Abstract
Maturation resistance and tolerogenic properties can be conferred on human and murine dendritic cells (DC), -crucial regulators of T cell responses, by exposure to rapamycin (RAPA), a ‘tolerance-sparing’ immunosuppressive agent. Mechanisms underlying this acquired unresponsiveness, typified by diminished functional responses to TLR or CD40 ligation, have not been identified. We report that in vitro and in vivo conditioning of murine myeloid (m) DC with RAPA elicits the de novo production of IL-1β by otherwise phenotypically immature DC. Interestingly, IL-1β production promotes overexpression of the transmembrane form of the IL-1R family member, IL-1R-like 1, also know as ST2 on RAPA-conditioned DC (RAPA-DC). ST2 is the recently-identified receptor for IL-33, a cytokine favoring Th type 2 (Th2) responses. In addition, transmembrane ST2, or ST2L, has been implicated as a potent negative regulator of TLR signaling. RAPA-DC generated from ST2−/− mice exhibited higher levels of costimulatory molecules (CD86) than wild-type RAPA-DC. Consistent with its regulatory function, IL-1β-induced ST2L expression suppressed the responsiveness of RAPA-DC to TLR or CD40 ligation. Thus, as a result of their de novo production of IL-1β, RAPA-DC upregulate ST2L and become refractory to pro-inflammatory, maturation-inducing stimuli. This work identifies a novel mechanism through which a clinically-important immunosuppressant impedes the capacity of DC to mature and consequently stimulate effector/adaptive T cell responses.
Keywords: Dendritic Cells, Cytokines Receptors, Cytokine, Tolerance/Suppression/Anergy, Signal Transduction
Introduction
The macrocyclic antibiotic rapamycin (RAPA)3 is a clinically-approved immunosuppressant (1, 2), and may have tolerance-sparing properties (3–5). Binding of RAPA to its intracellular receptor, the immunophilin FK506 binding protein 12, inhibits the activity of the mammalian target of rapamycin (mTOR). mTOR is a highly integrative serine/threonine kinase, and by mechanisms that are not fully understood, regulates critical physiological events, including responses to growth factors, mRNA translation, cell size, and growth (6–8).
The inhibitory activity of RAPA on antigen (Ag)-stimulated T lymphocytes is believed to result from its capacity to permit the transmission of signals from the TCR, but to inhibit signals through receptors that characteristically activate mTOR, such as CD28, and cytokine/growth factor receptors (9, 10). Accordingly, in the presence of RAPA, Ag-stimulated T cells undergo apoptosis or become anergic, even in the presence of co-stimulation or pro-inflammatory cytokines (4, 10). However, human and mouse regulatory T cells (Treg; both naturally-occurring CD4+CD25+Foxp3+ and Treg type-1 cells) are comparatively insensitive to RAPA, as there is evidence for their induction, expansion, and function in the presence of RAPA (11, 12).
Dendritic cells (DC) are critical regulators of immune reactivity (13, 14). Recently, we and others have shown, both in vitro and in vivo, that RAPA profoundly alters murine and human DC generation, maturation and T cell stimulatory function (15–18). Specifically, myeloid (m)DC generated in clinically-relevant concentrations of RAPA (RAPA-DC) are phenotypically immature, with weak ability to produce IL-12, and markedly inhibited T cell allostimulatory capacity (17, 18). Relatedly, both in vitro-generated RAPA-DC and DC from RAPA-treated mice show impaired responses to TLR4 and CD40 ligation (15, 17, 18). While poor stimulators of allogeneic CD4+ effector T cells, RAPA-DC enrich for potent alloAg-specific Treg (18). This contrasts with control (CTR) mDC that favor non-Treg activation/expansion, especially when matured (18). Moreover, when pulsed with alloAg and infused systemically, RAPA-DC, but not CTR-DC render alloAg-specific T cells hyporesponsive to donor and promote indefinite organ allograft survival (18, 19). Thus, in part by instilling maturation-resistance, RAPA enables DC to act as ‘negative cellular vaccines’ with tolerogenic potential (20, 21). However, the mechanisms underlying the resistance of RAPA-DC to inflammatory stimuli have not been defined.
In the course of studies to elucidate the molecular basis of the resistance of RAPA-DC to maturation, we have found that RAPA promotes the de novo production of IL-1β by otherwise phenotypically immature DC. This production of IL-1β drives the expression the transmembrane form of ST2, or ST2L (also known as IL-1R-Like 1), on the surface of RAPA-DC. In addition to being the recently-identified T cell surface receptor for IL-33, a cytokine that stimulates CD4+ Th2 responses (22), ST2L is expressed on LPS-stimulated macrophages and is as a potent negative regulator of TLR signaling, critical for endotoxin tolerance, i.e. the down-regulation of endotoxin-driven responses by APC after prior endotoxin exposure (23). Consistent with this regulatory capacity, IL-1β exposure and consequent increased ST2L expression inhibits the phenotypic and functional maturation of RAPA-DC to CpG and CD40 ligation. Thus, by inducing IL-1β production and upregulating ST2L expression, RAPA renders DC resistant to pro-inflammatory, maturation-inducing conditions, allowing them to retain their tolerogenic potential.
Materials and methods
Animals
Male C57BL/10 (B10; H2Kb), C3H/HeJ (C3H; H2Kk) and BALB/c (H2Kd) mice were from the Jackson Laboratory, Bar Harbor, ME and maintained in a specific pathogen-free facility at the University of Pittsburgh School of Medicine until use at 8–12 wks of age. Experiments were conducted under an institutional animal care and use committee-approved protocol in accordance with NIH guidelines. ST2-deficient IL1rl1−/−) BALB/c mice used as a source of bone marrow cells were housed at the University of Glasgow, under conditions approved by the Home Office (London, UK).
DC generation and stimulation
mDC were propagated from bone marrow (BM) cells for 7 days in GM-CSF and IL-4, as described (24). RAPA (10 ng/ml; Sigma, St. Louis, MO) was added to cultures two days after initial plating. Every 2 days, 80% of the culture supernatant was replaced with fresh cytokine-containing medium (with or without RAPA). On day (d) 4, non-adherent cells were removed. Some cultures also received IL-1R antagonist (a) (75 ng/ml; R&D Systems, Minneapolis, MN) on d6. In all experiments, non-adherent cells were harvested on d7 and CD11c+ DC positively selected (purity >97%) using anti-CD11c immunomagnetic beads (Miltenyi Biotec, Auburn, CA). Where indicated, DC were incubated for 18–22 h (0.5–1×106 cells/ml) with LPS (0.02 – 20 μg/ml; S. enteritidis; Sigma), CpG-oligodeoxynucleotide (0.02 – 20 μg/ml; ODN 1826: TCCATGACGTTCCTGACGTT; Invivogen, San Diego, CA), or agonistic α-murine CD40 monoclonal antibody (mAb) (HM40-3; 0.5 – 20 μg/ml; BD PharMingen, San Diego, CA).
DC mobilization in vivo and splenic DC isolation
B10 DCs were mobilized by i.p. administration of recombinant human fms-like tyrosine kinase-3 ligand (Flt3L; Amgen, Thousand Oaks, CA; 10 μg/d) for 10 d (d1-10) as described (15). Groups of animals also received RAPA (0.5 mg/kg/d i.p.; d3-10) in a vehicle of 51% polyethylene glycol 300, 5% polysorbate 80, and 5% ethanol (all from Sigma) (15). On d11, DC were isolated from collagenase-treated spleen preparations by density gradient centrifugation (16% wt/vol Histodenz; Sigma), then positively selected as described above.
Flow cytometry
Cell surface and intracellular Ag expression by DC was analyzed as described (24). Fluorophore-conjugated mAbs (clone designation) were used to detect expression of CD11c (N418), CD40 (3/23), CD86 (GL1), TLR2 (6C2; eBioscience, San Diego, CA), TLR4 (MTS510), TLR9 (M9.D6; eBioscience), I-Ak (11–5.2), I-Ab (AF6-120.1), I-E (14-4-4S) (eBioscience), or T1/ST2 (DJ8; MD Biosciences; Zurich, Switzerland). Appropriately-conjugated, isotype-matched IgGs served as controls. All reagents were from BD PharMingen, unless specified. Data were acquired with a LSR II flow cytometer (BD ImmunoCytometry Systems; San Jose, CA) and analyzed using the FlowJo 8.1.1 (Tree Star, Inc., Ashland, OR).
