 20 mg/ml, 1% Pharmalytes, and a trace amount of bromophenol blue). The samples were incubated at room temperature for 10 minutes on a shaker and centrifuged for 10 minutes at 16,000 × g. The supernatants were loaded onto a 13-cm immobilized pH gradient strip holder (GE Healthcare). Thirteen-centimeter immobilized pH gradient strips (pH 3 to 10) were put on the loaded samples and 1 ml of mineral oil was added. Isoelectric focusing was performed according to the protocol provided by the manufacturer (GE Healthcare). On completion of the isoelectric focusing, the strips were equilibrated in buffer 1 (TRIS-HCl [pH 8.8] at 50 mmol/L, containing urea at 6 mol/L, 30% glycerol, 2% sodium dodecyl sulfate [SDS], a trace amount of bromophenol blue, and DTT at 10 mg/ml) for 15 minutes and in buffer 2 (TRIS-HCl [pH 8.8] at 50 mmol/L, containing urea at 6 mol/L, 30% glycerol, 2% SDS, a trace amount of bromophenol blue, and DTT at 45 mg/ml) for 10 minutes with gentle agitation. The immobilized pH gradient strips were then rinsed in the SDS gel running buffer, transferred to SDS gel (10.5% SDS gel prepared with low-fluorescence glass plates), and sealed with 0.5% (wt/vol) agarose solution (in SDS gel running buffer). The electrophoresis was performed at room temperature until the dye fronts ran out of the gels. Image scans were performed immediately after the SDS–PAGE, using Typhoon TRIO (GE Healthcare). The scanned images were then analyzed with ImageQuant software (version 5.0; GE Healthcare), and subjected to in-gel analysis and cross-gel analysis, using DeCyder software version 6.0 (GE Healthcare). The ratio change in protein differential expression was obtained by in-gel DeCyder software analysis. The analytical gels were run in duplicate; a total of 150 μg of proteins per gel was loaded.
Spot Picking, Digestion, and Matrix-assisted Laser Desorption/Ionization Time-of-flight Mass Spectroscopy
Preparatory gels loaded with a total of 500 μg of proteins along with labeled proteins were run to pick spots. Protein spots were selected (cutoff, a 1.5-fold change on the 2D gels) with an Ettan Spot Picker (GE Healthcare) and subjected to in-gel trypsin digestion, peptide extraction, and desalting before matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy (MS) (ABI 4700; Applied Biosystems, Foster City, CA). A GPS Explorer (Applied Biosystems) workstation was used to search the database to match both MS and MS/MS data to proteins in the database. Spectral data were also converted into .dta files and searched against the International Protein Index (IPI) human nonredundant database, using the Open Mass Spectrometry Search Algorithm (OMSSA; free from NCBI, http://pubchem.ncbi.nlm.nih.gov/omssa/); the e-value cutoff was 0.01. All matched sequences were manually validated.
Protein Validation Approaches
ELISA.
Urine levels for kallikrein and uromodulin were assayed with commercially available ELISA kits used according to the manufacturer's instructions (human kallikrein/PSA DuoSet, cat. no. DY1344 [R&D Systems, Minneapolis, MN], which has a sensitivity of 0.03 ng/ml, exhibits linearity between 0.1 and 47.8 ng/ml, and has inter- and intraassay coefficients of variability of 15.3.0 and 10.6%, respectively; and human uromodulin, cat. no. M036020 [MD Biosciences, St. Paul, MN], which has a sensitivity of 6.62 ng/ml, exhibits linearity between 6.5 and 625 ng/ml, and has inter- and intraassay coefficients of variability of 12.0 and 7.6%, respectively). For urocortin-3, we developed an in-house ELISA using custom-made monoclonal antibodies raised against the C-terminal epitope of the published protein sequence. In preliminary assays, the sensitivity was 0.5 pg/ml, it exhibited linearity from 0.7 to 35 pg/ml, and had inter- and intraassay coefficients of variability of 14.7 and 9.6%, respectively.
Immunoblotting.
Urine sample proteins were centrifuged for 10 minutes at 13,000 rpm. After centrifugation, soluble protein content was measured with a Bio-Rad assay kit. Thirty micrograms of protein from each sample was diluted 1:1 in SDS–PAGE loading buffer and electrophoresed on 12% SDS–polyacrylamide gels (Invitrogen, Carlsbad, CA). Proteins were transferred to ImmobilonP membranes (Millipore, Bedford, MA) and blocked for 2 hours at room temperature with blocking buffer (4% nonfat milk powder) in TBST (TRIS base, 20 mmol/L; NaCl, 500 mmol/L; 0.05% Tween 20; pH 7.4). Protein bands were immunolocalized on Transblots with primary antibodies followed by horseradish peroxidase–conjugated secondary anti-rabbit or anti-mouse antibody and developed with SuperSignal West Pico chemiluminescent substrate (Pierce Biotechnology, Rockford, IL). Quantification of protein bands was performed in triplicate for each sample by determination of the relative optical density (ImageJ; National Institutes of Health, Bethesda, MD).
Turbidimetry.
Urinary orosomucoid-1 was analyzed in a particle-enhanced immunoturbidimetric assay as previously reported (21). The urinary creatinine level was measured for each sample, and urine orosomucoid-1 levels were corrected for corresponding urine creatinine concentration.
Statistical Methods, Definition of Biomarkers, and Sample Classification
Sensitivity and specificity were calculated on the basis of tabulating the number of correctly classified samples. Confidence intervals (95% CI) based on exact binomial calculations were determined with MedCalc (version 8.1.1.0; MedCalc Software, Mariakerke, Belgium; http://www.medcalc.be). Each receiver operating characteristic (ROC) plot was obtained by plotting all sensitivity values on the y axis against their equivalent 1 – specificity values on the x axis for all available thresholds (MedCalc Software). The area under the curve was evaluated, as it provides a single measure of overall accuracy that is not dependent on a particular threshold (22). The reported unadjusted P values were calculated on the basis of the natural logarithm-transformed intensities and the Gaussian approximation to the t distribution. Statistical adjustment for multiple testing was performed by the method described by Reiner and colleagues (23).
