Abstract
Since its discovery, the possible corelease of classic neurotransmitters from neurons has received much attention. Colocalization of monoamines and amino acidergic neurotransmitters [mainly glutamate and dopamine (DA) or serotonin] in mammalian neurons has been reported. However, few studies have dealt with the colocalization of DA and γ-aminobutyric acid (GABA) in neurons. With the aim of providing some insight into the colocalization of neurotransmitters during early vertebrate phylogeny, we studied GABA expression in dopaminergic neurons in the sea lamprey brain by using double-immunofluorescence methods with anti-DA and anti-GABA antibodies. Different degrees of colocalization of DA and GABA were observed in different dopaminergic brain nuclei. A high degree of colocalization (GABA in at least 25% of DA-immunoreactive neurons) was observed in populations of the caudal rhombencephalon, ventral isthmus, postoptic commissure nucleus, preoptic nucleus and in granule-like cells of the olfactory bulb. A new DA-immunoreactive striatal population that showed colocalization with GABA in about a quarter of its neurons was observed. In the periventricular hypothalamus, colocalization was observed in only a few cells, despite the abundance of DA- and GABA-immunoreactive neurons, and no double-labelled cells were observed in the paratubercular nucleus. The frequent colocalization of DA and GABA reveals that the dopaminergic populations of lampreys are more complex than previously reported. Double-labelled fibres or terminals were observed in different brain regions, suggesting possible corelease of DA and GABA by these lamprey neurons. The present results suggest that colocalization of DA and GABA in neurons appeared early in vertebrate evolution.
Keywords: agnathans, amino acidergic neurotransmitters, catecholamines, coexpression, cotransmission
Introduction
Experimental evidence refuting Dale’s principle (i.e. the notion that each neuron synthesizes and releases only one neurotransmitter) has accumulated over recent decades (see Strata & Harvey, 1999; Trudeau & Gutiérrez, 2007). Cotransmission by multiple neurotransmitters from the same neuron or axon terminal (and even from the same vesicle) is now well documented (see Hökfelt et al. 1992; Trudeau & Gutiérrez, 2007) and the postulate ‘one neuron, one neurotransmitter’ is probably the exception rather than the rule. The corelease of ‘classic’ neurotransmitters and a peptide, nucleotide, nitric oxide, neurotrophic factors or other molecules is well documented. However, the coexistence and corelease of two ‘classic’ neurotransmitters in a specific neuron has been less widely studied. Studies in recent years have focused on the cotransmission and corelease of γ-aminobutyric acid (GABA) and glycine (Lu et al. 2008), GABA and glutamate (Sandler & Smith, 1991; Gutiérrez, 2005), and glutamate and dopamine (DA) or serotonin (reviewed in Trudeau, 2004; and in Descarries et al. 2008). However, the coexistence of monoamines and GABA in the same cell and possible cofunctions of these neurotransmitters have been less well studied.
The coexistence of both DA and GABA, or their synthesizing enzymes, in the same cell has been previously reported in specific neuronal populations of a few vertebrate species. Colocalization of tyrosine hydroxylase (TH) (the rate-limiting enzyme of catecholamine synthesis) and glutamic acid decarboxylase (GAD) or GABA has been reported in some neurons in mammalian brains: in the olfactory bulb, diencephalon, mesencephalic central grey and cerebral cortex (Chronwall, 1985; Kosaka et al. 1985, 1987). Immunohistochemical and in-situ hybridization studies in the rat have also shown that a subset of DA neurons in the substantia nigra compacta contains GAD (the enzyme that catalyses GABA synthesis) (Hédou et al. 2000; González-Hernández et al. 2001). However, these results remain controversial as no immunoreactivity for GABA has been observed in these dopaminergic neurons (Kosaka et al. 1987; Sulzer et al. 1998). Unlike in mammals, the coexistence of DA or TH and GAD or GABA in central neurons in other vertebrate groups has been reported for only a few locations and species (Xenopus pituitary axons: de Rijk et al. 1992; dogfish spinal cord: Sueiro et al. 2004; snake olfactory bulb: Kosaka et al. 1991). Neurons containing both DA and GABA have also been reported in some invertebrates (Periplaneta: Distler, 1990; Aplysia: Díaz-Ríos et al. 2002; Díaz-Ríos & Miller, 2005).
Lampreys are important model organisms for the study of the neuronal basis of vertebrate locomotion (see Dubuc et al. 2008; Grillner et al. 2008). Neurotransmitter corelease may be important in the modulation and plasticity of neuronal networks at synaptic levels to enable interaction with various sets of receptors. We previously reported that, in lampreys, GABA was colocalized with DA in some spinal cord neurons (Rodicio et al. 2008). Other studies carried out in our laboratory on the sea lamprey brain also reported the colocalization of GABA and aspartate (Villar-Cerviño et al. 2008a), GABA and glycine (Villar-Cerviño et al. 2008b), and GABA and serotonin (Barreiro-Iglesias et al. 2009) in neurons of different nuclei. These studies suggest that the colocalization of ‘classical’ neurotransmitters is rather extended in extant jawless vertebrates. Accordingly, knowledge of colocalization of neurotransmitters in the different neuronal populations of lampreys appears important for a better understanding of the neurochemical basis of circuits underlying locomotion control and other brain functions.
To provide some new insight into the codistribution of different neurotransmitters in the sea lamprey brain, we studied the possible colocalization of DA and GABA in neurons by means of double-immunofluorescence methods. Results from the present investigation showed that DA and GABA are colocalized in subsets of neurons in different nuclei of the sea lamprey brain previously characterized as dopaminergic. Present and previous studies in our laboratory (Rodicio et al. 2008) indicate that lampreys are very useful vertebrate models for studies of the colocalization of ‘classical’ neurotransmitters.
Materials and methods
Upstream migrating adult sea lampreys (Petromyzon marinus L.; n = 5; about 75 cm in total body length) caught in the river Ulla (northwest Spain) and provided by a commercial supplier were used in this study. The lampreys were deeply anaesthetized with benzocaine (0.05% in freshwater), killed by decapitation and their brains dissected out and fixed immediately by immersion in 5% glutaraldehyde and 1% sodium metabisulphite in Tris-buffered saline (TBS) at pH 7.4 for 17 h. The brains were then embedded in Tissue Tek, frozen in liquid nitrogen-cooled isopentane and transversely sectioned on a cryostat (16 μm thick). All experiments conformed to the European Community guidelines on animal care and experimentation and were approved by the Ethics Committee of the University of Santiago de Compostela.