Microarrays
The University of Pittsburgh Genomics and Proteomics Core Laboratories conducted sample preparation and array hybridization. Total RNA, isolated using a total RNA isolation kit (BD Biosciences) from highly-purified, CD11c+ RAPA- and CTR-DC was processed to generate duplicates of biotinylated cRNA. Following cRNA hybridization to Affymetrix Mouse Genome 430A 2.0 GeneChips (Affymetrix, Inc., Santa Clara, CA), the microarrays were scanned using a GeneArray® 3000 scanner and signal intensities extracted with Microarray Analysis Suite (MAS) 5.0 (Affymetrix, Inc.). Differentially expressed genes were identified via the web-based gene expression analysis software, caGEDA implementing J5 analysis of data (data log2 and median normalization; threshold of 4; http://bioinformatics.upmc.edu/GE2/GEDA.html (25).
RNase protection assay (RPA)
RPA with 32P-uridine triphosphate–labeled anti-sense RNA probes to IL-1β, IL-1Ra, and GAPDH was performed as described in detail (26). Briefly, RNA was isolated from snap-frozen, purified DCs using a total RNA Isolation Kit (BD PharMingen). RPA was performed using the RiboQuant Multi-Probe RPA System (BD PharMingen) and cDNAs encoding mouse IL-1β, IL-1Ra and the housekeeping gene GAPDH were as templates. Quantification of bands was performed by densitometric assessment of scanned autoradiographs using Scion Image v1.63 software (NIH, Bethesda, MD). The signals from specific mRNA were normalized to the signals from housekeeping genes run on each lane to adjust for loading differences.
Quantitative RT-PCR (qRT-PCR)
DC RNA was extracted using TriReagent (Molecular Resource Center, Cincinnati, OH). and reversed transcribed using the iScript cDNA Synthesis Kit (Biorad, Hercules, CA). Replicate PCR reactions were performed using SYBR Green PCR Master Mix (Applied BioSystems; Foster City, CA), primers for ST2L – the membrane-bound form of ST2 (SuperArray, Frederick, MD) and IL-1β, IL-12p40, TLR9 and β-actin (designed by Primer Express Software, Applied BioSystems), then amplified on an ABI PRISM 7000 Sequence Detection System. Data were plotted using the manufacturer’s software as the ΔRn fluorescence signal versus cycle number. The cycle threshold number was determined as the cycle number at which the ΔRn crosses this threshold. Relative gene expression was determined by comparing to control, then normalized to β-actin mRNA using the comparative cycle threshold method (27).
ELISA
IL-1β concentrations in DC culture supernatants were quantified using the ELISA Ready-SET-Go! Kit (eBioscience).
Immunoblots
DC were lysed in 10 mM Tris HCl (pH 7.6), 158 mM NaCl, 5 mM EDTA (pH8.0), 1% sodium deoxycholate, 0.1% SDS, 1% Triton X-100, and the protease inhibitors phenylmethanesulfonyl fluoride [1mM], leupeptin [5μg/ml], aprotinin [5μg/ml], and sodium orthorovanadate [1mM]). All reagents were from Sigma. Debris was removed by centrifugation at 13,100×g, and 10–20 μg protein separated on 10% SDS-PAGE gels and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA). Immunoblotting was done by incubation with primary Abs, including rabbit polyclonal α-ST2 (1:1500; Abcam, Cambridge, MA), polyclonal α-phosphorylated ERK, p38, and JNK (1:1000; Cell Signaling Technology, Danvers, MA), polyclonal α-total ERK, p38, and JNK (1:1000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and mAb α-GAPDH (Novus Biologicals, Littleton, CO), followed by appropriate horseradish peroxidase-conjugated secondary Abs (Jackson ImmunoResearch, West Grove, PA). Visualization was conducted with Western Lighting (PerkinElmer, Waltham, MA), or in the case of the MAPK Abs, Super Signal West Pico (Pierce Chemical Co., Rockford, IL) chemiluminescent kits, and exposure to film. Densitometric assessment of bands was completed with Scion Image 1.63 (NIH).
EMSA
NF-κB and AP-1 DNA binding activity was measured as described (28), with γ-[32P]-labeled probes (NF-κB consensus; Promega, Madison, WI; AP-1 consensus and NF-κB and AP-1 mutants; Santa Cruz Biotechnology Inc., Santa Cruz, CA) and nuclear extracts from purified DC, following their stimulation with LPS, CpG, or α-CD40 for 20 min. Supershifts were completed with Abs to JunB and p50 (Santa Cruz Biotechnology Inc.).
MLR
Splenic T cells were purified by negative selection of non-T cells using α-CD11b, α-TER-119, α-Gr-1, α-I-A/I-E, α-B220, and α-Gr-1 mAbs (BD PharMingen) and removal via Mouse Depletion Dynabeads® (Dynal Biotech, Oslo, Norway). MLR were performed as described(15) using graded numbers of γ-irradiated (20 Gy) DC as stimulators. For the final 16–18 h, individual wells were pulse-labeled with 1 μCi 3[H] thymidine. Radioisotope incorporation was determined using a β scintillation counter. Results are expressed as mean c.p.m. ± 1 SEM calculated from triplicate wells.
Statistical analysis
Results are expressed as means ±1SEM or ±1SD, as indicated. The significance of differences between means was determined using the JMP IN 4.04 Statistical Package (SAS Institute Inc., Cary, NC) performing the Student’s ‘t’ or two-way ANOVA test. p<0.05 was considered significant.
Results
RAPA conditioning of BM-derived DC confers resistance to maturation following exposure to inflammatory stimuli
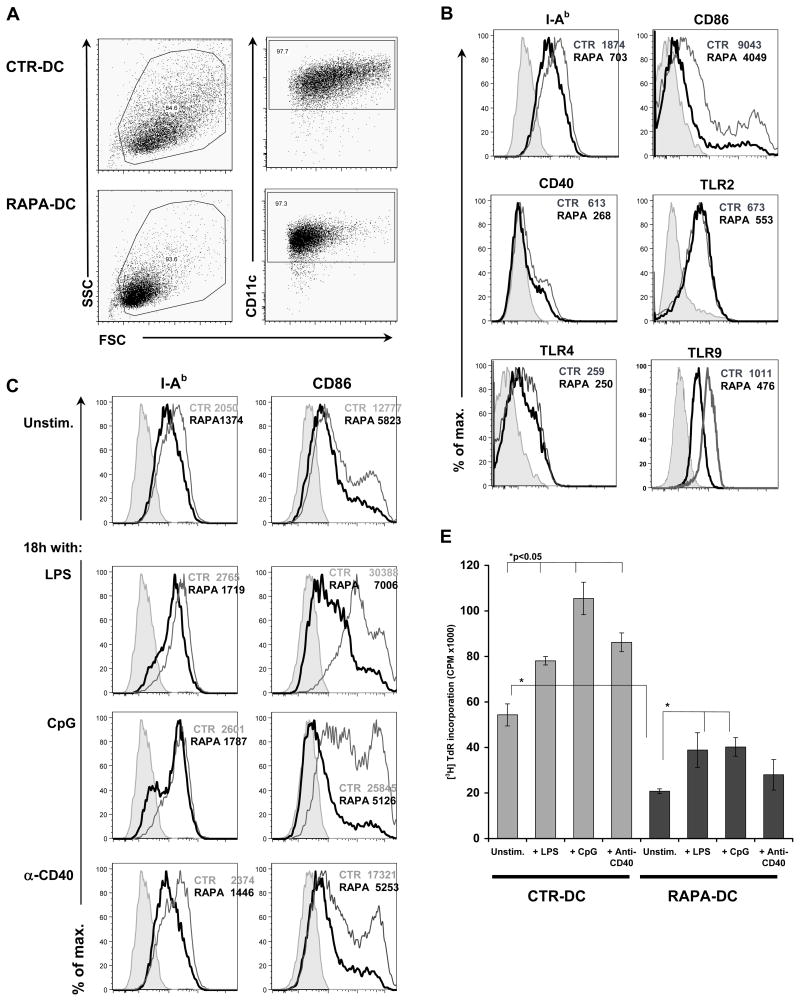
As reported previously (18), RAPA-DC propagated in vitro are a homogenous population of reduced size compared with CTR-DC (Figure 1A), consistent with the importance of mTOR in regulating cell size (7). When compared to CTR-DC, RAPA-DC also displayed lower levels of surface MHC class II and CD86, but did not differ significantly from controls in surface expression of TLR2, TLR4 and CD40 (Figure 1B). RAPA-DC also expressed TLR9 (by flow cytometry [intracellular; Figure 1B] and quantitative RT-PCR [qRT-PCR]; data not shown), although at slightly reduced levels compared with CTR-DC.
