Abstract
Rationale: Gold miners in South Africa undergo annual radiological screening for tuberculosis in an occupational health center of a gold mining company, but the optimal screening algorithm is unclear.
Objectives: To evaluate methods for active case detection of tuberculosis.
Methods: A sequential sample of miners attending annual medical examination was screened for tuberculosis using a symptom questionnaire, chest radiograph, and two sputum specimens for microscopy and culture.
Measurements and Main Results: There were 1,955 miners included in this study; all were male with a median age of 41 years (range, 20–61 yr). Presence of at least one of a trio of symptoms (new or worsening cough, night sweats, or weight loss) had similar sensitivity (29.4%) to either chest radiograph (25.5%) or sputum smear (25.5%). These sensitivities did not differ by HIV status. Presence of one or more elements of the symptom trio and/or new radiological abnormality substantially increased sensitivity to 49.0%. Specificity of the symptom trio was higher in HIV-uninfected (91.8%) than in HIV-infected persons (88.2%; P = 0.018). Specificity of chest radiography and smear were similar (98.7% and 99.0%, respectively) and did not differ by HIV status (both P values > 0.8).
Conclusions: In a population of gold miners who undergo regular radiological screening, the addition of chest radiography to symptom screening substantially improved the sensitivity and positive predictive value. HIV infection did not alter the sensitivity of the screening tool.
Keywords: tuberculosis, HIV infection, case finding
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
The World Health Organization Stop TB strategy includes tuberculosis case finding; however, the optimal tuberculosis screening algorithm and the impact of HIV on screening remain unclear.
What This Study Adds to the Field
The results of this study demonstrate that the addition of chest radiography to symptom screening significantly increases sensitivity with minimal loss of specificity and that the performance of tuberculosis screening is unaffected by HIV. These results will be used to inform international recommendations on the optimum tuberculosis screening strategy and encourage use of tuberculosis screening in high HIV prevalence settings.
Tuberculosis incidence has increased in most African countries, including those with well-run tuberculosis control programs, largely due to the HIV epidemic (1). The detection, treatment and cure of sputum smear–positive pulmonary tuberculosis is central to effective tuberculosis control. The World Health Organization's (WHO) Directly Observed Therapy, Short course (DOTS) strategy for tuberculosis control relied on detection of tuberculosis cases through self-presentation to health services with symptoms (2). This policy was based on studies undertaken in the pre-HIV era, which demonstrated that various active tuberculosis case-finding methods had small yield, generated large numbers of tuberculosis suspects requiring investigation, and that most patients detected by the screening programs had presented previously to a health service with respiratory symptoms (3–5).
In response to the HIV-associated tuberculosis epidemic, the WHO Stop TB program now includes intensive tuberculosis case finding as part of the strategy to reduce the burden of tuberculosis in HIV-infected individuals (6). Active case finding may contribute to tuberculosis control by reducing tuberculosis transmission through earlier diagnosis and treatment. However, the optimal tuberculosis screening strategies to be used in active case finding remain uncertain.
Since the early 1990s, the incidence of tuberculosis among gold miners working in South Africa has increased fourfold, as a result of the combined effects of the HIV epidemic and silicosis (7). The aim of this study was to determine the performance characteristics of various screening methods for the active case finding of pulmonary tuberculosis among the general population of miners and to investigate whether they differed by HIV status. Some of the results of these studies have been reported previously in the form of abstracts (8–10) and data from this study have been used in a previous publication describing the impact of HIV infection on tuberculosis prevalence and incidence in this population (11).
METHODS
Study Population and Site
All employees of the gold mining industry undergo annual medical examinations to determine fitness to work. These include radiological screening for tuberculosis and silicosis and a urine specimen for testing for glucose. We conducted this study at an occupational health center of a single gold mining company in the Free State Province, South Africa from July 2000 to January 2001. Some of the methods have been published previously (11). Briefly, alternate miners attending their annual medical examination were sampled; permanent employees were included and contractors were excluded.
All consenting miners underwent the following study procedures in addition to the routine mini–chest radiograph (100 mm × 100 mm): screening for tuberculosis with a symptom questionnaire (new or worsening cough and duration of cough, new or worsening sputum production, hemoptysis, night sweats, fever and duration of fever, and weight loss of more than 5 kg in previous 6 months) and collection of two sputum specimens for microscopy and culture 1 hour apart. The previous routine mini chest radiograph was obtained. The routinely collected urine specimen was used for HIV testing.
Individuals who had any symptom listed above, a new or changing radiological lesion compared with the previous chest radiograph, a positive sputum smear (positive defined as any of scanty, 1+, 2+, or 3+), or positive culture (hereafter referred to as the “screening” results) were defined as tuberculosis suspects. These tuberculosis suspects were referred for further investigation, wherein a standardized protocol included a standard size chest radiograph and three sputum specimens for microscopy, culture, and organism identification, referred to as the “review” results. The study team then reviewed all results and applied case definitions.
Mycobacteriology and HIV testing were as previously described, with sputum specimens examined using auramine staining and fluorochrome microscopy, and cultured on Löwenstein-Jensen media. Species identification was performed using the Genprobe polymerase chain reaction method for Mycobacterium tuberculosis and M. kansasii and standard biochemical tests for other species (12, 13). Radiography at screening and review were conducted by separate radiologists, both blinded to all other results. The mini chest radiograph was assessed for presence and grade of silicosis using a modified International Labour Organization scoring system (14).
Case Definitions
Due to the possibility of false-positive results at screening, the case definitions took into account results from both screening and review procedures, and a single positive sputum culture without supportive clinical evidence was not considered as evidence of tuberculosis. Participants were categorized as having definite pulmonary tuberculosis if they had at least one positive sputum culture with more than five colonies of M. tuberculosis and at least one of the following: compatible clinical features, compatible radiological features, or another sputum that was positive on culture. Participants were categorized as having presumed pulmonary tuberculosis if they had all of the following: compatible clinical features, no clinical response to 5 days of antibiotics before commencing tuberculosis treatment, response to tuberculosis treatment within 2 months, and were either smear positive or had new radiological abnormalities.