Immunofluorescence
Double-immunofluorescence experiments to study the colocalization of DA and GABA in sea lamprey neurons were performed as previously described (Rodicio et al. 2008). Briefly, sections were pretreated with 0.2% NaBH4 for 45 min and subsequently incubated for 3 days at 4 °C with a mixture of rabbit polyclonal anti-DA antiserum (1 : 900; H.W.M. Steinbusch, Maastricht, The Netherlands) and mouse monoclonal anti-GABA antibody (1 : 1200 clone GB-69; Sigma, St. Louis, MO, USA) in TBS containing 1% sodium metabisulphite, 15% normal goat serum and 0.2% Triton as detergent. After being rinsed in TBS, the samples were incubated for 1 h with a cocktail of Cy3-conjugated goat anti-rabbit immunoglobulin (1 : 200; Chemicon, Temecula, CA, USA) and fluorescein-conjugated goat anti-mouse immunoglobulin (1 : 50; Chemicon) in TBS, rinsed again and mounted with fluorescence anti-fade mounting medium (Vectashield; Vector, Burlingame, CA, USA).
Controls
The specificities of the GABA and DA antibodies have been well characterized by the suppliers. The monoclonal anti-GABA antibody does not cross-react with bovine serum albumin (BSA), l-α-aminobutyric acid, l-glutamic acid, l-aspartic acid, glycine, δ-aminovaleric acid, l-threonine, l-glutamine, taurine, putrescine, l-alanine or carnosine. The DA antiserum was raised against a DA-BSA conjugate and does cross-react with noradrenaline (< 10% cross-reaction) and with other monoamines (< 1% cross-reaction) (Steinbusch et al. 1991). No immunoreactivity was detected when the primary antibodies were omitted from the immunohistochemical processing. Control experiments (preadsorption with the corresponding antigens, GABA-BSA and DA-BSA conjugates, respectively, and western blots of lamprey brain protein extracts) previously carried out in our laboratory confirmed the specificity of these antibodies (anti-DA: Barreiro-Iglesias et al. 2008; anti-GABA: Villar-Cerviño et al. 2008b) in lamprey tissue.
Image acquisition and processing
Photomicrographs were taken with a spectral confocal laser scanning microscope (Leica TCS-SP2). Except when specified, photomicrographs are z-stack projections from confocal images. Data were acquired in the spectral confocal microscope by use of a narrow wavelength window tuned to the specific emission of each fluorescent marker (fluorescein isothiocyanate or Cy3). The photomicrographs were adjusted for brightness and contrast with Adobe Photoshop C.S.4 software.
Cell counts and measurements
The percentage of DA-immunoreactive (ir) cells that were also GABA-ir was calculated (n = 3). For quantitative analysis, only clearly stained neurons were counted in one of every three of the serial sections of each brain nucleus. As the size of DA cells did not vary greatly within the same population (although it may vary among different populations) and as determination of the total number of these cells was not the aim of the study, no correction factor was introduced. The percentage of DA-ir cells that also were GABA-ir was calculated for the brain nuclei that showed the highest degree of colocalization. To estimate cell size, the minor axis of 15 DA-ir/GABA-ir neurons was measured in each cell population in transverse sections with the aid of Leica Confocal software (Leica Microsystems, Milton Keynes, UK). Results are given in the text as mean ± SD.
Nomenclature
The nomenclature used in the present study for dopaminergic neuronal nuclei of the sea lamprey followed that used in a developmental study of the dopaminergic system carried out in our laboratory (Abalo et al. 2005), with slight modifications.
Results
General organization of the dopaminergic and GABAergic systems
Numerous DA-ir neuronal cell bodies are located in discrete groups distributed in the telencephalon, diencephalon and rhombencephalon of the sea lamprey (Abalo et al. 2005; Barreiro-Iglesias et al. 2008). Double DA/GABA immunolabelling revealed that GABAergic cell populations are much more numerous and widely distributed in the sea lamprey brain and most of them do not display DA-ir cells. Accordingly, colocalization of DA/GABA will be described on the basis of the DA-ir nuclei. The general organization of the dopaminergic and GABAergic immunoreactive systems in the sea lamprey has already been studied (DA: Abalo et al. 2005; GABA: Robertson et al. 2007) and its detailed study is beyond the scope of the present investigation. The topological organization of the dopaminergic populations in the adult sea lamprey and the location of DA/GABA double-immunolabelled neurons are schematically illustrated in Fig. 1. The following neuronal populations were considered: (i) the group of DA-ir cerebrospinal fluid-contacting (CSF-c) cells in the caudal rhombencephalon; (ii) the few DA-ir cells located in the ventral isthmus; (iii) the numerous DA-ir CSF-c cells of the mammillary nucleus [postinfundibular commissure nucleus of Abalo et al. (2005)]; (iv) the paratubercular nucleus [posterior tubercle nucleus of Abalo et al. (2005)]; (v) the numerous DA-ir CSF-c cells of the dorsal and ventral hypothalamic nuclei; (vi) the DA-ir cells of the postoptic commissure nucleus; (vii) the DA-ir cells of the preoptic nucleus; (viii) DA-ir neurons located in the striatum; and (ix) the population of DA-ir granule-like cells of the olfactory bulbs.
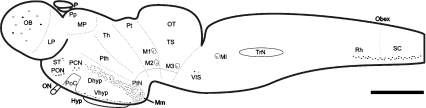
Fig. 1.
Schematic drawing of a lateral view of the brain of the sea lamprey showing the distribution of double-labelled DA-ir/GABA-ir cells (black dots) in comparison with the distribution of DA-ir cells (grey dots). M1-3 and MI, giant Müller cells; DHyp, dorsal hypothalamus; Hyp, hypophysis; LP, lateral pallium; Mm, mammillary nucleus; MP, medial pallium; OB, olfactory bulbs; ON, optic nerve; OT, optic tectum; P, pineal organ; PCN, postoptic commissure nucleus; PON, preoptic nucleus; PoC, postoptic commissure; Pp, parapineal organ; Pt, pretectum; PTh, prethalamus; PtN, paratubercular nucleus; Rh, rhombencephalon; SC, spinal cord; ST, striatum; Th, thalamus; TrN, trigeminal nucleus; TS, torus semicircularis; VIS, ventral isthmus; Vhyp, ventral hypothalamus. Scale bar: 1 mm.