RESULTS
A total of 120 subjects between the ages of 2 and 9 years were studied. Their demographic characteristics (Table 1) revealed that there were no significant differences in age, sex, ethnicity, or BMI distribution among the three groups, and that the frequency of asthma and allergies was also similar among the three groups. Furthermore, as expected from the study design, there were significant differences in several nocturnal polysomnography measures in the OSA group, compared with the other two groups, but no differences between PS and CO (Table 2).
TABLE 1.
DEMOGRAPHIC CHARACTERISTICS OF 60 CHILDREN WITH OBSTRUCTIVE SLEEP APNEA, 30 MATCHED CHILDREN WITH PRIMARY SNORING, AND 30 MATCHED CONTROL SUBJECTS
| OSA (n = 60) | PS (n = 30) | CO (n = 30) | |
|---|---|---|---|
| Mean age, yr | 6.6 ± 0.7 | 6.7 ± 0.4 | 6.6 ± 0.4 |
| Male sex, n | 32 | 16 | 16 |
| BMI, z-score | 1.18 ± 0.03 | 1.01 ± 0.04 | 0.98 ± 0.04 |
| Systolic blood pressure, mm Hg | 106.2 ± 4.1 | 103.2 ± 3.9 | 102.8 ± 3.8 |
| Diastolic blood pressure, mm Hg | 68.9 ± 2.7 | 66.8 ± 2.6 | 65.9 ± 3.1 |
| Ethnicity | |||
| African American | 20 | 10 | 10 |
| White non-Hispanic | 40 | 20 | 20 |
| Maternal educational attainment | |||
| College or higher | 38 | 19 | 20 |
| High school or lower | 22 | 11 | 10 |
| Maternal smoking | |||
| Yes | 23 | 11 | 9 |
| No | 37 | 19 | 11 |
| Asthma | 7 | 4 | 3 |
| Allergies |
12 |
6 |
6 |
Definition of abbreviations: BMI = body mass index; CO = control subjects; PS = children with primary snoring; OSA = obstructive sleep apnea.
TABLE 2.
POLYSOMNOGRAPHIC FINDINGS IN 60 CHILDREN WITH OBSTRUCTIVE SLEEP APNEA, 30 MATCHED CHILDREN WITH PRIMARY SNORING, AND 30 MATCHED CONTROL SUBJECTS
| OSA (n = 60) | PS (n = 30) | CO (n = 30) | |
|---|---|---|---|
| Sleep efficiency, % | 88.6 ± 6.0 | 91.6 ± 7.9 | 90.7 ± 8.8 |
| Sleep latency, min | 17.3 ± 14.8* | 24.7 ± 22.8 | 27.4 ± 19.9 |
| REM latency, min | 112.7 ± 49.1 | 121.1 ± 38.4 | 124.1 ± 47.7 |
| Stage 1, %TST | 10.6 ± 9.2 | 8.3 ± 4.9 | 7.8 ± 5.9 |
| Stage 2, %TST | 48.9 ± 6.6 | 42.4 ± 9.6 | 41.3 ± 9.6 |
| SWS, %TST | 19.6 ± 7.0* | 23.7 ± 6.4 | 25.2 ± 7.1 |
| REM, %TST | 22.4 ± 6.1 | 24.6 ± 7.1 | 26.6 ± 7.8 |
| Spontaneous arousal index, per hr TST | 4.4 ± 3.4† | 8.2 ± 2.8 | 7.5 ± 2.2 |
| Respiratory arousal index, per hr TST | 10.4 ± 10.1‡ | 1.8 ± 0.4 | 0.7 ± 0.5 |
| AHI, per hr TST | 12.4 ± 5.6‡ | 0.8 ± 0.4 | 0.4 ± 0.3 |
| Mean SpO2, % | 95.7 ± 1.9† | 97.8 ± 0.7 | 98.6 ± 0.4 |
| SpO2 nadir, % |
78.6 ± 6.3‡ |
93.8 ± 1.9 |
94.8 ± 1.4 |
Definition of abbreviations: AHI = apnea–hypopnea index; CO = control subjects; PS = children with primary snoring; OSA = obstructive sleep apnea; SpO2 = oxygen saturation as measured by pulse oximetry; SWS = slow wave sleep; TST = total sleep time.
P < 0.05, by two-way analysis of variance (ANOVA) for OSA versus CO or PS.
P < 0.01, by two-way ANOVA for OSA versus CO or PS.
P < 0.001, by two-way ANOVA for OSA versus CO or PS.
2D-DIGE Analysis
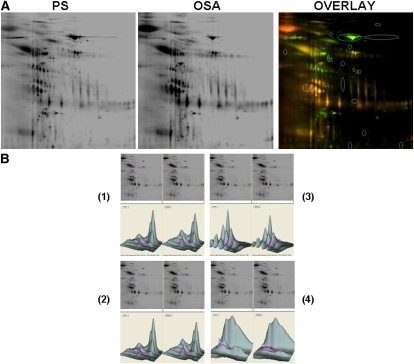
Urine samples were subjected to 2D-DIGE, as detailed in Methods. A total of 150 2D-DIGE experiments were performed such as to pair the 60 samples from OSA with either PS or CO, and also to pair CO and PS samples. In addition, 38 additional 2D-DIGE experiments were performed to ascertain the reproducibility and consistency of the findings. The overall number of detectable protein spots ranged from 713 to 789 in all urine samples (Figure 1). Analysis of differentially expressed species revealed the presence of 28 prominently differentially expressed spots, of which 23 were consistently present across all gels. Spots were resolved by loading large amounts of urinary proteins (500 μg) from each of the samples onto 2D gels, and then the 23 spots were further analyzed by in-gel trypsin digestion and matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy. These procedures were conducted on at least eight separate gels for each spot. We were able to consistently identify 12 nonredundant proteins. In addition to these, four additional proteins, namely gelsolin, perlecan, albumin, and immunoglobulin, were also confirmed as being differentially expressed, as previously reported (16). A list of the 12 novel nonredundant proteins that were identified with a high level of confidence on the basis of amino acid sequencing of 12 to 26 peptides per digested spot is presented in Table 3. On the basis of the 2D-DIGE quantification approach using DeCyder software, 3 of these 12 proteins were significantly decreased in OSA, and expression of the other 9 proteins was increased in OSA. There were no identifiable differences between PS and CO for these or other proteins.