Ethics Approval
Approval was obtained from the ethics committees of Anglogold Health Service, London School of Hygiene and Tropical Medicine, and University of the Witwatersrand. Written, or witnessed oral, informed consent was obtained from all participants.
Data Analysis
Data were analyzed using Stata 9.1 for Windows (Stata Corporation, College Station, TX). Fisher exact test was used to assess differences in demographic characteristics by HIV status, as well as differences in sensitivity and specificity by HIV status, previous tuberculosis, and silicosis. All confidence intervals (CIs) were exact, except those for odds ratios (ORs). Univariable and multivariable logistic regressions examined associations between symptoms and tuberculosis, adjusting for confounders.
Information on current or previous tuberculosis treatment was ascertained by cross-referencing with the company tuberculosis database. Those on tuberculosis treatment at time of screening were excluded from analysis. Those with a record of prior tuberculosis treatment that had been completed at any time before screening were classified as having previous tuberculosis.
We considered any positive mycobacterial culture, regardless of whether M. tuberculosis was identified, as defining a tuberculosis suspect who required further investigation. By contrast, in the analysis of “positive sputum culture” as a screening tool, we required that M. tuberculosis was specifically identified.
RESULTS
A total of 2,240 miners were invited to participate in the study, 262 (12%) of whom refused participation. Hence, 1,978 participants were screened for tuberculosis. Eighteen participants on tuberculosis treatment at the time of screening and five participants who were found to have nontuberculous mycobacterial disease on sputum culture during the study were excluded, leaving 1,955 participants in this analysis.
Participant Demographics
The demographic characteristics of these 1,955 participants are shown in Table 1. All participants were male, with a median age of 41 years (range, 20–61 yr) and a median employment duration of 19 years (range, 1–43 yr). Previous tuberculosis was reported by 9.8% of participants. Any silicosis (International Labour Association grade > 0/0) was diagnosed on chest radiograph for 26.0% of participants. HIV prevalence was 29.0% (95% CI, 27.0–31.0%). The HIV-infected participants were more likely to be younger, have a shorter duration of employment, be non–South African, and to live in a single-sex hostel; they were also more likely to report previous tuberculosis. HIV status was not associated with either working underground or with silicosis.
TABLE 1.
DEMOGRAPHIC CHARACTERISTICS OF STUDY PARTICIPANTS BY HIV STATUS (N = 1,955)
| HIV-uninfected (N = 1,388) |
HIV-infected (N = 567) |
||||
|---|---|---|---|---|---|
| Demographic Variable | N | % | N | % | P Value |
| Age group, years | |||||
| <30 | 70 | 5.0 | 41 | 7.2 | 0.001 |
| 30–39 | 470 | 33.9 | 234 | 41.3 | |
| 40–49 | 632 | 45.5 | 226 | 39.9 | |
| ≥50 | 216 | 15.6 | 66 | 11.6 | |
| Employment duration, yr | |||||
| <10 | 206 | 14.8 | 115 | 20.3 | <0.001 |
| 10–19 | 457 | 32.9 | 220 | 38.8 | |
| 20–29 | 559 | 40.3 | 176 | 31.0 | |
| ≥30 | 166 | 12.0 | 56 | 9.9 | |
| Country of origin | |||||
| South African | 729 | 52.5 | 250 | 44.1 | 0.001 |
| Non–South African | 659 | 47.5 | 317 | 55.9 | |
| Residence | |||||
| Non-hostel | 370 | 26.7 | 122 | 21.5 | 0.019 |
| Hostel | 1,018 | 73.3 | 445 | 78.5 | |
| Place of Work | |||||
| Surface | 93 | 6.7 | 43 | 7.6 | 0.494 |
| Underground | 1,295 | 93.3 | 524 | 92.4 | |
| Silicosis | |||||
| Absent | 1,002 | 72.2 | 416 | 73.4 | 0.751 |
| Early | 256 | 18.4 | 106 | 18.7 | |
| Advanced | 103 | 7.4 | 34 | 6.0 | |
| Missing | 27 | 1.9 | 11 | 1.9 | |
| Previous tuberculosis | |||||
| No | 1,280 | 92.2 | 484 | 85.4 | <0.001 |
| Yes |
108 |
7.8 |
83 |
14.6 |
|
P values are from a Fisher exact test for differences in characteristics by HIV status. Silicosis categories were: absent (International Labour Organization [ILO] grade: 0/0), early (ILO grade: 0/1 or 1/0), or advanced (ILO grade: 1/1, 2/2 or 3/3).
Prevalence of Positive Screening Criteria
The most common symptoms reported were night sweats (6.0%), weight loss (5.2%), and cough of any duration (2.5%); 10.7% of miners reported at least one symptom. All symptoms, except hemoptysis, were more common among HIV-infected participants, but there was strong evidence for an association with HIV status only for sputum production, night sweats, and weight loss (Table 2). Thirty-eight participants (1.9%) had a new or changing chest radiographic abnormality, with no difference by HIV status (P > 0.999). Thirty-two participants (1.6%) had at least one positive sputum smear at screening, again with no difference by HIV status (P = 0.844). Forty participants (2.0%) had at least one positive culture for M. tuberculosis at screening and this was more common among HIV-infected participants than HIV-uninfected participants (3.2% vs. 1.6%, respectively; P = 0.033).
TABLE 2.
