Abstract
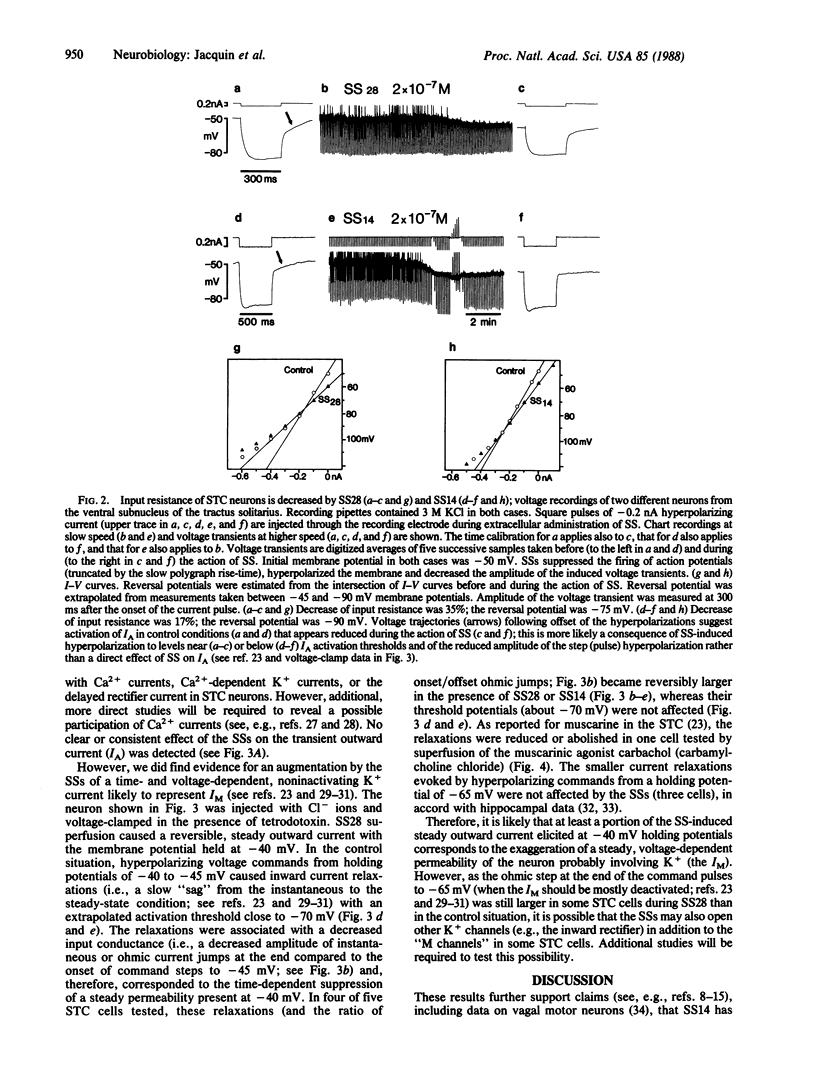
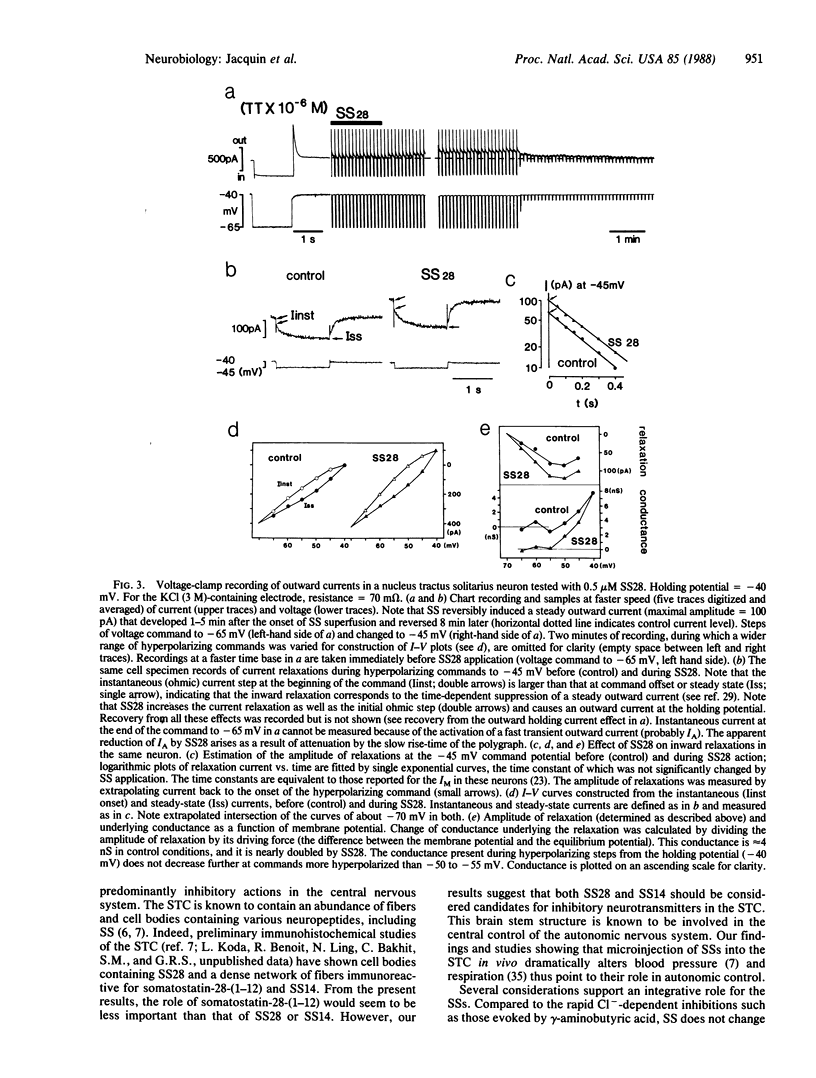
The synaptic function of somatostatin-containing fibers in the nervous system is controversial. Therefore, we used a slice preparation of the rat brain stem to test the electrophysiological effects of prosomatostatin-derived peptides on neurons of the solitary tract complex, which contains an abundance of somatostatin-containing fibers and cell bodies. Superfusion of both somatostatin-14 and somatostatin-28 (the precursor for somatostatin-14), but not somatostatin-28-(1-12) or -(1-10), predominantly inhibited spontaneous spike and subthreshold (probably synaptic) activity. In intracellular recordings, somatostatin-14 and -28 hyperpolarized most neurons in association with a slight (10-35%) but reproducible decrease in input resistance. These hyperpolarizing responses were augmented in depolarized cells and persisted in cells in which spontaneous inhibitory postsynaptic potentials became depolarizing after Cl- injection. These data suggest that somatostatin receptors regulate a K+ conductance. In voltage-clamp studies, somatostatin-28 and -14 induced a steady outward current and augmented the voltage-dependent, nonactivating outward K+ conductance (IM) shown to be blocked by activation of muscarinic cholinergic receptors. These results suggest (i) that somatostatin-containing elements in the solitary tract complex play an inhibitory role through the activation of postsynaptic permeability to potassium ions and (ii) that the same ion channel type may be coregulated by two neurotransmitter candidates, somatostatin and acetylcholine, through a reciprocal control mechanism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Spontaneous inhibitory post-synaptic potentials in hippocampus: mechanism for tonic inhibition. Brain Res. 1980 Oct 27;200(1):195–200. doi: 10.1016/0006-8993(80)91108-7. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980 Feb 14;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Champagnat J., Denavit-Saubie M., Siggins G. R. Rhythmic neuronal activities in the nucleus of the tractus solitarius isolated in vitro. Brain Res. 1983 Nov 28;280(1):155–159. doi: 10.1016/0006-8993(83)91184-8. [DOI] [PubMed] [Google Scholar]
- Champagnat J., Denavit-Saubié M., Grant K., Shen K. F. Organization of synaptic transmission in the mammalian solitary complex, studied in vitro. J Physiol. 1986 Dec;381:551–573. doi: 10.1113/jphysiol.1986.sp016343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagnat J., Denavit-Saubié M., Moyanova S., Rondouin G. Involvement of amino acids in periodic inhibitions of bulbar respiratory neurones. Brain Res. 1982 Apr 15;237(2):351–365. doi: 10.1016/0006-8993(82)90447-4. [DOI] [PubMed] [Google Scholar]