Figure 1.
(A) Two-dimensional differential in-gel electrophoresis (2D-DIGE) gels of urinary proteins of a child with obstructive sleep apnea (OSA) and a matched child with primary snoring (PS) and fluorescence image overlay. (B) Candidate spot analysis showing spot location on gel, differential intensity analysis for two spots showing increased expression in OSA (spots 1 and 2), and two spots in which decreased expression occurred (spots 3 and 4).
TABLE 3.
URINARY PROTEINS ALTERED IN PEDIATRIC OBSTRUCTIVE SLEEP APNEA
| Increased | Decreased |
|---|---|
| Uromodulin | Kallikrein |
| Urocortin-3 | Zinc finger protein-81 |
| Bikunin | Zinc finger protein-36/1 |
| Tenascin | |
| Human Tribbles homolog-2 | |
| Orosomucoid-2 | |
| α1-Microglobulin | |
| PCAF histone acetylase | |
| Prolyl hydroxylase domain |
Definition of abbreviation: PCAF = phorbol ester–inducible coactivator of the IRF family.
Protein Verification
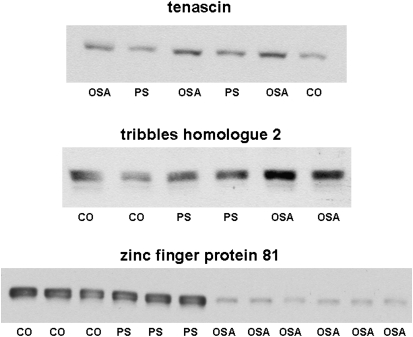
To confirm the identity of the candidate proteins that were differentially expressed in OSA urine samples, we employed either an immunoblotting or ELISA approach, using mostly commercially available antibodies. Western blots confirmed the increased expression of urinary proteins tenascin and tribbles homolog-2, and the decreased expression of zinc finger protein-81 (Figure 2).
Figure 2.
Representative Western blots for tenascin, tribbles homolog protein-2, and zinc finger protein-81 in urinary proteins from children with obstructive sleep apnea (OSA), those with primary snoring (PS), and healthy control subjects (CO).
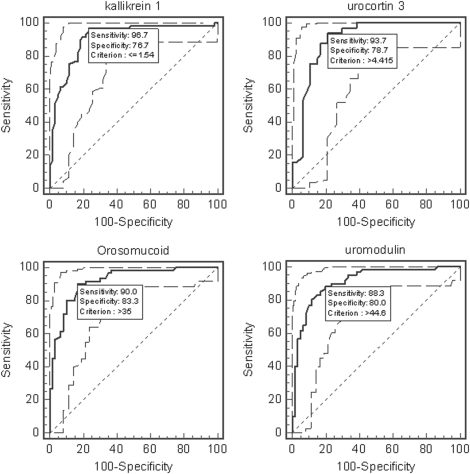
Uromodulin levels were increased in OSA (156.4 ± 13.0 ng/ml/mg creatinine compared with 29.1 ± 6.9 ng/ml/mg creatinine in CO [P < 0.0001] and 37.8 ± 10.6 ng/ml/mg creatinine in PS [P < 0.0001 vs. OSA]). ROC analyses revealed that uromodulin levels exceeding 44.6 pg/ml/mg creatinine achieved 88.3% sensitivity and 80.0% specificity to predict OSA (Figure 3).
Figure 3.
Receiver–operator curves for kallikrein-1, urocortin-3, orosomucoid, and uromodulin urine levels (after correction for corresponding urinary creatinine concentrations). Dashed lines indicate 95% confidence intervals.
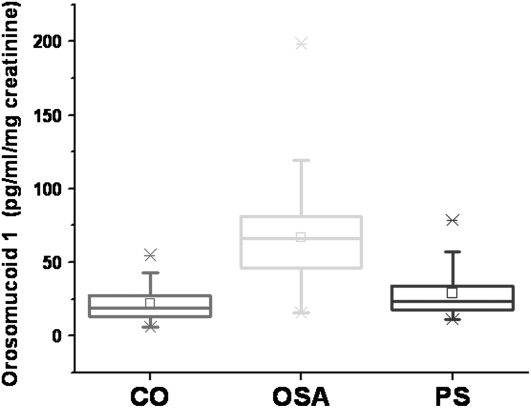
Orosomucoid-1 levels were measured in all urine samples. PS and CO orosomucoid urine levels were similar and were therefore merged. Their mean values were 25.0 ± 1.8 pg/ml/mg creatinine compared with 67.1 ± 3.9 pg/ml/mg creatinine in OSA samples (P < 0.0001; Figure 4). ROC analyses revealed that orosomucoid-1 levels greater than 35 pg/ml/mg creatinine achieved 90% sensitivity and 83.3% specificity to predict OSA (Figure 3).
Figure 4.
Box plots of urinary concentrations of orosomucoid-1 (after correction for corresponding urinary creatinine concentrations) in 30 control children (CO), 30 children with primary snoring (PS), and 60 children with obstructive sleep apnea (OSA). OSA versus PS or CO: P < 0.0001.
Kallikrein-1 urine levels were significantly reduced in the urine samples of children with OSA. Indeed, the kallikrein-1 level in control children was 3.32 ± 0.8 pg/ml/mg creatinine, 3.02 ± 0.6 pg/ml/mg creatinine in those with PS, and 0.85 ± 0.3 pg/ml/mg creatinine in those with OSA (P < 0.001). ROC analyses revealed that kallikrein-1 levels not exceeding 1.54 pg/ml/mg creatinine achieved 96.7% sensitivity and 76.7% specificity to predict OSA (Figure 3).
Urocortin-3 urine levels were significantly increased in OSA (6.9 ± 0.4 pg/ml/mg creatinine) compared with either PS or CO (3.8 ± 0.2 pg/ml/mg creatinine; P < 0.001). Of note, custom developed ELISAs for either urocortin-1 or urocortin-2, using commercially available antibodies, showed similar levels for these proteins in the urine samples of the three groups, definitively confirming the high specificity of the 2D-DIGE–MS approach. Furthermore, ROC analyses revealed that urocortin-3 levels not exceeding 4.4 pg/ml/mg creatinine achieved 93.7% sensitivity and 78.7% specificity to predict OSA (Figure 3).