PREVALENCE OF SYMPTOMS, CHEST RADIOGRAPH ABNORMALITIES, AND LABORATORY FINDINGS AT SCREENING, BY HIV STATUS (N = 1,955)
| HIV-uninfected (N = 1,388) |
HIV-infected (N = 567) |
||||
|---|---|---|---|---|---|
| Screening Method | N | % | N | % | P Value |
| Symptom | |||||
| New or worsening cough | |||||
| Any duration | 29 | 2.1 | 20 | 3.5 | 0.079 |
| >2 wk | 12 | 0.9 | 9 | 1.6 | 0.224 |
| >3 wk | 9 | 0.7 | 9 | 1.6 | 0.065 |
| New or worsening sputum production | 12 | 0.9 | 13 | 2.3 | 0.015 |
| Hemoptysis | 11 | 0.8 | 3 | 0.5 | 0.769 |
| Night sweats | 69 | 5.0 | 49 | 8.6 | 0.003 |
| Fever | |||||
| Any duration | 19 | 1.4 | 11 | 1.9 | 0.417 |
| >2 wk | 6 | 0.4 | 4 | 0.7 | 0.488 |
| >3 wk | 6 | 0.4 | 4 | 0.7 | 0.488 |
| Weight loss >5kg | 62 | 4.5 | 39 | 6.9 | 0.032 |
| At least one symptom | 133 | 9.6 | 75 | 13.2 | 0.023 |
| New or changing radiological abnormality | 27 | 1.9 | 11 | 1.9 | >0.999 |
| Smear positive | |||||
| First specimen | 19 | 1.4 | 10 | 1.8 | 0.538 |
| Second specimen | 22 | 1.6 | 6 | 1.1 | 0.529 |
| Combined | 22 | 1.6 | 10 | 1.8 | 0.844 |
| Culture positive | |||||
| First specimen | 18 | 1.3 | 16 | 2.8 | 0.034 |
| Second specimen | 20 | 1.4 | 13 | 2.3 | 0.181 |
| Combined |
22 |
1.6 |
18 |
3.2 |
0.033 |
P values are from a Fisher exact test for differences in prevalences by HIV status. Data on presence of cough, hemoptysis or night sweats were missing for each of three separate HIV-uninfected participants. Data on duration of cough were missing for five HIV-uninfected participants and one HIV-infected participant. Data on duration of fever were missing for three HIV-uninfected participants and two HIV-infected participants.
There were 282 participants (14.4%) with at least one positive screening criterion who hence required further investigation. The median time from screening visit to review visit among all tuberculosis suspects was 59 days (interquartile range, 43–77 d) due to the long delay in obtaining Löwenstein-Jensen culture results.
Characteristics of Tuberculosis Cases
There were 51 definite (20/51 [39.2%] HIV-infected) and no presumed cases of tuberculosis (see Table E1 in the online supplement). None of these tuberculosis cases were exclusively extrapulmonary. At screening, 15 of the 51 (29.4%) tuberculosis cases were symptomatic, 10 were asymptomatic with new or changing radiographic abnormalities (19.6%), and 26 were asymptomatic with no new or changing radiographic abnormalities (51.0%) (7 smear positive and 19 smear negative, detected by culture); these proportions did not differ by smear status (Fisher exact test P > 0.999). By the time of the review visit, this had changed to 22 of 47 tuberculosis cases being symptomatic (47%; review data were missing for four tuberculosis cases), 21 being asymptomatic with new or changing radiographic abnormalities (45%), and only 4 remaining asymptomatic with no new or changing radiographic abnormalities (9%; these 4 met case definitions by bacteriological confirmation). Only 13 of the 51 (25.5%) tuberculosis cases were smear positive at screening (8/31 for HIV-uninfected and 5/20 for HIV-infected; Fisher exact test P > 0.999). Ten of the 13 smear-positive and 17 of the 38 smear-negative cases at screening were smear positive at review, and so a total of 52.9% (27/51) of the tuberculosis cases were smear positive at review (18/31 for HIV-uninfected and 9/20 for HIV-infected; Fisher exact test P = 0.402).
Performance of Screening Methods
The usefulness of the various screening methods depends on their sensitivity and specificity, which may be expected to differ by HIV status. Hence, these are shown by HIV status in Table 3. Sensitivity did not differ by HIV status for any of the screening methods. The three most sensitive symptoms were: new or worsening cough, regardless of duration; night sweats; and weight loss. The combination of these three symptoms is referred to as the symptom trio. Presence of at least one of the symptom trio gave a sensitivity of 29.4%, which was similar to that of new or changing radiographic abnormalities (25.5%) or smear positivity (25.5%) and did not differ by HIV status. The sensitivity of culture positivity at screening was substantially higher at 78.4%, although this was not 100% because some of those who were culture negative at screening had positive cultures at the review visit. Specificity of the symptom trio (90.7%) was lower than for new or changing radiographic abnormalities (98.7%), smear positivity (99.0%), or culture positivity (100.0%). Specificity of the symptom trio was higher in HIV-uninfected participants than HIV-infected participants (91.8% vs. 88.2%; P = 0.018). The specificities of new or changing radiographic abnormalities, smear positivity, and culture positivity did not differ by HIV status.
TABLE 3.