- Champagnat J., Jacquin T., Richter D. W. Voltage-dependent currents in neurones of the nuclei of the solitary tract of rat brainstem slices. Pflugers Arch. 1986 Apr;406(4):372–379. doi: 10.1007/BF00590939. [DOI] [PubMed] [Google Scholar]
- Champagnat J., Siggins G. R., Koda L. Y., Denavit-Saubié M. Synaptic responses of neurons of the nucleus tractus solitarius in vitro. Brain Res. 1985 Jan 28;325(1-2):49–56. doi: 10.1016/0006-8993(85)90301-4. [DOI] [PubMed] [Google Scholar]
- Delfs J. R., Dichter M. A. Effects of somatostatin on mammalian cortical neurons in culture: physiological actions and unusual dose response characteristics. J Neurosci. 1983 Jun;3(6):1176–1188. doi: 10.1523/JNEUROSCI.03-06-01176.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd J., Kelly S. Is somatostatin an excitatory transmitter in the hippocampus? Nature. 1978 Jun 22;273(5664):674–675. doi: 10.1038/273674a0. [DOI] [PubMed] [Google Scholar]
- Gähwiler B. H., Brown D. A. GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Kalia M., Fuxe K., Agnati L. F., Hökfelt T., Härfstrand A. Somatostatin produces apnea and is localized in medullary respiratory nuclei: a possible role in apneic syndromes. Brain Res. 1984 Apr 2;296(2):339–344. doi: 10.1016/0006-8993(84)90070-2. [DOI] [PubMed] [Google Scholar]
- Kalia M., Fuxe K., Hökfelt T., Johansson O., Lang R., Ganten D., Cuello C., Terenius L. Distribution of neuropeptide immunoreactive nerve terminals within the subnuclei of the nucleus of the tractus solitarius of the rat. J Comp Neurol. 1984 Jan 20;222(3):409–444. doi: 10.1002/cne.902220308. [DOI] [PubMed] [Google Scholar]
- Luini A., Lewis D., Guild S., Schofield G., Weight F. Somatostatin, an inhibitor of ACTH secretion, decreases cytosolic free calcium and voltage-dependent calcium current in a pituitary cell line. J Neurosci. 1986 Nov;6(11):3128–3132. doi: 10.1523/JNEUROSCI.06-11-03128.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancillas J. R., Siggins G. R., Bloom F. E. Somatostatin selectively enhances acetylcholine-induced excitations in rat hippocampus and cortex. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7518–7521. doi: 10.1073/pnas.83.19.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison J. H., Benoit R., Magistretti P. J., Ling N., Bloom F. E. Immunohistochemical distribution of pro-somatostatin-related peptides in hippocampus. Neurosci Lett. 1982 Dec 30;34(2):137–142. doi: 10.1016/0304-3940(82)90165-3. [DOI] [PubMed] [Google Scholar]
