Abstract
Rationale: The risk of developing active tuberculosis in persons with latent Mycobacterium tuberculosis infection is substantially increased shortly after HIV-1 seroconversion. Immune responses in the lung are important to restrict the growth of M. tuberculosis to prevent the development of disease.
Objectives: To investigate innate and adaptive immune responses to M. tuberculosis in bronchoalveolar lavage from HIV-1–infected persons without active tuberculosis.
Methods: Peripheral blood was drawn and bronchoalveolar lavage (BAL) performed on healthy, HIV-1–uninfected (n = 21) and HIV-1–infected (n = 15) adults. Growth of M. tuberculosis was assessed in monocytes and alveolar macrophages. Cytokine expression by mycobacteria-specific CD4 and CD8 T cells was measured by intracellular cytokine staining or IFN-γ ELISpot.
Measurements and Main Results: Mycobacterial growth in monocytes or alveolar macrophages from HIV-1–infected and –uninfected persons did not differ. Total CD4 T-cell frequencies in BAL were lower in HIV-1–infected than in HIV-1–uninfected persons (P < 0.001). Mycobacteria (bacillus Calmette-Guérin)-specific CD4 T-cell responses in BAL were severely impaired: Frequencies of cells expressing IFN-γ or tumor necrosis factor (TNF)-α, as well as polyfunctional cells, expressing IFN-γ, TNF-α, and IL-2 together, were lower in HIV-1–infected persons than in uninfected controls (P < 0.01 for all).
Conclusions: In addition to a total CD4 T-cell deficit, the function of mycobacteria-specific CD4 T cells is significantly impaired in the lung of HIV-1–infected persons, which may account for the HIV-1–associated elevated risk for developing tuberculosis.
Keywords: HIV-1, tuberculosis, immunity mucosal, T-cells, macrophages
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
The risk of tuberculosis in HIV-infected persons is known to increase as the peripheral CD4 count declines. However, little is known of HIV-related immune defects in the lung, the initial site of mycobacterial infection.
What This Study Adds to the Field
This study compared lung innate and acquired immune responses in HIV-infected and HIV-uninfected persons living in an endemic area. We report a decreased frequency and function of mycobacteria-specific pulmonary CD4 T cells in HIV-1–infected persons.
Most close contacts of persons with active tuberculosis (TB) who become infected with Mycobacterium tuberculosis and develop an adaptive immune response against M. tuberculosis do not develop active TB (1, 2). The mechanisms of immunity against M. tuberculosis are not completely understood (3), but control of M. tuberculosis infection depends on a T-cell immune response comprising CD4 and CD8 cells (4–6). Th1 cytokines, including IFN-γ (5, 7, 8) and tumor necrosis factor (TNF)-α (9–11), are critical for effective immune responses. IL-2 may also be important, as this Th1 cytokine is required for secondary expansion of memory T cells (12). Furthermore, T cells that simultaneously express the three Th1 cytokines IFN-γ, TNF-α, and IL-2, referred to as polyfunctional T cells, have recently been associated with more effective control of intracellular infections (13), including M. tuberculosis (14).
Because of methodological difficulties, most studies of antimycobacterial immunity in humans have focused on peripheral blood rather than local mechanisms of infection and immune responses in the human lung. However, immunity at the site of infection may differ significantly from the periphery (15, 16) and may yield more relevant clues about mechanisms and immune components of protection (17). Detailed studies of human innate and adaptive immunity in the lung are important to enhance our understanding of protective immunity to M. tuberculosis. In immunocompetent persons with pulmonary TB, antigen-specific CD4 T cells accumulate in the lung (16, 18). This can form the basis for improved discrimination between active and latent TB when sampling bronchoalveolar lavage (BAL) cells for M. tuberculosis–specific T cells in low-incidence environments (19, 20).
Progressive loss of CD4 T cells and susceptibility to opportunistic infections like TB are hallmarks of HIV-1 infection. After HIV-1 seroconversion, the risk of active TB is greatly increased in persons who are latently infected with M. tuberculosis (21, 22). Furthermore, in contrast to the majority of other opportunistic infections, the risk of developing TB is substantially increased before CD4 T-cell loss is profound: a recent study reported that TB incidence doubled within the first year of HIV-1 infection (23).
Systemic T-cell responses against M. tuberculosis are impaired in HIV-1–infected adults (24–26). T cells play an important role in maintaining the integrity of granuloma formation in the human lung (27). Susceptibility to active TB in HIV-1–infected persons who are latently infected with M. tuberculosis is likely related to the inability of local immune mechanisms to control the M. tuberculosis infection. This includes dysregulation of the interaction between lymphocytes and alveolar macrophages.
However, the effect of underlying HIV-1 infection on the function and phenotype of pulmonary M. tuberculosis–specific T-cell responses has not been studied. We therefore investigated the differences in bronchoalveolar cell responses in patients with HIV-1 infection in comparison with HIV-1–uninfected persons.
METHODS
Participants
The study was approved by the Research Ethics Committees of the Universities of Cape Town (REC 381/2006) and Lübeck, Germany (05-096). Good clinical practice and ethical guidelines of the US Department of Health and Human Services and the South African Medical Research Council were adhered to; this included written, informed consent.
Participants were recruited at the Khayelitsha Site B Clinic, Cape Town, South Africa, an area with an extremely high TB incidence: 1,612/100,000 in the first quarter of 2008 (City of Cape Town). HIV-1–uninfected adults were invited to participate after attending voluntary testing and counseling for HIV-1 infection on the day of their negative test. HIV-1–infected persons were recruited at a subsequent visit to the HIV/AIDS clinic. All HIV-infected persons were offered HIV care according to South African national guidelines, which includes combined antiretroviral therapy (cART).
A symptom screen and physical examination were performed. Exclusion criteria included any symptoms suggestive of TB, a past history of TB or isoniazid preventive therapy, regularly smoking, pregnancy, chronic cardiovascular or metabolic illnesses, immunosuppressive medication, and an age less than 21 years. All participants had negative cultures for M. tuberculosis in BAL and had no radiological signs of lung disease. CD4 count and plasma viral load (in HIV-1–infected persons) were determined at the National Health Laboratory Service.
Bronchoalveolar Lavage and Blood Collection and Processing
Bronchoscopy and blood collection were performed before patients initiated cART. Bronchoscopy was conducted under local anesthesia with lidocaine as per British Thoracic Society guidelines (28). Additional conscious sedation with intravenous midazolam was given at the discretion of the operator. Standard flexible diagnostic bronchoscopy was performed, including a BAL of the middle lobe with 300 ml sterile saline instilled in 50-ml volumes as previously described (29). BAL fluid was stored on ice for a maximum of 30 minutes until bronchoalveolar lavage mononuclear cells (BALMC) were prepared as previously described (19), washed with phosphate-buffered saline, and strained through a 100-μm filter (Partec CellTrics; Partec Co., Münster, Germany).