Colocalization of DA and GABA in lamprey neurons
Double-immunofluorescence experiments revealed that DA and GABA immunoreactivities are colocalized in some neurons of most dopaminergic populations of the sea lamprey brain (arrows in Figs 2–4). The proportion of cells showing DA and GABA colocalization varied among the different dopaminergic populations. The dopaminergic populations of the brain that showed the highest degree of colocalization of both immunoreactivities (>25% of dopaminergic cells) were the caudal rhombencephalic cerebrospinal fluid-contacting population, ventral isthmus, postoptic commissure nucleus, preoptic nucleus, striatum and granule-like cells of the olfactory bulbs. In the mammillary and dorsal and ventral hypothalamic nuclei, very few of the DA-ir cells were also GABA-ir. Double-labelled cells were not observed in the paratubercular nucleus of any of the lampreys studied.
Fig. 2.
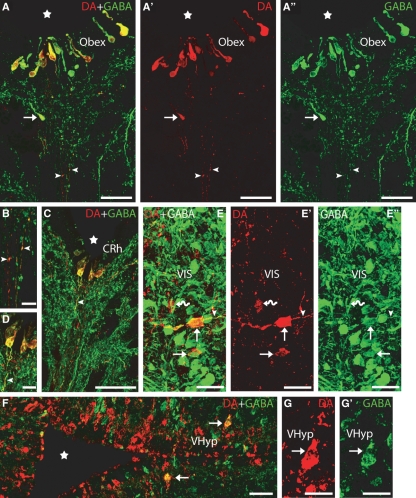
Transverse sections of the brain showing DA (red) and GABA (green) immunoreactivities. (A–A′′) Photomicrographs of a transverse section at the transition between the spinal cord and rhombencephalon (obex) showing double-labelled CSF-c cells in the ventral midline, close to the fourth ventricle. Note the presence of a DA-ir/GABA-ir cell ventral to the ependyma (arrow). Note also the presence of double-labelled thin fibres coursing in the raphe (arrowheads). (B) Detail of the double-labelled fibres of A. (C) Photomicrograph of a transverse section of the caudal rhombencephalon showing double-labelled CSF-c cells. Note the presence of a double-labelled process arising from a DA-ir/GABA-ir CSF-c cell (arrowhead). (D) Detail of C. (E–E′′) Photomicrographs of a transverse section of the rostral rhombencephalon showing double-labelled cells in the ventral isthmus (arrows). Note the presence of a dopaminergic cell not labelled by the anti-GABA antibody (curved arrow). Also note the presence of a double-labelled axonal-like process arising from one of the DA-ir/GABA-ir cells (arrowhead). (F) Photomicrograph of a transverse section of the ventral hypothalamus showing double-labelled cells (arrows) in addition to a number of singly GABA-ir and DA-ir cells. (G–G′) Detail of a double-labelled cell from F. Stars indicate the ventricle. Dorsal is up in A–E′ and right in F–G′. CRh, caudal rhombencephalon. For other abbreviations, see the legend to Fig. 1. Scale bars: 50 μm in A–A′′, C and F; 25 μm in E–E′′; and 12.5 μm in B, D and G–G′.
Fig. 4.
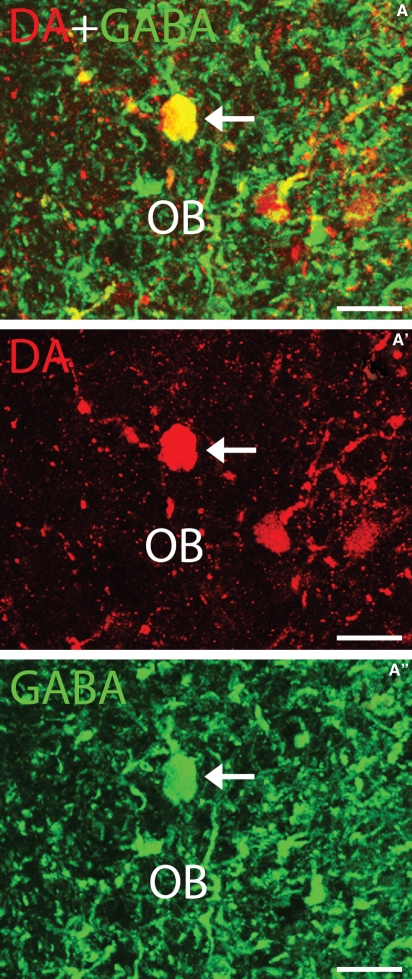
Transverse section of the sea lamprey olfactory bulb showing DA (red) and GABA (green) immunoreactivities. (A–A′′) Photomicrographs of a transverse section of the olfactory bulb showing a double-labelled granule-like cell (arrow). Dorsal is up in the figure. For abbreviations, see the legend to Fig. 1. Scale bars: 25 μm.
The group of DA-ir CSF-c cells of the caudal rhombencephalon was observed in the subependyma (arrow in Fig. 2A–A′′) near the ventral midline of the fourth ventricle (Fig. 2C and D). This group represents a rostral extension of the spinal DA-ir CSF-c population described by Rodicio et al. (2008) beyond the transition between the spinal cord and rhombencephalon (Fig. 2A–A′′ and B). These small cells (9.47 ± 1.43 μm in diameter) displayed an apical dendrite ending as a club on the ventricular surface and some of them also showed an axonal-like basal process arising from the opposite pole of the cell. All of the DA-ir cells (100%) in this nucleus were also labelled by the anti-GABA antibody. The axonal processes of these DA-ir/GABA-ir neurons extended ventrally and double labelling of these processes was often appreciable (arrowheads in Fig. 2A–D).
A small population of DA-ir cells was observed in the ventral isthmus (rostral rhombencephalon) (Fig. 2E–E′′). Approximately 58% of DA-ir cells in this region were also GABA-ir. The perikarya of these double-labelled cells were rounded or fusiform and 14.55 ± 1.49 μm in diameter. In some cells the axonal process was observed coursing laterally.
In the hypothalamus, three conspicuous DA-ir populations mostly consisting of small CSF-c cells were distinguishable: the mammillary nucleus located around the posterior infundibular recess and the ventral and dorsal hypothalamic nuclei. Double-labelled (DA-ir/GABA-ir) cells were scarce in these hypothalamic nuclei. In the mammillary nucleus very few DA-ir cells were GABA-ir (not shown); these were 12.62 ± 0.42 μm in diameter. In the dorsal hypothalamus most, if not all, of the DA-ir cells (8.97 ± 2.29 μm) were of CSF-c type. Occasional DA-ir cells in this nucleus displayed GABA immunoreactivity (not shown). Strongly stained DA-ir cells, mostly of CSF-c type, were observed in the ventral hypothalamic nucleus extending between the postoptic and infundibular recesses (Fig. 2F–G′). About 18% of the DA-ir cells of the ventral hypothalamic nucleus were also GABA-ir. The double-labelled perikarya of this nucleus were usually observed at some distance from the ventricle in the second or third parallel row of cells located away from the ependyma and most of them were not of CSF-c type (arrows in Fig. 2F–G′). These cells were spherical or fusiform in shape (11.54 ± 1.66 μm in diameter).
