Abstract
The aim of our study was to explore the fibre type composition of the human psoas major muscle at different levels of its origin, from the first lumbar to the fourth lumbar vertebra, and to compare the muscle fibre size and distribution of different fibre types between levels with respect to its complex postural and dynamic function. Muscle samples were collected from 15 young males (younger than 35 years). Serial transverse sections (5 μm) of the samples were cut by cryomicrotome. Type I, IIA and IIX muscle fibres were typed using myosin heavy chain identification. The serial sections were analysed using a light microscope with a magnitude of 100×. The differences between measurements were evaluated using a repeated-measures anova and Scheffé test for post-hoc analysis. Our study showed that the human psoas major muscle was composed of type I, IIA and IIX muscle fibres. It had a predominance of type IIA muscle fibres, whereas type I muscle fibres had the largest cross-sectional area. Type IIX muscle fibres were present as a far smaller percentage and had the smallest cross-sectional area. Moreover, the fibre type composition of the psoas major muscle was different between levels of its origin starting from the first lumbar to the fourth lumbar vertebra. We conclude that the fibre type composition of the psoas major muscle indicated its dynamic and postural functions, which supports the fact that it is the main flexor of the hip joint (dynamic function) and stabilizer of the lumbar spine, sacroiliac and hip joints (postural function). The cranial part of the psoas major muscle has a primarily postural role, whereas the caudal part of the muscle has a dynamic role.
Keywords: fibre types, human, immunohistochemistry, myosin heavy chain, origin, psoas major muscle
Introduction
Skeletal muscle consists of different fibre types characterized by their specific myosin heavy chain (MHC) isoforms. Nine MHC isoforms have been identified in mammalian skeletal muscle: MHC-β/I, MHC-IIa, MHC-IIx, MHC-IIb, MHC-embryonic, MHC-neonatal, MHC-α, MHC-extraocular and MHC-IIm (Sciotte & Morris, 2000). The fibre type composition can be determined by histochemical staining, which is used to identify the mATPase activity (Scott et al. 2001). More recently, through revealing the MHC transcript (mRNA) expression in fast fibres, fibres previously considered as type IIB were correctly identified as type IIX in humans (Smerdu et al. 1994; Pette & Staron, 1997; Hilber et al. 1999; Pette et al. 1999). Immunohistochemical analysis shows that in human limb muscles three MHC isoforms are expressed: MHC-I, MHC-IIa and MHC-IIx, i.e. types I, IIA and IIX (Staron, 1997). Moreover, human muscle fibres can co-express two different MHC isoforms. Such hybrid fibres almost always contain adjoining MHC isoforms (IIC = MHC-I + MHC-IIA and IIAX = MHC-IIA + MHC-IIX) (Scott et al. 2001). Functionally, the MHC isoform determines the mechanical properties of the fibres with MHC type I fibres that are characterized by a slow contractile speed and MHC type II fibres by a fast contractile speed. The metabolic properties of the MHC fibre subpopulations differ in general with regard to oxidative and glycolytic capacity, with MHC type II fibres primarily being glycolytic and easy to fatigue, and type I fibres being more oxidative and fatigue resistant (Andersen & Schiaffino, 1997). There is a relationship between the muscle fibre type proportion and its primary function (Marieb, 1989). Type I fibres are predominant in tonic muscles and tend to assume a postural role (Mannion et al. 1997; Boyd-Clark et al. 2001). In contrast, type II fibres are predominant in those muscles whose primary function is to perform fast movements such as some of the muscles of the extremities (Terzis et al. 2003; Srinivasan et al. 2007). Moreover, due to the differentiated functions and various roles of particular segments of the same muscle, it is possible that each segment has a different immunohistochemical profile (Lindman et al. 1990; Korfage & Van Eijden, 1999). The psoas major muscle has an important dynamic and postural function. It is the only muscle that connects the lumbar spine and lower limbs, and it therefore acts on the lumbar spine and lower limb (Williams & Newell, 2005). The psoas major muscle acts as a hip flexor and may assist in lateral rotation and abduction of the hip joint (Walther, 1981; Skyrme et al. 1999; Williams & Newell, 2005). It originates from the bodies of the lumbar vertebrae and therefore this muscle is generally considered to be an active postural muscle (Walther, 1981; Bogduk et al. 1992; Santaguida & McGill, 1995; Penning, 2000, 2002; Yoshio et al. 2002). The human psoas major muscle has a heterogeneous distribution of fibre types across the muscle, which have been reported in a number of studies (Johnson et al. 1973; Havenith et al. 1990; Zheng et al. 1992; Parkkola et al. 1993; Kimura, 2002). There are disagreements in the literature about whether the psoas major muscle is mostly composed of slow-twitch or fast-twitch fibres. The aim of this study was to determine the distribution and cross-sectional area (CSA) of different fibre types in human psoas major muscle. Furthermore, the purpose of this investigation was to determine the segmental morphology from L1 to L4 lumbar vertebra in order to explore the differences in structure between the upper and lower parts of the muscle. In order to investigate the expression pattern of all MHCs, we used the immunohistochemical method and morphometric analysis.