Figure 1. RAPA-conditioning of DC confers resistance to maturation following exposure to inflammatory ligands.
(A) BM-derived B10 myeloid DC were generated in the absence (control [CTR]-DC) or presence of 10 ng/ml RAPA (RAPA-DC) and purified to >97% purity by CD11c immunobead positive selection. Compared to CTR-DC (upper panels), RAPA-DC (lower panels) constituted a homogenous population of CD11c+ cells of reduced size, which (B) displayed reduced surface expression of MHC class II and CD86. (B) However, the expression of CD40, TLR2, and TLR4 RAPA-DC was not altered significantly. RAPA-DC were also positive for intracellular TLR9. (C) Following overnight incubation with 1 μg/ml LPS, 2 μg/ml CpG, or 5 μg/ml agonistic anti(α)-CD40 mAb, RAPA-DC did not increase MHC class II to levels found on CTR-DC and failed to upregulate CD86. (D) Upregulation of CD86 on RAPA-DC was inhibited over a range of concentrations of LPS, CpG and α-CD40. MFI = mean fluorescence intensity. The data are means ± 1SD from two independent experiments where CD11c+ cells were assessed. (E) Furthermore, RAPA-DC exposed to LPS (1 μg/ml), CpG (2 μg/ml), or α-CD40 mAb (5 μg/ml) displayed significantly reduced T cell allostimulatory capacity compared to similarly-stimulated CTR-DC. The data are means ± 1SD from sample replicates of one experiment representative of more than 4 performed. In B and C, histograms represent CD11c+ gated cells and numbers indicate MFI for each condition. Unstim. = unstimulated DC. Shaded area = isotype control. RAPA-DC are represented by thick black lines. In panel E, *=p<0.05 by Student’s ‘t’-test; the data shown are representative of more than 3 experiments performed.
RAPA exposure also significantly inhibited typical DC maturation following exposure to various inflammatory stimuli, including LPS, CpG, and agonistic α-CD40 mAb. Specifically, RAPA-DC failed to upregulate CD86 (Figure 1C) across various concentrations of these stimulants (Figure 1D) and maintained comparatively poor direct T cell allostimulatory capacity in MLR following 18h culture with LPS, CpG, or α-CD40 (Figure 1E). Marked impairment of RAPA-DC stimulatory ability was observed over a range of stimulator:responder cell ratios and with different doses of stimulators (LPS 0.2–20 μg/ml; CpG 0.2–20 μg/ml; CD40 0.5–20 μg/ml) (data not shown).
RAPA upregulates ST2 expression in DC
To compare gene expression between CTR- and RAPA-DC, and to identify negative regulators of DC maturation, total mRNA was isolated from purified DC and used to label Mouse Genome 430A 2.0 GeneChips (Affymetrix). Following extraction of signal intensities with Microarray Analysis Suite 5.0 (Affymetrix), 586 genes were determined to be differentially expressed between RAPA- and CTR-DC (data not shown), based on J5 analysis completed by the gene expression analysis software, caGEDA (25). Among the differentially regulated genes overexpressed in RAPA-DC was ST2L (J5 score = 4.17), an IL-1R family member (il1rl; Entrez GeneID: 17082; also know as T1/ST2, Fit-1, St2). In both rodents and humans, the ST2 gene encodes two isoforms of the ST2 protein, a soluble secreted form, sST2, and ST2L, a longer, transmembrane form (29–31). In addition to acting as the receptor for IL-33 (22), ST2L is a negative regulator of TLR4 signaling in macrophages (23). Relatedly, sST2 has also been shown to temper inflammatory responses (32).
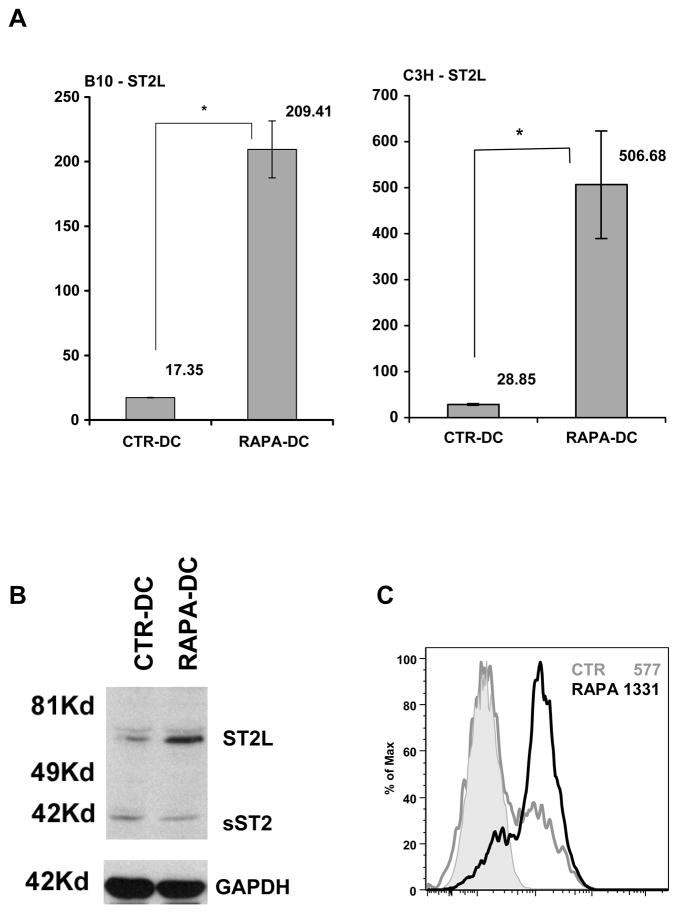
qRT-PCR (Figure 2A) Western blotting (Figure 2B) and flow cytometry (Figure 2C) confirmed modified expression of ST2 by RAPA-DC. The transmembrane form of ST2 (ST2L) was upregulated significantly in BM-derived RAPA-DC from several mouse strains (B10, C3H and BALB/c; Figure 2A–C and data not shown). The alternative isoform, soluble ST2 (sST2) was not expressed differently from CTR-DC in Western blots (Figure 2).
Figure 2. Overexpression of ST2L by RAPA-DC.
(A) CD11c+ DC propagated from B10 (left panel) or C3H (right panel) mice in the presence or absence of RAPA, were analyzed for ST2L expression by qRT-PCR using ST2L- and β-actin-specific probes. The data are means ± 1SD from sample replicates of one experiment, representative of more than 4 performed. Increased expression of ST2L protein by RAPA-DC was also established by (B) Western blotting, and (C) flow cytometric analysis. The data are representative of at least 3 independent experiments.