In the postoptic commissure nucleus, ventral (around the postoptic recess) and dorsal (Fig. 3A–A′′) DA-ir subpopulations were recognizable (Abalo et al. 2005; present results). Colocalization of DA and GABA was only observed in DA-ir cells (about 23% of cells) in the dorsal zone (arrow in Fig. 3A–A′′) and no double-labelled cells were observed in the ventral zone (not shown). Double-labelled perikarya were fusiform and small (10.46 ± 2.50 μm), some were CSF-c type and most of them had an axon arising from the basal pole.
Fig. 3.
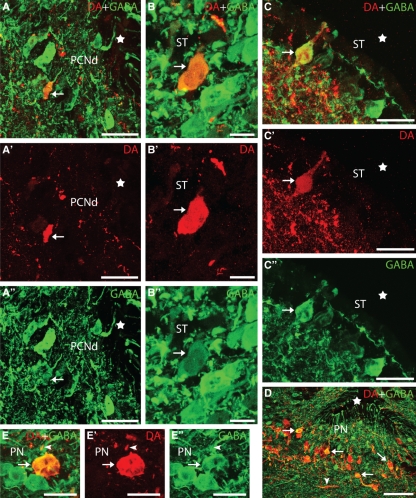
Transverse sections of the rostral diencephalon and telencephalon showing DA (red) and GABA (green) immunoreactivities. (A–A′′) Photomicrographs of a transverse section of the rostral diencephalon showing a double-labelled cell in the dorsal part of the postoptic commissure nucleus (arrows). (B–B′′) Photomicrographs of a transverse section of the striatum showing a double-labelled non-CSF-c cell of this region. (C–C′′) Photomicrographs of a transverse section of the striatum showing a double-labelled CSF-c cell. (D) Photomicrograph of a section of the preoptic region showing the high proportion of double-labelled (GABA/DA) dopaminergic cells. (E–E′′) Details of a double-labelled cell of the preoptic nucleus. Note the presence of a double-labelled terminal (arrowhead). Arrows indicate a double-labelled cell. Stars in figures indicate the impar telencephalic ventricle (A–C) and preoptic recess (D). Photomicrographs B–C′′ and E–E′′ are z-stack projections from eight confocal optical sections (0.5 μm thick). Dorsal is up in all figures. PCNd, dorsal postoptic commissure nucleus; PN, preoptic nucleus. For other abbreviations, see the legend to Fig. 1. Scale bars: 50 μm in A–A′′ and D; 12.5 μm in B–B′′; 15 μm in C–C′′; and 20 in E–E′′.
A small group of DA-ir cells (about 40 cells in each specimen) was observed in the striatum. No regional differences in DA cell distribution have been detected. Two types of DA-ir cells were distinguished on the basis of the appearance and location of their perikarya. The perikarya of the first type were located at some distance from the ventricle and were intermingled with GABAergic cells of the cell row characteristic of the lamprey striatum. Some DA-ir cells of this type were also GABA-ir (arrow in Fig. 3B–B′′) (about 25% of the DA-ir cells of this type) and their perikarya were fusiform or polygonal (12.51 ± 1.3 μm). The neurons of the second type were CSF-c cells (arrow in Fig. 3C–C′′) and all displayed colocalization of DA and GABA immunoreactivities. These scarce, oval or polygonal cells presented an apical dendrite protruding into the ventricle and perikarya were 7.95 ± 0.56 μm in diameter.
In the preoptic nucleus, the DA-ir cells occupied the caudal wall of the preoptic recess and were mostly observed in a periventricular location (Fig. 3D–E′′) dorsal to the optic chiasm but some were located in a more lateral position in the optic stalks (not shown). About 82% of the DA-ir cells located dorsal to the optic chiasm were also GABA-ir (arrows in Fig. 3D–E′′), whereas those in the optic stalks were not GABA-ir (not shown), which allowed us to differentiate two subpopulations of dopaminergic cells in this nucleus. The DA-ir/GABA-ir cells of the preoptic nucleus were 13.63 ± 2.47 μm in size and fusiform or polygonal in shape.
In the olfactory bulb, a population of DA-ir granule-like cells was observed in the inner cellular layer (Fig. 4A–A′′). About 26% of the DA-ir granule-like cells also exhibited GABA immunoreactivity. These DA-ir/GABA-ir cells contained rounded perikarya (11.47 ± 1.85 μm in size).
Colocalization of DA and GABA immunoreactivities in fibres and terminals
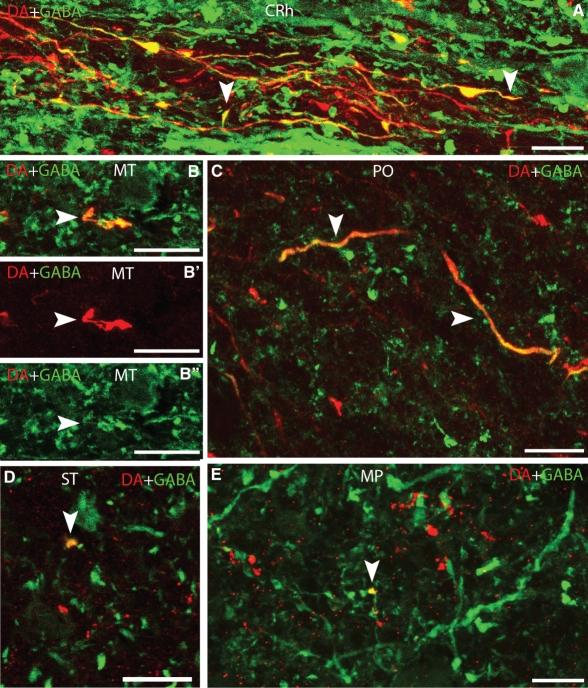
Representative brain regions were checked in order to observe the presence of fibres or terminals that were double labelled by the anti-DA and anti-GABA antibodies. To ensure the existence of double-labelled terminals, confocal photomicrographs (stacks 0.5 μm thick) were obtained at high magnification. In addition to the DA-ir/GABA-ir axonal processes arising from the CSF-c cells of the caudal rhombencephalon (see above; arrowheads in Figs 2C,D and 5A), double-labelled fibres or terminals were observed in the dorsal isthmic commissure, mesencephalic tegmentum (arrowhead in Fig. 5B–B′′), mammillary region, dorsal and ventral tuberal regions, preoptic region (arrowheads in Fig. 5C), striatum (arrowhead in Fig. 5D), lateral pallium, medial pallium (arrowhead in Fig. 5E) and olfactory bulb. In these regions, these fibres and terminals only represent a small proportion and most of the DA-ir fibres did not show double labelling (Fig. 5). Most double-labelled fibres and terminals were observed in lateral areas; however, commissural fibres were observed in the preoptic region (Fig. 5C).