Heparinized peripheral blood (40 ml) was collected by venipuncture and processed within 2 hours. Peripheral blood mononuclear cells (PBMC) were isolated by standard methods using density gradient centrifugation of blood layered on Ficoll-Paque Plus (GE Healthcare, Johannesburg, South Africa).
Infection Assay of Monocytes and Alveolar Macrophages with M. Tuberculosis
To isolate adherent cells, 5 × 105 PBMC were incubated for 60 minutes in plates at 37°C and washed three times with warm medium to remove nonadherent cells. Cytostaining revealed a purity of greater than 90% monocytes. An equivalent number (5 × 104) of BALMC to alveolar macrophages was plated. All cells were cultured in triplicate in 100 μl RPMI enriched with 10% heat-inactivated fetal calf serum (GIBCO, Mowbray, South Africa) and 100 U/ml penicillin G (Merck, Modderfontein, South Africa) in 96-well plates. The M. tuberculosis strain H37Rv was prepared as previously described (30) and cells were infected at a multiplicity of infection of 1:1. A new vial was thawed for every experiment. After 96 hours the supernatant was aspirated and cells lysed with 100 μl sodium dodecyl sulfate (Sigma-Aldrich, Johannesburg, South Africa). Four 10-fold serial dilutions of each lysate were prepared and plated onto Middelbrook 7H9 (Difco, Johannesburg, South Africa) with 10% acridine orange direct counts enrichment (BD Biosciences, Johannesburg, South Africa) and incubated at 37°C for 2 weeks. Colonies were counted as cfu per 100 μl lysate, which is equivalent to cfu per 5 × 104 initial monocytes or alveolar macrophages per 100 μl culture medium.
Antigens and Antibodies
Viable bacillus Calmette-Guérin (BCG), Danish strain 1331, was reconstituted from the vaccine vial (Statens Serum Institute, Copenhagen, Denmark) as previously described (31) and used at a multiplicity of infection of 1.2:1. M. tuberculosis purified protein derivative (PPD) (Statens Serum Institute) was used at 10 μg/ml; staphylococcal enterotoxin B (Sigma) was used at 10 μg/ml. For flow cytometry the following antibodies were used: anti-CD3 Pacific Blue (UCHT1), anti-CD4 PerCP-Cy5.5 (SK3), anti–IFN-γ AlexaFluor700 (B27), anti–IL-2 fluorescein isothiocyanate (FITC) (5344.111) and anti–TNF-α PE-Cy7 (MAb11). For surface marker expression fresh cells were stained with anti-CD3 Pacific Blue, anti-CD4 AlexaFluor 700 (RPA-T4), anti-CD8 PerCP-Cy5.5 (SK1), anti-CD45RA APC (HI100), and CD27 FITC (M T271); all antibodies were from BD Biosciences.
Cell Stimulation and Intracellular Cytokine Staining
Intracellular cytokine staining of BALMC was only performed on subjects from whom sufficient cells were available after performing the IFN-γ ELISpot and alveolar macrophage infection assays. Fresh PBMC or BALMC (1 × 106/ml) in RPMI containing 10% human AB+ serum were incubated with antigens for 7 hours. PBMC or BALMC incubated with medium alone or with staphylococcal enterotoxin B served as negative and positive controls, respectively. Brefeldin A (10 μg/ml, Sigma-Aldrich) was added for another 5 hours of incubation. After incubation cells were fixed with FACS Lysing Solution (BD Biosciences), and cryopreserved. Cells were thawed in batches, permeabilized with BD Perm/Wash buffer, and stained with fluorescent antibodies. For phenotypic analysis, freshly isolated PBMC or BALMC were stained with surface marker antibodies at 4°C for 20 minutes and fixed with 1.5% paraformaldehyde (Sigma-Aldrich).
Flow Cytometry
Stained cells were acquired on an LSR II flow cytometer (BD Biosciences) configured for three lasers and 12 detectors. Cell doublets were excluded using forward scatter–area versus forward scatter–height parameters. For every run, single stained mouse κ beads (BD Biosciences) were used to calculate compensation. Data analysis was performed with FlowJo software version 8.5.3 (TreeStar, Ashland, TX). The Boolean gate platform was used with individual cytokine gates to create all possible response pattern combinations.
IFN-γ ELISpot Assay
Responses to the M. tuberculosis–specific antigens early secreted antigenic target (ESAT)-6 and culture filtrate protein (CFP)-10 were measured in blood and BAL cells by IFN-γ ELISpot assay (MABTECH, Johannesburg, South Africa), as described elsewhere (19). Briefly, 2 × 105 PBMC or BALMC were left unstimulated (negative control) or were stimulated with recombinant ESAT-6, CFP-10 (5 μg/ml and 2.5 μg/ml, respectively; Proteix, Prague, Czech Republic) or anti-CD3 (positive control, MABTECH). For the purpose solely of classifying subjects a positive specific response was defined as 25 or more spot-forming cells (SFC) per million cells above the negative control and more than twice the SFC recorded for the negative control. High background discoloration, or artifact, precluding spot visualization in the negative control constituted an indeterminate result.
Data Analysis
The data analysis programs PESTLE (version 1.5.4) and SPICE (Simplified Presentation of Incredibly Complex Evaluations; version 4.1.6) were used to analyze flow cytometry data and generate graphical representations of T-cell responses using background-deducted flow cytometric data (both kindly provided by Mario Roederer, Vaccine Research Center, NIAID, NIH). Statistical tests between groups were performed with the Mann-Whitney U test, for paired data with the Wilcoxon signed rank test, and for 2 × 2 tables Fisher exact test of probability.
RESULTS
Participants
BALMC and PBMC were obtained from 15 HIV-1–infected and 21 HIV-1–uninfected persons. Participant demographics are shown in Table 1.
TABLE 1.