RAPA administration upregulates ST2L on splenic DC
Previously, we showed that splenic DC from RAPA-treated mice displayed impaired phenotypic maturation, pro-inflammatory cytokine production, T cell stimulatory capacity, and resistance to maturation after exposure to LPS (15). In the present studies, increased mRNA, protein, and surface expression of ST2(ST2L) were observed for splenic DC isolated from RAPA-treated animals (Figure 3). Specifically, when RAPA administration (1 mg/kg/d; d3 to 10) was combined with DC expansion/mobilization using Flt3L (10μg/day; d0 to 10), ST2L was overexpressed significantly (Figure 3A–B) and upregulated on the surface of purified splenic DC (Figure 3C), compared to DC isolated from vehicle-treated animals. Thus, both in vitro and in vivo, DC exposure/generation in the presence of RAPA leads to increased expression of the transmembrane form of ST2 (ST2L).
Figure 3. Administration of RAPA promotes overexpression of ST2L by splenic DC.

Purified splenic DC from RAPA-treated B10 mice (0.5 mg/kg for 7d) displayed increased expression of mRNA (A) and total ST2L protein (B). ST2L was also found to be increased on the surface of isolated CD11c+ splenic DC following 8d in vivo RAPA administration (C). Data are representative of at least 2 independent experiments.
Increased AP-1 activation in RAPA-DC
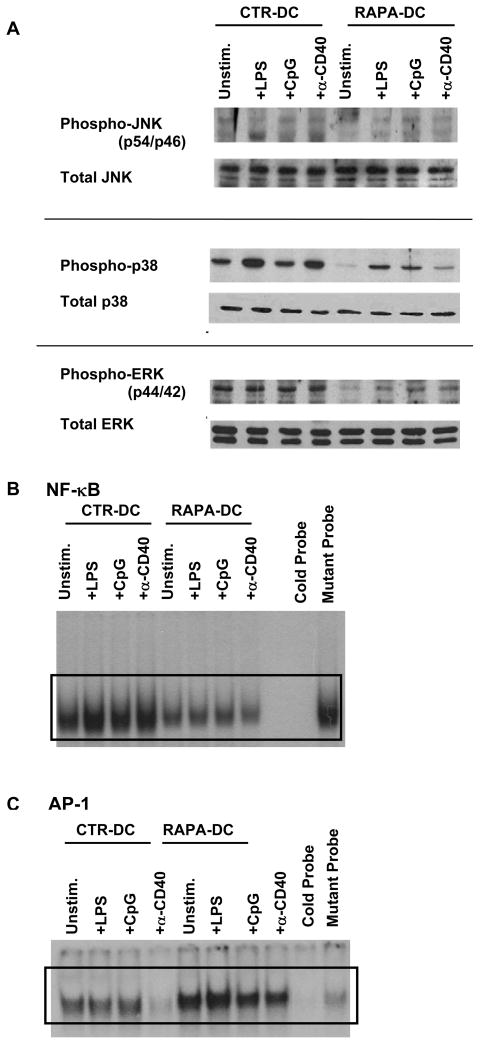
Early work showed that Fos/activator protein-1 (AP-1) activity regulates the expression of the ST2 gene in mouse NIH/3T3 fibroblasts (29). It has also been observed that exposure of the human lung fibroblast cell line WI-26 to RAPA results in rapid activation of JNK and activation of AP-1 (33). Relatedly, it is recognized that the ligation of DC receptors, including CD40, TLRs, or IL-1R, culminates in nuclear translocation of the transcription factor, NF-κB, or activation of MAPK pathways that can activate AP-1 (34, 35). Activation of MAPKs, including JNK, ERK, and p38, as well as NF-κB and AP-1 are inhibited by RAPA in coronary artery smooth muscle cells (36). Further, Brint et al (23) established that ST2 overexpression negatively regulates NF-κB DNA binding activity downstream of IL-1R and TLR4. Thus, to determine the status of MAPK, AP-1, and NF-κB activation in RAPA-DC, we assessed the level of MAPK phosphorylation in DC lysates and performed EMSA to assess AP-1 and NF-κB DNA binding activity by isolated nuclear proteins.
Similar to observations on RAPA-treated human lung fibroblasts (33), Western blot analysis of MAPK activation revealed that RAPA-DC,- either unstimulated or following 20 min exposure to LPS, CpG, or CD40 ligation had reduced phosphorylation of ERK and p38 (Figure 4A). Unstimulated RAPA-DC had similar levels to CTR-DC of JNK phosphorylation, but exhibited slightly inhibited JNK phosphorylation following exposure to inflammatory stimuli (Figure 4A). Consistent with our finding of reduced phenotypic and functional responses of RAPA-DC to danger signals/pro-inflammatory agents (Figure 1C and 1D) and the central role of NF-κB as a regulator of DC maturation and cellular inflammatory responses (37), little NF-κB DNA binding activity was detected in unstimulated RAPA-DC or after their exposure to LPS, CpG, or CD40 ligation (Figure 4B). Conversely, when compared with CTR-DC, RAPA-DC showed increased activation of AP-1, both before and after their stimulation with LPS, CpG or αCD40 (Figure 4C). These observations are in accordance with an ability of RAPA to promote AP-1 activation in other cells (33). As such, activation of AP-1 would be expected to facilitate ST2 expression (29). Likewise, our observation of suppressed NF-κB activation in RAPA-DC following their exposure to inflammatory stimuli is consistent with their maturation resistance, and correlates with the previously demonstrated ability of ST2 to negatively impact NF-κB activity downstream of TLR signaling (23).
Figure 4. Exposure of DC to RAPA inhibits NF-κB, but increases AP-1 DNA binding activity.
CTR- or RAPA-DC were purified following 7 day culture via positive selection using CD11c beads and then remained unstimulated (Unstim.) or were incubated with 1 μg/ml LPS, 2 μg/ml CpG, or 5 μg/ml agonistic α-CD40 mAb for 20 min, as indicated. (A) MAPK activation was determined by Western blot analysis of DC lysates for phosphorylated and total JNK, p38, and ERK. Blots shown are representative of three experiments with similar results. (B and C) Nuclear extracts from these cells were assessed for DNA binding activity in EMSA using 32P-labeled NF- κB (B) or AP-1 (C) probes. The data shown are representative of at least 2 independent experiments.
RAPA-induced IL-1β upregulates ST2L on RAPA-DC
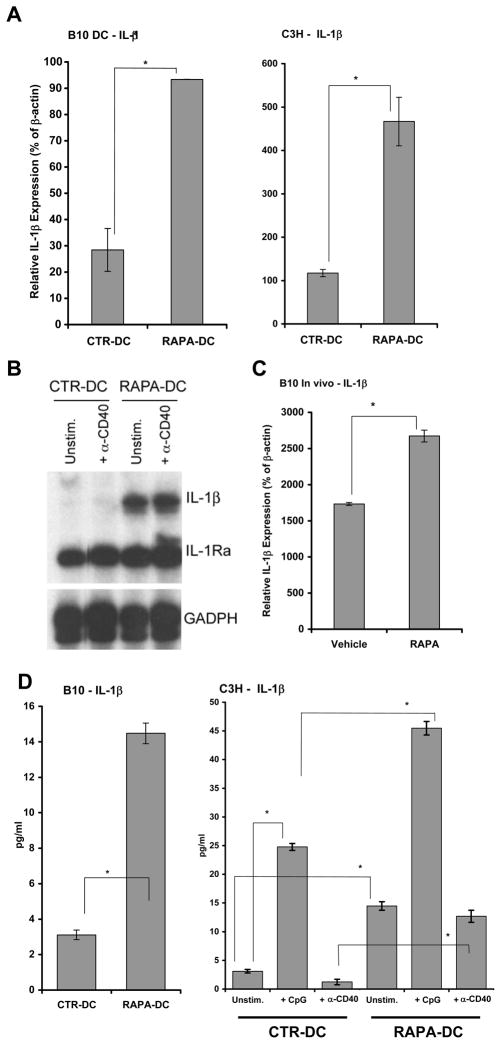
Inflammatory stimuli have been implicated in the induction of ST2 expression by various cell types. Leishmania major infection causes an increase in ST2L+ CD4+ T lymphocytes (Th2) at the site of infection (38). Likewise, LPS induces rapid surface expression of ST2L on peritoneal macrophages (23). Another pro-inflammatory stimulus known to both activate AP-1 (39) and to induce expression of ST2 in fibroblasts and macrophages is IL-1β (40, 41). Thus, we examined the possibility that RAPA increases the expression/production of IL-1β by DC. RAPA has not been reported previously to induce IL-1β, and is typically associated with reduced systemic or local inflammatory cytokines in transplant recipients or in autoimmune disease (42, 43). Surprisingly, based on these reports, and given the low CD86 expression and poor allostimulatory capacity of RAPA-DC (Figure 1), we found that RAPA-DC expressed increased IL-1β message (Figure 5A–B) and secreted significantly higher levels of IL-1β than CTR-DC, regardless of exposure to inflammatory stimuli (Figure 5D). Likewise, splenic DC from RAPA-treated animals also had significantly increased expression of IL-1β by qRT-PCR (Figure 5C).