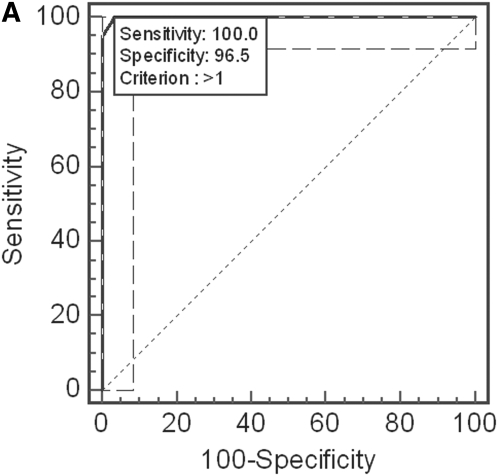
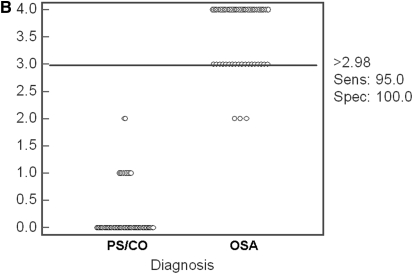
ROC analyses using more than one of the proteins measured by ELISA revealed that if all four proteins were employed, the presence of levels exceeding the cutoffs defined above for two or more of the proteins yielded 100% sensitivity and 96.5% specificity to predict OSA (Figure 5A). Moreover, the presence of levels exceeding the calculated cutoffs for three or more of the four proteins yielded a 95% sensitivity and 100% specificity to predict OSA (Figure 5B).
Figure 5.
(A) Receiver–operator curve for combinatorial analyses using one or more of the cutoff levels defined for kallikrein-1, urocortin-3, orosomucoid-1, and uromodulin urine levels (after correction for corresponding urinary creatinine concentrations). Use of two or more of these cutoffs yields a sensitivity of 100% and a specificity of 96.5%. Dashed lines indicate 95% confidence intervals. (B) Dot plot illustrating the discriminatory ability of three or more cutoff levels defined for kallikrein-1, urocortin-3, orosomucoid-1, and uromodulin urine levels (after correction for corresponding urinary creatinine concentrations). A sensitivity of 95% and a specificity of 100% were achieved.
DISCUSSION
This study shows that pediatric OSA is associated with reproducible alterations in the urinary proteome, and that such changes can potentially be used to screen children with habitual snoring in the future.
Some methodological issues deserve comment. In this study we obtained urine from the first void after sleep; we did not obtain a urinary sample before sleep and as such, did not assess overnight changes in urinary proteins. Such an approach could theoretically provide additional information as to whether the timing of the urine sample needs to be restricted to the first morning void, and could also shed light on whether changes in urinary candidate proteins occur specifically during sleep or not. Urine samples were processed using a standardized approach and the delay between collection and 2D-DIGE assay was similar, and did not exceed 14 days. However, we have not critically assessed whether longer waiting periods between sample collection and processing are possible. To reduce the potential effect of several known confounders, the three cohorts were matched for age, sex, ethnicity, and BMI, as well as for the presence of underlying inflammatory respiratory disorders such as allergic rhinitis or asthma. However, such a stringent design, which was intended to reduce variance and promote improved signal-to-noise ratios in urinary proteome differences, will require further validation in clinical referral pediatric populations for whom no exclusion criteria would be implemented. Obviously, under such field testing conditions, the potential effects on urinary marker patterns of coexisting diseases or the presence of acute intercurrent infections will need to be ascertained as well. An important advantage of our study design resided in the blinded nature of the 2D-DIGE and protein verification procedures, such that the actual phenotype, as determined from nocturnal polysomnography and corresponding to each urinary sample, was unknown to the investigators. Last, we did not proceed with protein enrichment procedures intended to increase the concentrations of less abundant proteins (24). Although such an approach can potentially identify a substantial number of less abundant proteins that would not emerge from the 2D-DIGE gels, affinity columns aiming to remove the most abundant proteins such as albumin, may also remove important proteins that are covalently bound. Of note, shotgun mass spectroscopic proteomic techniques that allow for dual sample simultaneous processing, and also afford unique sensitivity to the detection, may allow for deeper exploration of the urinary proteome in the future (25–27), including subfractions such as exosomes (28).
OSA-associated Urinary Proteins
Several uromodulin fragments were differentially excreted in the urine of patients with OSA, as evidenced from spot mass spectroscopic analyses. Uromodulin, which is identical to Tamm-Horsfall protein, is the most abundant protein in the urine of healthy individuals, and is expressed exclusively in the thick ascending limbs of Henle's loop. Urinary secretion occurs by proteolytic cleavage of the large N-terminal ectodomain from the glycosylphosphatidylinositol-anchored counterpart exposed at the lumenal cell surface of Henle's loop (29). Although the physiological role of uromodulin is uncertain, it is believed to be involved in the pathogenesis of various renal tubular disorders, where it may serve to stabilize the outer medulla in the face of injury, by decreasing inflammation, possibly through an effect on Toll-like receptor-4 (30, 31). Moreover, reduced urinary Tamm-Horsfall protein excretion is considered a reliable index of advanced distal tubular cell damage (32, 33). ELISA procedures further revealed increased concentrations of uromodulin-immunoreactive fragments in children with OSA, even after correction for urinary creatinine, and this highly consistent pattern was further exemplified by the highly favorable ROC characteristics of this assay.
Bikunin is a small proteoglycan, a member of the Kunitz-type or kunin family of serine protease inhibitors. Bikunin is encoded by an α-microglobulin–bikunin precursor protein that is proteolyzed, releasing α1-microglobulin and bikunin, two proteins with apparently unrelated functions. It is postulated that bikunin may play a role in the down-regulation of inflammatory responses; however, the putative increase in bikunin in the urine as inferred from 2D-DIGE will have to await further confirmatory studies (34). Development of a sensitive and reliable ELISA for bikunin is currently underway in our laboratory (35), and should permit more extensive exploration of the validity and reliability of bikunin quantitative assays in the screening of pediatric OSA.