CHARACTERISTICS OF PERSONS ENROLLED IN THE STUDY
| HIV-1–Infected | HIV-1–Uninfected | P Value | |
|---|---|---|---|
| N | 15 | 21 | |
| Sex: female/male, n | 11/4 | 13/8 | 0.564 |
| Age, mean yr (range) | 34.3 (24–51) | 32.7 (21–55) | 0.531 |
| CD4, median CD4 cells/μl (range) | 226 (61–595) | 786 (461–1,225) | < 0.001 |
| Plasma viral load, median RNA copies/ml, (range) | 22,000 (590–660,000) | NA | |
| BALMC/100 ml BALF, mean (range) | 11.6 × 106 (4.7–29.0) | 8.6 × 106 (1.9–28.5) | 0.14 |
| Alveolar macrophages, median % (IQR) | 79.7 (71.3–91.8) | 93.1 (84.6–96.0) | 0.023* |
| BAL lymphocytes, median % (IQR) | 20.3 (8.2–28.7) | 6.9 (4.0–15.5) | 0.023* |
| Response to ESAT-6/CFP-10, blood (positive/negative) | 10/5 | 11/10 | 0.190† |
| Indeterminate ELISpot assay on blood | 0 | 0 | |
| Response to ESAT-6/CFP-10, BAL (positive/negative) | 1/8 | 7/6 | 0.048† |
| Indeterminate ELISpot assay on BAL |
6 |
8 |
0.268† |
Definition of abbreviations: BAL = bronchoalveolar lavage; BALF = bronchoalveolar lavage fluid; BALMC = bronchoalveolar lavage mononuclear cells; CFP = culture filtrate protein; ESAT = early-secreted antigenic target; IQR = interquartile range; NA = not applicable.
Mann-Whitney U test.
Fisher exact test of probability.
Growth of Mycobacteria in Alveolar Macrophages from HIV-1–infected or HIV-1–uninfected Persons
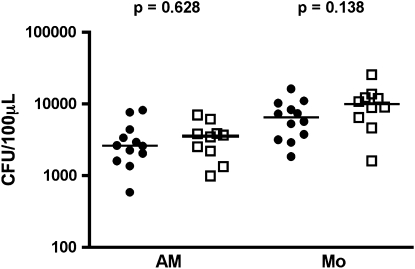
In humans, on aerosol infection with M. tuberculosis, the organisms are taken up by alveolar macrophages. As the risk of developing TB is already substantially increased before significant CD4 T-cell loss (21, 23, 32), we hypothesized that HIV-1 infection leads to impaired activation of macrophages, which, in turn, results in reduced control of mycobacterial replication. Bacterial counts were determined after culture of alveolar macrophages or immature monocytes from HIV-1–infected or –uninfected donors for 96 hours with M. tuberculosis. No differences in bacterial counts were observed between HIV-1–infected persons and –uninfected persons for either alveolar macrophages (median, 3,567 vs. 2,600 cfu/100 μl; P = 0.628) or immature monocytes (median, 9,875 vs. 6,467 cfu/100 μl; P = 0.138) (Figure 1).
Figure 1.
Growth of mycobacteria is unaffected by HIV-1 status. Adherence-purified peripheral monocytes (Mo) or alveolar macrophages (AM) were infected with Mycobacterium tuberculosis (strain H37Rv, multiplicity of infection 1:1) and cfu quantified after 96 hours. Solid circles, HIV-negative (HIV−); open squares, HIV-positive (HIV+). Horizontal lines represent the median. Differences were calculated using the Mann-Whitney U test.
CD4 T-Cells Are Depleted in BAL of HIV-1–infected Persons
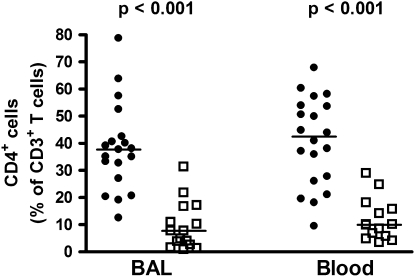
HIV-1 infection is associated with a significant and progressive depletion of CD4 T cells in the peripheral blood, and especially in the gastrointestinal tract (33, 34). We hypothesized that HIV-1 infection may also lead to depletion of CD4 T cells in the lung, another mucosal lymphoid tissue. The relative depletion of CD4 T cells in BAL from HIV-1–infected persons was determined by measuring the frequencies of CD4-expressing CD3+ T cells. CD4 T cells comprised a significantly lower frequency of BAL T cells in HIV-1–infected persons compared with –uninfected persons (median 7.7 vs. 37.7%; P < 0.001) (Figure 2). Similarly, a profound depletion of CD4 T cells was observed in peripheral blood from these HIV-1–infected persons when compared with the uninfected group (median, 10.0 .vs 42.4%; P < 0.001) (Figure 2).
Figure 2.
Relative frequencies of CD3 T cells expressing CD4 in bronchoalveolar lavage and blood from HIV-1–infected (HIV+) or –uninfected (HIV−) persons. Solid circles, HIV− ; open squares, HIV+. Horizontal lines represent the median. Significance was assessed by the Mann-Whitney U test.
BAL T-cell Memory Phenotype Is Unaffected by HIV-1 Infection
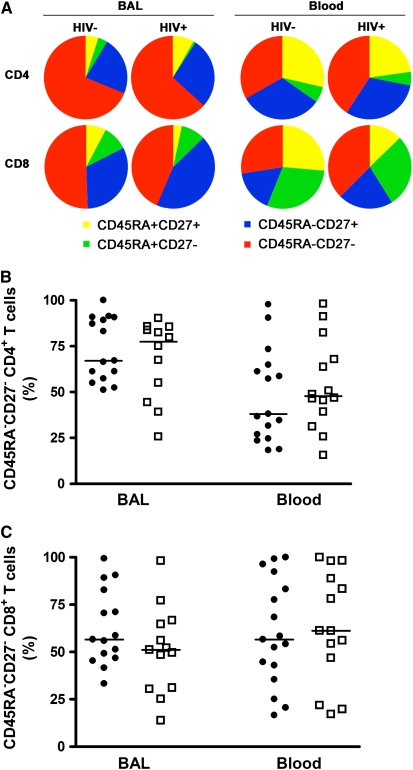
In HIV-1–infected persons the phenotype of peripheral blood HIV-1–specific T cells is skewed toward that of effector memory cells (35–38). To examine if the memory phenotype is altered in HIV-1 infection, we measured expression of CD45RA and CD27 on total CD4 and CD8 T cell subsets. In HIV-1–uninfected persons, 67.1% of BAL CD4 and 56.6% of CD8 T cells did not express CD45RA or CD27, typical of effector memory cells (Figure 3). Comparing the compartments, this group had a smaller proportion of peripheral blood CD4 T cells with this phenotype (38.1%; P = 0.013), but a similar proportion of CD8 T cells with the phenotype (56.5%; P = 0.389). No difference in memory phenotype of total BAL CD4 or CD8 T cells was shown when HIV-1–infected and –uninfected persons were compared (Figure 3A). Similarly, memory phenotypes of total CD4 or CD8 T cells in blood were not different in the subject groups (Figures 3B and 3C).