Figure 5. Exposure of DC to RAPA induces increased IL-1β expression.
(A) Significantly increased IL-1 β mRNA expression was observed for B10 (left panel) and C3H (right panel) CD11c+ RAPA-DC on d7 of culture by (A) qRT-PCR and (B) RPA. However, no corresponding increase was detected for IL-1R antagonist (IL-1Ra; B). Data are from single experiments representative of at least 3 separate experiments. (C) Significantly increased IL-1 β mRNA was also demonstrated for isolated CD11c+ splenic DC following 8d systemic administration of RAPA. Data are representative of at least 2 independent experiments. (D) Significantly increased IL-1 β secretion was also demonstrated for purified B10 (left panel) and C3H (right panel) RAPA-DC incubated for 18–22h (106 cells/ml) with or without CpG or α-CD40 mAb. All data are representative of at least 2 independent experiments.
It has been reported recently, that during their differentiation into DC, exposure of monocytes to IL-1β results in impaired DC maturation, reduced IL-12 production, and decreased ability to stimulate Ag-specific T cell responses (44). The authors, however, did not examine whether exposure of monocytes to IL-1β influenced subsequent ST2 expression by the DC. To verify whether RAPA-induced IL-1β expression by DC was responsible for the demonstrated increase in transmembrane ST2 (ST2L), we cultured RAPA-DC in the presence of IL-1R antagonist (IL-1Ra), that inhibits the activity of IL-1α and IL-1β by competitive, high avidity binding to the IL-1R. As shown in Figure 6, inhibiting the activity of secreted IL-1β, reduced the overall expression of ST2L (Figure 6A) and the level displayed on the surface of RAPA-DC compared to CTR-DC (Figure 6B). In addition, IL-1Ra treatment also reduced IL-1β mRNA in RAPA-DC, while increasing that for IL-12p40 (Figure 6C, D), consistent with IL-12 reduction in IL-1β-exposed DC (44). In total, these data suggest that RAPA-induced IL-1β drives autocrine/paracrine IL-1β production by RAPA-DC, and enhanced expression of ST2L on the cell surface.
Figure 6. IL-1 β produced by RAPA-DC regulates expression of ST2L and IL-12.
(A) Blocking IL-1 β activity by 24h treatment of RAPA-DC with the naturally-occurring IL-1 β receptor antagonist, IL-1Ra, significantly reduced overall ST2Lprotein expression, as determined by Western blotting of purified DC lysates (left panel). Data in the blot are from a single experiment representative of 2 experiments performed. (A, right panel) Densitometric analyses of ST2 bands were completed on scanned films from 2 independent experiments to generate the depicted arbitrary units relative to sample GAPDH levels (means ± 1SD). (B) RAPA-DC treated with IL-1Ra had reduced ST2L levels comparable to CTR-DC, as shown by flow cytometry. Histograms show CD11c+ gated cells and numbers indicate MFI for each culture condition. Shaded area = isotype control. RAPA-DC are represented by black lines and IL-1Ra-treated RAPA-DC by the dotted line. (C) RAPA-DC-produced IL-1 β appears to acts in an autocrine/paracrine fashion, given reduced IL-1 β mRNA expression by RAPA-DC following blocking of IL-1 β activity. (D) Blocking IL-1 β activity also increased the expression of IL-12p40 by RAPA-DC. B-D; data are representative of 3 independent experiments.
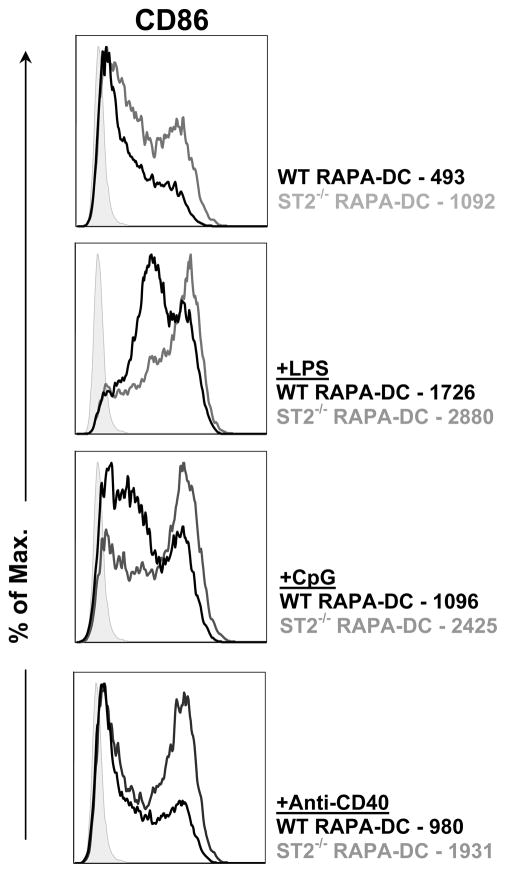
RAPA-DC from ST2-deficient mice exhibit higher levels of costimulatory molecule (CD86)expression
Comparison of RAPA-DC propagated from ST2-deficient BM compared with those propagated from wild-type mice revealed higher levels of expression of CD86 on the former cells (Figure 7). This was evident for unstimulated ST2−/− RAPA-DC and for LPS, CpG or anti-CD40-stimulated ST2−/− RAPA-DC. These data provide a causal link between upregulated ST2L expression on RAPA-DC and inhibition of CD86 expression.
Figure 7.
RAPA-DC from ST2-deficient mice exhibit higher levels of CD86. RAPA-DC were propagated from BM cells of wild-type (WT) or ST2-deficient BALB/c mice, as described in the Materials and Methods. Flow analysis revealed upregulation of CD86 expression on unstimulated and LPS, CpG and anti-CD40 stimulated RAPA-DC from ST2-deficient mice compared with RAPA-DC from wild-type controls. Figures denote MFI. Data are representative of 2 independent experiments.
