Abstract
Within the order Carnivora, the phylogeny of the red panda (Ailurus fulgens) is contentious, with morphological and molecular studies supporting a wide range of possible relationships, including close ties to procyonids, ursids, mustelids and mephitids. This study provides additional morphological data, including muscle maps, for the forelimb of Ailurus, based on the dissection of four cadavers from the National Zoological Park, Washington, DC, USA. The red panda forelimb is characterized by a number of primitive features, including the lack of m. rhomboideus profundus, a humeral insertion for m. cleidobrachialis, the presence of mm. brachioradialis, articularis humeri and coracobrachialis, a single muscle belly for m. extensor digitorum lateralis with tendons to digits III–V, four mm. lumbricales, and the presence of mm. flexor digitorum brevis manus, adductores digiti I, II and V, and abductor digiti I and V. Red pandas resemble Ailuropoda, mustelids and some procyonids in possessing a soft tissue origin of m. flexor digitorum superficialis. In addition, red pandas are similar to ursids and procyonids in having a variable presence of m. biceps brachii caput breve. Furthermore, Ailurus and some ursids lack m. rhomboideus capitis. The forelimb muscle maps from this study represent a valuable resource for analyzing the functional anatomy of fossil ailurids and some notes on the Miocene ailurid, Simocyon batalleri, are presented.
Keywords: carnivore evolution, forelimb, myology, red panda
Introduction
The red panda (Ailurus fulgens) (Cuvier, 1825) is an endangered carnivore currently restricted to the forests of the Himalayas and southern China. Weighing 3.7–6.2 kg, these adept climbers subsist primarily on bamboo leaves and shoots (Roberts & Gittleman, 1984; Johnson et al. 1988; Reid et al. 1991; Wei et al. 1999). The phylogeny of the red panda has long been the subject of debate. Morphological and molecular studies have supported a wide range of possible relationships (Table 1). Due to these conflicting accounts, the red panda has been classified as an ursid, a procyonid or within its own family, the Ailuridae. For the purposes of this study, Ailuridae will be used to refer to the red panda and its fossil relatives (sensu Wallace & Wang, 2004; Wozencraft, 2005; Salesa et al. 2006).
Table 1.
Phylogenetic hypotheses for the position of Ailurus
| Ailurus+UrsidaeTodd & Pressman (1968),Hunt (1974),Bugge (1978),Wozencraft (1989a,b;) Decker & Wozencraft (1991),Zhang & Shi (1991) | Ailurus + ProcyonidaeMivart (1885),Hollister (1915),Gregory (1936),O’Brien et al. (1985),Goldman et al. (1989),Wayne et al. (1989),Pecon Slattery & O’Brien (1995),Dragoo & Honeycutt (1997) |
| Ailurus + (Ursidae+Otariidae)Ginsburg (1982) | Ailurus+ (Procyonidae +Mustelidae)Fulton & Strobeck (2006) |
| Ailurus+ (Ursidae +Pinnipeds)Wyss & Flynn (1993),Vrana et al. (1994) | Ailurus+ (Procyonidae + Mephitidae + Mustelidae)Bininda-Emonds et al. (1999),Flynn et al. (2005) |
| Ailurus+ (Ursidae + Procyonidae)Zhang & Ryder (1993) | Ailurus+ MephitidaeFlynn et al. (2000),Delisle & Strobeck (2005) |
Few soft tissue features have been utilized in the aforementioned phylogenetic analyses and the myology of the red panda remains largely undocumented. Carlsson (1925) dissected a young male and Davis (1964) incorporated Carlsson’s data and ‘partial dissections’ of Ailurus in his classic monograph on the giant panda. More recently, a number of investigators studied the muscles associated with the radial sesamoid (Inaba & Takahashi, 1996; Endo et al. 2001; Antón et al. 2006), whereas Fisher et al. (2008) analyzed the muscles of the hindlimb. This study augments the soft tissue data for Ailurus, including muscle maps and detailed descriptions of the forelimb muscles. The features of the red panda forelimb are compared with other carnivores and the functional anatomy of the fossil ailurid, Simocyon batalleri, is discussed (Salesa et al. 2008).
Materials and methods
Dissections were conducted on the left and right forelimbs of four captive-born red pandas (A. fulgens). These specimens were also the subject of a previous study on the hindlimb (Fisher et al. 2008). The pandas lived at the National Zoological Park in Washington, DC and included an adult male of unknown age (USNM 597647), an adult female of unknown age (USNM 525292), a 12-year-old female (USNM 597645) and a 5-month-old female (USNM 597646). Following necropsy, the specimens were fresh-frozen and stored at the Osteopreparatory Laboratory at the Museum Support Center of the National Museum of Natural History (USNM) (Suitland, MD, USA).
Dissections took place at the Arizona College of Osteopathic Medicine (Midwestern University) and Arizona State University. Digital photographs were taken at each level of the dissection using a Nikon Coolpix 5400 or a Canon Powershot S3 IS. In addition, to assist with muscle mapping, digital photographs were taken of the forelimb skeleton of a male red panda (USNM 305770, captive-born). The scapula was photographed in medial and lateral views, the humerus in lateral, medial, cranial and caudal views, the articulated radius/ulna in caudomedial and craniolateral views, and an articulated manus in dorsal and palmar views. Muscle origins and insertions were recorded on transparency film overlying these photographs, producing muscle maps.
Data from the forelimb dissections were compared with previous accounts of this species (Carlsson, 1925; Davis, 1964; Inaba & Takahashi, 1996; Endo et al. 2001; Antón et al. 2006) and other carnivores (Tables 2 and 3). The terminology in this report conforms to the standards of the Nomina Anatomica Veterinaria (Waibl et al. 2005). However, the term mm. flexores breves profundi (Čihák, 1972) has been adopted to describe the group of muscles designated as the m. flexor digiti I brevis and mm. interossei by Waibl et al. (2005).
Table 2.
The sources of data for the Suborder Caniformia (taxonomy after Wozencraft, 2005)
If the number of specimens is described in the reference, this information is provided in the superscript.
Table 3.
The sources of data for the Suborder Feliformia (taxonomy after Wozencraft, 2005)
If the number of specimens is described in the reference, this information is provided in the superscript.
The figures in this study illustrate variations that were observed in four or more of the eight limbs dissected. In addition, it should be noted that the neck muscles mentioned in the forelimb muscle descriptions (e.g. mm. scalenii, mm. sternocephalicus pars mastoidea et occipitalis) will be fully described and illustrated in an upcoming study of the axial musculature of the red panda.
Extrinsic muscles of the forelimb
M. trapezius
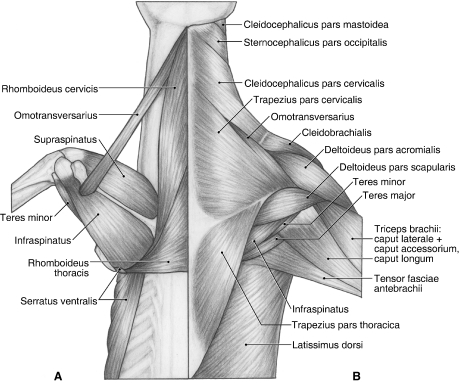
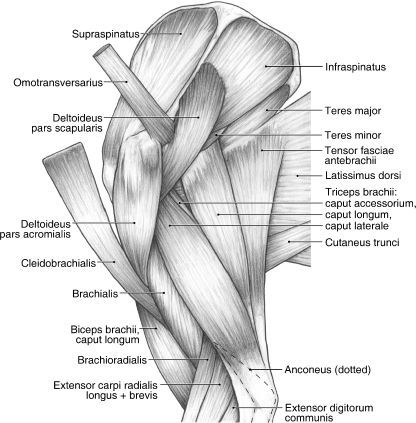
M. trapezius originates from the ligamentum nuchae and supraspinous ligament overlying the C3–T7 spinous processes, and the thoracolumbar fascia (Fig. 1B). It is composed of a distinct pars cervicalis and pars thoracica. M. trapezius pars cervicalis arises from the ligamentum nuchae at the level of the C3–T1 vertebrae (Fig. 1B). It lies superficial to the mm. rhomboideus cervicis et thoracis (Fig. 1A). The fibers of m. trapezius pars cervicalis course caudoventrally to insert via fleshy fibers onto the spine of the scapula and the suprahamate process of the acromion (Fig. 2A). In its distal third, its cranial fibers are fused with m. omotransversarius (Fig. 1B).
Fig. 1.
Dorsal view of the back and shoulder in Ailurus. (A) Deep view; (B) superficial view.
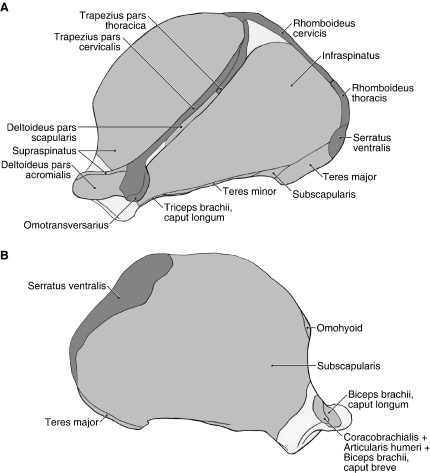
Fig. 2.
Scapula muscle maps for Ailurus (left side), including the origin of m. deltoideus pars scapularis from the scapular spine (present in four limbs). (A) Lateral view; (B) medial view.
M. trapezius pars thoracica arises from the supraspinous ligament overlying the T1–T7 vertebrae, and the thoracolumbar fascia (Fig. 1B). It lies superficial to m. rhomboideus thoracis (Fig. 1A) and is fused on its deep surface with m. latissimus dorsi (Fig. 1B). Its fibers course cranioventrally to insert via a small aponeurosis onto the proximal third of the scapular spine and the fascia of m. infraspinatus (Fig. 2A). In two of the limbs dissected, m. trapezius pars thoracica also inserted onto the fascia overlying m. supraspinatus. When the neck is fixed, m. trapezius pars cervicalis moves the scapula dorsad and craniad. When the scapula is fixed, unilateral contraction of the pars cervicalis laterally flexes the neck. M. trapezius pars thoracis moves the scapula dorsad and caudad. Both the pars cervicalis and pars thoracis help stabilize the scapula.
M. rhomboideus cervicis
M. rhomboideus cervicis originates from the ligamentum nuchae at the level of the C1–C7 vertebrae (Fig. 1A). Its fibers run caudoventrally to insert via an aponeurosis onto the cranial angle and vertebral border of the scapula, cranial to m. rhomboideus thoracis (Fig. 2A). Near its point of insertion, it is fused on its deep aspect with m. serratus ventralis. When the neck is fixed, m. rhomboideus cervicis moves the scapula dorsad and craniad. When the scapula is fixed, unilateral contraction of m. rhomboideus cervicis laterally flexes the neck, whereas bilateral contraction extends the neck. M. rhomboideus cervicis also fixes the scapula on the body wall.
M. rhomboideus thoracis
M. rhomboideus thoracis is entirely separable from m. rhomboideus cervicis (Fig. 1A). Its vertebral levels of origin varied in the limbs dissected, ranging from the spinous processes of T1–T3, T1–T4 or T2–T4. Its fibers course transversely to insert via a thin aponeurosis along the vertebral border of the scapula, adjacent to m. serratus ventralis (Fig. 2A). The aponeurosis of m. rhomboideus thoracis also inserts onto the fascia overlying mm. infraspinatus and teres major. M. rhomboideus thoracis draws the scapula dorsad and mediad and fixes the scapula on the body wall.
M. serratus ventralis
M. serratus ventralis originates from the wing of the atlas, the transverse processes of all seven cervical vertebrae and ribs 1–7 (Figs 1A and 3B). The fascicles arising in the neck and those arising from ribs 1–3 lie deep to the mm. scalenii. At the caudal angle of the scapula, m. serratus ventralis inserts via a stout tendon onto the lateral and medial surfaces of scapula as well as the vertebral border (Fig. 2A). Moving cranially, the insertion is on the medial and vertebral borders of the scapula (Fig. 2B). Near the cranial angle, m. serratus ventralis is fused extensively with m. rhomboideus cervicis. M. serratus ventralis works with m. pectoralis profundus to support the trunk on the forelimbs. When the forelimb is free, the cervical fibers of m. serratus ventralis retract, whereas the thoracic fibers advance, the limb. When the forelimb is fixed, the cervical fibers laterally flex (with unilateral contraction) or extend (with bilateral contraction) the neck, whereas the thoracic fibers propel the trunk craniad. The thoracic fibers can also assist in forced inspiration.
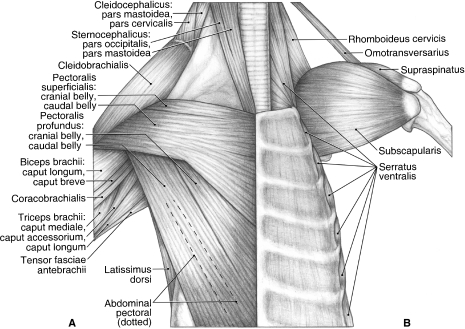
Fig. 3.