Fig. 5.
Photomicrographs of transverse sections of the sea lamprey brain showing DA (red) and GABA (green) immunoreactivities in fibres or terminals (arrowheads). (A) Caudal rhombencephalon. (B–B′′) Mesencephalic tegmentum. (C) Preoptic region. (D) Striatum. (E) Medial pallium. B–B′′ and E are z-stack projections from four confocal optical sections (0.5 μm thick). D is a 0.5-μm-thick confocal optical section. Dorsal is up in all figures. MT, mesencephalic tegmentum; PO, preoptic region. For other abbreviations, see the legend to Figs 1 and 2. Scale bars: 12 μm in A, C–E and 18 μm in B–B′′.
Discussion
The coexistence of DA and GABA immunoreactivities in neurons of several nuclei of the sea lamprey brain is reported here for the first time. In addition, the results revealed the presence of two new types of DA-ir neurons in the striatum, one consisting of a few cells located away from the ventricle and the other of a few periventricular CSF-c cells. CSF-c cells, including DA-ir (Pierre et al. 1997; Pombal et al. 1997; Abalo et al. 2005; Barreiro-Iglesias et al. 2008) and GABA-ir (Meléndez-Ferro et al. 2002, 2003; Robertson et al. 2007) cells, are very abundant in the lamprey brain. It has been proposed that this type of cell modulates the activity of their target neurons in response to chemical variations in the cerebrospinal fluid (Vigh et al. 2004). The presence of striatal dopaminergic cells has not previously been reported in lampreys (river lamprey: Pierre et al. 1997; Pombal et al. 1997; sea lamprey: Abalo et al. 2005). The different results in sea lamprey may be attributed to the use of large, mature animals in the present study. It is not clear whether the absence of similar cells in the river lamprey is due to interspecific differences or to the use of animals in earlier stages of sexual maturation. The appearance of cell populations immunoreactive to galanin specifically associated with the latter stages of sexual maturation has been reported in the trout telencephalon (Rodríguez et al. 2003) and something similar may also occur with dopaminergic populations during lamprey maturation.
Colocalization of DA and GABA was observed in most DA-ir nuclei of the lamprey brain
The present results showed colocalization of DA and GABA in neurons of most of the dopaminergic nuclei of the lamprey brain, and in some of these nuclei colocalization was observed in all or most of the dopaminergic cells. Previous studies have reported the coexistence of DA and GABA (or their synthesizing enzymes) in neurons of some vertebrates (mammals: Kosaka et al. 1987; Gall et al. 1987; Betarbet et al. 1997; Hédou et al. 2000; González-Hernández et al. 2001; a snake: Kosaka et al. 1991; Xenopus: de Rijk et al. 1992; dogfish shark: Sueiro et al. 2004) and invertebrates (Periplaneta: Distler, 1990; Aplysia: Díaz-Ríos et al. 2002; Díaz-Ríos & Miller, 2005). Together with the results of these studies, the present results indicate that colocalization of DA and GABA in neurons appeared early on during vertebrate evolution.
In the sea lamprey rhombencephalon, all of the dopaminergic cells of the caudal rhombencephalic nucleus and about half of the scarce cells located in the ventral isthmic region were also GABAergic. The presence of dopaminergic descending afferents to the spinal cord from the caudal rhombencephalic nucleus has previously been reported by our group (Barreiro-Iglesias et al. 2008). Accordingly, these cells also appear to be a source of GABAergic descending projections to the spinal cord in lampreys, although this hypothesis must be tested experimentally. The presence of both dopaminergic and DA-ir/GABA-ir cells in the ventral isthmus appears to be characteristic of lampreys, as the dopaminergic cells of the isthmus in lampreys do not correspond to the locus coeruleus of other vertebrates, which is noradrenergic (Abalo et al. 2005).
A few DA-ir/GABA-ir neurons were observed in the dopaminergic populations of the lamprey hypothalamus. In mammals, scarce dopaminergic neurons of the arcuate nucleus also colocalize DA and GABA (review in Chronwall, 1985) and they appeared to be the source of the DA-ir/GABA-ir tubero-hypophyseal axons that innervate the pituitary neurointermediate lobe (Vuillez et al. 1987; Schimchowitsch et al. 1991). Colocalization of DA and GABA has also been reported in amphibian pituitary terminals originating in the suprachiasmatic nucleus (de Rijk et al. 1992), although GABA could not be detected in the neuronal cell bodies (Tuinhof et al. 1994). Although these results suggest the existence of similar patterns of DA/GABA colocalization in hypothalamic neurons of different vertebrates, no studies have been carried out to investigate whether or not the hypothalamic DA-ir/GABA-ir neurons innervate the lamprey pituitary. However, a high degree of DA and GABA colocalization was observed in dopaminergic cells of the sea lamprey preoptic nucleus, and about a quarter of the dopaminergic neurons in the dorsal part of the postoptic commissure nucleus also express GABA. As far as we are aware, colocalization of DA and GABA has not been reported in cells of the preoptic region of other vertebrates. Although the presence of these preoptic cells may represent a specialization characteristic of lampreys, other hypotheses cannot be ruled out as similar studies have not been carried out with other vertebrates.
The present report is the first description of the presence of DA-ir/GABA-ir cells in the olfactory bulb of lampreys. The olfactory bulb of sea lampreys contains five types of GABAergic neurons (Meléndez-Ferro et al. 2001) but colocalization of DA and GABA was only observed in granule-like cells of the inner cellular layer. In mammals, TH and GAD or GABA immunoreactivities have been reported to colocalize in some neurons of the olfactory bulb (rat: Kosaka et al. 1985, 1987; Gall et al. 1987; hamster: Kosaka et al. 1988). The presence of TH-ir/GABA-ir cells has been also reported in the olfactory bulb of a snake (Kosaka et al. 1991). Accordingly, these studies suggest that the local circuit dopaminergic/GABAergic cells of the olfactory bulbs appeared early on in phylogeny, before the separation of agnathans and gnathostomes. However, it is not clear whether the dopaminergic/GABAergic cell types (granule-like cells) found in lamprey olfactory bulbs correspond to those reported in land vertebrates. In mammals, the two types of double-labelled cells were mainly located in the periglomerular region and external plexiform layer (rat: Kosaka et al. 1985; Gall et al. 1987; hamster: Kosaka et al. 1988), whereas no double-labelled cells were observed in the periglomerular region of lamprey. In a snake, two types of double-labelled (GABA/TH) cells of the olfactory bulbs were mostly located in periglomerular regions but also in mitral/plexiform layers (Kosaka et al. 1991). Thus, the type of dopaminergic/GABAergic cell present in the olfactory bulb of the sea lamprey appears to differ from those observed in mammals and snakes.