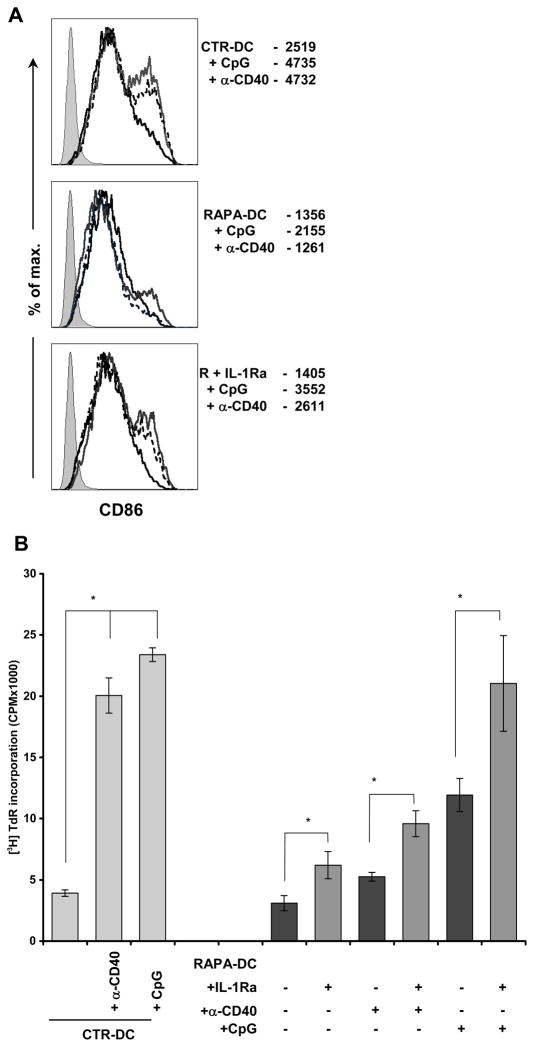
IL-1Ra-treated RAPA-DC display increased responsiveness to TLR and CD40 ligation
Given the observed marked resistance of RAPA-DC to TLR and CD40 ligation, and the demonstrated role of ST2L as a negative regulator of TLR signaling (23), we assessed the influence of decreased ST2L by IL-1Ra treatment on RAPA-DC function. Following overnight exposure to LPS, CpG, or α-CD40, IL-1Ra-treated RAPA-DC showed increased maturity, both phenotypically and functionally. Specifically, we found that inhibition of IL-1β activity resulted in increased CD86 expression following TLR9 or CD40 ligation (Figure 8A), and significantly increased T cell stimulatory ability (Figure 8B). IL-1Ra-treated RAPA-DC exposed to LPS also displayed increased CD86 expression and allostimulatory capacity (data not shown), although the increase in alloreactivity did not reach statistical significance. Also, none of the inflammatory stimuli induced equivalent maturation of IL-1Ra-treated RAPA-DC compared to that of CTR-DC, suggesting that additional, negative regulators of DC function or deficiencies in inflammatory signaling exist in RAPA-DC. Nevertheless, these findings support the ability of IL-1β-induced ST2 to impede inflammatory signaling in RAPA-DC.
Figure 8. IL-1β-induced ST2 promotes resistance to maturation.
(A) Blocking the influence of IL-1 β on RAPA-DC by treatment with IL-1Ra not only reduced ST2L expression, but also increased phenotypic maturation following 18h stimulation with CpG or α-CD40 mAb, as shown by increased CD86 expression. For CTR-DC (top panel), RAPA-DC (middle panel), and RAPA-DC + IL-1Ra (R+IL-1Ra; lower panel), unstimulated DC are represented by the thick black lines, CpG-exposed DC by grey lines, and α-CD40 mAb-treated DC by the dashed lines. (B) In addition, IL-1Ra-treated RAPA-DC displayed increased functional maturation, as evidenced by elevated allogeneic T cell stimulatory capacity. Data are representative of 2 independent experiments.
Discussion
While previous studies have shown that classic immunosuppressive drugs (corticosteroids and calcineurin inhibitors) and anti-inflammatory agents, such as aspirin, target DC function through inhibition of NF-κB activation (45–47), few reports have addressed the molecular basis of maturation resistance in pharmacologically-modified DC. In this study, we have extended previous observations on the maturation resistance and tolerogenic properties of RAPA-conditioned DC (15, 16, 18, 48). Our findings reveal that, both in vitro and in vivo, exposure of murine myeloid DC to RAPA increases their expression of the transmembrane form of Toll/IL-1 receptor (TIR) superfamily member ST2, a recognized negative regulator of TLR signaling in endotoxin-stimulated macrophages (23, 49). The observation that upregulation of transmembrane ST2 (ST2L) is associated with an enhanced production of IL-1β by RAPA-DC, and that inhibition of IL-1β activity reduces ST2L expression implicates IL-1β as a negative regulator of the maturation of these cells. This role of IL-1β is consistent with a recent report of the ability of IL-1β to markedly impair DC maturation by an unknown mechanism (44).
To our knowledge, there have been no previous investigations of ST2 expression by freshly-isolated or cultured DC. ST2 is highly homologous to the IL-1R. As discussed, it exists in both soluble and membrane-bound forms, with ST2L expressed primarily by hematopoietic cells and soluble ST2 (sST2) predominantly by fibroblasts (50). In addition to macrophages, ST2L mRNA expression has been demonstrated in T and B lymphocytes, mast cells and erythroid and BM stem cells (29). ST2L is expressed preferentially on Th2 cells (51, 52) and plays a functional role in the generation of important Th2 effector functions (53, 54). Recently, a ligand for ST2 has been described. Thus, the IL-1 family member IL-33 has been shown to drive production of Th2 cytokines by in vitro-polarized Th2 cells following ligation of the ST2 receptor (22). Preliminary experiments that we have conducted with IL-33 show that, in response to this ST2 ligand, RAPA-DC signal through NF-κB and MAPKs, and upregulate the message for IL-1β (data not shown).
Our attention was drawn to the potential role of ST2 as a negative regulator of RAPA-DC maturation following gene expression (microarray) profiling of highly-purified cells in which ST2L was consistently and significantly upregulated. Most members of the TIR superfamily initiate immunity via activation of NF-κB, leading in turn, to production of pro-inflammatory cytokines. By contrast, ST2 inhibits NF-κB activation through IL-1R and TLR2, 4 and 9. The present findings are consistent with those of Brint et al (23) who defined the ability of overexpressed ST2 in murine macrophages to sequester the critical TLR signaling adapters MyD88 and MyD88-like (Mal) and, consequently, to negatively regulate TLR4 and TLR9 signaling. The physiological relevance of this negative regulation was emphasized by the findings that ST2-deficient macrophages produced increased IL-12 in response to CpG stimulation, and that ST2 was necessary for endotoxin tolerance.
An ability of ST2 to modulate CD40 signaling, suggested by our findings, has not been described previously, and leads us to speculate that tumor necrosis factor receptor-associated factor-6 (TRAF-6), an important common signaling adapter between TLR and CD40 signaling (55), may also be sequestrated in RAPA-DC. Based on the present observations, it may also be suggested that the inhibitory effect of IL-1β on DC maturation and function described by Makino et al (44) may be the result of IL-1β-induced upregulation of ST2L. An inhibitory effect of IL-1β on IL-12 production, as observed in the present studies, and its ability to induce ST2, would be consistent with the importance of ST2 in favoring Th2 responses (23, 54).
The RAPA-induced increase in IL-1β, overexpression of ST2L, and IL-1β-dependent resistance to TLR- and CD40 ligation-induced maturation that we observed was confirmed using LPS-resistant C3H/HeJ RAPA-DC, ruling out any possibility that LPS contamination of administered RAPA or recombinant IL-1Ra facilitated the observed ST2L upregulation or altered DC responses.
Inflammatory activation of cells of the innate immune system, including DC, is often described as a double-edged sword (56), with the potential to facilitate effective removal of pathogens, or, if not tempered, to promote chronic inflammatory states. These risks are offset by mechanisms that tightly regulate TLR and other inflammatory signals (49). Pro-inflammatory stimuli, such as LPS, act in a regulatory loop, in which initial activation of the TLR also causes the induction of negative regulators. Thus, in addition to upregulation of ST2L, LPS stimulation of TLR4 drives the expression/production of MyD88s, suppressors of cytokine signaling (SOCS) and other regulators (49). A novel aspect of the current findings is that although RAPA-DC produce IL-1β, inducing ST2L, they never mature into CD86hi, potent allostimulatory cells. Thus, DC conditioning with RAPA and the consequent induction of ST2L, establishes a barrier to functional maturation of the cells, preserving their tolerogenic phenotype (20). This might be expected to decrease/minimize any potential risk of host sensitization using RAPA-DC as negative cellular vaccines. The findings support continued evaluation of RAPA as a tolerance-spring/promoting immunosuppressant and the further assessment of maturation-resistant DCs as tolerogenic vectors (18, 57–59) in immune-mediated inflammatory conditions.