α1-Microglobulin (A1M) is a 27-kD glycoprotein that is present in various body fluids; its exact biological function is unknown. Urinary A1M has emerged as a reliable and noninvasive, inexpensive diagnostic alternative for the diagnosis and monitoring of urinary tract disorders and, more importantly, as a useful indicator for the early detection of tubular disorders (36, 37). On the basis of previous findings (16) and the current findings, in which children with OSA were found to have increased albuminuria, it is reasonable to assume that the intermittent hypoxia and globally increased oxidative stress and inflammatory processes activated by OSA (38) may lead to mild renal dysfunction. Under such circumstances, increased urinary secretion of A1M would be expected to occur, and should prompt further investigation specifically to assess renal and tubular function in patients with sleep apnea.
It has become apparent that OSA may play a causative role in the initiation and propagation of atherosclerosis (3, 39). Inflammatory pathways activated by intermittent hypoxia during sleep and sleep fragmentation are prominent contributors to endothelial dysfunction and atheroma formation. Human tribbles homolog-2 is one such inflammatory protein and has been shown to localize within unstable areas of atheroma plaque and also to down-regulate IL-10 activity (40). Interestingly, we have previously reported on the reversible reductions in plasma IL-10 levels associated with OSA in nonobese children as well as the presence of significant endothelial dysfunction in otherwise healthy children with OSA (41–43). In this study, both 2D-DIGE and subsequent immunoblots of urinary proteins clearly showed increased concentrations of human tribbles homolog-2 protein in the urine of children with OSA. Of note, human tribbles homolog-2 may also play a role in down-regulating the differentiation of preadipocytes (44). Similarly, we found increased tenascin levels in OSA urine samples (see Figure 2). Tenascin belongs to a group of matrix proteins that appear to play important roles in cancer, inflammation, and atherosclerosis. Although the mechanisms leading to the putative increase in tenascin expression and excretion in urine remain to be established, the increased presence of this protein in OSA should further raise the awareness to the large-scale inflammatory responses elicited by this disease (38).
Orosomucoid, or α1-acid glycoprotein, is an extensively glycosylated (45%) serum protein, with five N-glycans being attached to the human protein (45, 46). It is also negatively charged (pI = 2.7–3.2) because of the presence of sialic acids (12% of the carbohydrate moiety). Orosomucoid is an acute-phase protein that is synthesized primarily by hepatocytes, but extrahepatic synthesis has also been reported. The serum concentration of orosomucoid rises two to five times during an acute-phase response (47). Its glycosylation pattern can also change depending on the type of inflammation (48). Orosomucoids have been implicated in the modulation of a variety of immune responses including neutrophil and platelet activation and endothelial function and permeability (49–53), and have also been associated with longevity (54). More recently, orosomucoids have also been proposed as markers of low-grade systemic inflammation such as in obesity (55), and to serve as reliable biomarkers of kidney dysfunction in diabetic patients (56, 57). The increased concentrations of orosomucoids in the urine samples of children with OSA would further suggest and confirm the underlying activation of inflammatory pathways in this disease (1, 3, 38, 39, 41, 42). Furthermore, orosomucoid levels enabled favorable ROC characteristics as a putative urine protein biomarker, as defined in this proteomic study.
PCAF (phorbol ester–inducible coactivator of the IRF family) histone acetylase is a 400-kD protein that appears to play a role in the signaling mechanisms associated with DNA damage and oxidation (58). Because we could not verify this protein using alternative methods to the 2D-DIGE–mass spectroscopy approaches, the significance of PCAF histone acetylase increases in urine remains unclear. Similarly, we found increases in prolyl hydroxylases domain proteins in the urine. These proteins are acutely down-regulated by hypoxia, and as such allow for stabilization of hypoxia-inducible factor-α (HIF-α), but are markedly up-regulated under chronic hypoxic conditions, such as might occur in OSA. Such increases in prolyl hydroxylases during chronic hypoxic conditions will trigger HIFα desensitization, a feedback mechanism that is needed to protect cells against cell death, and furthermore to enable adaptation to chronic hypoxia (59).
Interestingly, kallikrein-1 levels were reduced in the urine of children with OSA, suggesting that vasodilator mechanisms may be overcome by the presence of sleep apnea. Indeed, in rats exposed to chronic hypoxia, significant reductions in kallikrein will emerge and may play a role in the preservation of renal function (60). More recently, we have shown that rats exposed to intermittent hypoxia during sleep will develop marked reductions in renal kallikrein concentrations with marked increases in α-antitrypsin, a well-known enzyme that degrades kallikrein, possibly aiming to prevent systemic hypertension, a frequent consequence of OSA in children (61, 62). Although the functional implications of such findings remain elusive, the reductions in kallikrein in urine samples emerged as a potentially valuable tool in the prediction of OSA. It remains unclear, however, whether ROC characteristics and the area under the curve will further improve when kallikrein levels are combined with other differentially expressed proteins into an expanded diagnostic cluster. Our preliminary data using the four proteins assayed with ELISA would certainly support this hypothesis.
Zinc finger domains are common, relatively small protein motifs that fold around one or more zinc ions. In addition to their role as a DNA-binding module, zinc fingers have been shown to mediate protein–protein and protein–lipid interactions. The small zinc-ligating domain, often found in clusters containing fingers with different binding specificities, can facilitate multiple, often independent intermolecular interactions between nucleic acids and proteins. We found substantial and consistent reductions in the urinary expression of two of the zinc-binding proteins, including zinc finger protein-36/1, which appears to be an important suppressor of vascular endothelial growth factor (VEGF) expression (63, 64). Considering the previously reported increases in serum VEGF levels in pediatric and adult OSA (65, 66), it is likely that degradation and urinary excretion of zinc finger binding proteins may occur in the context of intermittent hypoxia, thereby relieving the posttranslational regulation of VEGF and other hypoxia-sensitive genes, and enabling their increased expression.