Figure 3.
Analysis of memory phenotypes of CD4 and CD8 T cells in bronchoalveolar lavage (BAL) and blood. (A) Relative proportions of CD45RA and/or CD27 expressing CD4 (upper plots) and CD8 (lower plots) T cells in BAL or blood from HIV-1–infected (HIV+) or –uninfected (HIV−) persons. Relative proportions of CD45RA−CD27− effector memory (B) CD4 or (C) CD8 T cells in BAL and blood from HIV-1–infected or –uninfected persons. Solid circles, HIV− ; open squares, HIV+. Horizontal lines represent the median. No significant differences were observed (Mann-Whitney U test).
Mycobacteria-Specific Th1 Cells Are Reduced in BAL from HIV-1–infected Persons
To study the local immune response to mycobacteria in the lung, we measured the magnitude and functional quality of PPD- or BCG-specific CD4 T cells in BAL and blood by intracellular cytokine staining. Mycobacteria-specific CD4 T-cell responses in both compartments comprised multiple subsets of cells expressing combinations of IFN-γ, TNF-α, and/or IL-2 (Figure 4; see Figure E1 in the online supplement). In HIV-1–negative persons the frequency of PPD or BCG-specific IFN-γ–expressing CD4 T cells detected in BAL exceeded those detected in blood (Figures 5A and 5B). By contrast, in HIV-1–infected persons, PPD-specific responses in blood and BAL were not different. BCG-specific responses were also not markedly different between compartments in HIV-1–infected persons.
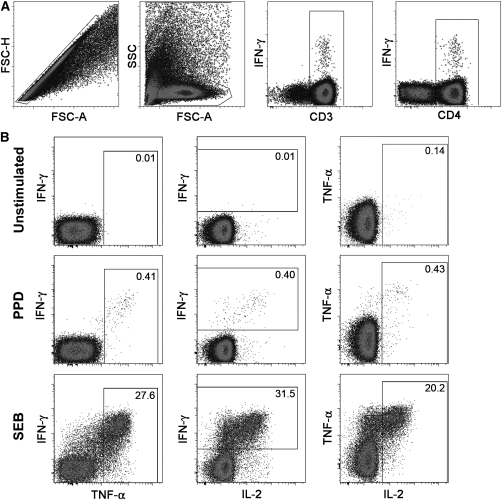
Figure 4.
Flow cytometric analysis of T-cell cytokine production in bronchoalveolar lavage (BAL). Representative dot plots from a single individual are shown. (A) Gating strategy used to identify CD4 and CD8 T cells (from left to right). Cell doublets were excluded using forward scatter–area versus scatter–height parameters. Subsequently lymphocytes, then CD3+ T cells, and then CD4+ T cells were selected. (B) Detection of IFN-γ, tumor necrosis factor (TNF)-α and/or IL-2-expressing CD4 T cells in unstimulated (top), purified protein derivative–stimulated (middle), or staphylococcal enterotoxin B–stimulated (bottom) BAL mononuclear cells. The percentage of cells falling into the respective gates is indicated in each plot.
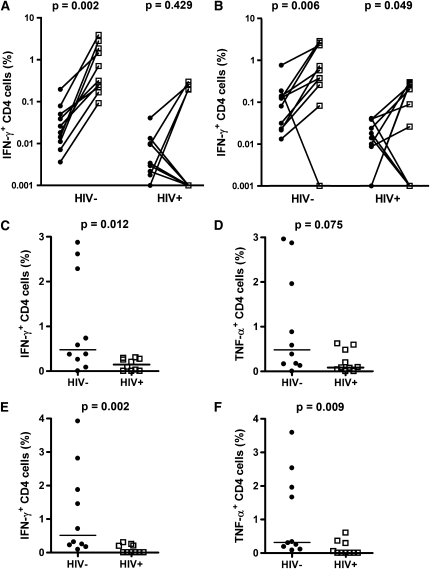
Figure 5.
Mycobacteria-specific T-cell responses in bronchoalveolar lavage (BAL) are impaired in HIV-1–infected persons. Paired blood and BAL frequencies of (A) purified protein derivative (PPD)-specific or (B) Bacillus Calmette-Guérin (BCG)-specific CD4 T cells expressing IFN-γ in HIV-1–uninfected (HIV−) or HIV-1–infected (HIV+) persons. Differences were calculated with the Wilcoxon signed rank test. Zeros were set to a value of 0.001. (C–F) BAL frequencies of mycobacteria-specific Th1 CD4 T cells in uninfected or HIV-1–infected persons. Frequencies of (C and D) PPD-specific or (E and F) BCG-specific CD4 T cells in BAL expressing (C and E) IFN-γ or (D and F) tumor necrosis factor (TNF)-α. Solid circles, blood; open squares, BAL. Horizontal lines represent the median. Background values (unstimulated cells) were subtracted for each antigen-stimulated measurement. Differences were calculated using the Mann-Whitney U test.
Next, the frequency of BAL CD4 T cells expressing IFN-γ or TNF-α on PPD or BCG stimulation in HIV-1–infected and –uninfected persons were compared. The frequencies of PPD-specific (median, 0.146 vs. 0.477%; P = 0.012) and BCG-specific (median, 0.0 vs. 0.513%; P = 0.002) IFN-γ–producing CD4 T cells in BAL from the HIV-1–infected group were lower compared with the uninfected group (Figures 5C and 5E). A trend of lower frequency of PPD-specific TNF-α–expressing cells was also evident in HIV-1–infected persons (Figure 5D). However, BCG-specific CD4 T cells expressing TNF-α were lower in HIV-1–infected persons (median 0.0 vs. 0.316%; P = 0.009; Figure 5F). In BAL samples from many subjects high background levels were observed in the FITC channel, presumably due to autofluorescence (data not shown). This artifact precluded reliable quantification of monofunctional IL-2–expressing BAL CD4 T cells.
The depletion of mycobacteria-specific CD4 T-cell responses in peripheral blood from HIV-1–infected persons is well described (24–26). We also observed significantly reduced frequencies of PPD- and BCG-specific cytokine-producing CD4 T cells in peripheral blood (data not shown).