- Mueller A. L., Kunkel D. D., Schwartzkroin P. A. Electrophysiological actions of somatostatin (SRIF) in hippocampus: an in vitro study. Cell Mol Neurobiol. 1986 Dec;6(4):363–379. doi: 10.1007/BF00711406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Williams J. T. On the potassium conductance increased by opioids in rat locus coeruleus neurones. J Physiol. 1985 Jul;364:265–280. doi: 10.1113/jphysiol.1985.sp015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Williams J. T., Surprenant A., Christie M. J. Mu and delta receptors belong to a family of receptors that are coupled to potassium channels. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5487–5491. doi: 10.1073/pnas.84.15.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olpe H. R., Balcar V. J., Bittiger H., Rink H., Sieber P. Central actions of somatostatin. Eur J Pharmacol. 1980 May 2;63(2-3):127–133. doi: 10.1016/0014-2999(80)90436-7. [DOI] [PubMed] [Google Scholar]
- Oomura Y., Mizuno Y. Effect of somatostatin on the vagal motor neuron in the rat. Brain Res Bull. 1986 Sep;17(3):397–401. doi: 10.1016/0361-9230(86)90245-5. [DOI] [PubMed] [Google Scholar]
- Pittman Q. J., Siggins G. R. Somatostatin hyperpolarizes hippocampal pyramidal cells in vitro. Brain Res. 1981 Sep 28;221(2):402–408. doi: 10.1016/0006-8993(81)90791-5. [DOI] [PubMed] [Google Scholar]
- Randić M., Miletić V. Depressant actions of methionine-enkephalin and somatostatin in cat dorsal horn neurones activated by noxious stimuli. Brain Res. 1978 Aug 18;152(1):196–202. doi: 10.1016/0006-8993(78)90148-8. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Perrin M. H., Rivier J. E., Vale W. High affinity binding sites for a somatostatin-28 analog in rat brain. Life Sci. 1981 May 11;28(19):2191–2198. doi: 10.1016/0024-3205(81)90628-7. [DOI] [PubMed] [Google Scholar]
- Richter D. W. Generation and maintenance of the respiratory rhythm. J Exp Biol. 1982 Oct;100:93–107. doi: 10.1242/jeb.100.1.93. [DOI] [PubMed] [Google Scholar]
- Siggins G. R., McGinty J. F., Morrison J. H., Pittman Q. J., Zieglgänsberger W., Magistretti P. J., Gruol D. L. The role of neuropeptides in the hippocampal formation. Adv Biochem Psychopharmacol. 1982;33:413–422. [PubMed] [Google Scholar]
- Trussell L. O., Jackson M. B. Adenosine-activated potassium conductance in cultured striatal neurons. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4857–4861. doi: 10.1073/pnas.82.14.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoo A., Yoshii M., Narahashi T. Block of calcium channels by enkephalin and somatostatin in neuroblastoma-glioma hybrid NG108-15 cells. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9832–9836. doi: 10.1073/pnas.83.24.9832. [DOI] [PMC free article] [PubMed] [Google Scholar]