SENSITIVITY AND SPECIFICITY OF SYMPTOMS AND INVESTIGATIONS AT SCREENING FOR ACTIVE CASE FINDING OF PULMONARY TUBERCULOSIS, BY HIV STATUS
| Sensitivity (95% CI) |
Specificity (95% CI) |
|||||
|---|---|---|---|---|---|---|
| Screening Method | HIV-uninfected (N = 31) | HIV-infected (N = 20) | P Value | HIV-uninfected (N = 1347) | HIV-infected (N = 544) | P Value |
| Symptom | ||||||
| New or worsening cough | ||||||
| Any duration | 16.1 (5.5–33.7) | 15.0 (3.2–37.9) | >0.999 | 98.6 (97.8–99.1) | 97.1 (95.3–98.3) | 0.036 |
| >2 wk | 9.7 (2.0–25.8) | 5.0 (0.1–24.9) | >0.999 | 99.3 (98.7–99.7) | 98.5 (97.1–99.4) | 0.108 |
| >3 wk | 6.5 (0.8–21.4) | 5.0 (0.1–24.9) | >0.999 | 99.5 (98.9–99.8) | 98.5 (97.1–99.4) | 0.045 |
| New or worsening sputum production | 16.1 (5.5–33.7) | 0.0 (0.0–16.8) | 0.143 | 99.5 (98.9–99.8) | 97.6 (95.9–98.7) | 0.001 |
| Hemoptysis | 6.5 (0.8–21.4) | 0.0 (0.0–16.8) | 0.514 | 99.3 (98.7–99.7) | 99.4 (98.4–99.9) | 1 |
| Night sweats | 25.8 (11.9–44.6) | 25.0 (8.7–49.1) | >0.999 | 95.6 (94.4–96.6) | 92.1 (89.5–94.2) | 0.003 |
| Fever | ||||||
| Any duration | 6.5 (0.8–21.4) | 0.0 (0.0–16.8) | 0.514 | 99 (98.3–99.4) | 98.3 (96.9–99.2) | 0.257 |
| >2 wk | 6.5 (0.8–21.4) | 0.0 (0.0–16.8) | 0.514 | 99.7 (99.2–99.9) | 99.3 (98.1–99.8) | 0.238 |
| >3 wk | 6.5 (0.8–21.4) | 0.0 (0.0–16.8) | 0.514 | 99.7 (99.2–99.9) | 99.3 (98.1–99.8) | 0.238 |
| Weight loss >5kg | 19.4 (7.5–37.5) | 10.0 (1.2–31.7) | 0.456 | 95.9 (94.7–96.9) | 93.6 (91.2–95.5) | 0.032 |
| ≥1 symptom | 29 (14.2–48.0) | 30.0 (11.9–54.3) | >0.999 | 91.3 (89.7–92.8) | 87.9 (84.8–90.5) | 0.025 |
| Symptom trio* | 29 (14.2–48.0) | 30.0 (11.9–54.3) | >0.999 | 91.8 (90.2–93.2) | 88.2 (85.2–90.8) | 0.018 |
| New or changing radiological abnormality | 25.8 (11.9–44.6) | 25.0 (8.7–49.1) | >0.999 | 98.6 (97.8–99.1) | 98.9 (97.6–99.6) | 0.824 |
| Smear positive | ||||||
| First specimen | 22.6 (9.6–41.1) | 25.0 (8.7–49.1) | >0.999 | 99.1 (98.4–99.5) | 99.1 (97.9–99.7) | > 0.999 |
| Second specimen | 25.8 (11.9–44.6) | 20.0 (5.7–43.7) | 0.743 | 99.0 (98.3–99.4) | 99.6 (98.7–100.0) | 0.176 |
| Combined | 25.8 (11.9–44.6) | 25.0 (8.7–49.1) | >0.999 | 99.0 (98.3–99.4) | 99.1 (97.9–99.7) | > 0.999 |
| Culture positive | ||||||
| First specimen | 58.1 (39.1–75.5) | 80.0 (56.3–94.3) | 0.135 | 100.0 (99.7–100.0) | 100.0 (99.3–100.0) | — |
| Second specimen | 64.5 (45.4–80.8) | 65.0 (40.8–84.6) | >0.999 | 100.0 (99.7–100.0) | 100.0 (99.3–100.0) | — |
| Combined |
71 (52.0–85.8) |
90.0 (68.3–98.8) |
0.166 |
100.0 (99.7–100.0) |
100.0 (99.3–100.0) |
— |
Definition of abbreviation: CI = confidence interval.
There were 13 participants who were missing data on at least one of these screening methods; they have been removed from this analysis.
At least one of new or worsening cough (any duration), night sweats, or weight loss.
Combining the results from two sputum smears did not greatly increase the sensitivity from that of the first or second sputum smear alone (combining HIV-infected and HIV-uninfected in Table 3: 25.5% vs. 23.5% and 23.5%, respectively). In contrast, combining the results from two cultures increased the sensitivity from 66.7% and 64.7% (first and second cultures, respectively) to 78.4%. Specificities were unchanged by combining either two sputum smear results or two culture results (Table 3). There were 19 participants with positive smear results at screening who did not meet case definition for tuberculosis, none of whom had any positive cultures at screening or review; of these, 5 were scanty on one of the smears (the other smear being negative) and 14 were scanty on both smears. In contrast, for the 13 participants with positive smear results at screening who did fulfill case definitions, 2 were scanty on one of the smears, 3 were scanty on both smears, 7 were 1+ on both smears, and 1 participant was 3+ on both smears.
Some participants reported more than one symptom (4.8% of HIV-infected and 2.6% of HIV-uninfected; P = 0.017). Hence, logistic regression was used to determine which symptoms were independently predictive of tuberculosis (Table 4). In a multivariable logistic regression of all six symptoms, there were strong associations between tuberculosis and both new or worsening cough and night sweats; these associations were not confounded by HIV, previous tuberculosis, or any silicosis (Table 4). A separate multivariable logistic regression containing just new or worsening cough and night sweats showed that these associations remained strong (OR for cough, 3.6; 95% CI, 1.3–9.8; OR for night sweats, 3.7; 95% CI, 1.6–8.3). Dropping weight loss from the symptom trio reduced sensitivity from 29.4 to 27.5% and increased specificity from 90.7 to 93.6%. As the focus of this article is active case finding and this symptom trio has been reported in many other articles, loss of weight was retained in the symptom combination under evaluation.
TABLE 4.
LOGISTIC REGRESSION ANALYSIS OF SYMPTOMS AT SCREENING AS PREDICTORS OF PULMONARY TUBERCULOSIS (N = 1,914)
| Univariable |
Multivariable |
|||||
|---|---|---|---|---|---|---|
| Predictor of Pulmonary Tuberculosis | OR | 95% CI | P Value | OR | 95% CI | P Value |
| New or worsening cough | 8.48 | (3.75–19.20) | <0.001 | 3.72 | (0.90–15.39) | 0.069 |
| New or worsening sputum production | 10.02 | (3.60–27.85) | <0.001 | 0.93 | (0.17–4.90) | 0.927 |
| Hemoptysis | 6.30 | (1.37–28.89) | 0.018 | 1.12 | (0.19–6.67) | 0.902 |
| Night sweats | 5.79 | (2.99–11.20) | <0.001 | 3.63 | (1.55–8.52) | 0.003 |
| Fever | 2.67 | (0.62–11.54) | 0.187 | 0.46 | (0.08–2.56) | 0.379 |
| Weight loss | 3.62 | (1.65–7.93) | 0.001 | 1.32 | (0.45–3.84) | 0.612 |
| HIV | 1.60 | (0.90–2.83) | 0.108 | 1.46 | (0.80–2.64) | 0.215 |
| Previous tuberculosis | 1.22 | (0.52–2.91) | 0.647 | 0.74 | (0.29–1.91) | 0.535 |
| Any silicosis |
1.57 |
(0.87–2.81) |
0.131 |
1.55 |
(0.84–2.85) |
0.162 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio.