In total, evidence has accumulated that the immunosuppressant RAPA can modify the properties of DC and T cells towards conditions that may favor tolerance induction. This is typically accounted for by its ability to impede directly Ag-stimulated T cells and to facilitate the induction of anergy or apoptosis of effector T cells, while apparently preserving the expansion/induction/function of Treg. Nonetheless, we have demonstrated in previous (15) and the present studies, that both in vitro and in vivo, RAPA reduces the T cell stimulatory function of DC and confers marked resistance to DC maturation following their exposure to inflammatory conditions. We now identify a novel mechanism through which RAPA inhibits DC stimulatory capacity and provide insight into the previously reported intriguing capability of IL-1β to reduce DC maturation and their T cell stimulatory function.
Supplementary Material
Acknowledgments
We thank Dr. Jon Cardinal and Ximei Peng for technical assistance with the EMSA assays and Dr. Ryan Fischer for assistance with DC culture. The authors also thank Ms. Miriam Freeman for excellent administrative support.
Footnotes
Non-standard abbreviations used in this paper: a, antagonist; α, anti; B10, C57BL/10; C3H, C3H/HeJ; CTR, control; d, day; DC, dendritic cells; flt3L, fms-like tyrosine kinase-3 ligand; m, myeloid; mTOR, mammalian target of rapamycin; q, quantitative; RAPA, rapamycin; RPA, RNase protection assay; Treg, regulatory T cells; Th2, T helper type 2 cell; TIR, Toll/IL-1R
Conflict-of-interest disclosure
The authors declare no competing financial interests.
This work was supported by National Institutes of Health (NIH) grants R01AI41011 and R01AI60994 (A.W.T). H.R.T was supported by non-concurrent fellowships from the American Society of Transplantation and the NIH (T32CA082084 and F32AI072940). T.L.S. was supported by an NIH research training fellowship (T32CA082084).
Publisher's Disclaimer: “This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.”
References
- 1.Sehgal SN. Rapamune (RAPA, rapamycin, sirolimus): mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem. 1998;31:335–340. doi: 10.1016/s0009-9120(98)00045-9. [DOI] [PubMed] [Google Scholar]
- 2.Kahan BD, Camardo JS. Rapamycin: clinical results and future opportunities. Transplantation. 2001;72:1181–1193. doi: 10.1097/00007890-200110150-00001. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med. 1999;5:1298–1302. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 4.Wells AD, Li XC, Li Y, Walsh MC, Zheng XX, Wu Z, Nunez G, Tang A, Sayegh M, Hancock WW, Strom TB, Turka LA. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999;5:1303–1307. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- 5.Blaha P, Bigenzahn S, Koporc Z, Schmid M, Langer F, Selzer E, Bergmeister H, Wrba F, Kurtz J, Kiss C, Roth E, Muehlbacher F, Sykes M, Wekerle T. The influence of immunosuppressive drugs on tolerance induction through bone marrow transplantation with costimulation blockade. Blood. 2003;101:2886–2893. doi: 10.1182/blood-2002-10-3014. [DOI] [PubMed] [Google Scholar]
- 6.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 7.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Gao W, Lu Y, El Essawy B, Oukka M, Kuchroo VK, Strom TB. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transplant. 2007;7:1722–1732. doi: 10.1111/j.1600-6143.2007.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai JH, Tan TH. CD28 signaling causes a sustained down-regulation of I kappa B alpha which can be prevented by the immunosuppressant rapamycin. J Biol Chem. 1994;269:30077–30080. [PubMed] [Google Scholar]
- 10.Mondino A, Mueller DL. mTOR at the crossroads of T cell proliferation and tolerance. Semin Immunol. 2007;19:162–172. doi: 10.1016/j.smim.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 12.Coenen JJ, Koenen HJ, van Rijssen E, Hilbrands LB, Joosten I. Rapamycin, and not cyclosporin A, preserves the highly suppressive CD27+ subset of human CD4+CD25+ regulatory T cells. Blood. 2006;107:1018–1023. doi: 10.1182/blood-2005-07-3032. [DOI] [PubMed] [Google Scholar]
- 13.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 14.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 15.Hackstein H, Taner T, Zahorchak AF, Morelli AE, Logar AJ, Gessner A, Thomson AW. Rapamycin inhibits IL-4-induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood. 2003;101:4457–4463. doi: 10.1182/blood-2002-11-3370. [DOI] [PubMed] [Google Scholar]
- 16.Woltman AM, van der Kooij SW, Coffer PJ, Offringa R, Daha MR, van Kooten C. Rapamycin specifically interferes with GM-CSF signaling in human dendritic cells, leading to apoptosis via increased p27KIP1 expression. Blood. 2003;101:1439–1445. doi: 10.1182/blood-2002-06-1688. [DOI] [PubMed] [Google Scholar]
- 17.Monti P, Mercalli A, Leone BE, Valerio DC, Allavena P, Piemonti L. Rapamycin impairs antigen uptake of human dendritic cells. Transplantation. 2003;75:137–145. doi: 10.1097/00007890-200301150-00025. [DOI] [PubMed] [Google Scholar]
- 18.Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol. 2007;178:7018–7031. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 19.Taner T, Hackstein H, Wang Z, Morelli AE, Thomson AW. Rapamycin-treated, alloantigen-pulsed host dendritic cells induce Ag-specific T cell regulation and prolong graft survival. Am J Transplant. 2005;5:228–236. doi: 10.1046/j.1600-6143.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- 20.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 21.Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol. 2004;4:24–35. doi: 10.1038/nri1256. [DOI] [PubMed] [Google Scholar]
- 22.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Brint EK, Xu D, Liu H, Dunne A, McKenzie AN, O’Neill LA, Liew FY. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol. 2004;5:373–379. doi: 10.1038/ni1050. [DOI] [PubMed] [Google Scholar]
- 24.Morelli AE, Zahorchak AF, Larregina AT, Colvin BL, Logar AJ, Takayama T, Falo LD, Thomson AW. Cytokine production by mouse myeloid dendritic cells in relation to differentiation and terminal maturation induced by lipopolysaccharide or CD40 ligation. Blood. 2001;98:1512–1523. doi: 10.1182/blood.v98.5.1512. [DOI] [PubMed] [Google Scholar]
- 25.Patel S, Lyons-Weiler J. caGEDA: a web application for the integrated analysis of global gene expression patterns in cancer. Appl Bioinformatics. 2004;3:49–62. doi: 10.2165/00822942-200403010-00007. [DOI] [PubMed] [Google Scholar]
- 26.Morelli AE, Larregina AT, Ganster RW, Zahorchak AF, Plowey JM, Takayama T, Logar AJ, Robbins PD, Falo LD, Thomson AW. Recombinant adenovirus induces maturation of dendritic cells via an NF-kappaB-dependent pathway. J Virol. 2000;74:9617–9628. doi: 10.1128/jvi.74.20.9617-9628.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal Biochem. 2000;285:194–204. doi: 10.1006/abio.2000.4753. [DOI] [PubMed] [Google Scholar]
- 28.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergers G, Reikerstorfer A, Braselmann S, Graninger P, Busslinger M. Alternative promoter usage of the Fos-responsive gene Fit-1 generates mRNA isoforms coding for either secreted or membrane-bound proteins related to the IL-1 receptor. EMBO J. 1994;13:1176–1188. doi: 10.1002/j.1460-2075.1994.tb06367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwahana H, Yanagisawa K, Ito-Kosaka A, Kuroiwa K, Tago K, Komatsu N, Katashima R, Itakura M, Tominaga S. Different promoter usage and multiple transcription initiation sites of the interleukin-1 receptor-related human ST2 gene in UT-7 and TM12 cells. Eur J Biochem. 1999;264:397–406. doi: 10.1046/j.1432-1327.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Tago K, Io K, Kuroiwa K, Arai T, Iwahana H, Tominaga S, Yanagisawa K. The cloning and nucleotide sequence of human ST2 cDNA. Genomics. 2000;67:284–290. doi: 10.1006/geno.2000.6269. [DOI] [PubMed] [Google Scholar]