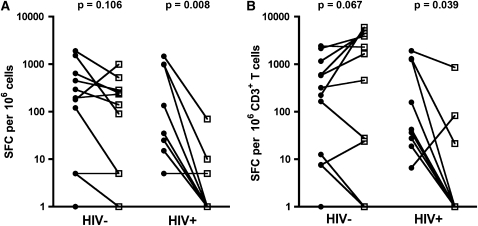
Next, the ELISpot assay was used to quantify ESAT-6– and CFP-10–specific IFN-γ–producing CD4 T cells in BAL and blood. Equivalent counts were detected in these two compartments from HIV-1–uninfected persons (median in BAL 90 and in blood 180 SFC per million cells, respectively; P = 0.148; Figure 6A). By contrast, ESAT-6/CFP-10–specific IFN-γ responses were significantly lower in BAL compared with blood from HIV-1–infected persons (median BAL 0 vs. blood, 35 SFC per million cells; P = 0.008; Figure 6A). These findings were not different when ELISpot counts were normalized to site-specific CD3 frequencies (Figure 6B).
Figure 6.
Early-secreted antigenic target-6 and culture filtrate protein-10–specific responses, measured by IFN-γ ELISpot assay in bronchoalveolar lavage (BAL) mononuclear cells and peripheral blood mononuclear cells. (A) Paired BAL or blood frequencies of IFN-γ–producing T cells from HIV-1–infected (HIV+) and HIV-1–uninfected (HIV−) persons. (B) IFN-γ T-cell responses normalized to CD3 frequencies in BAL or blood. Zeros were set to a value of 1. Solid circles, blood; open squares, BAL. Differences were calculated using the Wilcoxon signed rank test. SFC = spot-forming cells.
The Frequency of Polyfunctional CD4 T Cells in BAL Is Reduced in HIV-1–infected Persons
Bronchoalveolar HIV-1–specific CD4 cells have been shown to be more polyfunctional, coexpressing IFN-γ, IL-2, and TNF-α, than peripheral HIV-1–specific CD4 cells (29). We compared the functionality of PPD- and BCG-specific CD4 T cells in these two compartments. Autofluorescence did not confound the measurement of polyfunctional T cells, which coexpress IFN-γ, TNF-α, and IL-2, as no background staining in the FITC channel in unstimulated BAL cells affected the measurement of polyfunctional cells. Specific polyfunctional CD4 T cells could be reliably detected after stimulation of BAL cells with mycobacterial antigens. Cells coexpressing IFN-γ, TNF-α, and IL-2 were virtually undetectable in unstimulated BAL cells (Figure E2). Higher frequencies of BCG-specific polyfunctional CD4 T cells were detected in BAL compared with blood in HIV-1–uninfected persons (median 0.120 vs. 0.002%; P = 0.004; data not shown). The PPD-specific response showed a similar trend (median 0.187 vs. 0.0635%; P = 0.084; data not shown).
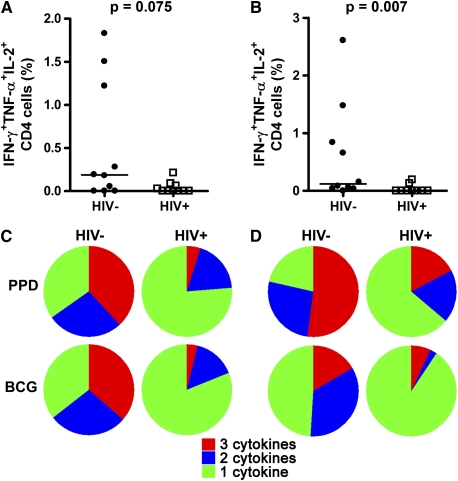
We next compared the frequency of polyfunctional mycobacteria-specific CD4 T cells in HIV-1–infected and –uninfected persons. Frequencies of PPD-specific polyfunctional BAL CD4 T cells tended to be lower in HIV-1–infected persons (median, 0.0 vs. 0.187%; P = 0.075; Figure 7A). When BCG was used as recall antigen, a lower BAL frequency of polyfunctional CD4 T cells was clearly observed in the HIV-1–infected group (median, 0.0 vs. 0.120%; P = 0.007, Figure 7B).
Figure 7.
Bronchoalveolar lavage (BAL) frequencies of mycobacteria-specific polyfunctional CD4 T cells are reduced in HIV-1 infection. Frequencies of (A) purified protein derivative (PPD)-specific or (B) bacillus Calmette-Guérin (BCG)-specific CD4 T cells in BAL from HIV-1–uninfected (HIV−) or HIV-1–infected (HIV+) persons. Horizontal lines represent the median. Differences were calculated using the Mann-Whitney U test. (C and D) Pie charts represent the median proportions of PPD- or BCG-specific polyfunctional (cells producing three cytokines, red), bifunctional (cells producing two cytokines, blue) and monofunctional (cells producing one cytokine, green) out of the total cytokine CD4 T-cell response. (C) BAL CD4 T-cell response. (D) Blood CD4 T-cell response.
The frequency of PPD- and BCG-specific polyfunctional CD4 T cells in peripheral blood was also lower in HIV-1–infected persons when compared with uninfected controls (PPD, median 0.011 vs. 0.064%; P = 0.0152; BCG, median, 0.001 vs. 0.009%; P = 0.023; data not shown).
Next, we compared the relative proportions of PPD- or BCG-specific monofunctional (cells producing one cytokine), bifunctional (two cytokines), and polyfunctional CD4 T cells (three cytokines) in the two compartments. PPD- and BCG-specific BAL cells from HIV-1–uninfected persons were predominantly bi- or polyfunctional, whereas more than 75% of these cells were monofunctional in HIV-1–infected persons (Figure 7C). A similar pattern was observed for mycobacteria-specific CD4 T cells from blood; cells from HIV-1–infected persons were predominantly monofunctional, whereas most cells from HIV-1–negative persons coexpressed two or three cytokines (Figure 7D). Interestingly, we observed a different pattern of cytokine expression between PPD- and BCG-stimulated peripheral blood CD4 T cells. The PPD-specific response appeared to be more polyfunctional in both HIV-1–infected and –uninfected persons than the BCG-specific response (Figure 7D). This was not observed for BAL responses.
DISCUSSION
To determine the impact of HIV-1 infection on the pulmonary CD4 T-cell response to mycobacteria in the lungs, we measured the immunological response of local and systemic innate and adaptive cells in HIV-1–infected and HIV-1–uninfected persons from an area of high tuberculosis incidence. Four major points emerged: (1) there is an equivalent growth of M. tuberculosis in alveolar macrophages from HIV-1–infected and HIV-1–uninfected persons, (2) the reduction in relative frequencies of CD4 T cells in the lung and in the peripheral blood of HIV-1–infected persons is similar, (3) proportion of BAL cells expressing the T-cell memory phenotype were not different in HIV-1–infected or HIV-1–uninfected persons, and (4) the frequency and functional quality of mycobacteria-specific pulmonary CD4 T cells is impaired in HIV-1–infected persons.