DA and GABA are colocalized in cells of the striatum of the adult lamprey brain
The present results revealed the presence of DA-ir cells in the striatum, where about a quarter of those non-CSF-c cells and all of those CSF-c cells were also GABA-ir. In mammals, the striatum also harbours a population of dopaminergic cells, all of non-CSF-c type, the vast majority of which are similar to medium-sized spiny striatal interneurons and express the GAD65 enzyme (reviewed by Huot & Parent, 2007). The striatum is the largest integrative component of the basal ganglia. The presence of a dopaminergic/GABAergic neuronal population in the lamprey striatum (present results) suggests that the neurochemical organization of the basal ganglia is quite well conserved between lampreys and mammals. In mammals, the major source of DA in the striatum is the substantia nigra, whereas there is no similar dopaminergic midbrain nucleus in lampreys. The dopaminergic input to the lamprey striatum originates from the posterior tubercle nucleus (i.e. paratubercular nucleus), which has been suggested to be homologous to the mammalian substantia nigra (Pombal et al. 1997). The motor deficits arising after striatal depletion of DA are qualitatively similar in lampreys and mammals, and the role of the dopaminergic innervation of the striatum appears to be conserved throughout vertebrate evolution (Thompson et al. 2008). However, our study shows no GABA immunoreactivity in the DA-ir cells of the sea lamprey paratubercular nucleus, whereas the coexistence of TH and GAD (Hédou et al. 2000; González-Hernández et al. 2001) and DA and GABA (González-Hernández et al. 2001) has been reported in some substantia nigra neurons of mammals.
Functional considerations on the colocalization of DA and GABA in brain neurons
The presence of DA and GABA immunoreactivities in fibres or terminals in different regions of the lamprey brain suggests the corelease of both neurotransmitters by some lamprey neurons distributed through various brain regions. Although several studies have shown colocalization of GABAergic and catecholaminergic markers (see above), functional studies on these cells are scant. Functional evidence of the corelease of the fast-acting inhibitory neurotransmitter GABA and DA by periglomerular cells of the rat olfactory bulb has recently been reported (Maher & Westbrook, 2008). The functional significance of the coexistence of DA, GABA and neuropeptide Y in axon terminals for the regulation of alpha-melanophore-stimulating hormone release has also been investigated in Xenopus pituitary, where all three neurotransmitters appear to act as fast inhibitory neurotransmitters in an additive way but with differential actions (Leenders et al. 1993). The results of these studies may also apply to other cells that display colocalization of GABA and DA, including those of lampreys, although this must be tested in further physiological studies.
It is now well established that axons of dopaminergic neurons can exhibit varicosities or terminals with synaptic membrane specialization and terminals without junctional complexes, which are morphologically distinguishable by electron microscopy (see Descarries et al. 2008). The transmitter that is released from these non-junctional or non-synaptic terminals (asynaptic) may diffuse into the extracellular space and reach remote targets, allowing for effects at a distance or volume transmission. The finding that a proportion of the monoaminergic neurons of rat were immunoreactive for glutamate provided indirect evidence that central nervous system (CNS) monoamine neurons may use glutamate as a cotransmitter, a hypothesis also supported by physiological evidence on the nigrostriatal system in vivo and in culture (Sulzer et al. 1998). For those dopaminergic neurons expressing glutamate it has been proposed that the non-synaptic terminals may be specialized for the release of DA, whereas the terminals with synaptic junctions may constitute the site of glutamate release (Trudeau & Gutiérrez, 2007; Descarries et al. 2008). Another possibility is the corelease of both neurotransmitters at the same synaptic terminals (Descarries et al. 2008). Similarly, one or both of these mechanisms of neurotransmitter release may apply to neurons containing DA and GABA. The extensive colocalization of DA and GABA observed in neurons of different brain nuclei makes sea lampreys a potential vertebrate model for testing these hypotheses and studying cotransmission by ‘classical neurotransmitters’.
Conclusions
Colocalization of DA and GABA occurs in dopaminergic cells and fibres of various CNS regions of lampreys. Comparison of the present results in the sea lamprey with results of studies in other animals suggests that colocalization of these two neurotransmitters is an ancient characteristic of dopaminergic cells. Together with previous reports on colocalization of GABA and DA in the spinal cord (Rodicio et al. 2008) and of GABA and serotonin in the brain (Barreiro-Iglesias et al. 2009), the present results show that the coexpression of classical neurotransmitters in monoaminergic CNS populations in lampreys is far more extensive and complex than previously reported. Lampreys potentially constitute a very interesting animal model for exploring cotransmission and the functions of dual release of classical neurotransmitters in the brain of vertebrates.
Acknowledgments
This study was financially supported by the Ministerio de Educación y Ciencia (BFU2004-01080), the Xunta de Galicia (PGIDIT05PXIC20004PN) and a predoctoral contract (María Barbeito contract) from the Xunta de Galicia to A.B.-I. We thank the Consellería de Medio Ambiente (Dirección Xeral de Montes e Medio Ambiente Natural) of the Xunta de Galicia for kind permission to capture the animals used in this study. We also thank the Servicio de Microscopía Electrónica and Mercedes Rivas Cascallar (University of Santiago de Compostela) for confocal microscope facilities and assistance.
Author contributions
A.B.-I. contributed to the concept/design of the study, acquisition of experimental data, data analysis/interpretation and drafting of the manuscript. V.V.-C. contributed to the acquisition of experimental data. R.A. contributed to the data analysis/interpretation and critical revision of the article. M.C.R. contributed to the concept/design of the study, data analysis/interpretation, critical revision and final approval of the article.