Materials and methods
Materials
Samples of the psoas major muscles used in this study were obtained from 15 male subjects (aged between 18 and 35 years) (at the Department of Forensic Medicine, University Hospital, Rijeka) who had undergone sudden accidental death. Muscle samples were taken within 24 h of the onset of death. None of them had a history of neuromuscular disease or any other disease that could alter muscle fibre type composition. The local ethics committee of the School of Medicine, University of Rijeka approved this study. Four muscle samples (1 × 1 × 1 cm in size) were taken from the right and left sides of the body. Muscle specimens were taken at 1 cm from the spine in a lateral position at four different levels: the level of the first, second, third and fourth lumbar vertebral bodies. The tissue specimens were frozen in liquid nitrogen and stored at −80 °C until they were processed for immunohistochemistry.
Methods
Immunohistochemistry
Serial muscle cryosections (CM1850, Leica, Wien, Austria) were prepared on 5-μm-thick glass slides. To identify MHC isoforms, three monoclonal antibodies were used: BA-F8 (1 : 200), specific to the slow or MHC-I isoform; SC-71 (1 : 500), specific to the MHC-IIa isoform; and BF-35 (undiluted), specific to all MHC isoforms except MHC-IIx. BA-F8 antibody stains slow, type I muscle fibres. SC-71 antibody stains both fast fibre types, types IIA and IIX, but with a different intensity. We considered the fibres that stained more intensively as type IIA. BF-35 antibody stains all fibre types except IIX. Therefore, we considered unstained fibres as type IIX (Fig. 1). These monoclonal antibodies were kindly provided by Prof. Stefano Schiaffino (University of Padua, Italy). The slides were incubated overnight with primary antibodies at 4 °C in a humidified environment. After washing them in phosphate-buffered saline –Tween 20 for 3 × 5 min, the sections were incubated at room temperature (22 °C) for 15 min with biotinylated anti-mouse IgG and then for 15 min with peroxidase-labelled streptavidin (Dako, Copenhagen, Denmark). After washing the sections with phosphate-buffered saline, the bound antigen–antibody complex was visualized with 3,3′-diaminobenzidine in chromogen solution (Dako).
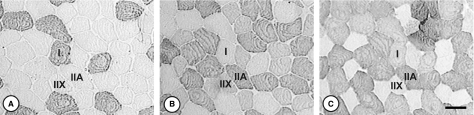
Fig. 1.
Identification of the muscle fibre types with monoclonal antibodies against MHC isoforms: (A) BA-F8 (MHC-I; 1 : 200) stains slow, type I muscle fibres; (B) SC-71 (MHC-IIa, 1 : 500) stains both fast fibre types, type IIA and IIX, but with different intensity; and (C) BF-35 (specific to all MHC isoforms except MHC-IIx; undiluted) stains all fibre types except IIX. Therefore, we considered unstained fibres to be type IIX. Scale bar: 50 μm.
Fibre typing and morphometry
The five fields of view of the muscle samples obtained from the right and left sides of the body were examined under a microscope (BX50; Olympus, Tokyo, Japan) and photographed at a magnification of 100× using a video camera (SSC-DC58AP; Sony, Tokyo, Japan) connected to a personal computer. The photographic images were analysed using an image analysis system that employed issa v.2.5 software (V.A.M.S., Zagreb, Croatia). The percentage of fibre types, fibre type distribution and fibre CSA were calculated.
Statistics
statistica 7.1 computer software (StatSoft Inc., Tulsa, OK, USA) was used for statistical analysis. The results are displayed as the mean and 95% confidence interval. A repeated-measures anova was used to make a comparison between the groups. The Scheffé test was used post-hoc to identify specific differences between groups. Statistical significance was set at P < 0.05 and P < 0.01.
Results
Fibre type composition of the psoas major muscle
In our study the psoas major muscle was mostly composed of type IIA fibres (49.77%), type I fibres were present at 40.15% and type IIX fibres were present at 10.08%. Type I fibres had the largest CSA (2973.28 μm2), type IIA fibres had a CSA of 2644.65 μm2 and type IIX fibres had the smallest CSA (2106.80 μm2). Detailed analysis of the left and right muscles showed a significantly higher percentage of type IIA fibres compared with type I fibres (F = 1.6629; P < 0.01) and type IIX fibres (F = 1.6629; P < 0.01). Moreover, type I fibres were present as a significantly higher percentage than type IIX fibres (F = 1.6629; P < 0.01). Furthermore, a detailed analysis of both muscles showed a significantly larger CSA of type I fibres than type IIA fibres (F = 7.8063; P < 0.01) and type IIX fibres (F = 7.8063; P < 0.01). Type IIA fibres had a significantly larger CSA than type IIX fibres (F = 7.8063; P < 0.01) (Table 1).