There were 41 participants who had missing data on at least one of these predictors; they were removed from the analysis. All variables were included in the multivariable analysis.
Sensitivities of the four screening methods did not differ by presence or absence of silicosis (data not shown; all P values > 0.3) or reported previous tuberculosis (data not shown; all P values > 0.15). Specificities of both symptom trio and new or changing radiographic abnormalities were lower in the presence of silicosis than in its absence (87.7% vs. 91.9%, P = 0.008; and 97.1% vs. 99.3%, P < 0.001, respectively). Specificity of smear did not differ by presence or absence of silicosis (P > 0.999). Specificity of the symptom trio was lower in the presence of reported previous tuberculosis than in its absence (83.2% vs. 91.6%; P = 0.001), whereas the specificities of new or changing radiographic abnormalities and sputum smear did not differ by presence or absence of reported previous tuberculosis (P > 0.1).
The addition of various combinations of new or changing radiographic abnormalities, smear, and/or culture to the symptom trio may substantially improve its sensitivity, without major loss of specificity. The performances of various combinations are given in Table 5. Combining symptom trio with either new or changing radiographic abnormalities or smear positivity gives an almost additive increase in sensitivity, with very little decrease in specificity. The combination of symptom trio, new or changing radiographic abnormalities, smear, and culture gives a very high sensitivity (98.0%) with reasonable specificity (88.6%).
TABLE 5.
SENSITIVITY AND SPECIFICITY OF COMBINATIONS OF SYMPTOMS AND INVESTIGATIONS AT SCREENING FOR THE ACTIVE CASE FINDING OF PULMONARY TUBERCULOSIS
| Combinations of Screening Methods | Prevalence of Criterion (N = 1,953) | Sensitivity (95% CI) (N = 51) | Specificity (95% CI) (N = 1,902) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|
| Symptom trio* | 199 (10.2%) | 29.4 (17.5–43.8) | 90.3 (88.9–91.6) | 7.5 (4.3–12.1) | 97.9 (97.2–98.6) |
| New chest radiograph abnormality | 38 (2.0%) | 25.5 (14.3–39.6) | 98.7 (98.1–99.1) | 34.2 (19.6–51.4) | 98.0 (97.3–98.6) |
| Smear positive | 32 (1.6%) | 25.5 (14.3–39.6) | 99.0 (98.4–99.4) | 40.6 (23.7–59.4) | 98.0 (97.3–98.6) |
| Culture positive | 40 (2.1%) | 78.4 (64.7–88.7) | 100.0 (99.8–100.0) | 100.0 (91.2–100.0) | 99.4 (99.0–99.7) |
| Symptom trio and/or CXR | 226 (11.6%) | 49.0 (34.8–63.4) | 89.4 (88.0–90.8) | 11.1 (7.3–15.9) | 98.5 (97.8–99.0) |
| Symptom trio and/or smear positive | 224 (11.5%) | 47.1 (32.9–61.5) | 89.5 (88.0–90.8) | 10.7 (7.0–15.5) | 98.4 (97.7–99.0) |
| Symptom trio and/or CXR and/or smear positive | 248 (12.7%) | 62.7 (48.1–75.9) | 88.6 (87.1–90.0) | 12.9 (9.0–17.7) | 98.9 (98.3–99.3) |
| Symptom trio and/or smear positive and/or culture positive | 245 (12.5%) | 88.2 (76.1–95.6) | 89.5 (88.0–90.8) | 18.4 (13.7–23.8) | 99.6 (99.2–99.9) |
| Symptom trio and/or CXR and/or smear positive and/or culture positive |
266 (13.6%) |
98.0 (89.6–100.0) |
88.6 (87.1–90.0) |
18.8 (14.3–24.0) |
99.9 (99.7–100.0) |
Definition of abbreviations: CI = confidence interval; CXR = new or changing radiological abnormality; NPV = negative predictive value; PPV = positive predictive value.
There were two participants who were missing data on at least one of these screening methods; they have been removed from this analysis.
At least one of new or worsening cough (any duration), night sweats, or weight loss.
The positive and negative predictive values are given in Table 5. The positive predictive values of symptom trio, new or changing radiographic abnormalities, and smear positivity were low (all <41%), which would result in a large number of tuberculosis suspects being unnecessarily investigated further. The negative predictive values were all very high (lowest = 97.9%).
Sensitivity of symptom trio did not differ between the 13 smear-positives at screening (30.8%) and the 38 smear-negatives (29.0%; P > 0.999). Sensitivity of new or changing radiographic abnormalities was higher among the 13 smear-positives (38.5%) than the 38 smear-negatives (21.1%), but the difference was not statistically significant (P = 0.274), possibly due to low power.
DISCUSSION
In order for community-based, active case finding for tuberculosis to be efficient in high HIV prevalence settings, the performance of the algorithm used for tuberculosis screening should be unaffected by HIV status. For active tuberculosis case finding the screening algorithm should have a high sensitivity, to ensure that as few tuberculosis cases as possible are missed, and a high specificity, to minimize the number of tuberculosis suspects investigated inappropriately for tuberculosis, thereby reducing costs and the burden on the laboratories.
The main findings of this study are that adding chest radiography (defined as new or changing radiographic abnormalities) to symptom screening substantially improved the sensitivity of the screening tool, compared with symptom screening alone, as seen in some other studies (15, 16), and that tuberculosis screening was not substantially affected by HIV status.