- 32.Hayakawa H, Hayakawa M, Kume A, Tominaga SI. Soluble ST2 blocks IL-33 signaling in allergic airway inflammation. J Biol Chem. 2007 doi: 10.1074/jbc.M704916200. [DOI] [PubMed] [Google Scholar]
- 33.Poulalhon N, Farge D, Roos N, Tacheau C, Neuzillet C, Michel L, Mauviel A, Verrecchia F. Modulation of collagen and MMP-1 gene expression in fibroblasts by the immunosuppressive drug rapamycin. A direct role as an antifibrotic agent? J Biol Chem. 2006;281:33045–33052. doi: 10.1074/jbc.M606366200. [DOI] [PubMed] [Google Scholar]
- 34.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–328. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 35.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 36.Omura T, Yoshiyama M, Izumi Y, Kim S, Matsumoto R, Enomoto S, Kusuyama T, Nishiya D, Nakamura Y, Akioka K, Iwao H, Takeuchi K, Yoshikawa J. Involvement of c-Jun NH2 terminal kinase and p38MAPK in rapamycin-mediated inhibition of neointimal formation in rat carotid arteries. J Cardiovasc Pharmacol. 2005;46:519–525. doi: 10.1097/01.fjc.0000179001.00779.a5. [DOI] [PubMed] [Google Scholar]
- 37.Li Q, I, Verma M. NF-[kappa]B regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 38.Kropf P, Bickle Q, Herath S, Klemenz R, Muller I. Organ-specific distribution of CD4+ T1/ST2+ Th2 cells in Leishmania major infection. Eur J Immunol. 2002;32:2450–2459. doi: 10.1002/1521-4141(200209)32:9<2450::AID-IMMU2450>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 39.Muegge K, Vila M, Gusella GL, Musso T, Herrlich P, Stein B, Durum SK. Interleukin 1 induction of the c-jun promoter. Proc Natl Acad Sci U S A. 1993;90:7054–7058. doi: 10.1073/pnas.90.15.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laursen NB, Kessler R, Fr √ ∂ hli E, Klemenz R. Effects of ras transformation on the induction of the IL-1 receptor related T1 gene in response to mitogens, anisomycin, IL-1 and TNFalpha. Oncogene. 1998;16:575–586. doi: 10.1038/sj.onc.1201522. [DOI] [PubMed] [Google Scholar]
- 41.Oshikawa K, Yanagisawa K, Tominaga S, Sugiyama Y. ST2 protein induced by inflammatory stimuli can modulate acute lung inflammation. Biochem Biophys Res Commun. 2002;299:18–24. doi: 10.1016/s0006-291x(02)02578-0. [DOI] [PubMed] [Google Scholar]
- 42.Maeda K, Shioi T, Kosugi R, Yoshida Y, Takahashi K, Machida Y, Izumi T. Rapamycin ameliorates experimental autoimmune myocarditis. Int Heart J. 2005;46:513–530. doi: 10.1536/ihj.46.513. [DOI] [PubMed] [Google Scholar]
- 43.Wasowska B, Wieder KJ, Hancock WW, Zheng XX, Berse B, Binder J, Strom TB, Kupiec-Weglinski JW. Cytokine and alloantibody networks in long term cardiac allografts in rat recipients treated with rapamycin. J Immunol. 1996;156:395–404. [PubMed] [Google Scholar]
- 44.Makino M, Maeda Y, Mukai T, Kaufmann SH. Impaired maturation and function of dendritic cells by mycobacteria through IL-1beta. Eur J Immunol. 2006;36:1443–1452. doi: 10.1002/eji.200535727. [DOI] [PubMed] [Google Scholar]
- 45.Lee JI, Ganster RW, Geller DA, Burckart GJ, Thomson AW, Lu L. Cyclosporine A inhibits the expression of costimulatory molecules on in vitro-generated dendritic cells: association with reduced nuclear translocation of nuclear factor kappa B. Transplantation. 1999;68:1255–1263. doi: 10.1097/00007890-199911150-00007. [DOI] [PubMed] [Google Scholar]
- 46.Matasic R, Dietz AB, Vuk-Pavlovic S. Dexamethasone inhibits dendritic cell maturation by redirecting differentiation of a subset of cells. J Leukoc Biol. 1999;66:909–914. doi: 10.1002/jlb.66.6.909. [DOI] [PubMed] [Google Scholar]
- 47.Hackstein H, Morelli AE, Larregina AT, Ganster RW, Papworth GD, Logar AJ, Watkins SC, Falo LD, Thomson AW. Aspirin inhibits in vitro maturation and in vivo immunostimulatory function of murine myeloid dendritic cells. J Immunol. 2001;166:7053–7062. doi: 10.4049/jimmunol.166.12.7053. [DOI] [PubMed] [Google Scholar]
- 48.Chiang PH, Wang L, Bonham CA, Liang X, Fung JJ, Lu L, Qian S. Mechanistic insights into impaired dendritic cell function by rapamycin: inhibition of Jak2/Stat4 signaling pathway. J Immunol. 2004;172:1355–1363. doi: 10.4049/jimmunol.172.3.1355. [DOI] [PubMed] [Google Scholar]
- 49.Liew FY, Xu D, Brint EK, O’Neill AL. Negative regulation of Toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 50.Gachter T, Werenskiold AK, Klemenz R. Transcription of the interleukin-1 receptor-related T1 gene is initiated at different promoters in mast cells and fibroblasts. J Biol Chem. 1996;271:124–129. doi: 10.1074/jbc.271.1.124. [DOI] [PubMed] [Google Scholar]
- 51.Xu D, Chan WL, Leung BP, Huang F, Wheeler R, Piedrafita D, Robinson JH, Liew FY. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J Exp Med. 1998;187:787–794. doi: 10.1084/jem.187.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lohning M, Stroehmann A, Coyle AJ, Grogan JL, Lin S, Gutierrez-Ramos JC, Levinson D, Radbruch A, Kamradt T. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc Natl Acad Sci U S A. 1998;95:6930–6935. doi: 10.1073/pnas.95.12.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coyle AJ, Lloyd C, Tian J, Nguyen T, Erikkson C, Wang L, Ottoson P, Persson P, Delaney T, Lehar S, Lin S, Poisson L, Meisel C, Kamradt T, Bjerke T, Levinson D, Gutierrez-Ramos JC. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J Exp Med. 1999;190:895–902. doi: 10.1084/jem.190.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J Exp Med. 2000;191:1069–1076. doi: 10.1084/jem.191.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kobayashi T, Walsh PT, Walsh MC, Speirs KM, Chiffoleau E, King CG, Hancock WW, Caamano JH, Hunter CA, Scott P, Turka LA, Choi Y. TRAF6 is a critical factor for dendritic cell maturation and development. Immunity. 2003;19:353–363. doi: 10.1016/s1074-7613(03)00230-9. [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi KS, Flavell RA. Shielding the double-edged sword: negative regulation of the innate immune system. J Leukoc Biol. 2004;75:428–433. doi: 10.1189/jlb.0703321. [DOI] [PubMed] [Google Scholar]
- 57.Mirenda V, Berton I, Read J, Cook T, Smith J, Dorling A, Lechler RI. Modified dendritic cells coexpressing self and allogeneic major histocompatability complex molecules: an efficient way to induce indirect pathway regulation. J Am Soc Nephrol. 2004;15:987–997. doi: 10.1097/01.asn.0000119575.98696.1d. [DOI] [PubMed] [Google Scholar]
- 58.Yates SF, Paterson AM, Nolan KF, Cobbold SP, Saunders NJ, Waldmann H, Fairchild PJ. Induction of regulatory T cells and dominant tolerance by dendritic cells incapable of full activation. J Immunol. 2007;179:967–976. doi: 10.4049/jimmunol.179.2.967. [DOI] [PubMed] [Google Scholar]
- 59.Woltman AM, van der Kooij SW, de Fijter JW, van Kooten C. Maturation-resistant dendritic cells induce hyporesponsiveness in alloreactive CD45RA+ and CD45RO+ T-cell populations. Am J Transplant. 2006;6:2580–2591. doi: 10.1111/j.1600-6143.2006.01520.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.