Ventral view of the chest and shoulder in Ailurus, including m. biceps brachii caput breve (present in four limbs) and a small, partially separable cranial belly of m. pectoralis superficialis (present in six limbs). (A) Superficial view; (B) deep view.
M. latissimus dorsi
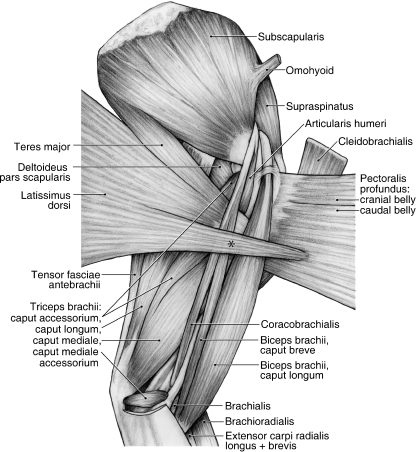
M. latissimus dorsi originates from the spinous processes of the thoracic vertebrae, the ribs and the thoracolumbar fascia (Figs 1B and 3A). Whereas the fascial origin of m. latissimus dorsi was constant, the vertebral and rib levels varied among the specimens dissected. The muscle originated from the T4–T7 vertebrae and ribs 9–12 in four limbs, T4–T8 and ribs 9–13 in two limbs, and T4–T8 and ribs 10–12 in two limbs. M. latissimus dorsi has two major insertions. Superficially, fibers course toward the brachium and insert onto a shared aponeurosis with the caudal belly of m. pectoralis profundus (Fig. 4). This aponeurosis then comes to lie superficial to m. biceps brachii. In addition, the deep fibers of m. latissimus dorsi fuse with m. teres major to form a conjoined tendon that inserts onto the teres major tuberosity on the medial aspect of the proximal humerus (Figs 4, 5 and 6A,C). When the forelimb is advanced and fixed, m. latissimus dorsi draws the trunk craniad. When the limb is free, m. latissimus dorsi retracts the forelimb.
Fig. 4.
Medial view of the left shoulder and brachium in Ailurus, including m. biceps brachii caput breve (present in four limbs). *Fibers of m. latissimus dorsi coursing superficially to join m. pectoralis profundus.
Fig. 5.
Lateral view of the left shoulder and brachium in Ailurus, including the origin of m. deltoideus pars scapularis from the scapular spine (present in four limbs).
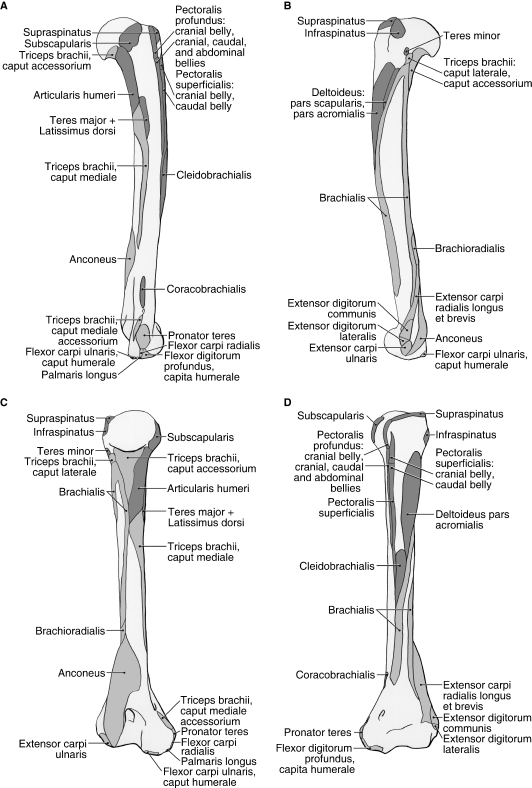
Fig. 6.
Humerus muscle maps for Ailurus (left side), including insertions of the cranial and caudal bellies of m. pectoralis superficialis (present in six limbs). (A) medial view; (B) lateral view; (C) caudal view; (D) cranial view.
M. omotransversarius
This long, thin muscle originates from the wing of the atlas. M. omotransversarius fuses with the distal third of m. trapezius pars cervicalis (Fig. 1B) and inserts onto the fascia overlying m. deltoideus pars acromialis and the suprahamate process of the acromion (Figs 2A and 5). When the scapula is fixed, m. omotransversarius laterally flexes the neck. When the neck is fixed, m. omotransversarius draws the scapula craniad, thus advancing the forelimb.
M. brachiocephalicus
M. brachiocephalicus consists of three parts: m. cleidobrachialis, m. cleidocephalicus pars cervicalis and m. cleidocephalicus pars mastoidea.
M. cleidobrachialis originates from a very faint intersectio clavicularis (Figs 1B and 3A). It courses just cranial to m. omotransversarius and fans out to cover the lateral and cranial aspects of the shoulder. At the level of the glenohumeral joint, the fascia enclosing m. cleidobrachialis becomes fused with the fascia enclosing m. pectoralis superficialis. M. cleidobrachialis inserts via fleshy fibers onto the cranial aspect of the midshaft of the humerus, just distal to mm. pectoralis superficialis (Fig. 6A,D). In three limbs, m. cleidobrachialis also inserted partially onto m. brachialis and, in one of these instances, the attachment also extended onto the surface of m. deltoideus pars acromialis.
M. cleidocephalicus pars cervicalis shares the fiber direction of m. cleidobrachialis and, apart from the faint intersectio clavicularis, these two muscles appear as a continuous sheet of muscle (Figs 1B and 3A). M. cleidocephalicus pars cervicalis lies superficial to mm. splenius capitis, rhomboideus cervicis and omotransversarius. It arises from the ligamentum nuchae from the level of the occipital bone to the C3 vertebra and inserts onto the intersectio clavicularis (Fig. 1B). M. cleidocephalicus pars cervicalis is fused slightly with m. sternocephalicus pars occipitalis (Fig. 1B). In addition, at the intersectio clavicularis, m. cleidocephalicus pars cervicalis fuses with m. cleidocephalicus pars mastoidea (Fig. 3A). Otherwise, the two muscles are completely separable. M. cleidocephalicus pars cervicalis is much more robust than the pars mastoidea.
M. cleidocephalicus pars mastoidea is a thin, strap-like muscle similar in appearance to m. omotransversarius. It arises from the mastoid process, immediately adjacent to m. sternocephalicus pars mastoidea, and inserts on the intersectio clavicularis, deep to the belly of m. cleidocephalicus pars cervicalis (Figs 1B and 3A). Apart from their common attachment to the intersectio clavicularis, m. cleidocephalicus pars mastoidea is completely separable from the pars cervicalis.
M. brachiocephalicus advances the forelimb; however, when the forelimbs are fixed, it ventrally flexes the neck (with bilateral contraction) or laterally flexes the neck (with unilateral contraction).
M. pectoralis superficialis
M. pectoralis superficialis originates from the manubrium as well as the first, second and cranial half of the third sternebrae (Fig. 3A). In all but two limbs, there was a small, partially separable cranial belly (Fig. 3A). At the level of the glenohumeral joint, the fascia enclosing m. pectoralis superficialis fused with that covering m. cleidobrachialis. M. pectoralis superficialis inserts along an elevated ridge on the humeral shaft as well as via an aponeurosis onto the fascia overlying m. biceps brachii (Fig. 6A,D). M. pectoralis superficialis adducts the forelimb or shifts the trunk towards an abducted limb. It may also assist in advancing or retracting the forelimb, depending on the intial position of the forelimb relative to the trunk.
M. pectoralis profundus
M. pectoralis profundus consists of cranial, caudal and abdominal bellies (Fig. 3A). The cranial and caudal bellies are separable throughout much of their length but share a few fibers at their origin and contribute to a common aponeurosis of insertion. The cranial belly of m. pectoralis profundus originates from the second to fifth sternebrae (Fig. 3A). It lies deep to m. supraspinatus as it crosses the glenohumeral joint and inserts onto the dorsal and medial aspects of the greater tubercle (Fig. 6A,D). In addition, it gives rise to a shared aponeurosis with the caudal belly. The caudal belly originates from the fifth to ninth sternebrae (Fig. 3A). It gives rise to a shared aponeurosis with m. latissimus dorsi to insert onto the fascia overlying m. biceps brachii. In addition, it gives rise to an aponeurosis, formed in concert with the cranial belly, which is then joined on its deep aspect by the abdominal belly. This shared aponeurosis courses immediately superficial to the m. biceps brachii tendon and inserts onto the cranial aspect of the greater tubercle and a ridge extending distally onto the humeral shaft (Fig. 6A,D).
Located deep to the caudal belly of m. pectoralis profundus, the long, strap-like abdominal belly arises from fascia lying superficial to m. rectus abdominis (Fig. 3A). The belly courses through the axilla and into the arm, where its fibers insert onto the deep aspect of the shared aponeurosis of the cranial and caudal bellies. M. pectoralis profundus works with mm. serratus ventralis to support the trunk on the forelimbs. In addition, it retracts the free limb and draws the trunk craniad when the forelimb is advanced and fixed.
Shoulder
M. deltoideus
M. deltoideus consists of a distinct pars acromialis and pars scapularis (Figs 1B and 5). M. deltoideus pars acromialis is the larger belly and is fusiform in shape. It originates from the hamate process of the acromion and the fascia overlying m. supraspinatus (Figs 2A and 5). It is completely separable from m. deltoideus pars scapularis and inserts via fleshy fibers onto the deltoid crest (Fig. 6B,D). M. deltoideus pars scapularis originates from the fascia covering m. infraspinatus and therefore indirectly takes origin from the scapular spine (Figs 1B and 5). In four of the limbs dissected, m. deltoideus pars scapularis also had a bony origin off the spine near its midpoint (Figs 2A and 5). In two limbs, there was also a partial origin off the suprahamate process of the acromion. M. deltoideus pars scapularis courses deep to the pars acromialis to reach the humerus (Fig. 5). It gives rise to a very thin aponeurosis to insert onto the deltoid crest and onto the fascia overlying m. triceps brachii caput laterale (Fig. 6B). M. deltoideus flexes and abducts the glenohumeral joint.
M. supraspinatus
M. supraspinatus takes origin from the supraspinous fossa and the cranial aspects of the scapular spine and hamate process of the acromion (Figs 1A, 2A, 3B and 5). As the muscle crosses the glenohumeral joint, it is fused on its deep surface with the joint capsule. M. supraspinatus inserts via a stout tendon onto the dorsal aspect of the greater tubercle, superficial to the tendon of the cranial belly of m. pectoralis profundus (Figs 5 and 6A–D). M. supraspinatus extends and stabilizes the glenohumeral joint.
M. infraspinatus
M. infraspinatus arises from the infraspinous fossa, scapular neck, m. teres minor tendon of origin and the caudal aspect of the scapular spine and suprahamate process of the acromion (Figs 1A, 2A and 5). The muscle inserts via a stout tendon onto a prominent tubercle on the lateral aspect of the greater tubercle, distal and caudal to the insertion of m. supraspinatus (Fig. 6B–D). A depression marks the attachment of its tendon onto this tubercle. M. infraspinatus stabilizes the glenohumeral joint and laterally rotates the humerus; it may also assist in extension or flexion of the glenohumeral joint, depending on the position of the humeral head relative to the glenoid cavity.
M. teres minor
This diminutive muscle has a thin, tendinous origin off the distal half of the caudal border of the scapula (Figs 1A,B, 2A and 5). Some fibers of m. infraspinatus take origin from the surface of this tendon. The tendon of m. teres minor inserts into a small depression, distal to the prominent tubercle marking the insertion of m. infraspinatus (Fig. 6B,C). M. teres minor flexes the glenohumeral joint.
M. teres major
M. teres major originates from the surface of m. subscapularis and the proximal half of the caudal border of the scapula (Figs 1B, 2A,B, 4 and 5). In two limbs, m. teres major also took origin from the surface of m. infraspinatus. M. teres major fuses with m. latissimus dorsi to form a flattened, conjoined tendon (Figs 4 and 5). The tendon passes deep to mm. coracobrachialis and biceps brachii (Fig. 4) to insert onto the teres major tuberosity of the humerus (Fig. 6A,C). M. teres major flexes the glenohumeral joint.
M. subscapularis
M. subscapularis originates from the subscapular fossa and the caudal border of the scapula (Figs 2A,B, 3B and 4). In one limb, m. subscapularis wrapped around the cranial edge of the scapula and also took origin from the surface of m. supraspinatus. The tendon of m. subscapularis passes deep to mm. coracobrachialis and articularis humeri (Fig. 4), fuses with the joint capsule of the glenohumeral joint, and then inserts extensively onto the lesser tubercle of the humerus (Figs 3B and 6A,C,D). M. subscapularis adducts and stabilizes the glenohumeral joint; it may also assist in flexion or extension of this joint.