The role of alveolar macrophages in control of M. tuberculosis is still incompletely understood. Macrophages cocultured with autologous CD8 T cells from persons with high levels of exposure to M. tuberculosis may have enhanced capacity to limit mycobacterial growth (4). In this study, persons were recruited from a geographic region of high TB incidence, where the majority of persons have been exposed to M. tuberculosis. We found no difference in mycobacterial growth in monocytes or alveolar macrophages from HIV-1–infected and –uninfected persons. This finding is in agreement with previous studies reporting similar alveolar macrophage function in healthy controls and HIV-1–infected subjects (39–41). Notably, our results are consistent with those reported by Day and colleagues (39), who showed equivalent in vitro growth of M. tuberculosis in alveolar macrophages from healthy and HIV-1–infected subjects with peripheral CD4 counts less than 200 cells/μl. However, it has been shown that in vitro HIV-1 infection of human alveolar macrophages reduces M. tuberculosis–mediated TNF-α release and apoptosis (42). This may be an additional factor leading to the greater susceptibility of HIV-1–infected persons to develop TB. Additional studies are required to investigate this further.
The finding of reduced BAL CD4 T-cell frequencies in HIV-1–infected persons is consistent with other reports showing that HIV-1 infection is associated with depletion of CD4 cells at mucosal tissues, such as in the gastrointestinal tract (33). Another study found no depletion of CCR5+ memory CD4 T cells in BAL from HIV-1–infected persons, compared with healthy controls (29). These authors argued that quantification of total CD4 cells is confounded by the inverted CD4/CD8 ratios at mucosal sites, such as the gut, compared with blood. We did not observe inverted CD4/CD8 ratios in BAL. We also excluded smokers and persons with signs or symptoms of lung disease, as these are known to be common confounders when quantifying pulmonary cells (29). The median CD4 count in the HIV-1–infected group was relatively low, at 226 CD4 cells/μl. Although all HIV-1–infected participants were asymptomatic at the time of their study visit a considerable proportion of participants may have had progressive HIV-1 infection. Unfortunately we were unable to estimate the date of HIV-1 infection for participants in our study.
No differences in memory phenotypes of CD4 and CD8 T cells in BAL between HIV-1–infected and –uninfected persons were found; memory phenotypes of peripheral blood CD4 and CD8 T-cell populations from infected and uninfected subjects were also not different, as reported previously (37). Thus, HIV-1 infection may reduce CD4 T-cell frequencies, but does not appear to affect distributions of CD4 and CD8 phenotype.
The elevated risk of developing active TB on HIV-1 infection (23) suggests that mycobacteria-specific immunity is undermined early in the course of HIV-1 infection. Functional impairment of the mycobacteria-specific CD4 T-cell response in peripheral blood from HIV-1–infected persons has been well described (24, 25) and may persist during cART (26). A recent longitudinal study also showed that acute HIV-1 infection induced a rapid depletion of M. tuberculosis–specific responses in persons who remained free of TB symptoms (43).
Our study indicates that mycobacteria-specific pulmonary CD4 T cells, expressing IFNγ and TNFα, are depleted in HIV-1–infected persons. HIV-1–infected persons also presented with reduced BAL CD4 T-cell responses to the antigens ESAT-6/CFP-10, PPD, and BCG, compared with HIV-1–uninfected persons. Moreover, the relative enrichment of PPD- and BCG-specific CD4 T cells in BAL compared with blood from HIV-1–uninfected persons is consistent with previous studies showing that M. tuberculosis–specific Th1 cells localize to the site of infection in PPD-positive subjects (6, 16, 18). Our observation that BAL responses to PPD and BCG in the HIV-1–infected group were not higher than those in blood support the finding that lung T-cell responses are impaired in HIV-1–infected persons. When ESAT-6 and CFP-10 peptide pools were used as recall antigens, similar frequencies of specific CD4 T-cell responses were detected in BAL and blood from HIV-1–uninfected persons. In HIV-1–infected persons ESAT-6/CFP-10–specific responses were lower in BAL compared with blood.
The measurement of specific T-cell frequencies in BAL was limited by a lower sensitivity of detection in those individuals with low BAL CD4 T-cell numbers. This limitation was thus particularly relevant to data from the HIV-1–infected group, who had lower CD4 T-cell frequencies, and may be an important confounder in the comparisons of specific responses in BAL and blood from HIV-1–infected persons. Multiple other factors may contribute to the contrasting results when using the crude antigens, PPD/BCG, and the peptide antigens, ESAT-6/CFP-10. These antigen preparations may detect different subsets of T cells. T cells recognizing ESAT-6 and CFP-10 will only be present in M. tuberculosis–infected persons, whereas PPD and BCG contain a multiplicity of antigens resulting in higher immunogenicity and their stimulation allows detection of T cells induced by BCG vaccination, M. tuberculosis infection, and/or exposure to environmental mycobacteria.
Several studies have suggested that polyfunctional T cells may more effectively control intracellular infections than monofunctional T cells (13, 44, 45), including M. tuberculosis (14). We demonstrate that polyfunctional, mycobacteria-specific cells are markedly depleted in BAL from HIV-1–infected subjects with no evidence of TB. These findings point to a significant impairment of mycobacteria-specific T cells in the lungs of persons with HIV-1 infection, which may contribute to the significantly elevated risk of infected persons to develop tuberculosis (21, 22).
The relatively small number of subject samples, particularly for intracellular cytokine staining experiments, constitutes a limitation of this study. Ideally, larger sample sizes may have allowed more rigorous comparisons of groups. However, given the appreciable risk associated with bronchoscopy, especially in HIV-1–infected persons, larger sample sizes were judged not to be justifiable. We did not separate the data from ESAT-6/CFP-10–reactive and –nonreactive persons in our analyses, mostly due to the small sample sizes in our study. IFN-γ ELISpot assay reactivity in PBMC to ESAT-6 and/or CFP-10 was observed in 10 of 15 HIV-1–infected (66.7%) and 11 of 21 HIV-1–uninfected (52.4%) subjects. When using BCG or PPD as recall antigens in assays on persons from our high TB incidence setting, we typically observe no association between magnitude or functional profiles of T-cell responses and reactivity to RD-1 proteins.
In summary, significantly impaired numbers and/or function of M. tuberculosis–specific alveolar CD4 T cells, rather than function of alveolar macrophages, are associated with the increased susceptibility of persons with HIV-1 infection to develop TB and argue in favor of the earlier introduction of antiretroviral therapy in HIV-infected persons if TB is to be averted.