Table 1.
Fibre type composition of the left and right human psoas major muscle.
| Percentage |
CSA (μm2) |
|||
|---|---|---|---|---|
| Fibre type | Left muscle | Right muscle | Left muscle | Right muscle |
| Type I | 40.09 ± 3.19† | 40.21 ± 4.42† | 2939.57 ± 798.70* | 3006.99 ± 663.32* |
| Type IIA | 50.58 ± 6.58* | 48.95 ± 6.34* | 2656.80 ± 616.32† | 2632.50 ± 466.89† |
| Type IIX | 9.33 ± 6.25 | 10.84 ± 5.06 | 1980.21 ± 578.97 | 2233.38 ± 643.37 |
Values are mean ± SD.
Significantly different from the rest of the fibres of the same muscle (P < 0.01).
Significantly different from the type IIX fibres of the same muscle (P < 0.01).
Fibre type composition of the psoas major muscle with regard to the level of its origin from the first to the fourth lumbar vertebra
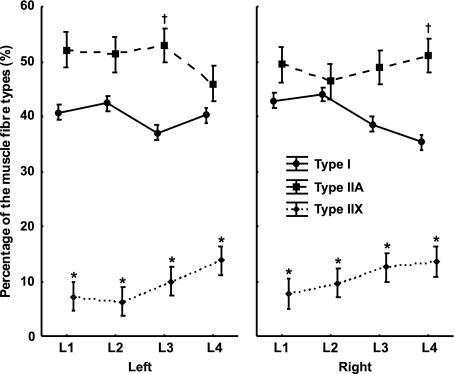
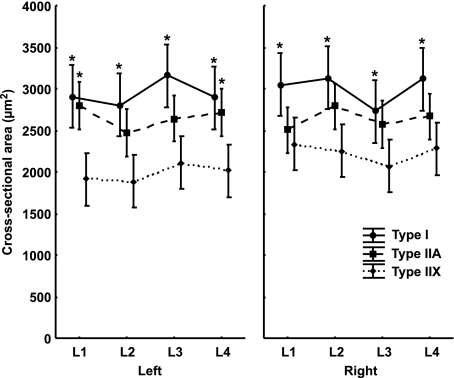
Type IIA fibres were present as the highest percentage, type I fibres were present as a smaller percentage than type IIA fibres and type IIX fibres were present as the smallest percentage at all levels of origin in the left as well as in the right psoas major muscle. The percentage of type IIA fibres in the left muscle remained unchanged between the first and third lumbar vertebra and then decreased at the level of the fourth lumbar vertebra, whereas in the right muscle the percentage increased from the level of the second to the fourth lumbar vertebra. In the left muscle the percentage of type I fibres remained unchanged at all levels of origin. Moreover, their percentage in the right muscle decreased between the level of the second to the fourth lumbar vertebra. The percentage of type IIX fibres in both muscles increased between the first and fourth lumbar vertebra. A detailed analysis showed that, in both muscles at all levels of origin, the percentage of type I and IIA fibres was significantly higher than the percentage of type IIX fibres (F = 3.7148; P < 0.01). In the left muscle at the level of the third lumbar vertebra, the percentage of type IIA fibres was significantly higher than the percentage of type I fibres (F = 3.7148; P < 0.01). In the right muscle at the level of the fourth lumbar vertebra, the percentage of type IIA fibres was significantly higher than the percentage of type I fibres (F = 3.7148; P < 0.01) (Fig. 2). An analysis of the CSA of type I, IIA and IIX fibres showed that, in both muscles, type I fibres had the largest CSA, type IIA fibres had a smaller CSA than type I fibres and type IIX fibres had the smallest CSA at all levels of origin. A detailed analysis of the left muscle showed a significantly larger CSA of type I fibres than type IIX fibres (F = 4.3649; P < 0.01) at all levels of origin. At the level of the first and fourth lumbar vertebra, type IIA fibres had a significantly larger CSA than type IIX fibres (F = 4.3649; P < 0.01). A detailed analysis of the right muscle showed, at all levels of origin, a significantly larger CSA of type I fibres than type IIX fibres (F = 4.3649; P < 0.01) (Fig. 3).
Fig. 2.