Caudal brachium
M. triceps brachii
In the red panda, five heads are present, including the capita longum, laterale, mediale, accessorium and mediale accessorium (Figs 4 and 5). The primary action of these muscles is to extend the elbow joint; however, the caput longum can also flex the glenohumeral joint. In addition, m. triceps brachii assists in stabilizing the elbow joint when standing.
Caput longum
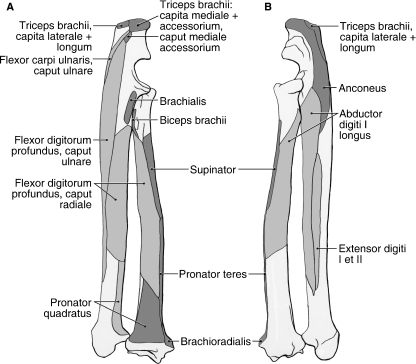
The long head originates from the distal third of the caudal border of the scapula and scapular neck (Figs 1B, 2A and 5). The belly of the long head is entirely separable from those of the other mm. triceps. At the level of the elbow joint, it forms a short tendon that fuses with the tendon of the caput laterale (Fig. 5). This conjoined tendon inserts onto the proximal and lateral aspects of the olecranon process (Fig. 7A,B), and is continuous with the fascia covering the flexor antebrachium and lateral half of the extensor antebrachium (Figs 4 and 5).
Fig. 7.
Radius and ulna muscle maps for Ailurus (left side). (A) Caudomedial view; (B) craniolateral view.
Caput laterale
The lateral head of m. triceps brachii originates from the caudolateral aspect of the humeral neck (Figs 1B, 5 and 6B,C). Its belly is extensively fused with that of the caput accessorium in the proximal two-thirds of the brachium. Distally, it gives rise to a tendon that joins that of the caput longum to insert on the proximal and lateral aspects of the olecranon process (Figs 5 and 7A,B). In addition, the tendon becomes continuous with the fascia covering the flexor antebrachium and lateral half of the extensor antebrachium (Figs 4 and 5).
Caput mediale
The medial head originates from the caudomedial aspect of the humeral shaft (Figs 4 and 6A,C). In two limbs, a very small bundle of fibers was shared between the caput mediale and caput longum. In the distal third of the brachium, the caput mediale fuses with the caput accessorium (Fig. 4). These heads then insert together via mixed tendinous and fleshy fibers onto the proximal and medial aspects of the olecranon process (Figs 4 and 7A). In addition, the tendinous fibers become continuous with the fascia covering the flexor antebrachium (Fig. 4).
Caput accessorium
The accessory belly of m. triceps brachii originates from the caudal aspect of the humeral neck (Figs 4, 5 and 6A,C). In one limb, the origin, reduced in size, was limited to the proximal portion of the mapped area. In addition, the caput accessorium was partially subdivided in one limb. In the proximal two-thirds of the brachium, the belly of the caput accessorium is fused with that of the caput laterale. In the distal third of the brachium, it fuses with the caput mediale (Fig. 4). These two heads then insert via mixed tendinous and fleshy fibers onto the proximal and medial aspects of the olecranon process (Figs 4 and 7A). In addition, the tendinous fibers become continuous with the fascia covering the flexor antebrachium (Fig. 4).
Caput mediale accessorium
In addition to the four larger bellies, a small belly is also located distal to the caput mediale (Fig. 4). This belly originates from the caudomedial aspect of humerus, distal to the entepicondylar foramen, and the ulnar nerve travels deep to it (Figs 4 and 6A,C). It is entirely separable from the other bellies of mm. triceps brachii, crosses the elbow joint medially and inserts via fleshy fibers onto the medial aspect of the olecranon process, distal to the conjoined insertion of the capita mediale et accessorium (Fig. 7A).
M. anconeus
M. anconeus lies deep to the mm. triceps brachii capita laterale et accessorium and originates from the caudal aspect of the distal third of the humerus (Fig. 6A,C). The muscle crosses lateral to the elbow joint, deep to the m. triceps brachii caput laterale (Figs 5 and 8). It fuses with the elbow joint capsule and fans out to insert extensively onto the lateral aspect of the olecranon process and proximal ulnar shaft (Fig. 7B). M. anconeus extends the elbow joint; contraction of this muscle may also tense the joint capsule, thus preventing its entrapment between the humerus and ulna.
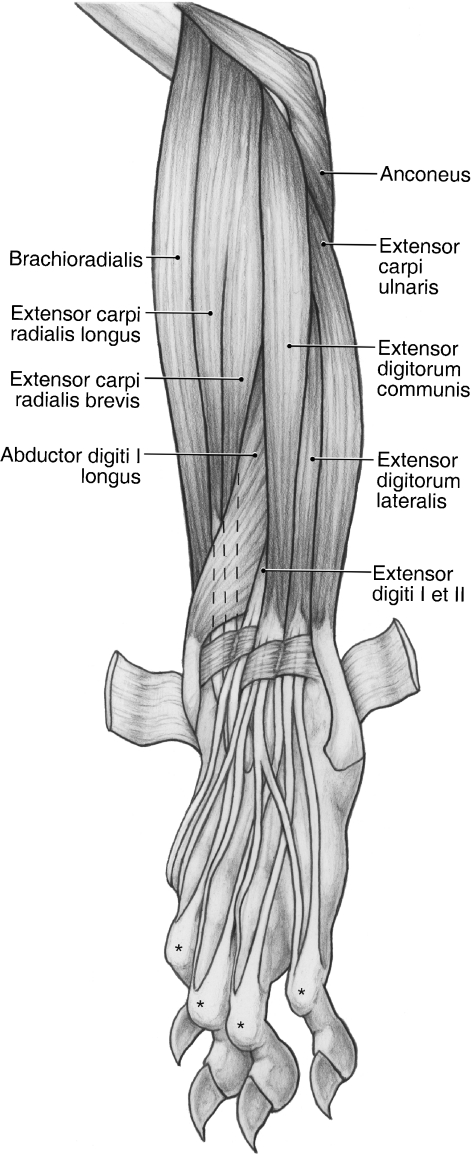
Fig. 8.
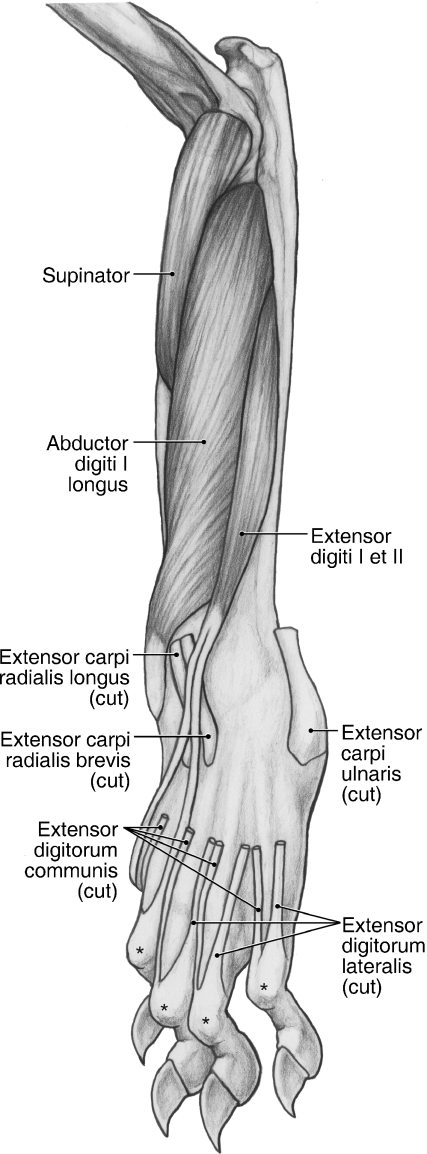
Superficial dissection of the craniolateral antebrachium and dorsal manus in the left forelimb of Ailurus. *The extensor expansions.
M. tensor fasciae antebrachii
M. tensor fascia antebrachii has soft tissue origins and insertions. It originates from a tendinous raphe on the superficial surface of mm. latissimus dorsi and teres major (Fig. 5). It fuses with m. cutaneus trunci on the medial aspect of the distal brachium to form an aponeurosis that becomes continuous with the antebrachial fascia. M. tensor fasciae antebrachii tenses the antebrachial fascia and assists in extending the elbow joint.
Cranial brachium
M. articularis humeri
M. articularis humeri originates from the coracoid process via a thin tendon shared with mm. coracobrachialis and biceps brachii caput breve (Figs 2B and 4). This tendon courses superficial to the tendon of m. subscapularis (Fig. 4). M. articularis humeri inserts via fleshy fibers onto the caudomedial aspect of the proximal humeral shaft (Fig. 6A,C). M. articularis humeri stabilizes and weakly extends the glenohumeral joint.
M. coracobrachialis
M. coracobrachialis extends along the entire length of the brachium (Fig. 4). It originates from the coracoid process via a thin tendon shared with mm. articularis humeri and biceps brachii caput breve (Figs 2B and 4). This tendon of origin courses superficial to the tendon of m. subscapularis (Fig. 4). M. coracobrachialis inserts via fleshy and tendinous fibers onto the craniomedial aspect of the humerus, proximal to the entepicondylar foramen (Figs 4 and 6A,D). Its point of insertion is marked by a small raised area. M. coracobrachialis adducts and extends the glenohumeral joint.
M. biceps brachii
M. biceps brachii caput longum was present in all of the limbs dissected. This belly originates via a tendon from the supraglenoid tubercle (Figs 2B and 4). Its tendon courses within the bicipital groove, deep to a thin transverse humeral ligament (Fig. 4). Its muscle belly is fusiform and gives rise to a bicipital aponeurosis that is continuous with the fascia covering the cranial antebrachium (Figs 4 and 5). However, its main tendon of insertion dives deep to insert onto the radial tuberosity (Fig. 7A).
In four limbs, m. biceps brachii caput breve was also present (Figs 3A and 4). The slender caput breve arises in common with the shared tendon of mm. coracobrachialis and articularis humeri from the coracoid process (Figs 2B and 4). Just proximal to its point of insertion, it fuses with the caput longum to form a stout tendon inserting onto the radial tuberosity (Fig. 7A). M. biceps brachii extends the glenohumeral joint and flexes the elbow joint. In addition, it stabilizes the elbow joint when standing.
M. brachialis
M. brachialis has an extensive origin from the caudolateral aspect of the humerus (Figs 5 and 6B,D). In one limb, m. brachialis took partial origin from the deep surface of the lateral head of m. triceps brachii, whereas in another, m. brachialis took partial origin from the deep aspects of mm. extensor carpi radialis longus and brachioradialis. The muscle belly of m. brachialis courses in spiral-like fashion towards the cranial aspect of the brachium so that it comes to cross the elbow joint deep to m. biceps brachii (Fig. 5). In the distal third of the brachium, the muscle lies deep to mm. brachioradialis and extensor carpi radialis longus et brevis (Fig. 5). The deep aspect of m. brachialis is fused with the elbow joint capsule and its tendon inserts onto the proximal ulna (Fig. 7A). M. brachialis flexes the elbow joint.
Caudal antebrachium
M. brachioradialis
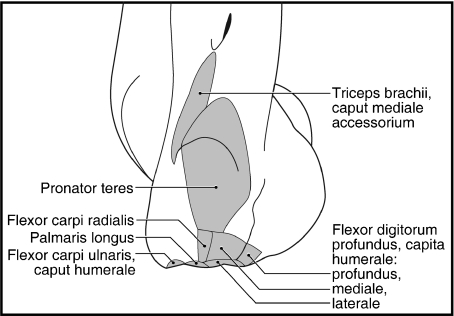
M. brachioradialis originates from the lateral supracondylar ridge, proximal to the common origin of mm. extensor carpi radialis longus et brevis (Figs 5, 6B,C and 8). In one limb, m. brachioradialis was fused with m. extensor carpi radialis longus proximally, whereas in three limbs, it was fused slightly with m. brachialis on its cranial and lateral aspects. M. brachioradialis inserts via fleshy fibers onto the surface of m. pronator teres and onto the distal radius (Figs 7A,B and 11). M. brachioradialis supinates the forearm.
Fig. 11.