This study was conducted before antiretroviral therapy was available in South Africa outside of private clinics or clinical trials and so it is extremely unlikely that any of the participants would have been taking antiretroviral therapy. CD4 counts were not determined among HIV-infected participants in this study, preventing investigation of the impact of immunosuppression. Although the number of tuberculosis cases was relatively small, the sensitivities were generally similar by HIV status, suggesting that low statistical power to detect differences was unlikely to have biased the conclusions. Specificity was lower in HIV-infected than HIV-uninfected participants; this may be because a greater proportion of HIV-infected than HIV-uninfected participants who did not have tuberculosis nonetheless had symptoms and new or changing radiographic abnormalities suggestive of tuberculosis.
In this study, around half of undiagnosed tuberculosis cases (26 of 51) found by active case finding were both asymptomatic and with no new or changing radiological abnormalities at screening. However, at the review visit, disease progression was clear with only four cases still asymptomatic with no new or changing radiological abnormalities (but confirmed bacteriologically). In addition, at screening only 13 of 51 tuberculosis cases were smear positive and these 13 had a low degree of smear positivity (5 were scanty, 7 were 1+, and only 1 was 3+). Yet, in the interval between screening and review, 17 of the 38 smear-negative cases became smear positive. These results are similar to those from a study screening 4,668 employees of 22 businesses in Harare, Zimbabwe for prevalent tuberculosis disease. This study detected active tuberculosis in 27 employees (0.5%), 11 of whom were detected only through culture, yet by follow-up, symptoms had developed in all but two participants (17). This provides strong evidence that active case finding contributes to tuberculosis control by finding cases earlier and so reducing the duration of infectiousness. It also suggests that during active case finding, scanty positives should be investigated further, as many of these may be tuberculosis cases in early stages of disease.
Chest radiography was used extensively for tuberculosis case finding in Europe, Canada, Japan, and the United States during the 20th century and contributed to the control of tuberculosis in these countries (18). Consistent with the results from this study, a tuberculosis survey done in two Cape Town communities found that the addition of chest radiography to symptom screening increased the sensitivity from 69% for symptom screening alone to 90% for combined symptom and chest radiography screening (16). Although radiographic facilities are scarce in resource-limited settings, affordable, mobile, digital chest radiographic units are becoming available that will enable mass tuberculosis screening in some resource-limited settings, with appropriate infrastructure, and this should improve the performance of active tuberculosis case finding when combined with symptom screening. Although the combination of symptoms, chest radiography, and sputum microscopy and culture provides the best sensitivity and further improves the positive predictive value, it would not be generalizable as microscopy services are already overloaded and few resource-limited countries have culture facilities.
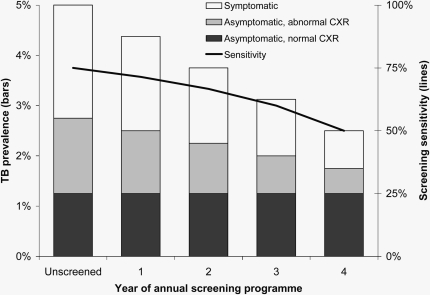
It is notable that in this study the sensitivity of new or changing radiographic abnormalities alone (25.5%) was considerably lower than that reported in other populations (15, 16) but is similar to that reported in a study of screening for tuberculosis in adults with advanced HIV infection (19). This low sensitivity may in part reflect the difficulties of identifying tuberculosis against a background of occupational lung disease in this mining population or a lower sensitivity of mini chest radiographs compared with standard size chest radiographs. Alternatively this observation could indicate that sensitivity may change with time when any given screening tool is repeatedly applied to the same population. Figure 1 illustrates how such a situation could arise if a substantial fraction of undetected tuberculosis remains active but untreated for a substantially longer period than the interval between successive screens. This demonstrates that secular changes in the sensitivity of a screening tool can be expected if it is used repeatedly in the same population. The decreasing sensitivity should not be taken as an indication to stop the screening program, but rather that the program is working by reducing the proportion of true active tuberculosis cases that are detectable using the screening tool after successive rounds of screening (assuming correct subsequent treatment). Another possible, but highly speculative, explanation is that the selective pressure of approximately 50 years of radiological screening in this population has pushed the circulating tuberculosis strains toward ones that are less readily detectable by radiological screening.
Figure 1.
Schematic of the likely impact of an annual active case-finding program of symptom screen and chest radiograph on tuberculosis prevalence and sensitivity of the screening. Prevalent tuberculosis is divided into symptomatic tuberculosis disease, asymptomatic tuberculosis disease with radiographic abnormalities, and asymptomatic tuberculosis disease with no radiographic abnormalities. Assumptions: (1) Tuberculosis prevalence at final year is 2.5% and is 30% symptomatic, 20% asymptomatic with radiographic abnormalities, and 50% asymptomatic with no radiographic abnormalities, as reported in this article. (2) This is assumed to represent an open cohort of miners who are regularly screened for tuberculosis when joining the workforce and then at regular intervals after this. Hence, prevalence of tuberculosis disease that is asymptomatic with no radiographic abnormalities has remained static in the presence of the screening program. (3) The screening program has reduced the prevalence of symptomatic disease and asymptomatic disease with radiographic abnormalities at the same constant rate, producing the same linear trend in declining prevalence. (4) Sensitivity of the screening program is defined as the proportion of disease that is symptomatic plus the proportion of disease that is asymptomatic with radiographic abnormalities (i.e., it is the proportion of all true active disease that can be detected by symptom and radiographic screening). (5) For illustrative purposes, it is assumed that tuberculosis prevalence was 5% before the screening program. CRX = new or changing radiological abnormality; TB = tuberculosis.