Supplementary Material
Acknowledgments
The authors thank the study participants. They also thank the staff at Ubuntu Clinic for assistance in recruitment, and Prof. Mark Nicol for providing the M. tuberculosis strain, H37Rv.
Supported by the German Research Foundation grant DFG SCHE1556 (B.K.) and German National Respiratory Society (D.G.P.). T.J.S. and R.J.W. are funded by the Wellcome Trust (072070, 080929, 084323). W.H. has additional support from the Aeras Global TB Vaccine Foundation, Gates Foundation, and the NIH (RO1-AI-065653 and NO1-AI-70022). R.J.W. also has support from the MRC (UK) and European Union.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200907-1011OC on September 24, 2009
Conflict of Interest Statement: B.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.J.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.W. holds a patent, United States 10/994191, confirmation number 1197, “Recombinant adenylate cyclase of Bordetella sp. for diagnostic and immunomonitoring uses, method of diagnosing or immunomonitoring using said recombinant adenylate cyclase, and kit for diagnosing or immunomonitoring comprising said recombinant adenylate cyclase” from Institut Pasteur. C.L.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.D. holds a patent, United States 10/994191, confirmation number 1197, “Recombinant adenylate cyclase of Bordetella sp. for diagnostic and immunomonitoring uses, method of diagnosing or immunomonitoring using said recombinant adenylate cyclase, and kit for diagnosing or immunomonitoring comprising said recombinant adenylate cyclase” from Institut Pasteur. W.A.H. received up to $1,000 from GlaxoSmithKline Bio in consultancy fees, up to $1,000 from NFID in lecture fees, more than $100,001 from the Aeras Global TB Foundation in sponsored grants, and more than $100,001 from the Gates Foundation in sponsored grants. C.L. received $1,001–$5,000 from Pfizer for organizing symposia, $1,001–$5,000 from AstraZeneca in lecture honorarium, $1,001–$5,000 from Oxfordimunotec in lecture honorarium, $1,001–$5,000 from Gilead Science in lecture honorarium, and up to $1,000 from Bayer Healthcare in lecture honorarium, and holds a patent, United States 10/994191, confirmation number 1197, “Recombinant adenylate cyclase of Bordetella sp. for diagnostic and immunomonitoring uses, method of diagnosing or immunomonitoring using said recombinant adenylate cyclase, and kit for diagnosing or immunomonitoring comprising said recombinant adenylate cyclase” from Institut Pasteur. R.J.W. holds a patent, United States 10/994191, confirmation number 1197, “Recombinant adenylate cyclase of Bordetella sp. for diagnostic and immunomonitoring uses, method of diagnosing or immunomonitoring using said recombinant adenylate cyclase, and kit for diagnosing or immunomonitoring comprising said recombinant adenylate cyclase” from Institut Pasteur.
References
- 1.Bates JH. Transmission and pathogenesis of tuberculosis. Clin Chest Med 1980;1:167–174. [PubMed] [Google Scholar]
- 2.Cailleaux-Cezar M, de A Melo D, Xavier GM, de Salles CL, de Mello FC, Ruffino-Netto A, Golub JE, Efron A, Chaisson RE, Conde MB. Tuberculosis incidence among contacts of active pulmonary tuberculosis. Int J Tuberc Lung Dis 2009;13:190–195. [PMC free article] [PubMed] [Google Scholar]
- 3.Chan J, Flynn J. The immunological aspects of latency in tuberculosis. Clin Immunol 2004;110:2–12. [DOI] [PubMed] [Google Scholar]
- 4.Carranza C, Juarez E, Torres M, Ellner JJ, Sada E, Schwander SK. Mycobacterium tuberculosis growth control by lung macrophages and CD8 cells from patient contacts. Am J Respir Crit Care Med 2006;173:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orme IM, Roberts AD, Griffin JP, Abrams JS. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J Immunol 1993;151:518–525. [PubMed] [Google Scholar]
- 6.Walrath J, Zukowski L, Krywiak A, Silver RF. Resident Th1-like effector memory cells in pulmonary recall responses to Mycobacterium tuberculosis. Am J Respir Cell Mol Biol 2005;33:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med 1993;178:2249–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ottenhoff TH, Kumararatne D, Casanova JL. Novel human immunodeficiencies reveal the essential role of type-I cytokines in immunity to intracellular bacteria. Immunol Today 1998;19:491–494. [DOI] [PubMed] [Google Scholar]
- 9.Chan J, Xing Y, Magliozzo RS, Bloom BR. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med 1992;175:1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, Schreiber R, Mak TW, Bloom BR. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 1995;2:561–572. [DOI] [PubMed] [Google Scholar]
- 11.Flesch IE, Kaufmann SH. Activation of tuberculostatic macrophage functions by gamma interferon, interleukin-4, and tumor necrosis factor. Infect Immun 1990;58:2675–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 2006;441:890–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 2007;13:843–850. [DOI] [PubMed] [Google Scholar]
- 14.Forbes EK, Sander C, Ronan EO, McShane H, Hill AVS, Beverley PCL, Tchilian EZ. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol 2008;181:4955–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breen RA, Janossy G, Barry SM, Cropley I, Johnson MA, Lipman MC. Detection of mycobacterial antigen responses in lung but not blood in HIV-tuberculosis co-infected subjects. AIDS 2006;20:1330–1332. [DOI] [PubMed] [Google Scholar]
- 16.Silver RF, Zukowski L, Kotake S, Li Q, Pozuelo F, Krywiak A, Larkin R. Recruitment of antigen-specific Th1-like responses to the human lung following bronchoscopic segmental challenge with purified protein derivative of Mycobacterium tuberculosis. Am J Respir Cell Mol Biol 2003;29:117–123. [DOI] [PubMed] [Google Scholar]
- 17.Dheda K, Chang JS, Breen RA, Kim LU, Haddock JA, Huggett JF, Johnson MA, Rook GA, Zumla A. In vivo and in vitro studies of a novel cytokine, interleukin 4delta2, in pulmonary tuberculosis. Am J Respir Crit Care Med 2005;172:501–508. [DOI] [PubMed] [Google Scholar]
- 18.Barry S, Breen R, Lipman M, Johnson M, Janossy G. Impaired antigen-specific CD4(+) T lymphocyte responses in cavitary tuberculosis. Tuberculosis (Edinb) 2009;89:48–53. [DOI] [PubMed] [Google Scholar]