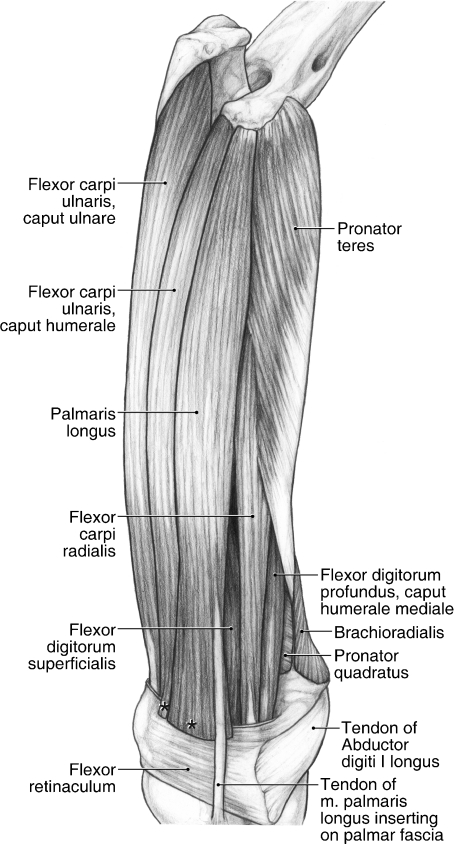
Superficial dissection of the caudomedial antebrachium of the left forelimb in Ailurus, including fusion of m. pronator teres in its proximal two-thirds with m. flexor carpi radialis (present in six limbs) and separable mm. flexor carpi ulnaris capita humerale et ulnare (present in seven limbs). *The cut edge of mm. flexor carpi ulnaris caput humerale (present in four limbs) and palmaris longus where they insert onto the superficial palmar fascia.
M. extensor carpi radialis longus
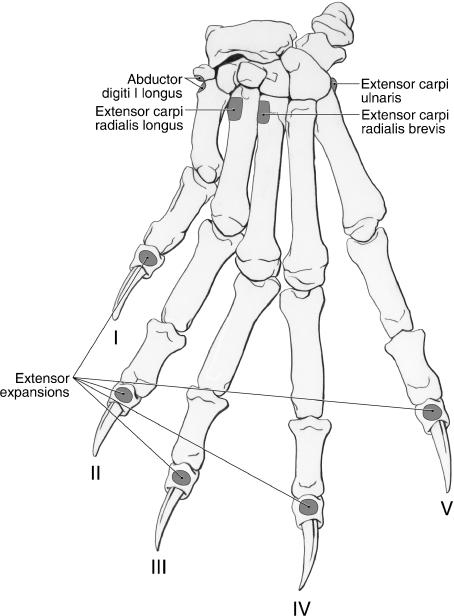
M. extensor carpi radialis longus shares a common origin with m. extensor carpi radialis brevis from the lateral supracondylar ridge and lateral epicondyle (Figs 5, 6B,D and 8) and the bellies of the two muscles only became separable in the middle of the antebrachium (Fig. 8). However, in one limb, these muscles were entirely independent. M. extensor carpi radialis longus gives rise to a tendon in the distal antebrachium (Fig. 8). The tendon courses medial to the tendon of m. extensor carpi radialis brevis and deep to m. abductor digiti I longus and the extensor retinaculum. Proximal to the carpal joint, the tendon of mm. extensor carpi radialis longus courses deep to a shared retinaculum with the tendon of m. extensor carpi radialis brevis (Fig. 8). It then passes deep to the digit I tendon of m. extensor digiti I et II to insert on the dorsomedial aspect of the base of the second metacarpal (Figs 8–10). M. extensor carpi radialis longus extends the carpal joint.
Fig. 10.
Deep dissection of the craniolateral antebrachium and dorsal manus in the left forelimb of Ailurus. *The extensor expansions.
M. extensor carpi radialis brevis
As noted above, with one exception, m. extensor carpi radialis brevis shares a common origin with m. extensor carpi radialis longus from the lateral supracondylar ridge and lateral epicondyle (Figs 5, 6B,D and 8). The two muscles become separable in the middle of the antebrachium and m. extensor carpi radialis brevis gives rise to a tendon in the distal third (Fig. 8). This tendon travels lateral to the tendon of m. extensor carpi radialis longus and courses with this tendon, deep to a shared retinaculum (Fig. 8). It then travels deep to the tendons of m. extensor digiti I et II and the medial two tendons of m. extensor digitorum communis to insert on the dorsomedial aspect of the base of the third metacarpal (Figs 8–10). M. extensor carpi radialis brevis extends the carpal joint.
M. extensor digitorum communis
M. extensor digitorum communis takes origin from the lateral epicondyle (Figs 6B,D and 8). In two limbs, m. digitorum communis was completely fused with m. extensor digitorum lateralis at its origin. In addition, in one of these limbs, the common extensor took partial origin from the surface of m. extensor carpi radialis brevis. Immediately proximal to the extensor retinaculum, m. extensor digitorum communis gives rise to three tendons (Fig. 8). These tendons course within a separate retinaculum, medial to that of m. extensor digitorum lateralis (Fig. 8).
The medial tendon of m. extensor digitorum communis courses superficial to the digit II tendon of m. extensor digiti I et II, passes dorsomedial to the metacarpophalangeal joint of digit II and contributes to the extensor expansion of that digit (Figs 8–10). The middle tendon courses dorsomedial to the metacarpophalangeal joint of digit III and contributes to its extensor expansion (Figs 8–10). In one limb, this tendon also contributed a small slip to the medial tendon of m. extensor digitorum communis, just proximal to the metacarpophalangeal joint. The lateral tendon of m. extensor digitorum communis is rather broad compared with its counterparts. In most limbs, it divided at the level of the base of the fourth metacarpal (Fig. 8); however, in two limbs, this division took place deep to the extensor retinaculum. The two resulting tendons course dorsomedial to the metacarpophalangeal joints of digits IV and V, respectively, and contribute to the extensor expansions (Figs 8–10). M. extensor digitorum communis extends the carpal joint and the metacarpophalangeal and interphalangeal joints of digits II to V.
M. extensor digitorum lateralis
M. extensor digitorum lateralis originates from the lateral surface of the lateral epicondyle (Figs 6B,D and 8). In two limbs, it was fused at its origin with m. extensor digitorum communis. Immediately proximal to the extensor retinaculum, m. extensor digitorum lateralis gives rise to three tendons (Fig. 8). The tendons course deep to their own retinaculum, lateral to the one housing the tendons of m. extensor digitorum communis (Fig. 8). However, in one limb, two tendons arose at the level of the retinaculum, whereas the third arose in the metacarpus.
The medial tendon of m. extensor digitorum lateralis courses deep to the communis tendons to digits IV and V, passes dorsolateral to the metacarpophalangeal joint of digit III, and contributes to the extensor expansion of that digit (Figs 8–10). The middle tendon courses deep to the communis tendon to digit V, passes dorsolateral to the metacarpophalangeal joint of digit IV, and contributes to its extensor expansion (Figs 8–10). The lateral tendon passes dorsolateral to the metacarpophalangeal joint of digit V and helps form the extensor expansion of that digit (Figs 8–10). M. extensor digitorum lateralis extends the carpal joint and the metacarpophalangeal and interphalangeal joints of digits III to V.
M. extensor carpi ulnaris
M. extensor carpi ulnaris orginates from the lateral epicondyle (Fig. 6B,C). It gives rise to a tendon at the level of the extensor retinaculum (Fig. 8). After coursing deep to the retinaculum, the tendon inserts broadly onto the palmolateral aspect of the base of the fifth metacarpal (Figs 8–10 and 13) and the lateral aspect of the pisiform (not visible on the palmar manus map). M. extensor carpi ulnaris abducts the carpal joint. In addition, it further extends an extended carpus but further flexes the carpus if it is in a flexed position.
Fig. 13.
Palmar manus muscle map for Ailurus, including the separate insertions of mm. flexor carpi ulnaris capita humerale et ulnare (present in seven limbs) (left side).
M. supinator
This muscle lies deep in the extensor compartment (Fig. 10). It originates from the annular and radiohumeral ligaments connecting the lateral epicondyle to the radius and is fused with m. abductori digiti I longus. In four limbs, m. supinator was also fused with the m. flexor digitorum profundus caput radiale. The belly of m. supinator wraps around the radius, dives deep to m. pronator teres and inserts on the craniomedial aspect of the proximal half of the radius (Fig. 7A,B). In addition, it inserts partially onto the fascia overlying m. pronator teres. As its name implies, this muscle supinates the forearm.
M. abductor digiti I longus
M. abductor digiti I longus lies deep to mm. extensor digitorum communis and extensor digitorum lateralis (Figs 8 and 10). It is fused on its medial aspect with m. supinator. The abductor’s extensive muscle belly occupies the groove between the radius and ulna (Fig. 10). It originates from the craniolateral aspect of the ulna and radius, as well as the intervening interosseous membrane (Fig. 7B). The muscle belly wraps around the distal radius, coursing superficial to the tendons of mm. extensor carpi radialis brevis et longus, and becomes tendinous at the level of the carpal joint (Fig. 10). The tendon courses deep to the main extensor retinaculum and rapidly fans out to insert, via two strong bands, onto the radial sesamoid and the dorsomedial aspect of the base of the first metacarpal (Figs 9 and 13). M. abductor digiti I longus abducts the carpal joint and the carpometacarpal joint of the pollex. This muscle may also assist in supination (Antón et al. 2006).
Fig. 9.
Dorsal manus muscle map for Ailurus (left side).
M. extensor digiti I et II
M. extensor digiti I et II lies deep to mm. extensor digitorum lateralis and extensor carpi ulnaris (Figs 8 and 10). It originates from the fascia covering m. abductor digiti I longus and the craniolateral aspect of the ulnar shaft (Figs 7B and 10). Immediately proximal to the carpal joint, it gives rise to two tendons. The tendons course within a groove on the distal radius, deep to a separate retinaculum (Fig. 8). The tendon to digit I courses superficial to the tendons of mm. extensor carpi radialis brevis et longus, passes dorsal to the metacarpophalangeal joint of digit I and forms the extensor expansion of this digit (Figs 9 and 10). The tendon to digit II, equal in size, courses superficial to the tendon of m. extensor carpi radialis brevis and deep to the digit II tendon of m. extensor digitorum communis (Fig. 8). The tendon then courses dorsolateral to the metacarpophalangeal joint of digit II to contribute to the extensor expansion of that digit (Figs 8–10). M. extensor digiti I et II extends the carpal joint and the metacarpophalangeal and interphalangeal joints of digits I and II.
Cranial antebrachium
M. pronator teres
M. pronator teres is fused along its proximal two-thirds (six limbs) or proximal half (two limbs) with m. flexor carpi radialis (Fig. 11). Covered on its superficial surface by a sheet of tendon, it originates from the cranial aspect of the medial epicondyle of the humerus (Figs 6A,C,D, 11 and 12). M. supinator partially inserts onto the superficial, tendinous surface of m. pronator teres. M. pronator teres inserts via tendinous fibers onto the distal half of the radius and a ridge marks its insertion (Fig. 7A,B). M. pronator teres pronates the forearm.
Fig. 12.
Close-up view of the left medial epicondyle muscle map in Ailurus.
M. flexor carpi radialis
M. flexor carpi radialis originates from the medial epicondyle (Figs 6A,C, 11 and 12). It is fused proximally with m. pronator teres (Fig. 11). In addition, m. flexor carpi radialis was also fused with m. flexor digitorum profundus caput humerale mediale in four limbs. In one limb, m. flexor carpi radialis was composed of two heads of origin that rapidly fused to form a single belly in the proximal third of the antebrachium. M. flexor carpi radialis gives rise to a stout tendon at the level of the flexor retinaculum (Fig. 11). This tendon courses through its own retinaculum, passes deep to the radial sesamoid and inserts onto the base of the second and third metacarpals (Fig. 13). M. flexor carpi radialis flexes the carpal joint.
M. palmaris longus
M. palmaris longus originates from the medial epicondyle via a thin tendon (Figs 6A,C, 11 and 12). This muscle can be fused with the m. flexor digitorum profundus caput laterale (two limbs), m. flexor digitorum profundus caput humerale mediale (five limbs) or both (one limb). The belly of m. palmaris longus fans out as it approaches the carpus and fleshy fibers insert superficially onto the palmar fascia overlying the pisiform (Fig. 11). In three of the limbs dissected, there was a clear tendon branching off to insert onto the radial sesamoid and, in an additional limb, there was a slight fascial attachment to the radial sesamoid, although this did not constitute a separate tendon of attachment. However, in all cases, the main tendon of m. palmaris longus coursed deep to the superficial layer of connective tissue spanning the radial sesamoid and pisiform (from which m. palmaris brevis took origin) to insert onto the palmar fascia (Fig. 11). M. palmaris longus flexes the carpal joint. When it does insert on the radial sesamoid, m. palmaris longus may adduct or stabilize the sesamoid.
M. flexor carpi ulnaris
M. flexor carpi ulnaris includes a caput humerale and caput ulnare. The caput humerale originates from the medial epicondyle (Figs 6A,C, 11 and 12), whereas the caput ulnare originates from the caudomedial aspect of the ulna, including part of the olecranon process (Figs 7A and 11). The bellies were entirely independent in all but one limb (Fig. 11); in this exceptional limb, they were fused in the distal quarter of the antebrachium. The caput humerale has a mixed fleshy and tendinous insertion onto the medial and palmar aspects of the pisiform, whereas the caput ulnare inserts onto its lateral and palmar aspects (Fig. 13). In four limbs, the caput humerale also inserted via fleshy fibers onto the superficial layer of connective tissue spanning the pisiform and radial sesamoid (Fig. 11). M. flexor carpi ulnaris flexes and abducts the carpal joint.