The sensitivity of symptom screening for active tuberculosis case finding varies markedly in different communities, workplace settings, and in HIV clinic patients (Table 6) (20, 21). The explanation for the large differences in sensitivity and specificity of symptom screening seen in these studies, all based in Southern Africa, is likely to be multifactorial and may include: differences in study populations, such as mining versus general populations and the proportion HIV-infected; the screening algorithms used; and the case definitions of tuberculosis used. For example, four of the seven studies (including this study) used follow-up data when applying case definitions to reduce the problem of false-negative cultures, whereas the other three were restricted to screening data only. One of the seven studies used spoligotyping to reduce the potential problem of laboratory cross-contamination. In addition, the presence or absence of routine screening for tuberculosis in the population is likely to play an important role, although currently this is mostly restricted to mining populations.
TABLE 6.
PERFORMANCE OF TUBERCULOSIS SYMPTOM SCREENING IN DIFFERENT SOUTHERN AFRICAN SETTINGS
| Author, Year | Location | N | HIV Prevalence (%) | Symptoms Included | Gold Standard | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|---|
| Communities | |||||||
| Corbett, 2008 (20) | Harare | 8,979 | 21 | Cough, weight loss, fever, hemoptysis, night sweats | Case definitions applied* | 77 | 89 |
| den Boon, 2006 (16) | Cape Town | 1,170 | <12† | Cough, hemoptysis, night sweats, fever, weight loss | Positive smear or culture at screening | 69 | 68 |
| Wood, 2007 (21) | Cape Town | 762 | 23 | Cough, night sweats, loss of appetite, loss of weight | 2 positive smears or cultures at screening | 33 | 74‡ |
| Workplaces | |||||||
| Corbett, 2007 (17) | Harare factory workers | 4,668 | 19 | Cough, fever, hemoptysis, night sweats, unintentional weight loss | Case definitions applied* | 59 | — |
| Lewis§ | South African gold miners | 1,905 | 29 | New or worsening cough (any duration), night sweats, weight loss | Case definitions applied* | 31 | 90 |
| HIV clinics | |||||||
| Mohammed, 2004 (19) | Cape Town HIV clinic, WHO stage III or IV | 129 | 100 | >1 of measured weight loss, cough, night sweats, or fever | Positive screening culture and symptoms or CXR | 100 | 88.1 |
| Day, 2006 (15)‖ |
South African gold miners |
899 |
100 |
Cough, night sweats, weight loss |
Case definitions applied* |
59 |
76 |
Definition of abbreviations: CXR = chest radiographic abnormalities; WHO = World Health Organization.
Case definitions used screening and follow-up results from clinical examination, chest radiographic abnormalities, smear, and culture.
HIV testing was not done; HIV prevalence in this population was assumed to be less than the 12% reported from local antenatal clinics.
Not able to calculate specificity as proportion with one or more symptom that did not have tuberculosis was not specified. Specificity for loss of weight reported as it had the highest specificity.
This study.
Results did not differ by CD4 count.
The generalizability of our results may be limited because gold miners are regularly screened for tuberculosis, which would reduce the prevalence of undiagnosed active disease, and the definition of cough was restricted to a “new or worsening cough” regardless of duration. However, the minimal impact of HIV status, silicosis, or previous tuberculosis on the performance of the screening methods is likely to be generalizable. The generalizability of our findings concerning the value of chest radiography as a screening tool may be questioned because (1) silicosis is prevalent, and (2) chest radiography results were classified as new or changing abnormalities in comparison to a previous chest radiograph. However, silicosis is slowly progressive over many years (22), whereas radiological changes of active tuberculosis evolve over 6 to 12 months (17). Therefore, comparing the screening radiograph to a film taken about 1 year earlier, new changes would be much more likely to represent TB than silicosis. Hence, the results are more generalizable than may be first assumed.
We do not know reasons for nonparticipation in this study; however, it was done in conjunction with a study of sexually transmitted infections that required examination of genitalia and we suspect this was the main reason for nonparticipation. This is unlikely to have biased our results and the uptake of 88% is reasonable. The strengths of this study include a gold standard definition of tuberculosis based on culture, a large sample size, and wide inclusion criteria.
Conclusions
The addition of chest radiography to symptom screening substantially improved the sensitivity of the screening tool in this population of gold miners. Furthermore, our findings suggest that the usefulness of this screening strategy will be retained in high HIV prevalence settings. Community-wide active case finding for tuberculosis may contribute to reducing tuberculosis transmission by reducing the duration of infectiousness. Secular changes in the sensitivity of the strategy should be anticipated if repeated screening rounds are performed. Further research is required to develop more sensitive, field-friendly tuberculosis screening tools.
Supplementary Material
Acknowledgments
The authors thank the Safety in Mines Research Advisory Committee in South Africa for funding this study (SIMHEALTH 705), the miners who participated, and the mine company for cooperation. They also thank Vicky Moloi for coordinating participant recruitment and specimen collection.
Supported by the Safety in Mines Research Advisory Committee in South Africa (SIMHEALTH 705). G.J.C. and K.L.F. are partially funded and J.J.L. is fully funded by the Consortium to Respond Effectively to the AIDS/TB Epidemic (CREATE), which is funded by the Bill and Melinda Gates Foundation. A.D.G. is supported by a UK Department of Health Public Health Career Scientist award. E.L.C. is funded by the Wellcome Trust.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200806-846OC on September 10, 2009
Conflict of Interest Statement: J.J.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.C.'s spouse/life partner is an employee of Enviroserv, and received $1,001–$5,000 for serving as an expert advisor to the Global Fund Local Authority for Price Water House Coopers and up to $1,000 for serving as alternate for board of trustees member from South African Business Coalition On HIV/AIDS. J.H.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.L.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.D.G. received up to $1,000 for contribution to expenses for travel to a conference in 2007 from Roche. R.J.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.L.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.J.C. was employed by Anglogold Health Services and seconded to Aurum Health Research, which conducted the study.