In summary, morning urine samples from 60 children fulfilling the polysomnographic and clinical criteria of OSA revealed selective and consistent alterations in specific components of the urinary proteome as assessed by 2D-DIGE followed by mass spectroscopy, when compared with samples from children with either habitual snoring or healthy nonsnoring control subjects. For those four proteins for which quantitative assessments were possible for the whole cohort, ROC analyses for each of the proteins were globally sensitive and specific in identification of OSA, and furthermore, combinatorial approaches yielded highly predictive performances. These findings further suggest that prospective studies in expanded cohorts should be contemplated to enable more extensive assessment and validation of urinary protein clusters as a diagnostic tool in children with habitual snoring.
Supported by National Institutes of Health grants HL-065270 and HL-086662 (D.G.), an investigator-initiated grant from Merck Company (L.K.G.), and a sleep fellowship from Jazz Pharmaceuticals (R.B.).
Originally Published in Press as DOI: 10.1164/rccm.200905-0765OC on September 24, 2009
Conflict of Interest Statement: D.G. serves on the National Speaker Bureau for Merck Company and has received honoraria for lectures on pediatric asthma not exceeding $10,000 for 2007 and 2008. S.J. received $1,001–$5,000 for a workshop at SOFT from Roche Diagnostics, $50,000–$100,000 in sponsored grants from Bio Site Corporation for developing assays for analysis of drugs, $10,001–$50,000 from Siemes Corporation in sponsored grants for developing ELISAs for biomarkers, and is a shareholder in PGXL Technologies, LLC, with $50,001–$100,000 in stock ownership or options. A.B.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.K.-G. received more than $100,001 from AstraZeneca and more than $100,001 from Merck in industry-sponsored grants and is the recipient of an investigator-initiated grant from Merck Company on the treatment of sleep apnea in children. R.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. O.S.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Sans Capdevila O, Kheirandish-Gozal L, Dayyat E, Gozal D. Pediatric obstructive sleep apnea: complications, management, and long-term outcomes. Proc Am Thorac Soc 2008;5:274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gozal D. Obstructive sleep apnea in children: implications for the developing central nervous system. Semin Pediatr Neurol 2008;15:100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gozal D, Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. Am J Respir Crit Care Med 2008;177:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Brien LM, Mervis CB, Holbrook CR, Bruner JL, Smith NH, McNally N, McClimment MC, Gozal D. Neurobehavioral correlates of sleep-disordered breathing in children. J Sleep Res 2004;13:165–172. [DOI] [PubMed] [Google Scholar]
- 5.Ferreira AM, Clemente V, Gozal D, Gomes A, Pissarra C, César H, Coelho I, Silva CF, Azevedo MHP. Snoring in Portuguese primary school children. Pediatrics 2000;106:E64. [DOI] [PubMed] [Google Scholar]
- 6.O'Brien LM, Holbrook CR, Mervis CB, Klaus CJ, Bruner J, Raffield TJ, Rutherford J, Mehl RC, Wang M, Tuell A, et al. Sleep and neurobehavioral characteristics in 5–7-year-old hyperactive children. Pediatrics 2003;111:554–563. [DOI] [PubMed] [Google Scholar]
- 7.Urschitz MS, Guenther A, Eitner S, Urschitz-Duprat PM, Schlaud M, Ipsiroglu OS, Poets CF. Risk factors and natural history of habitual snoring. Chest 2004;126:790–800. [DOI] [PubMed] [Google Scholar]
- 8.Kaditis AG, Finder J, Alexopoulos EI, Starantzis K, Tanou K, Gampeta S, Agorogiannis E, Christodoulou S, Pantazidou A, Gourgoulianis K, et al. Sleep-disordered breathing in 3,680 Greek children. Pediatr Pulmonol 2004;37:499–509. [DOI] [PubMed] [Google Scholar]
- 9.Rosen CL, Larkin EK, Kirchner HL, Emancipator JL, Bivins SF, Surovec SA, Martin RJ, Redline S. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr 2003;142:383–389. [DOI] [PubMed] [Google Scholar]
- 10.Montgomery-Downs HE, O'Brien LM, Holbrook CR, Gozal D. Snoring and sleep-disordered breathing in young children: subjective and objective correlates. Sleep 2004;27:87–94. [DOI] [PubMed] [Google Scholar]
- 11.Montgomery-Downs HE, Gozal D. Sleep habits and risk factors for sleep-disordered breathing in infants and young toddlers in Louisville, Kentucky. Sleep Med 2006;7:211–219. [DOI] [PubMed] [Google Scholar]
- 12.Carroll JL, McColley SA, Marcus CL, Curtis S, Loughlin GM. Inability of clinical history to distinguish primary snoring from obstructive sleep apnea syndrome in children. Chest 1995;108:610–618. [DOI] [PubMed] [Google Scholar]
- 13.Wang RC, Elkins TP, Keech D, Wauquier A, Hubbard D. Accuracy of clinical evaluation in pediatric obstructive sleep apnea. Otolaryngol Head Neck Surg 1998;118:69–73. [DOI] [PubMed] [Google Scholar]
- 14.Bhattacharjee R, Dayyat E, Kheirandish-Gozal L, Capdevila OS, Gozal D. Nocturnal polysomnographic characteristics of habitually snoring children initially referred to pediatric ENT or sleep clinics. Sleep Med. 2009;10:1031–1034. [DOI] [PubMed] [Google Scholar]
- 15.Schechter MS; Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome. Technical report: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2002;109:e69. [DOI] [PubMed] [Google Scholar]
- 16.Krishna J, Shah ZA, Merchant M, Klein JB, Gozal D. Urinary protein expression patterns in children with sleep-disordered breathing: preliminary findings. Sleep Med 2006;7:221–227. [DOI] [PubMed] [Google Scholar]
- 17.Larbi NB, Jefferies C. 2D-DIGE: comparative proteomics of cellular signaling pathways. Methods Mol Biol 2009;517:1–28. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery-Downs HE, O'Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics 2006;117:741–753. [DOI] [PubMed] [Google Scholar]
- 19.Rechstschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: Brain Information Services/Brain Research Institute, University of California; 1968.