M. flexor digitorum superficialis
M. flexor digitorum superficialis arises in the distal antebrachium from the superficial, tendinous surface of the capita humerale laterale et ulnare of m. flexor digitorum profundus (Fig. 14). Therefore, it lacks a bony site of origin. The muscle fibers of m. flexor digitorum superficialis extend into the carpal tunnel (Fig. 14) and, within the tunnel, the muscle gives rise to three tendons of equal size, serving digits II to IV (Fig. 15A,B). These tendons divide at the metacarpophalangeal joint to allow the m. flexor digitorum profundus tendons to course to the distal phalanx (Fig. 15B). The superficialis tendons insert onto the palmar aspects of the middle phalanges of digits II to IV (Fig. 13). M. flexor digitorum superficialis flexes the carpal joint and the metacarpophalangeal and proximal interphalangeal joints of digits II to V.
Fig. 14.
Deep dissection of the caudomedial antebrachium of the left forelimb in Ailurus.
Fig. 15.
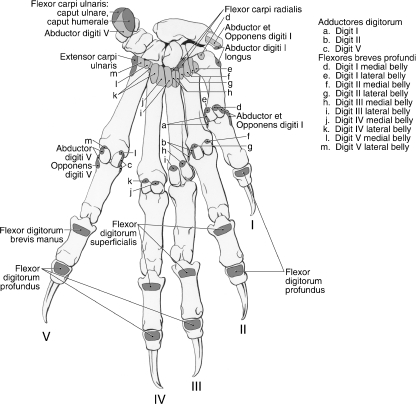
Palmar views of the manus in Ailurus (left side). (A) First (superficial) layer; (B) second layer; (C) third layer; (D) fourth (deep) layer, including two subdivisions in the medial belly of m. flexor brevis profundus digiti III (present in six limbs). *The superficial connective tissue spanning the pisiform and radial sesamoid.
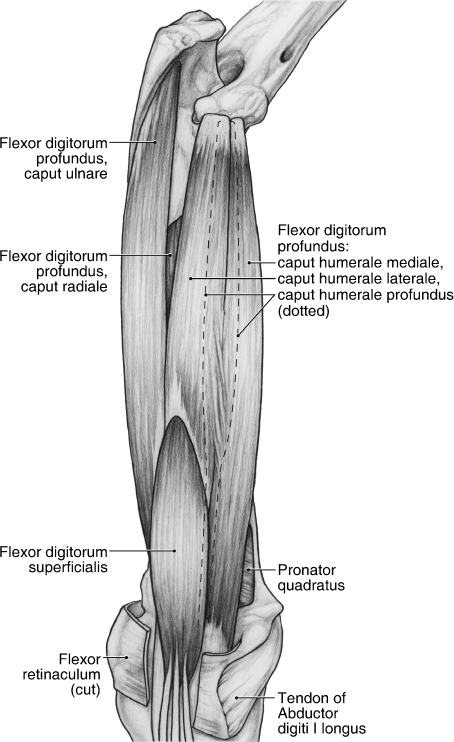
M. flexor digitorum profundus
M. flexor digitorum profundus is composed of three humeral, one ulnar and one radial belly of origin (Fig. 14). The three humeral bellies include the caput humerale mediale, caput humerale laterale and caput humerale profundus (Fig. 14). The caput humerale mediale originates via a tendon from a divot on the craniomedial aspect of the medial epicondyle (Fig. 12). Immediately distal to this, the caput humerale laterale originates via a stout tendon from a second divot on the medial epicondyle (Fig. 12). Finally, the caput humerale profundus originates via a tendon from a raised area of bone on the cranial aspect of the medial epicondyle (Fig. 12). In two of the limbs dissected, the capita humerale laterale et profundus were fused at their origin. In the middle of the antebrachium, the caput humerale laterale fuses with the caput humerale mediale. In addition, the caput humerale mediale fused with m. flexor carpi radialis in four of the limbs dissected.
The remaining heads of m. flexor digitorum profundus include the caput ulnare, arising from the ulna (Figs 7A and 14), and the caput radiale, arising from the ulna, interosseous membrane and radius (Figs 7A and 14). In two limbs, the capita ulnare et radiale were extensively fused. As noted above, m. flexor digitorum superficialis takes fleshy origin from the superficial tendinous surface of the caput ulnare and caput humerale laterale (Fig. 14).
In four of the limbs, the capita humerale, ulnare et radiale fused immediately proximal to the carpal tunnel and collectively gave rise to a common tendon. However, in the remaining limbs, the capita humerale gave rise to a distinct tendon in the distal third of the antebrachium that eventually joined the main profundus tendon just proximal to the carpal tunnel. In the tunnel, the common tendon courses deep to the tendons of m. flexor digitorum superficialis and into the manus (Fig. 15A,B). At the level of the metacarpal bases, five distinct tendons arise (Fig. 15B). These tendons course between two sesamoid bones located on the palmar aspect of the metacarpophalangeal joints and then through the bifurcated superficialis tendons to insert on the palmar aspect of the distal phalanges of digits I to V (Figs 13 and 15A,B). M. flexor digitorum profundus flexes the carpal joint and the metacarpophalangeal and interphalangeal joints of digits I to V.
M. pronator quadratus
M. pronator quadratus is situated in the distal third of the antebrachium, deep to m. flexor digitorum profundus (Fig. 14). It originates from the distal third of the ulna and inserts on the distal third of the radius (Fig. 7A). Its deep aspect is intimately fused with the distal third of the interosseous membrane. M. pronator quadratus pronates the forearm.
Manus
Superficial muscles
M. palmaris brevis
M. palmaris brevis originates from the palmar fascia overlying the hypothenar muscles. Its transverse muscle fibers form a very faint layer coursing superficial to the hypothenar eminence and inserting onto a superficial layer of connective tissue spanning the distance between the pisiform and radial sesamoid (Fig. 15A). This superficial layer of tissue is distinct from the flexor retinaculum, which is a thickened band of fascia that is located on a deeper plane (Fig. 15B). M. palmaris brevis tenses the skin of the palm and may assist in maintaining grip.
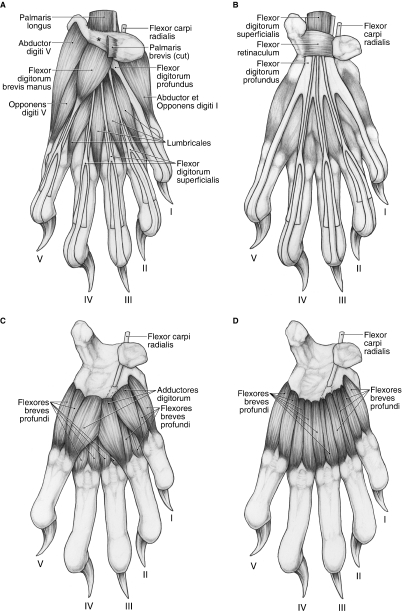
M. flexor digitorum brevis manus
This muscle belly arises from the flexor retinaculum (Fig. 15A). It gives rise to a thin tendon that courses superficial to the profundus tendon to digit V. At the level of the metacarpophalangeal joint, it bifurcates and surrounds the profundus tendon (Fig. 15A). The medial and lateral slips eventually course deep to the profundus tendon, fuse in the midline and insert on the palmar aspect of the base of the middle phalanx (Fig. 13). M. flexor digitorum brevis manus flexes the metacarpophalangeal and proximal interphalangeal joints of digit V.
Hypothenar eminence
M. abductor digiti V
This muscle arises from the palmar aspect of the pisiform (Figs 13 and 15A). It gives rise to a tendon that travels deep to the tendon of the m. opponens digiti V brevis (Fig. 15A). The tendon then courses palmolateral to the metacarpophalangeal joint of digit V to insert on the lateral sesamoid and the palmolateral aspect of the base of the proximal phalanx of digit V (Fig. 13). M. abductor digiti V abducts digit V and weakly flexes its metacarpophalangeal joint.
M. opponens digiti V
M. opponens digiti V arises from the flexor retinaculum, deep to m. flexor brevis digitorum manus (Fig. 15A). It gives rise to a tendon that courses superficial to the tendon of the abductor digiti V, crosses the metacarpophalangeal joint palmolaterally and inserts on the palmolateral aspect of the base of the proximal phalanx (Figs 13 and 15A). M. opponens digiti V brevis flexes the metacarpophalangeal joint of digit V.
Thenar eminence
Mm. abductor et opponens digiti I
These muscles are intimately fused and originate from the palmomedial aspect of the radial sesamoid and its cartilage, a superficial layer of palmar fascia spanning the pisiform and radial sesamoid, and the flexor retinaculum (Fig. 15A). The fused muscle mass gives rise to tendinous fibers that course palmomedial to the metacarpophalangeal joint of digit I to insert onto the medial sesamoid and medial aspect of the base of proximal phalanx (Fig. 13). Mm. abductor et opponens digiti I flex the metacarpophalangeal joint of the pollex.
Central compartment
Mm. lumbricales
The four mm. lumbricales originate from the palmar surface of the common tendon of m. flexor digitorum profundus (Fig. 15A). The bellies of the mm. lumbricales occupy the spaces between adjacent long flexor tendons and each m. lumbricalis contributes to the extensor expansion of its respective digit (Fig. 15A). However, in three limbs, m. lumbricalis I originated more proximally, from the flexor retinaculum, whereas in two limbs, m. lumbricalis III originated from the dorsal surface of the profundus tendon. In addition, in one limb, m. lumbricalis III was composed of two muscle bellies that converged distally to form a single tendon of insertion. The mm. lumbricales flex the metacarpophalangeal joints and weakly extend the interphalangeal joints of digits II to V. In addition, mm. lumbricales II et III weakly abduct, whereas mm. lumbricales IV et V weakly adduct, their respective digits.
Mm. adductores digitorum
The mm. adductores digitorum serve digits I, II and V, and lie superficial to the mm. flexores breves profundi (Fig. 15C). The adductors are fused proximally and share a common origin from the transverse carpal ligament. Mm. adductor digiti I et II course lateral to their respective metacarpophalangeal joints and insert onto the metacarpophalangeal joint capsule, lateral sesamoid and lateral aspect of the base of proximal phalanx (Figs 13 and 15C). M. adductor digiti V courses medial to the metacarpophalangeal joint of digit V and inserts onto the metacarpophalangeal joint capsule, medial sesamoid and medial aspect of the base of the proximal phalanx (Figs 13 and 15C). In two limbs, m. adductor digiti V inserted onto the medial aspect of the first metacarpal. The mm. adductors digitorum adduct digits I, II and V, and weakly flex the metacarpophalangeal joints of these digits.
Mm. flexores breves profundi
There is a medial and lateral belly associated with each digit, comprising the mm. flexores breves profundi (Fig. 15C,D). A great deal of fusion is present among these bellies, especially among the muscles associated with digits III and IV. The mm. flexores breves profundi flex the metacarpophalangeal joints and, depending on their position relative to the central axis of the manus, abduct or adduct the digits. In addition, most of these muscles extend distally, beyond the sesamoids, to contribute to the extensor expansions. In these cases, the mm. flexores breves profundi also help to extend the interphalangeal joints.
Mm. flexores breves profundi digiti I
The medial belly originates from the palmar aspect of the base of the first metacarpal (Fig. 13). It inserts onto the medial sesamoid at the first metacarpophalangeal joint (Figs 13 and 15D). The lateral belly originates from the transverse carpal ligament and palmar aspect of the first metacarpal base (Fig. 13). It inserts onto the lateral sesamoid at the first metacarpophalangeal joint (Figs 13 and 15D).
Mm. flexores breves profundi digiti II
The medial belly is composed of two partially separable bellies that originate from the base of the first and second metacarpals, respectively (Fig. 13). The bellies fuse distally to insert onto the medial sesamoid at the second metacarpophalangeal joint (Figs 13 and 15D). The lateral belly originates from the second metacarpal base and inserts onto the lateral sesamoid at the second metacarpophalangeal joint and onto the extensor expansion of digit II (Fig. 13).
Mm. flexores breves profundi digiti III
The medial belly arises from the base of the third metacarpal and inserts onto the medial sesamoid at the third metacarpophalangeal joint and onto the extensor expansion of digiti III (Fig. 13). This belly had two subdivisions in six of the limbs dissected (Fig. 15D). The lateral belly originates from the base of the third metacarpal and inserts onto the lateral sesamoid at the third metacarpophalangeal joint and the extensor expansion of digiti III (Fig. 13). In one limb, the lateral belly also had two subdivisions.