- 19.Jafari C, Ernst M, Kalsdorf B, Greinert U, Diel R, Kirsten D, Marienfeld K, Lalvani A, Lange C. Rapid diagnosis of smear-negative tuberculosis by bronchoalveolar lavage enzyme-linked immunospot. Am J Respir Crit Care Med 2006;174:1048–1054. [DOI] [PubMed] [Google Scholar]
- 20.Jafari C, Ernst M, Strassburg A, Greinert U, Kalsdorf B, Kirsten D, Lange C. Local immunodiagnosis of pulmonary tuberculosis by enzyme-linked immunospot. Eur Respir J 2008;31:261–265. [DOI] [PubMed] [Google Scholar]
- 21.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet 2002;359:2059–2064. [DOI] [PubMed] [Google Scholar]
- 22.Lawn SD, Butera ST, Shinnick TM. Tuberculosis unleashed: the impact of human immunodeficiency virus infection on the host granulomatous response to Mycobacterium tuberculosis. Microbes Infect 2002;4:635–646. [DOI] [PubMed] [Google Scholar]
- 23.Sonnenberg P, Glynn JR, Fielding K, Murray J, Godfrey-Faussett P, Shearer S. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J Infect Dis 2005;191:150–158. [DOI] [PubMed] [Google Scholar]
- 24.Zhang M, Gong J, Iyer DV, Jones BE, Modlin RL, Barnes PF. T cell cytokine responses in persons with tuberculosis and human immunodeficiency virus infection. J Clin Invest 1994;94:2435–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elliott AM, Hurst TJ, Balyeku MN, Quigley MA, Kaleebu P, French N, Biryahwaho B, Whitworth JA, Dockrell HM, Hayes RJ. The immune response to Mycobacterium tuberculosis in HIV-infected and uninfected adults in Uganda: application of a whole blood cytokine assay in an epidemiological study. Int J Tuberc Lung Dis 1999;3:239–247. [PubMed] [Google Scholar]
- 26.Sutherland R, Yang H, Scriba TJ, Ondondo B, Robinson N, Conlon C, Suttill A, McShane H, Fidler S, McMichael A, et al. Impaired IFN-gamma-secreting capacity in mycobacterial antigen-specific CD4 T cells during chronic HIV-1 infection despite long-term HAART. AIDS 2006;20:821–829. [DOI] [PubMed] [Google Scholar]
- 27.Kaufmann SH. New issues in tuberculosis. Ann Rheum Dis 2004;63:ii50–ii56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor ML, Noble PW, White B, Wise R, Liu MC, Bochner BS. Extensive surface phenotyping of alveolar macrophages in interstitial lung disease. Clin Immunol 2000;94:33–41. [DOI] [PubMed] [Google Scholar]
- 29.Brenchley JM, Knox KS, Asher AI, Price DA, Kohli LM, Gostick E, Hill BJ, Hage CA, Brahmi Z, Khoruts A, et al. High frequencies of polyfunctional HIV-specific T cells are associated with preservation of mucosal CD4 T cells in bronchoalveolar lavage. Mucosal Immunol 2008;1:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newton SM, Smith RJ, Wilkinson KA, Nicol MP, Garton NJ, Staples KJ, Stewart GR, Wain JR, Martineau AR, Fandrich S, et al. A deletion defining a common Asian lineage of Mycobacterium tuberculosis associates with immune subversion. Proc Natl Acad Sci USA 2006;103:15594–15598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanekom WA, Hughes J, Mavinkurve M, Mendillo M, Watkins M, Gamieldien H, Gelderbloem SJ, Sidibana M, Mansoor N, Davids V, et al. Novel application of a whole blood intracellular cytokine detection assay to quantitate specific T-cell frequency in field studies. J Immunol Methods 2004;291:185–195. [DOI] [PubMed] [Google Scholar]
- 32.Lawn SD, Myer L, Edwards D, Bekker LG, Wood R. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS 2009; Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 33.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med 2004;200:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, Casazza JP, Kuruppu J, Kunstman K, Wolinsky S, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature 2002;417:95–98. [DOI] [PubMed] [Google Scholar]
- 35.Harari A, Petitpierre S, Vallelian F, Pantaleo G. Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1-infected subjects with progressive disease: changes after antiretroviral therapy. Blood 2004;103:966–972. [DOI] [PubMed] [Google Scholar]
- 36.Zimmerli SC, Harari A, Cellerai C, Vallelian F, Bart PA, Pantaleo G. HIV-1-specific IFN-gamma/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc Natl Acad Sci USA 2005;102:7239–7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmer BE, Boritz E, Wilson CC. Effects of sustained HIV-1 plasma viremia on HIV-1 Gag-specific CD4+ T cell maturation and function. J Immunol 2004;172:3337–3347. [DOI] [PubMed] [Google Scholar]
- 38.Scriba TJ, Zhang HT, Brown HL, Oxenius A, Tamm N, Fidler S, Fox J, Weber JN, Klenerman P, Day CL, et al. HIV-1-specific CD4+ T lymphocyte turnover and activation increase upon viral rebound. J Clin Invest 2005;115:443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Day RB, Wang Y, Knox KS, Pasula R, Martin WJ II, Twigg HL III. Alveolar macrophages from HIV-infected subjects are resistant to Mycobacterium tuberculosis in vitro. Am J Respir Cell Mol Biol 2004;30:403–410. [DOI] [PubMed] [Google Scholar]
- 40.Mwandumba HC, Russell DG, Nyirenda MH, Anderson J, White SA, Molyneux ME, Squire SB. Mycobacterium tuberculosis resides in nonacidified vacuoles in endocytically competent alveolar macrophages from patients with tuberculosis and HIV infection. J Immunol 2004;172:4592–4598. [DOI] [PubMed] [Google Scholar]
- 41.Elssner A, Carter JE, Yunger TM, Wewers MD. HIV-1 infection does not impair human alveolar macrophage phagocytic function unless combined with cigarette smoking. Chest 2004;125:1071–1076. [DOI] [PubMed] [Google Scholar]
- 42.Patel NR, Zhu J, Tachado SD, Zhang J, Wan Z, Saukkonen J, Koziel H. HIV impairs TNF-alpha mediated macrophage apoptotic response to Mycobacterium tuberculosis. J Immunol 2007;179:6973–6980. [DOI] [PubMed] [Google Scholar]
- 43.Geldmacher C, Schuetz A, Ngwenyama N, Casazza JP, Sanga E, Saathoff E, Boehme C, Geis S, Maboko L, Singh M, et al. Early depletion of Mycobacterium tuberculosis-specific T helper 1 cell responses after HIV-1 infection. J Infect Dis 2008;198:1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J Virol 2007;81:8468–8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 2006;107:4781–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.