References
- 1.Corbett EL, Marston B, Churchyard GJ, De Cock KM. Tuberculosis in sub-Saharan Africa: opportunities, challenges, and change in the era of antiretroviral treatment. Lancet 2006;367:926–937. [DOI] [PubMed] [Google Scholar]
- 2.Dye C, Watt CJ, Bleed D. Low access to a highly effective therapy: a challenge for international tuberculosis control. Bull World Health Organ 2002;80:437–444. [PMC free article] [PubMed] [Google Scholar]
- 3.Banerji D, Andersen S. A sociological study of awareness of symptoms among persons with pulmonary tuberculosis. Bull World Health Organ 1963;29:665–683. [PMC free article] [PubMed] [Google Scholar]
- 4.Nagpaul DR, Vishwanath MK, Dwarakanath G. A socio-epidemiological study of out-patients attending a city tuberculosis clinic in India to judge the place of specialized centres in a tuberculosis control programme. Bull World Health Organ 1970;43:17–34. [PMC free article] [PubMed] [Google Scholar]
- 5.Nyunt UT, Ko Gyi U, Kahn G, Than Tin D, Aye UB. Tuberculosis baseline survey in Burma in 1972. Tubercle 1974;55:313–325. [DOI] [PubMed] [Google Scholar]
- 6.Stop TB Partnership and WHO. 2006. Global Plan to Stop TB 2006–2015. [Accessed Apr 16, 2008] Available from: www.stoptb.org/globalplan/
- 7.Churchyard GJ, Kleinschmidt I, Corbett EL, Mulder D, De Cock KM. Mycobacterial disease in South African gold miners in the era of HIV infection. Int J Tuberc Lung Dis 1999;3:791–798. [PubMed] [Google Scholar]
- 8.Charalambous S, Moloi V, Grant AD, Seabi O, Mangenene N, Rankhakile MK, Churchyard GJ, Corbett EL. 2002. Impact of HIV on prevalent (active) TB disease in South African gold miners: implications for duration of infectivity, case-finding and TB transmission [abstract no. MoOrC1099]. XIV International AIDS Conference, Barcelona, Spain.
- 9.Churchyard GJ, Charalambous S, Moloi V, Fielding KL, Day JH, Grant AD, Hayes RJ, Corbett EL. 2002. Population based screening for active tuberculosis in a community with a high prevalence of TB. Presented at the 33rd World Conference of Lung Health, Montreal, Canada, 6–10 October, 2002. Abstract no. 041-PD.
- 10.Corbett EL, Charalambous S, Moloi V, Fielding KL, Grant AD, Dye C, De Cock KM, Hayes RJ, Williams BG, Churchyard GJ. HIV and the duration of infectivity before diagnosis of TB disease: implications for TB transmission and control in high HIV prevalence areas [abstract]. Int J Tuberc Lung Dis 2003;7:S137. [Google Scholar]
- 11.Corbett EL, Charalambous S, Moloi VM, Fielding K, Grant AD, Dye C, De Cock KM, Hayes RJ, Williams BG, Churchyard GJ. Human immunodeficiency virus and the prevalence of undiagnosed tuberculosis in African gold miners. Am J Respir Crit Care Med 2004;170:673–679. [DOI] [PubMed] [Google Scholar]
- 12.Corbett EL, Churchyard GJ, Clayton TC, Williams BG, Mulder D, Hayes RJ, De Cock KM. HIV infection and silicosis: the impact of two potent risk factors on the incidence of mycobacterial disease in South African miners. AIDS 2000;14:2759–2768. [DOI] [PubMed] [Google Scholar]
- 13.Sterne JA, Turner AC, Connell JA, Parry JV, Fine PE, Ponnighaus JM, Nyasulu S, Mkandwire PK. Human immunodeficiency virus: GACPAT and GACELISA as diagnostic tests for antibodies in urine. Trans R Soc Trop Med Hyg 1993;87:181–183. [DOI] [PubMed] [Google Scholar]
- 14.International Labour Office. Guidelines for the use of ILO international classification of radiographs of pneumoconiosis. Series 22: Occupational Safety and Health. Geneva, Switzerland: International Labour Office; 1981.
- 15.Day JH, Charalambous S, Fielding KL, Hayes RJ, Churchyard GJ, Grant AD. Screening for tuberculosis prior to isoniazid preventive therapy among HIV-infected gold miners in South Africa. Int J Tuberc Lung Dis 2006;10:523–529. [PubMed] [Google Scholar]
- 16.den Boon S, White NW, van Lill SW, Borgdorff MW, Verver S, Lombard CJ, Bateman ED, Irusen E, Enarson DA, Beyers N. An evaluation of symptom and chest radiographic screening in tuberculosis prevalence surveys. Int J Tuberc Lung Dis 2006;10:876–882. [PubMed] [Google Scholar]
- 17.Corbett EL, Bandason T, Cheung YB, Munyati S, Godfrey-Faussett P, Hayes R, Churchyard G, Butterworth A, Mason P. Epidemiology of tuberculosis in a high HIV prevalence population provided with enhanced diagnosis of symptomatic disease. PLoS Med 2007;4:e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golub JE, Mohan CI, Comstock GW, Chaisson RE. Active case finding of tuberculosis: historical perspective and future prospects. Int J Tuberc Lung Dis 2005;9:1183–1203. [PMC free article] [PubMed] [Google Scholar]
- 19.Mohammed A, Ehrlich R, Wood R, Cilliers F, Maartens G. Screening for tuberculosis in adults with advanced HIV infection prior to preventive therapy. Int J Tuberc Lung Dis 2004;8:792–795. [PubMed] [Google Scholar]
- 20.Corbett EL, Zezai A, Cheung YB, Bandason T, Dauya E, Munyati SS, Butterworth AE, Rusikaniko S, Churchyard GJ, Mungofa S, et al. Provider-initiated symptom screening for tuberculosis: diagnostic value and the impact of HIV. Bull World Health Organ. (In press) [DOI] [PMC free article] [PubMed]
- 21.Wood R, Middelkoop K, Myer L, Grant AD, Whitelaw A, Lawn SD, Kaplan G, Huebner R, McIntyre J, Bekker LG. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med 2007;175:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cowie RL. The influence of silicosis on deteriorating lung function in gold miners. Chest 1998;113:340–343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.