Mm. flexores breves profundi digiti IV
The medial belly originates from the base of the fourth metacarpal and inserts onto the medial sesamoid at the fourth metacarpophalangeal joint and the extensor expansion of digit IV (Fig. 13). In most cases, the lateral belly originates from the bases of the fourth and fifth metacarpals; however, the belly arose from the fourth metacarpal alone in two limbs. The lateral belly inserts onto the lateral sesamoid at the fourth metacarpophalangeal joint and the extensor expansion of digit IV (Fig. 13).
Mm. flexores breves profundi digiti V
The medial belly originates from the base of the fifth metacarpal and inserts onto the medial sesamoid of the fifth metacarpophalangeal joint and the extensor expansion of digit V (Fig. 13). The lateral belly originates from the base of the fifth metacarpal and inserts onto the lateral sesamoid of the fifth metacarpophalangeal joint (Fig. 13). In one limb, this belly also inserted onto the extensor expansion of digit V.
Concluding remarks
Intraspecific variation in Ailurus
Similar to the hindlimb (Fisher et al. 2008), the muscles and tendons of the red panda forelimb display a considerable range of variation. Once again, this demonstrates the need for larger samples when utilizing soft tissue characters in phylogenetic analyses. Apart from its smaller size, the forelimb morphology of the 5-month-old female resembled that of the adults. Similarly, most of the previous accounts of the red panda forelimb fall within the range of variation documented in this study (Carlsson, 1925; Endo et al. 2001; Antón et al. 2006). However, Carlsson (1925) describes an occipital origin for m. trapezius pars cervicalis, whereas this muscle arose more distally, from the C3 vertebral level, in this study (Fig. 1). Carlsson (1925) also describes mm. teres minor and infraspinatus as inseparable, whereas these muscles were distinct in the eight limbs dissected for this study (Figs 1 and 5). In contrast to the five bellies of mm. triceps brachii noted here (Figs 4 and 5), Carlsson (1925) describes three bellies in her specimen. Moreover, Carlsson (1925) does not describe an abdominal belly of m. pectoralis profundus, m. palmaris brevis or m. opponens digiti V, although these muscles were constant in the present study (Figs 3 and 15A).
More recently, Inaba & Takahashi (1996), Endo et al. (2001) and Antón et al. (2006) documented the muscles associated with the radial sesamoid of Ailurus. In comparing these accounts with the current study, the morphology of mm. flexor digitorum brevis manus and palmaris longus appears to vary. In the two specimens dissected by Antón et al. (2006, p. 760), m. flexor digitorum brevis manus inserted on the ‘proximal epiphysis of the fifth metacarpal’; however, in the four specimens dissected for this study, the muscle was constant in supplying the superficialis tendon to digit V and inserting onto the middle phalanx (Figs 13 and 15A). Carlsson (1925) also observed an insertion onto the middle phalanx in her specimen. Furthermore, in three of the eight limbs dissected for this study, m. palmaris longus sent a tendon to insert on the radial sesamoid, similar to that described in the two specimens dissected by Antón et al. (2006). However, this tendon was not present in the remaining five limbs. Lastly, in this study, the main tendon of m. palmaris longus was constant in diving deep to the superficial layer of palmar fascia spanning the radial sesamoid and pisiform (Fig. 15A); however, Antón et al. (2006) describe the main tendon as coursing deep to the flexor retinaculum. In all of the limbs dissected in this study, it was clear that the flexor retinaculum, holding the long flexor tendons in place, was located deep to the tendon of m. palmaris longus (Fig. 11).
Primitive retentions in Ailurus
The myology of the red panda forelimb is largely characterized by primitive retentions. These primitive traits include the lack of m. rhomboideus profundus, a humeral insertion for m. cleidobrachialis, the presence of mm. brachioradialis, articularis humeri and coracobrachialis, a single muscle belly for m. extensor digitorum lateralis with tendons to digits III–V, four mm. lumbricales, and the presence of mm. flexor digitorum brevis manus, adductores digiti I, II and V, and abductor digiti I and V (Table 4). Unfortunately, the terminology associated with the short digital flexors suffers from a lack of standardization, making it difficult to conduct a comprehensive, family-level comparison. However, according to Windle & Parsons (1897), the majority of carnivores retain a full complement of mm. flexores digitorum breves, and this is the case in Ailurus (Fig. 15D). As data on these muscles are limited at the species and genus levels, they are not included in Tables 4 and 5.
Table 4.
Distribution of forelimb traits in the red panda and other caniforms
| Ailurus | Procyonidae | Ursidae | Mustelidae | Canidae | |
|---|---|---|---|---|---|
| Rhomboideus capitis | Absent* | Present | Present (variable in Ursus americanus) | Present | Present |
| Rhomboideus profundus | Absent | Absent (except Potos) | Absent | Present | Absent |
| Insertion of cleidobrachialis | Humerus | Humerus | Humerus | Humerus (and forearm in Ictonyx) | Humerus (forearm in Lycaon) |
| Brachioradialis | Present | Present | Present | Present | Absent or reduced |
| Articularis humeri | Present | Present | Present | Variable | Present |
| Coracobrachialis | Present | Absent (variable in Potos) | Present | Variable | Absent |
| Biceps brachii caput breve | Variable* | Variable | Variable | Absent | Absent |
| Extensor digitorum lateralis | Single muscle belly | Single muscle belly (except some Nasua) | Single muscle belly | Single muscle belly (except Aonyx) | Single muscle belly |
| Extensor digitorum lateralis | To digits III–V | To digits III–V | To digits III–V (IV and V in Ailuropoda) | To digits III–Vor IV-V | To digits III–V or IV–V |
| Origin of flexor digitorum superficialis | FDP* | Variable | Humerus (FDP in Ailuropoda) | FDP | Humerus |
| Lumbricales | n = 4 | n = 4 | n = 4 | n = 4 (3 in Aonyx) | n = 3 |
| Flexor digitorum brevis manus | Present | Present | Absent (except Ursus arctos) | Absent (except Mustela putorius) | Absent or reduced |
| Adductores digitorum | To digits I, II, V | Typically to digits I, II, V | Typically to digits I, II, V | Typically todigits I, II, V | To digits I, II, V (I, II, IV in Canis aureus) |
| Abductor digiti I | Present (fused with opponens) | Present | Present (except Ursus maritimus) | Present | Present |
| Abductor digiti V | Present | Present | Present | Present | Present |
The traits that have been identified as derived in red pandas.
Comparative caniform data are derived from the references listed in Table 2.
FDP, flexor digitorum profundus.
Table 5.
Distribution of forelimb traits in the feliforms
| Felidae | Hyaenidae | Herpestidae | Viverridae | Eupleridae | Nandiniidae | |
|---|---|---|---|---|---|---|
| Rhomboideus capitis | Present | Absent (variable in Hyaena hyaena) | Present | Absent (variable in Paradoxurus and Genetta) | Absent | No data |
| Rhomboideus profundus | Absent | No data | No data | Absent | Absent | No data |
| Insertion of cleidobrachialis | Forearm | Variable | Forearm | Variable | Forearm | No data |
| Brachioradialis | Present (except Acinonyx) | Absent or reduced | Present | Present | Present | Present |
| Articularis humeri | Present | Present | Present | Present (variable in Genetta) | Present | No data |
| Coracobrachialis | Absent | Absent | Absent | Absent (variable in Genetta) | Absent or reduced | Reduced |
| Biceps brachii caput breve | Absent | Absent | Absent | Absent (variable in Paradoxurus) | Absent | Absent |
| Extensor digitorum lateralis | Single muscle belly (except Panthera leo and Acinonyx) | Two muscle bellies (except Proteles) | Single muscle belly | Single muscle belly | Single muscle belly | No data |
| Extensor digitorum lateralis | To digits II–V, III–V or IV–V | To digits IV–V | To digits III–V or IV–V | To digits III–V or IV–V | To digits III–V | No data |
| Origin of flexor digitorum superficialis | FDP and palmaris longus (FDP in Acinonyx) | Humerus (but fused with FDP) | No data | FDP | No data | No data |
| Lumbricales | n = 4 | n = 2, 3 or 4 | n = 4 | n = 4 | No data | No data |
| Flexor digitorum brevis manus | Present | Present (variable in Hyaena) | Present | Present | No data | No data |
| Adductores digitorum | To digits I, II, V | To digits II, V (III, V in Crocuta) | To digits I, II, V | To digits I, II, V | To digits I, II, V | No data |
| Abductor digiti I | Typically present | Absent | Present | Present | Present | No data |
| Abductor digiti V | Present | Present | Present | Present | No data | Present |
Data are derived from the references listed in Table 3.
FDP, flexor digitorum profundus.
Of the aforementioned primitive traits, the retention of mm. abductor digiti V appears to be constant in carnivores (Fig. 15A; Tables 4 and 5). In addition, the majority of carnivores, including Ailurus, lack m. rhomboideus profundus and retain mm. brachioradialis, articularis humeris, adductores digiti I, II and V, abductor digiti I, and four mm. lumbricales (Figs 4, 8 and 15A,C; Tables 4 and 5). Although red pandas resemble most caniforms in retaining a humeral insertion for m. cleidobrachialis, a derived, distal insertion is more commonly seen in feliforms (Fig. 5; Tables 4 and 5). In contrast, red pandas, procyonids and many feliforms retain m. flexor digitorum brevis manus, whereas other caniforms have lost this muscle (Fig. 15A; Tables 4 and 5).
Mm. extensor digitorum lateralis and coracobrachialis are particularly variable in carnviores. For m. extensor digitorum lateralis, the primitive condition, as seen in the red panda (nine out of 10 limbs), includes tendons to digits III–V (Fig. 8; Tables 4 and 5). Most carnivores have a reduced number of tendons but the domestic cat can have an additional tendon to digit II (Getty, 1975a). Finally, as m. coracobrachialis is present in other members of the Fereuungulata (sensu Waddell et al. 1999), such as perissodactyls and artiodactyls, its presence in some carnivores, including Ailurus, is likely to be primitive (Fig. 4; Table 4) (Gratiolet, 1867; Macalister, 1873c; Beddard & Treves, 1889; Windle & Parsons, 1901; Campbell, 1935, 1936; Getty, 1975b; Sisson, 1975; Macdonald et al. 1985; Nickel et al. 1986; Smuts & Bezuidenhout, 1987; Kneepkens et al. 1989; Fisher et al. 2007).
Traits shared with Ailuropoda, procyonids and mustelids
M. flexor digitorum superficialis takes origin from the surface of m. flexor digitorum profundus in Ailurus, Ailuropoda and the mustelids (Fig. 14; Table 4) (Macalister, 1873a,b; Mackintosh, 1875–1877; Davis, 1964). In canids, a humeral origin is constant; however, the origin of this muscle is variable in procyonids, arising from m. flexor digitorum profundus in Nasua and most Procyon specimens but taking origin from the humerus in Potos and some Procyon limbs (Table 4) (Beswick-Perrin, 1871; Mackintosh, 1875–1877; Allen, 1882; Windle, 1888; Getty, 1975a; McClearn, 1984, 1985; Nickel et al. 1986; Evans, 1993; Kainer & McCracken, 2003). Among the feliforms, a soft tissue origin for m. flexor digitorum superficialis is found in felids and viverrids, whereas a humeral origin is present in hyaenids (Table 5) (Devis, 1868; Macalister, 1873b; Ross, 1876; Watson & Young, 1879; Watson, 1882; Young & Robinson, 1889; Mivart, 1900; Carlsson, 1925; Getty, 1975a; Gilbert, 2002).
In perissodactyls and artiodactyls, a humeral origin for m. flexor digitorum superficialis is the rule. Although the muscle can sometimes have a secondary origin from the surface of m. flexor digitorum profundus, a medial epicondyle origin is constant in these groups (Gratiolet, 1867; Beddard & Treves, 1889; Windle & Parsons, 1901; Kajava, 1923; Campbell, 1935, 1936; Getty, 1975b; Sisson, 1975; Nickel et al. 1986; Kneepkens et al. 1989; Fisher et al. 2007). The exception is Camelus, which has an entirely tendinous superficial flexor that is confined to the manus (Smuts & Bezuidenhout, 1987). This evidence suggests that a soft tissue origin for m. flexor digitorum superficialis is a derived feature in carnivores. In Ailurus, this is particularly true, as the muscle arises in the distal third of the antebrachium (Fig. 14).
Traits shared with ursids and procyonids
The presence of m. biceps brachii caput breve is variable in Ailurus. It was present in the two limbs dissected by Carlsson (1925) and in four of the eight limbs dissected in this study (Fig. 4). Although this muscle is absent in mustelids and canids, it is variably present in ursids and procyonids (Table 4). In bears, it is present in Ursus maritimus and Tremarctos ornatus, variable in U. arctos and U. americanus, and absent in Ailuropoda (Haughton, 1867a; Shepherd, 1883; Kelley, 1888; Windle & Parsons, 1897; Davis, 1964). Among procyonids, it is present in Potos, variable in Procyon and absent in Nasua and Bassaricyon (Beswick-Perrin, 1871; Mackintosh, 1875–1877; Allen, 1882; Windle, 1888; Windle & Parsons, 1897; Carlsson, 1925). Interestingly, although variable in Paradoxurus (Windle & Parsons, 1897), the caput breve appears to be absent in all other feliforms (Table 5).