- 20.Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep 1992;15:173–184. [PubMed] [Google Scholar]
- 21.Christiansen MS, Blirup-Jensen S, Foged L, Larsen M, Magid E. A particle-enhanced turbidimetric immunoassay for quantitative determination of orosomucoid in urine: development, validation and reference values. Clin Chem Lab Med 2004;42:1168–1177. [DOI] [PubMed] [Google Scholar]
- 22.DeLeo J. Receiver operating characteristic laboratory (ROCLAB): software for developing decision strategies that account for uncertainty. In: Ayyub BM, editor. Proceedings of the second international symposium on uncertainty modeling and analysis. Los Alamitos, CA: IEEE Computer Society Press; 1993. pp. 318–325.
- 23.Reiner A, Yekutieli D, Benjamini Y. Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics 2003;19:368–375. [DOI] [PubMed] [Google Scholar]
- 24.Zolotarjova N, Martosella J, Nicol G, Bailey J, Boyes BE, Barrett WC. Differences among techniques for high-abundant protein depletion. Proteomics 2005;5:3304–3313. [DOI] [PubMed] [Google Scholar]
- 25.Liu H, Sadygov RG, Yates JR III. A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal Chem 2004;76:4193–4201. [DOI] [PubMed] [Google Scholar]
- 26.Old WM, Meyer-Arendt K, Aveline-Wolf L, Pierce KG, Mendoza A, Sevinsky JR, Resing KA, Ahn NG. Comparison of label-free methods for quantifying human proteins by shotgun proteomics. Mol Cell Proteomics 2005;4:1487–1502. [DOI] [PubMed] [Google Scholar]
- 27.Nesvizhskii AI, Vitek O, Aebersold R. Analysis and validation of proteomic data generated by tandem mass spectrometry. Nat Methods 2007;4:787–797. [DOI] [PubMed] [Google Scholar]
- 28.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA 2004;101:13368–13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukuoka S, Kobayashi K. Analysis of the C-terminal structure of urinary Tamm-Horsfall protein reveals that the release of the glycosyl phosphatidylinositol–anchored counterpart from the kidney occurs by phenylalanine-specific proteolysis. Biochem Biophys Res Commun 2001;289:1044–1048. [DOI] [PubMed] [Google Scholar]
- 30.El-Achkar TM, Wu XR, Rauchman M, McCracken R, Kiefer S, Dagher PC. Tamm-Horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering TLR4 expression. Am J Physiol Renal Physiol 2008;295:F534–F544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serafini-Cessi F, Malagolini N, Cavallone D. Tamm-Horsfall glycoprotein: biology and clinical relevance. Am J Kidney Dis 2003;42:658–676. [DOI] [PubMed] [Google Scholar]
- 32.Thornley C, Dawnay A, Cattell WR. Human Tamm-Horsfall glycoprotein: urinary and plasma levels in normal subjects and patients with renal disease determined by a fully validated radioimmunoassay. Clin Sci (Lond) 1985;68:529–535. [DOI] [PubMed] [Google Scholar]
- 33.Lynn KL, Marshall RD. Excretion of Tamm-Horsfall glycoprotein in renal disease. Clin Nephrol 1984;22:253–257. [PubMed] [Google Scholar]
- 34.Pugia MJ, Valdes R Jr, Jortani SA. Bikunin (urinary trypsin inhibitor): structure, biological relevance, and measurement. Adv Clin Chem 2007;44:223–245. [DOI] [PubMed] [Google Scholar]
- 35.Pugia MJ, Jortani SA, Basu M, Sommer R, Kuo HH, Murphy S, Williamson D, Vranish J, Boyle PJ, Budzinski D, Valdes R Jr, Basu SC. Immunological evaluation of urinary trypsin inhibitors in blood and urine: role of N- and O-linked glycoproteins. Glycoconj J 2007;24:5–15. [DOI] [PubMed] [Google Scholar]
- 36.Penders J, Delanghe JR. α1-Microglobulin: clinical laboratory aspects and applications. Clin Chim Acta 2004;346:107–118. [DOI] [PubMed] [Google Scholar]
- 37.Guder WG, Hofmann W. Clinical role of urinary low molecular weight proteins: their diagnostic and prognostic implications. Scand J Clin Lab Invest Suppl 2008;241:95–98. [DOI] [PubMed] [Google Scholar]
- 38.Khalyfa A, Capdevila OS, Buazza MO, Serpero LD, Kheirandish-Gozal L, Gozal D. Genome-wide gene expression profiling in children with non-obese obstructive sleep apnea. Sleep Med 2009;10:75–86. [DOI] [PubMed] [Google Scholar]
- 39.Bhattacharjee R, Kheirandish-Gozal L, Pillar G, Gozal D. Cardiovascular complications of obstructive sleep apnea syndrome: evidence from children. Prog Cardiovasc Dis 2009;51:416–433. [DOI] [PubMed] [Google Scholar]
- 40.Deng J, James CH, Patel L, Smith A, Burnand KG, Rahmoune H, Lamb JR, Davis B. Human tribbles homologue 2 is expressed in unstable regions of carotid plaques and regulates macrophage IL-10 in vitro. Clin Sci (Lond) 2009;116:241–248. [DOI] [PubMed] [Google Scholar]
- 41.Gozal D, Capdevila OS, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am J Respir Crit Care Med 2008;177:1142–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gozal D, Serpero LD, Sans Capdevila O, Kheirandish-Gozal L. Systemic inflammation in non-obese children with obstructive sleep apnea. Sleep Med 2008;9:254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gozal D, Kheirandish-Gozal L, Serpero LD, Sans Capdevila O, Dayyat E. Obstructive sleep apnea and endothelial function in school-aged nonobese children: effect of adenotonsillectomy. Circulation 2007;116:2307–2314. [DOI] [PubMed] [Google Scholar]
- 44.Naiki T, Saijou E, Miyaoka Y, Sekine K, Miyajima A. TRB2, a mouse Tribbles ortholog, suppresses adipocyte differentiation by inhibiting AKT and C/EBPβ. J Biol Chem 2007;282:24075–24082. [DOI] [PubMed] [Google Scholar]