References
- Abalo XM, Villar-Cheda B, Anadón R, et al. Development of the dopamine-immunoreactive system in the central nervous system of the sea lamprey. Brain Res Bull. 2005;66:560–564. doi: 10.1016/j.brainresbull.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Barreiro-Iglesias A, Villar-Cerviño V, Anadón R, et al. Descending brain-spinal cord projections in a primitive vertebrate, the lamprey: cerebrospinal fluid-contacting and dopaminergic neurons. J Comp Neurol. 2008;511:711–723. doi: 10.1002/cne.21863. [DOI] [PubMed] [Google Scholar]
- Barreiro-Iglesias A, Cornide-Petronio ME, Anadón R, et al. Serotonin and GABA are colocalized in restricted groups of neurons in the larval sea lamprey brain: insights into the early evolution of neurotransmitter colocalization in vertebrates. J Anat. 2009;215:435–443. doi: 10.1111/j.1469-7580.2009.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betarbet R, Turner R, Chockkan V, et al. Dopaminergic neurons intrinsic to the primate striatum. J Neurosci. 1997;17:6761–6768. doi: 10.1523/JNEUROSCI.17-17-06761.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronwall BM. Anatomy and physiology of the neuroendocrine arcuate nucleus. Peptides. 1985;6:1–11. doi: 10.1016/0196-9781(85)90128-7. [DOI] [PubMed] [Google Scholar]
- Descarries L, Bérubé-Carrière N, Riad M, et al. Glutamate in dopamine neurons: synaptic versus diffuse transmission. Brain Res Rev. 2008;58:290–302. doi: 10.1016/j.brainresrev.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Díaz-Ríos M, Miller MW. Rapid dopaminergic signaling by interneurons that contain markers for catecholamines and GABA in the feeding circuitry of Aplysia. J Neurophysiol. 2005;93:2142–2156. doi: 10.1152/jn.00003.2004. [DOI] [PubMed] [Google Scholar]
- Díaz-Ríos M, Oyola E, Miller MW. Colocalization of gamma-aminobutyric acid-like immunoreactivity and catecholamines in the feeding network of Aplysia californica. J Comp Neurol. 2002;445:29–46. doi: 10.1002/cne.10152. [DOI] [PubMed] [Google Scholar]
- Distler P. Synaptic connections of dopamine-immunoreactive neurons in the antennal lobes of Periplaneta americana. Colocalization with GABA-like immunoreactivity. Histochemistry. 1990;93:401–408. doi: 10.1007/BF00315858. [DOI] [PubMed] [Google Scholar]
- Dubuc R, Brocard F, Antri M, et al. Initiation of locomotion in lampreys. Brain Res Rev. 2008;57:172–182. doi: 10.1016/j.brainresrev.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Gall CM, Hendry SH, Seroogy KB, et al. Evidence for coexistence of GABA and dopamine in neurons of the rat olfactory bulb. J Comp Neurol. 1987;266:307–318. doi: 10.1002/cne.902660302. [DOI] [PubMed] [Google Scholar]
- González-Hernández T, Barroso-Chinea P, Acevedo A, et al. Colocalization of tyrosine hydroxylase and GAD65 mRNA in mesostriatal neurons. Eur J Neurosci. 2001;13:57–67. [PubMed] [Google Scholar]
- Grillner S, Wallén P, Saitoh K, et al. Neural bases of goal-directed locomotion in vertebrates – an overview. Brain Res Rev. 2008;57:2–12. doi: 10.1016/j.brainresrev.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Gutiérrez R. The dual glutamatergic-GABAergic phenotype of hippocampal granule cells. Trends Neurosci. 2005;28:297–303. doi: 10.1016/j.tins.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Hédou G, Chasserot-Golaz S, Kemmel V, et al. Immunohistochemical studies of the localization of neurons containing the enzyme that synthesizes dopamine, GABA, or gamma-hydroxybutyrate in the rat substantia nigra and striatum. J Comp Neurol. 2000;426:549–560. doi: 10.1002/1096-9861(20001030)426:4<549::aid-cne4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Arvidsson U, Bean A, et al. Colocalization of neuropeptides and classical neurotransmitters – functional significance. Clin Neuropharmacol. 1992;15(Suppl 1 pt A):309A–310A. doi: 10.1097/00002826-199201001-00160. [DOI] [PubMed] [Google Scholar]
- Huot P, Parent A. Dopaminergic neurons intrinsic to the striatum. J Neurochem. 2007;101:1441–1447. doi: 10.1111/j.1471-4159.2006.04430.x. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Hataguchi Y, Hama K, et al. Coexistence of immunoreactivities for glutamate decarboxylase and tyrosine hydroxylase in some neurons in the periglomerular region of the rat main olfactory bulb: possible coexistence of gamma-aminobutyric acid (GABA) and dopamine. Brain Res. 1985;343:166–171. doi: 10.1016/0006-8993(85)91172-2. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Kosaka K, Hataguchi Y, et al. Catecholaminergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactivities in various brain regions of the rat. Exp Brain Res. 1987;66:191–210. doi: 10.1007/BF00236215. [DOI] [PubMed] [Google Scholar]
- Kosaka K, Hama K, Nagatsu I, et al. Possible coexistence of amino acid (gamma-aminobutyric acid), amine (dopamine) and peptide (substance P); neurons containing immunoreactivities for glutamic acid decarboxylase, tyrosine hydroxylase and substance P in the hamster main olfactory bulb. Exp Brain Res. 1988;71:633–642. doi: 10.1007/BF00248757. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Kosaka K, Nagatsu I. Tyrosine hydroxylase-like immunoreactive neurons in the olfactory bulb of the snake, Elaphe quadrivirgata, with special reference to the colocalization of tyrosine hydroxylase- and GABA-like immunoreactivities. Exp Brain Res. 1991;87:353–362. doi: 10.1007/BF00231852. [DOI] [PubMed] [Google Scholar]
- Leenders HJ, de Koning HP, Ponten SP, et al. Differential effects of coexisting dopamine, GABA and NPY on alpha-MSH secretion from melanotrope cells of Xenopus laevis. Life Sci. 1993;52:1969–1975. doi: 10.1016/0024-3205(93)90638-j. [DOI] [PubMed] [Google Scholar]
- Lu T, Rubio ME, Trussell LO. Glycinergic transmission shaped by the corelease of GABA in a mammalian auditory synapse. Neuron. 2008;57:524–535. doi: 10.1016/j.neuron.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Maher BJ, Westbrook GL. Co-transmission of dopamine and GABA in periglomerular cells. J Neurophysiol. 2008;99:1559–1564. doi: 10.1152/jn.00636.2007. [DOI] [PubMed] [Google Scholar]