Apart from Camelus, perissodactyls and artiodactyls are characterized by a single belly of m. biceps brachii, the caput longum (Gratiolet, 1867; Macalister, 1873c; Beddard & Treves, 1889; Windle & Parsons, 1901; Campbell, 1935, 1936; Getty, 1975b; Sisson, 1975; Nickel et al. 1986; Smuts & Bezuidenhout, 1987; Kneepkens et al. 1989; Fisher et al. 2007). Although many ungulates have reduced lateral digits and an associated reduction of the distal forelimb musculature, the caput breve is also absent in ungulates (e.g. hippos, tragulids, rhinos, tapirs and some suids) that have retained weight-bearing lateral digits and well-developed forelimb muscles, including the intrinsic muscles of the manus. Considering that the immediate outgroup, the feliforms, also lack the caput breve, as do basal caniforms (e.g. canids), the variable presence of a caput breve in Ailurus is most likely to be derived.
Traits shared with ursids only
Among caniforms, the red panda resembles some ursids in lacking m. rhomboideus capitis (Table 4). Although most bears do have a belly arising from the occipital bone, this attachment was missing in two of the four American black bears discussed by Windle & Parsons (1897). The lack of an occipital attachment is most likely a derived feature in red pandas, as a cranial origin is found in all other caniforms, felids and herpestids, and is variable in Paradoxurus, Genetta and Hyaena hyaena (Tables 4 and 5) (Haughton, 1867b,c,e; Devis, 1868; Beswick-Perrin, 1871; Macalister, 1873a,b; Mackintosh, 1875–1877; Ross, 1876; Watson & Young, 1879; Young, 1880; Allen, 1882; Watson, 1882; Kelley, 1888; Windle, 1888; Young & Robinson, 1889; Windle & Parsons, 1897; Mivart, 1900; Davis, 1964; Taylor, 1974; Getty, 1975a; Nickel et al. 1986; Evans, 1993; Kainer & McCracken, 2003). Evidence from other members of the Fereuungulata provides some support for this interpretation. Although data for rhinos are not available, m. rhomboideus capitis is absent in tapirids and equids (Windle & Parsons, 1901; Campbell, 1935, 1936; Sisson, 1975; Nickel et al. 1986). Similarly, most artiodactyls lack this muscle, although it is variably present in hippos and present in pigs, Pecari, Hyemoschus and Odocoileus (Gratiolet, 1867; Macalister, 1873c; Beddard & Treves, 1889; Windle & Parsons, 1901; Campbell, 1935, 1936; Dalzell, 1970; Getty, 1975b; Sisson, 1975; Nickel et al. 1986; Smuts & Bezuidenhout, 1987; Kneepkens et al. 1989; Fisher et al. 2007).
Functional anatomy of S. batalleri
Salesa et al. (2008) recently analyzed the postcranial skeleton of the Miocene ailurid, S. batalleri, from Batallones-1 in Spain, utilizing the existing literature on carnivore myology to gain insights into its functional anatomy. As the current study provides muscle maps and dissection data for Ailurus, the closest living relative to Simocyon, a few additional notes on this fossil ailurid are presented here.
The coracoid process is relatively robust in S. batalleri and Salesa et al. (2008) note that it is the site of origin for mm. coracobrachialis and biceps brachii caput breve. In Ailurus, the coracoid process also serves as the origin for m. articularis humeri, which stabilizes and weakly extends the glenohumeral joint (Figs 2B and 4). As most carnivores retain this muscle, including the red panda, m. articularis humeri was probably present in S. batalleri (Tables 4 and 5). In addition, Salesa et al. (2008) argue that m. coracobrachialis flexed the elbow joint in S. batalleri; however, this muscle inserts onto the distal humerus in Ailurus and probably acted solely on the glenohumeral joint in Simocyon (Fig. 6A,D).
Salesa et al. (2008) indicate that the accessory head of m. triceps brachii originates on the medial aspect of the humeral neck, inferior to the articular head. However, this area is more closely associated with m. articularis humeri in Ailurus (Fig. 6A), the origin of the m. triceps brachii caput accessorium being located more caudally (Fig. 6C). In addition, Salesa et al. (2008) state that m. flexor digitorum superficialis originates from the medial epicondyle of the humerus; however, this muscle has a soft tissue origin in Ailurus, taking origin from the surface of m. flexor digitorum profundus in the distal third of the antebrachium (Fig. 14). As noted above, a soft tissue origin is also present in Ailuropoda, mustelids and some procyonids (Table 4). Thus, the purported superficialis scar on the medial epicondyle of S. batalleri is probably associated with one of the bellies of m. flexor digitorum profundus (Fig. 12).
Lastly, Salesa et al. (2008), citing Antón et al. (2006), indicate that m. flexor digitorum brevis manus inserts on the proximal epiphysis of metacarpal V in Ailurus. However, this muscle was constant in providing the superficialis tendon to digit V and inserting onto the base of the middle phalanx in the four specimens dissected for this study and in the specimen dissected by Carlsson (1925) (Figs 13 and 15A). Thus, rather than medially rotating the metacarpal, in most cases, this muscle would flex the metacarpophalangeal and proximal interphalangeal joints of digit V in Ailurus and, possibly, S. batalleri.
Conclusion
This study provides additional soft tissue features that can be incorporated in future phylogenetic studies of carnivores (Tables 4 and 5). In addition, the forelimb muscle maps provide a unique resource for those analyzing the functional anatomy of fossil ailurids. The forelimb muscles of Ailurus are characterized by a number of primitive retentions (Table 4); this was also the case in the hindlimb (see Table 3 in Fisher et al. 2008). Although not as informative as derived traits, the retention of many primitive features is consistent with the hypothesis that red pandas occupy a basal position among the caniforms. Features that are most likely to be derived in the forelimb of Ailurus include the soft tissue origin of m. flexor digitorum superficialis, the variable presence of m. biceps brachii caput breve and the lack of m. rhomboideus capitis. When combined with data from the hindlimb (Fisher et al. 2008), derived features in the limb musculature provide equal support for a close relationship to ursids (four traits) or procyonids (four traits). Dissections of the axial musculature in red pandas are ongoing and may provide additional characters to help elucidate their phylogeny. In addition, data for the Mephitidae are needed in order to test the proposal that skunks are closely related to red pandas (Flynn et al. 2000; Delisle & Strobeck, 2005).
Acknowledgments
R.E.F. would like to thank Jim Mead and Richard Thorington (National Museum of Natural History, Smithsonian Institution) for granting permission to work on the red panda specimens. In addition, the authors thank Linda Gordon, Dave Schmidt and Helen Kafka (National Museum of Natural History, Smithsonian Institution) for facilitating the shipment of the specimens to Arizona. Special thanks to Charley Potter and John Ososky (National Museum of Natural History, Smithsonian Institution) for their continued support and to Autumn Ervin (Arizona State University) and Kerr Whitfield (University of Arizona, College of Medicine–Phoenix in Partnership with Arizona State University) for their assistance in translating Carlsson (1925). R.E.F. would also like to thank Linda Walters (Midwestern University) for assisting with the dissection of specimen USNM 597647. Finally, the authors thank the two reviewers for their comments. All of the figures in this manuscript were produced by B.A. Funding for this project was provided by a Midwestern University Research Grant (R.E.F.) and a Midwestern University Summer Research Fellowship (J.H.). R.E.F. is a Research Collaborator in the Division of Mammals, National Museum of Natural History, Smithsonian Institution.
References
- Allen H. The muscles of the limbs of the raccoon (Procyon lotor) Proc Acad Nat Sci Philadelphia. 1882:115–144. [Google Scholar]
- Antón M, Salesa MJ, Pastor J, et al. Implications of the functional anatomy of the hand and forearm of Ailurus fulgens (Carnivora, Ailuridae) for the evolution of the ‘false-thumb’ in pandas. J Anat. 2006;209:757–764. doi: 10.1111/j.1469-7580.2006.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beddard FE. On the visceral and muscular anatomy of Cryptoprocta ferox. Proc Zool Soc Lond. 1895:430–437. [Google Scholar]
- Beddard FE, Treves F. On the anatomy of Rhinoceros sumatrensis. Proc Zool Soc Lond. 1889;1:7–25. [Google Scholar]
- Beswick-Perrin J. On the myology of the limbs of the Kinkajou (Cercoleptes caudivolvulus) Proc Zool Soc Lond. 1871:547–559. [Google Scholar]
- Bininda-Emonds OR, Gittleman JL, Purvis A. Building large trees by combining phylogenetic information: a complete phylogeny of the extant Carnivora (Mammalia) Biol Rev. 1999;74:143–175. doi: 10.1017/s0006323199005307. [DOI] [PubMed] [Google Scholar]
- Bugge J. The cephalic arterial system in carnivores, with special reference to the systematic classification. Acta Anat. 1978;101:45–61. doi: 10.1159/000144948. [DOI] [PubMed] [Google Scholar]
- Campbell B. Baltimore: Johns Hopkins University; The Comparative Myology of the Hippopotamus, Pig, and Tapir. Ph.D. Dissertation. [Google Scholar]
- Campbell B. The comparative myology of the forelimb of the hippopotamus, pig and tapir. Am J Anat. 1936;59:201–247. [Google Scholar]
- Carlsson A. Uber Ailurus fulgens. Acta Zool. 1925;6:269–305. [Google Scholar]
- Čihák R. Ontogenesis of the skeleton and intrinsic muscles of the human hand and foot. Adv Anat Embryol Cell Biol. 1972;46:5–194. [PubMed] [Google Scholar]
- Cuvier F. Histoire naturelle des Mammifères, avec des figures originales, colorées, desinées d’après des animaux vivants. Paris: Bouasse-Lebel & Basset Réunies s.d; 1825. [Google Scholar]
- Dalzell BB. Descriptive and Functional Morphology of the Extrinsic Shoulder Musculature in Artiodactyls. Berkeley: University of California; 1970. Masters Thesis. [Google Scholar]
- Davis DD. The shoulder architecture of bears and other carnivores. Fieldana Zool. 1949;31:285–305. [Google Scholar]
- Davis DD. The Giant Panda: A Morphological Study of Evolutionary Mechanisms. Chicago: Chicago Natural History Museum; 1964. [Google Scholar]
- Decker DM, Wozencraft WC. Phylogenetic analysis of recent procyonid genera. J Mammal. 1991;72:42–55. [Google Scholar]
- Delisle I, Strobeck C. A phylogeny of the Caniformia (order Carnivora) based on 12 complete protein-coding mitochondrial genes. Mol Phylogenet Evol. 2005;37:192–201. doi: 10.1016/j.ympev.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Devis CW. Notes on the myology of Viverra civetta. J Anat Physiol. 1868;2:207–217. [PMC free article] [PubMed] [Google Scholar]
- Dragoo JW, Honeycutt RL. Systematics of mustelid-like carnivores. J Mammal. 1997;78:426–443. [Google Scholar]
- Endo H, Sasaki M, Kogiku H, et al. Radial sesamoid bone as a part of the manipulation system in the lesser panda (Ailurus fulgens) Ann Anat. 2001;183:181–184. doi: 10.1016/S0940-9602(01)80045-5. [DOI] [PubMed] [Google Scholar]
- Evans HE. Miller’s Anatomy of the Dog. Philadelphia: Saunders, an imprint of Elsevier; 1993. [Google Scholar]
- Fisher RE, Scott K, Naples VL. Fore limb myology of the pygmy hippopotamus (Choeropsis liberiensis) Anat Rec. 2007;290:673–693. doi: 10.1002/ar.20531. [DOI] [PubMed] [Google Scholar]