- 45.Fournier T, Medjoubi NN, Porquet D. Alpha-1-acid glycoprotein. Biochim Biophys Acta 2000;1482:157–171. [DOI] [PubMed] [Google Scholar]
- 46.Schmid K, Nimerg RB, Kimura A, Yamaguchi H, Binette JP. The carbohydrate units of human plasma α1-acid glycoprotein. Biochim Biophys Acta 1977;492:291–302. [DOI] [PubMed] [Google Scholar]
- 47.Mackiewicz A, Ganapathi MK, Schultz D, Kushner I. Monokines regulate glycosylation of acute-phase proteins. J Exp Med 1987;166:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Graaf TW, van der Stelt ME, Anbergen MG, van Dijk W. Inflammation-induced expression of sialyl Lewis X–containing glycan structures on α1-acid glycoprotein (orosomucoid) in human sera. J Exp Med 1993;177:657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Costello MJ, Gewurz H, Siegel JN. Inhibition of neutrophil activation by α1-acid glycoprotein. Clin Exp Immunol 1984;55:465–472. [PMC free article] [PubMed] [Google Scholar]
- 50.Andersen P, Godal HC. The antiheparin effect of α1-acid glycoprotein probably due to steric hindrance of the heparin–thrombin interaction. Thromb Res 1979;15:857–868. [DOI] [PubMed] [Google Scholar]
- 51.Boncela J, Papiewska I, Fijalkowska I, Walkowiak B, Cierniewski CS. Acute phase protein α1-acid glycoprotein interacts with plasminogen activator inhibitor type 1 and stabilizes its inhibitory activity. J Biol Chem 2001;276:35305–35311. [DOI] [PubMed] [Google Scholar]
- 52.Sörensson J, Matejka GL, Ohlson M, Haraldsson B. Human endothelial cells produce orosomucoid, an important component of the capillary barrier. Am J Physiol 1999;276:H530–H534. [DOI] [PubMed] [Google Scholar]
- 53.Benedek IH, Blouin RA, McNamara PJ. Serum protein binding and the role of increased α1-acid glycoprotein in moderately obese male subjects. Br J Clin Pharmacol 1984;18:941–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raynaud-Simon A, Lafont S, Berr C, Dartigues JF, Le Bouc Y. Orosomucoid: a mortality risk factor in elderly people living in the community? Clin Nutr 2002;21:45–50. [DOI] [PubMed] [Google Scholar]
- 55.Maachi M, Piéroni L, Bruckert E, Jardel C, Fellahi S, Hainque B, Capeau J, Bastard JP. Systemic low-grade inflammation is related to both circulating and adipose tissue TNFα, leptin and IL-6 levels in obese women. Int J Obes Relat Metab Disord 2004;28:993–997. [DOI] [PubMed] [Google Scholar]
- 56.Christiansen MS, Iversen K, Larsen CT, Goetze JP, Hommel E, Mølvig J, Pedersen BK, Magid E, Feldt-Rasmussen B. Increased urinary orosomucoid excretion: a proposed marker for inflammation and endothelial dysfunction in patients with type 2 diabetes. Scand J Clin Lab Invest 2009;69:272–281. [DOI] [PubMed] [Google Scholar]
- 57.Jiang H, Guan G, Zhang R, Liu G, Liu H, Hou X, Cheng J. Increased urinary excretion of orosomucoid is a risk predictor of diabetic nephropathy. Nephrology (Carlton) 2009;14:332–337. [DOI] [PubMed] [Google Scholar]
- 58.Vassilev A, Yamauchi J, Kotani T, Prives C, Avantaggiati ML, Qin J, Nakatani Y. The 400 kDa subunit of the PCAF histone acetylase complex belongs to the ATM superfamily. Mol Cell 1998;2:869–875. [DOI] [PubMed] [Google Scholar]
- 59.Ginouvès A, Ilc K, Macías N, Pouysségur J, Berra E. PHDs overactivation during chronic hypoxia “desensitizes” HIFα and protects cells from necrosis. Proc Natl Acad Sci USA 2008;105:4745–4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen CF, Chen LW, Chien CT, Wu MS, Tsai TJ. Renal kallikrein in chronic hypoxic rats. Clin Exp Pharmacol Physiol 1996;23:819–824. [DOI] [PubMed] [Google Scholar]
- 61.Thongboonkerd V, Gozal E, Sachleben LR Jr, Arthur JM, Pierce WM, Cai J, Chao J, Bader M, Pesquero JB, Gozal D, et al. Proteomic analysis reveals alterations in the renal kallikrein pathway during hypoxia-induced hypertension. J Biol Chem 2002;277:34708–34716. [DOI] [PubMed] [Google Scholar]
- 62.Amin R, Somers VK, McConnell K, Willging P, Myer C, Sherman M, McPhail G, Morgenthal A, Fenchel M, Bean J, et al. Activity-adjusted 24-hour ambulatory blood pressure and cardiac remodeling in children with sleep disordered breathing. Hypertension 2008;51:84–91. [DOI] [PubMed] [Google Scholar]
- 63.Bell SE, Sanchez MJ, Spasic-Boskovic O, Santalucia T, Gambardella L, Burton GJ, Murphy JJ, Norton JD, Clark AR, Turner M. The RNA binding protein Zfp36l1 is required for normal vascularisation and post-transcriptionally regulates VEGF expression. Dev Dyn 2006;235:3144–3155. [DOI] [PubMed] [Google Scholar]
- 64.Kwon HS, Shin HC, Kim JS. Suppression of vascular endothelial growth factor expression at the transcriptional and post-transcriptional levels. Nucleic Acids Res 2005;33:e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gozal D, Lipton AJ, Jones KL. Circulating vascular endothelial growth factor levels in patients with obstructive sleep apnea. Sleep 2002;25:59–65. [DOI] [PubMed] [Google Scholar]
- 66.Lavie L, Kraiczi H, Hefetz A, Ghandour H, Perelman A, Hedner J, Lavie P. Plasma vascular endothelial growth factor in sleep apnea syndrome: effects of nasal continuous positive air pressure treatment. Am J Respir Crit Care Med 2002;165:1624–1628. [DOI] [PubMed] [Google Scholar]