- Meléndez-Ferro M, Pérez-Costas E, Rodríguez-Muñoz R, et al. GABA immunoreactivity in the olfactory bulbs of the adult sea lamprey Petromyzon marinus L. Brain Res. 2001;893:253–260. doi: 10.1016/s0006-8993(00)03316-3. [DOI] [PubMed] [Google Scholar]
- Meléndez-Ferro M, Pérez-Costas E, Villar-Cheda B, et al. Ontogeny of gamma-aminobutyric acid-immunoreactive neuronal populations in the forebrain and midbrain of the sea lamprey. J Comp Neurol. 2002;446:360–376. doi: 10.1002/cne.10209. [DOI] [PubMed] [Google Scholar]
- Meléndez-Ferro M, Pérez-Costas E, Villar-Cheda B, et al. Ontogeny of gamma-aminobutyric acid-immunoreactive neurons in the rhombencephalon and spinal cord of the sea lamprey. J Comp Neurol. 2003;464:17–35. doi: 10.1002/cne.10773. [DOI] [PubMed] [Google Scholar]
- Pierre J, Mahouche M, Suderevskaya EI, et al. Immunocytochemical localization of dopamine and its synthetic enzymes in the central nervous system of the lamprey Lampetra fluviatilis. J Comp Neurol. 1997;380:119–135. [PubMed] [Google Scholar]
- Pombal MA, El Manira A, Grillner S. Afferents of the lamprey striatum with special reference to the dopaminergic system: a combined tracing and immunohistochemical study. J Comp Neurol. 1997;386:71–91. [PubMed] [Google Scholar]
- de Rijk EP, van Strien FJ, Roubos EW. Demonstration of coexisting catecholamine (dopamine), amino acid (GABA), and peptide (NPY) involved in inhibition of melanotrope cell activity in Xenopus laevis: a quantitative ultrastructural, freeze-substitution immunocytochemical study. J Neurosci. 1992;12:864–871. doi: 10.1523/JNEUROSCI.12-03-00864.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B, Auclair F, Ménard A, et al. GABA distribution in lamprey is phylogenetically conserved. J Comp Neurol. 2007;503:47–63. doi: 10.1002/cne.21348. [DOI] [PubMed] [Google Scholar]
- Rodicio MC, Villar-Cerviño V, Barreiro-Iglesias A, et al. Colocalization of dopamine and GABA in spinal cord neurones in the sea lamprey. Brain Res Bull. 2008;76:45–49. doi: 10.1016/j.brainresbull.2007.10.062. [DOI] [PubMed] [Google Scholar]
- Rodríguez MA, Anadón R, Rodríguez-Moldes I. Development of galanin-like immunoreactivity in the brain of the brown trout (Salmo trutta fario), with some observations on sexual dimorphism. J Comp Neurol. 2003;465:263–285. doi: 10.1002/cne.10832. [DOI] [PubMed] [Google Scholar]
- Sandler R, Smith AD. Coexistence of GABA and glutamate in mossy fiber terminals of the primate hippocampus: an ultrastructural study. J Comp Neurol. 1991;8:177–192. doi: 10.1002/cne.903030202. [DOI] [PubMed] [Google Scholar]
- Schimchowitsch S, Vuillez P, Tappaz ML, et al. Systematic presence of GABA-immunoreactivity in the tubero-infundibular and tubero-hypophyseal dopaminergic axonal systems: an ultrastructural immunogold study on several mammals. Exp Brain Res. 1991;83:575–586. doi: 10.1007/BF00229836. [DOI] [PubMed] [Google Scholar]
- Steinbusch HWM, Van Vliet SP, Bol JGJM, et al. Development and application of antibodies to primary (DA, L-Dopa) and secondary (cGMP) messengers: a technical report. In: Calas A, Eugène D, editors. Neurocytochemical Methods. Vol. 58. Berlin: Springer Verlag; 1991. pp. 1–27. [Google Scholar]
- Strata P, Harvey R. Dale’s principle. Brain Res Bull. 1999;50:349–350. doi: 10.1016/s0361-9230(99)00100-8. [DOI] [PubMed] [Google Scholar]
- Sueiro C, Carrera I, Molist P, et al. Distribution and development of glutamic acid decarboxylase immunoreactivity in the spinal cord of the dogfish Scyliorhinus canicula (elasmobranchs) J Comp Neurol. 2004;478:189–206. doi: 10.1002/cne.20285. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Joyce MP, Lin L, et al. Dopamine neurons make glutamatergic synapses in vitro. J Neurosci. 1998;18:4588–4602. doi: 10.1523/JNEUROSCI.18-12-04588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RH, Ménard A, Pombal M, et al. Forebrain dopamine depletion impairs motor behavior in lamprey. Eur J Neurosci. 2008;27:1452–1460. doi: 10.1111/j.1460-9568.2008.06125.x. [DOI] [PubMed] [Google Scholar]
- Trudeau LE. Glutamate co-transmission as an emerging concept in monoamine neuron function. J Psychiatry Neurosci. 2004;29:296–310. [PMC free article] [PubMed] [Google Scholar]
- Trudeau LE, Gutiérrez R. On cotransmission & neurotransmitter phenotype plasticity. Mol Interv. 2007;7:138–146. doi: 10.1124/mi.7.3.5. [DOI] [PubMed] [Google Scholar]
- Tuinhof R, González A, Smeets WJ, et al. Central control of melanotrope cells of Xenopus laevis. Eur J Morphol. 1994;32:307–310. [PubMed] [Google Scholar]
- Vigh B, Manzano e Silva MJ, Frank CL, et al. The system of cerebrospinal fluid-contacting neurons. Its supposed role in the nonsynaptic signal transmission of the brain. Histol Histopathol. 2004;19:607–628. doi: 10.14670/HH-19.607. [DOI] [PubMed] [Google Scholar]
- Villar-Cerviño V, Barreiro-Iglesias A, Anadón R, et al. Aspartate immunoreactivity in the telencephalon of the adult sea lamprey: comparison with GABA immunoreactivity. Brain Res Bull. 2008a;75:246–250. doi: 10.1016/j.brainresbull.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Villar-Cerviño V, Barreiro-Iglesias A, Anadón R, et al. Distribution of glycine immunoreactivity in the brain of adult sea lamprey (Petromyzon marinus). Its supposed role in the nonsynaptic signal transmission of the brain. J Comp Neurol. 2008b;507:1441–1463. doi: 10.1002/cne.21634. [DOI] [PubMed] [Google Scholar]
- Vuillez P, Pérez SC, Stoeckel ME. Colocalization of GABA and tyrosine hydroxylase immunoreactivities in the axons innervating the neurointermediate lobe of the rat pituitary: an ultrastructural immunogold study. Neurosci Lett. 1987;18:53–58. doi: 10.1016/0304-3940(87)90671-9. [DOI] [PubMed] [Google Scholar]