- Fisher RE, Adrian B, Elrod C, et al. The phylogeny of the red panda (Ailurus fulgens): evidence from the hindlimb. J Anat. 2008;213:607–628. doi: 10.1111/j.1469-7580.2008.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JJ, Nedbal MA, Dragoo JW, et al. Whence the red panda? Mol Phylogenet Evol. 2000;17:190–199. doi: 10.1006/mpev.2000.0819. [DOI] [PubMed] [Google Scholar]
- Flynn JJ, Finarelli JA, Zehr S, et al. Molecular phylogeny of the Carnivora (Mammalia): assessing the impact of increased sampling on resolving enigmatic relationships. Syst Biol. 2005;54:317–337. doi: 10.1080/10635150590923326. [DOI] [PubMed] [Google Scholar]
- Fulton TL, Strobeck C. Molecular phylogeny of the Arctoidea (Carnivora): effect of missing data on supertree and supermatrix analyses of multiple gene data sets. Mol Phylogenet Evol. 2006;41:165–181. doi: 10.1016/j.ympev.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Getty R. Carnivore myology. In: Getty R, editor. Sisson & Grossman’s The Anatomy of Domestic Animals. Philadelphia: W.B. Saunders Company; 1975a. pp. 1507–1537. [Google Scholar]
- Getty R. Ruminant myology. In: Getty R, editor. Sisson and Grossman’s The Anatomy of the Domestic Animals. Philadelphia: W. B. Saunders Company; 1975b. pp. 791–860. [Google Scholar]
- Gilbert SG. Pictorial Anatomy of the Cat. Toronto: University of Toronto Press; 2002. [Google Scholar]
- Ginsburg L. Sur la position systematique du petit panda, Ailurus fulgens (Carnivora, Mammalia) Geobios. 1982;6:247–258. [Google Scholar]
- Goldman D, Giri PR, O’Brien SJ. Molecular genetic-distance estimates among the ursidae as indicated by one- and two-dimensional protein electrophoresis. Evolution. 1989;43:282–295. doi: 10.1111/j.1558-5646.1989.tb04228.x. [DOI] [PubMed] [Google Scholar]
- Gratiolet L-P. Rechereches sur l’anatomie de l’hippopotame. Paris: Victor Masson et Fils; 1867. [Google Scholar]
- Gregory WK. On the phylogenetic relationships of the giant panda (Ailuropoda) to other arctoid carnivora. Am Mus Novit. 1936;878:1–29. [Google Scholar]
- Haughton S. On the muscles of the Virginian bear. Proc R Ir Acad Ser 2. 1867a;ix:508–511. [Google Scholar]
- Haughton S. On the muscular anatomy of the badger. Proc R Ir Acad Ser 2. 1867b;ix:507–508. [Google Scholar]
- Haughton S. On the muscular anatomy of the Irish terrier, as compared with that of the Australian dingo. Proc R Ir Acad Ser 2. 1867c;ix:504–507. [Google Scholar]
- Haughton S. On the muscular anatomy of the lion. Proc R Ir Acad Ser 2. 1867d;ix:85–93. [Google Scholar]
- Haughton S. On the muscular anatomy of the otter (Lutra vulgaris) Proc R Ir Acad Ser 2. 1867e;ix:511–515. [Google Scholar]
- Hollister N. The genera and subgenera of raccoons and their allies. Proc US Natl Mus. 1915;49:143–150. [Google Scholar]
- Hunt RM. The auditory bulla in carnivora: an anatomical basis for reappraisal of carnivore evolution. J Morphol. 1974;143:21–76. doi: 10.1002/jmor.1051430103. [DOI] [PubMed] [Google Scholar]
- Inaba T, Takahashi KW. An anatomical study of the psuedothumb and skeleton on the red panda (Ailurus fulgens) Jpn J Zoo Wildl Med. 1996;1:87–92. [Google Scholar]
- Johnson KG, Schaller GB, Jinchu H. Comparative behavior of red and giant pandas in the Wolong Reserve, China. J Mammal. 1988;69:552–564. [Google Scholar]
- Kainer RA, McCracken TO. Dog Anatomy: A Coloring Atlas. Jackson: Teton New Media; 2003. [Google Scholar]
- Kajava Y. Die volare handmusckulatur der huftiere. Acta Soc Med Fenn Duodecim. 1923;4:1–184. [Google Scholar]
- Kelley EA. Notes on the myology of Ursus maritimus. Proc Acad Nat Sci Philadelphia. 1888:141–154. [Google Scholar]
- Kneepkens A, Badoux D, MacDonald A. Descriptive and comparative myology of the forelimb of the babirusa (Babyrousa babyrussa L. 1758) Anat Histol Embryol. 1989;18:349–365. doi: 10.1111/j.1439-0264.1989.tb00609.x. [DOI] [PubMed] [Google Scholar]
- Macalister A. On the arrangement of the pronator muscles in the limbs of vertebrate animals. J Anat Physiol. 1869;3(Pt 2):335–340. [PMC free article] [PubMed] [Google Scholar]
- Macalister A. On the anatomy of Aonyx. Proc R Ir Acad Ser 2. 1873a;1:539–547. [Google Scholar]
- Macalister A. The muscular anatomy of the civet and tyra. Proc R Ir Acad Ser 2. 1873b;1:506–513. [Google Scholar]
- Macalister A. The anatomy of Chaeropsis liberiensis. Proc R Ir Acad Ser 2. 1873c;1:494–500. [Google Scholar]
- Macdonald AA, Kneepkens AFLM, Kolfschoten TV, et al. Comparative anatomy of the limb musculature of some Suina. Fortschr Zool. 1985;30:95–97. [Google Scholar]
- Mackintosh HW. Notes on the myology of the coati-mondi (Nasua narica and N. fusca) and common marten (Martes foina) Proc R Ir Acad Ser 2. 1875;ii:48–55. –1877. [Google Scholar]
- McClearn D. Myological and Biomechanical Studies of Mammalian Limbs. Cambridge: Harvard University; 1984. Ph.D. Dissertation. [Google Scholar]
- McClearn D. Anatomy of raccoon (Procyon lotor) and coati (Nasua narica and N. nasua) forearm and leg muscles: relations between fiber length, moment-arm length, and joint angle excursion. J Morphol. 1985;183:87–115. doi: 10.1002/jmor.1051830106. [DOI] [PubMed] [Google Scholar]
- Mivart SG. On the anatomy, classification, and distribution of the Arctoidea. Proc Zool Soc Lond. 1885:340–404. [Google Scholar]
- Mivart SG. The Cat: An Introduction to the Study of Backboned Animals, Especially Mammals. New York: Charles Scribner’s Sons; 1900. [Google Scholar]
- Murie J. On the female generative organs, viscera, and fleshy parts of Hyaena brunnea, Thunberg. Trans Zool Soc Lond. 1867;vii:503–512. [Google Scholar]
- Nickel R, Schummer A, Seiferle E, et al. The Locomotor System of the Domestic Animals. I. New York: Springer-Verlag; 1986. The anatomy of domestic animals. [Google Scholar]
- O’Brien SJ, Nash WG, Wildt DE, et al. A molecular solution to the riddle of the giant panda’s phylogeny. Nature. 1985;317:140–144. doi: 10.1038/317140a0. [DOI] [PubMed] [Google Scholar]
- Pecon Slattery J, O’Brien SJ. Molecular phylogeny of the red panda (Ailurus fulgens) J Hered. 1995;86:413–422. doi: 10.1093/oxfordjournals.jhered.a111615. [DOI] [PubMed] [Google Scholar]
- Reid D, Jinchu H, Yan H. Ecology of the red panda Ailurus fulgens in the Wolong Reserve, China. J Zool (Lond) 1991;225:347–364. [Google Scholar]
- Roberts MS, Gittleman JL. Ailurus fulgens. Mammal Species. 1984;222:1–8. [Google Scholar]
- Ross FO. Myology of the cheetah, or hunting leopard of India (Felis jubata) Proc R Ir Acad Ser 2. 1876;3:23–32. [Google Scholar]
- Salesa M, Anton M, Peigne S, et al. Evidence of a false thumb in a fossil carnivore clarifies the evolution of pandas. Proc Natl Acad Sci USA. 2006;103:379–382. doi: 10.1073/pnas.0504899102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salesa MJ, Anton M, Peigne S, et al. Functional anatomy and biomechanics of the postcranial skeleton of Simocyon batalleri (Viret, 1929) (Carnivora, Ailuridae) from the Late Miocene of Spain. Zool J Linn Soc. 2008;152:593–621. [Google Scholar]
- Shepherd FJ. Short notes on the myology of the American black bear (Ursus americanus) J Anat Physiol. 1883;18:103–117. [PMC free article] [PubMed] [Google Scholar]
- Sisson S. Equine myology. In: Getty R, editor. Sisson and Grossman’s The Anatomy of the Domestic Animals. Philadelphia: W.B. Saunders Company; 1975. pp. 376–453. [Google Scholar]
- Smuts MM, Bezuidenhout AJ. Anatomy of the Dromedary. Oxford: Clarendon Press; 1987. [Google Scholar]
- Taylor ME. The functional anatomy of the forelimb of some African Viverridae (Carnivora) J Morphol. 1974;143:307–331. doi: 10.1002/jmor.1051430305. [DOI] [PubMed] [Google Scholar]
- Todd NB, Pressman SR. The karyotype of the lesser panda (Ailurus fulgens) and general remarks on the phylogeny and affinities of the panda. Carnivore Genet Newsl. 1968;5:105–108. [Google Scholar]
- Vrana PB, Milinkovitch MC, Powell JR, et al. Higher level relationships of the arctoid carnivora based on sequence data and “total evidence”. Mol Phylogenet Evol. 1994;3:47–58. doi: 10.1006/mpev.1994.1006. [DOI] [PubMed] [Google Scholar]
- Waddell PJ, Cao Y, Hauf J, et al. Using novel phylogenetic methods to evaluate mammalian mtDNA, including amino acid-invariant sites-LogDet plus site stripping, to detect internal conflicts in the data, with special reference to the positions of hedgehog, armadillo, and elephant. Syst Biol. 1999;48:31–53. doi: 10.1080/106351599260427. [DOI] [PubMed] [Google Scholar]
- Waibl H, Gasse H, Hashimoto Y, et al. Nomina Anatomica Veterinaria. Hannover: International Committee on Veterinary Gross Anatomical Nomenclature, World Association of Veterinary Anatomists; 2005. [Google Scholar]
- Wallace SC, Wang X. Two new carnivores from an unusual late Tertiary forest biota in eastern North America. Nature. 2004;431:556–559. doi: 10.1038/nature02819. [DOI] [PubMed] [Google Scholar]
- Watson M. On the muscular anatomy of Proteles as compared with that of Hyaena and Viverra. Proc Zool Soc Lond. 1882:579–586. [Google Scholar]
- Watson M, Young AH. On the anatomy of Hyaena crocuta (H. maculata) Proc Zool Soc Lond. 1879:79–107. [Google Scholar]
- Wayne RK, Benveniste RE, Janczewski DN, et al. Molecular and biochemical evolution of the carnivora. In: Gittleman JL, editor. Carnivore Behavior, Ecology, and Evolution. Ithaca, NY: Cornell University Press; 1989. pp. 465–494. [Google Scholar]
- Wei F, Feng Z, Wang Z, et al. Use of the nutrients in bamboo by the red panda (Ailurus fulgens) J Zool (Lond) 1999;248:535–541. [Google Scholar]
- Windle BCA. Notes on the limb myology of Procyon cancrivorus and of the Ursidae. J Anat Physiol. 1888;23:81–89. [PMC free article] [PubMed] [Google Scholar]
- Windle BCA, Parsons FG. On the myology of the terrestrial carnivora. Part I: muscles of the head, neck, and fore-limb. Proc Zool Soc Lond. 1897:370–409. [Google Scholar]
- Windle BCA, Parsons FG. On the muscles of the Ungulata. Part I. Muscles of the head, neck, and fore-limb. Proc Zool Soc Lond. 1901;2:656–704. [Google Scholar]
- Wozencraft WC. Classification of the recent carnivora. In: Gittleman JL, editor. Carnivore Behavior, Ecology, and Evolution. Ithaca, NY: Cornell University Press; 1989a. pp. 569–593. [Google Scholar]
- Wozencraft WC. The phylogeny of the recent carnivora. In: Gittleman JL, editor. Carnivore Behavior, Ecology, and Evolution. Ithaca, NY: Cornell University Press; 1989b. pp. 495–535. [Google Scholar]
- Wozencraft WC. Order: Carnivora. In: Wilson DE, Reeder DM, editors. Mammal Species of the World : A Taxonomic and Geographic Reference. Baltimore: The Johns Hopkins University Press; 2005. pp. 532–628. [Google Scholar]
- Wyss AR, Flynn JJ. A phylogenetic analysis and definition of the Carnivora. In: Szalay FS, Novacek MJ, McKenna MC, editors. Mammal Phylogeny: Placentals. New York: Springer-Verlag; 1993. pp. 32–52. [Google Scholar]
- Young AH. Myology of Viverra civetta. J Anat Physiol. 1880;14:166–177. [PMC free article] [PubMed] [Google Scholar]
- Young AH, Robinson A. On the anatomy of Hyaena striata. Part II. J Anat Physiol. 1889;23:187–200. [PMC free article] [PubMed] [Google Scholar]
- Zhang Y-P, Ryder OA. Mitochondrial DNA sequence evolution in the Arctoidea. Proc Natl Acad Sci USA. 1993;90:9557–9561. doi: 10.1073/pnas.90.20.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y-P, Shi L-M. Riddle of the giant panda. Nature. 1991;352:573. doi: 10.1038/352573a0. [DOI] [PubMed] [Google Scholar]