The percentage of the muscle fibre types of the human psoas major muscle with regard to body side (left and right) and the levels of origin of the muscle (L1–L4 segment of the lumbar spine). Values are mean ± 0.95 confidence interval. *Significantly different from the rest of the fibres of the same muscle at the same level of origin (P < 0.01). †Significantly different from the type I fibres of the same muscle at the same level of origin (P < 0.01).
Fig. 3.
The CSA of the muscle fibre types of the human psoas major muscle with regard to body side (left and right) and the levels of origin of the muscle (L1–L4 segment of the lumbar spine). Values are mean ± 0.95 confidence interval. *Significantly different from the type IIX fibres of the same muscle at the same level of origin (P < 0.01).
Discussion
Fibre type composition of the psoas major muscle
In this study, we typed immunohistochemically different muscle fibres using three monoclonal antibodies specific for the MHC-I isoform, MHC-II isoform and all MHC isoforms except MHC-IIX as described in Materials and methods. In this way, three types of fibres in human muscle could be determined: type I, type IIA and type IIX (Nikolic et al. 2001). Immunohistochemically, it is almost impossible to determine hybrid fibres clearly as they co-express two MHC isoforms in different proportions. Therefore, hybrid fibres that have a predominance of the MHC-IIa isoform cannot be distinguished from pure IIA fibres, whereas hybrid fibres that have a predominance of the MHC-IIx isoform cannot be distinguished from pure IIX fibres. Consequently, we aligned those fibres to either type IIA or type IIX fibres. Such hybrid fibres could only be identified by analysing single muscle fibres with SDS-PAGE (Trappe et al. 2007), which could not be performed in our study. Our research showed a predominance of type IIA muscle fibres, a smaller percentage of type I fibres and the smallest percentage of type IIX fibres in the psoas major muscle (Table 1). Our results partly differed from current knowledge regarding the percentage of muscle fibre types. Although very little data about the fibre type composition of the psoas major muscle can be found within the literature, previous studies suggested a predominance of type I fibres in the psoas major muscle (Havenith et al. 1990; Zheng et al. 1992; Parkkola et al. 1993) or at least an equal percentage of both type I and II fibres (Johnson et al. 1973). Our research showed a predominance of type IIA muscle fibres and, when added together with the percentage of type IIX muscle fibres, we found a total of 60% of type II muscle fibres compared with 40% of type I muscle fibres. This partly corresponded to the results presented by Kimura (2002). In his study, type 1 fibres were present at 42%, type 2 at 33% and type 3 at 25%. Type 1 fibres are slow-twitch, whereas types 2 and 3 are fast-twitch fibres. When type 2 and 3 fibres were added together, there was a predominance of fast-twitch fibres (58% compared with 42% slow-twitch fibres) (Kimura, 2002). It is not clear if the above-mentioned differences are the product of different research methods or perhaps the different number of subjects involved in studies (Johnson et al. 1973; Havenith et al. 1990; Parkkola et al. 1993; Kimura, 2002). However, the fact that our research showed a predominance of fast-twitch fibres in the psoas major muscle points to its dynamic function. In this study, we also analysed the CSAs of muscle fibres of the psoas major muscle. According to our results, in the psoas major muscle type I muscle fibres had the largest, type IIA fibres had smaller and type IIX fibres had the smallest CSA. Our results did not completely correspond with current knowledge about the fibre type composition of the psoas major muscle but they did partly correspond with the results of Kimura (2002). He also found a larger CSA of slow-twitch fibres in contrast to fast-twitch fibres. However, Parkkola et al. (1993) described type I fibres as slow fibres with a small cross-sectional diameter and type II fibres as fast fibres with a large cross-sectional diameter, which does not conform to the hypothesis that a muscle adapts to its task with a change of diameter or CSA of muscle fibres and not only with the number of certain fibre types. The fact that type I fibres had a significantly larger CSA than type II fibres and that the percentage of type I fibres was considerably high, points to the postural function of the psoas major muscle. Our results therefore indicated the complex dynamic (Walther, 1981; Bogduk et al. 1992; Skyrme et al. 1999; Williams & Newell, 2005) and postural (Walther, 1981; Bogduk et al. 1992; Santaguida & McGill, 1995; Penning, 2000, 2002; Yoshio et al. 2002) functions of the psoas major muscle.
Differences in the fibre type composition of the psoas major muscle with regard to the level of its origin from the first to fourth lumbar vertebra
The psoas major is a long muscle that lies on either side of the lumbar vertebral column. It has a complex origin. The muscle inserts on the anterior surfaces of the transverse processes of all of the lumbar vertebrae and on the side of the bodies of two adjoining vertebrae and their intervertebral disc starting from the twelfth thoracic towards the fifth lumbar vertebra. The muscle converges at a tendon that, after joining the iliacus muscle, becomes attached to the lesser trochanter of the femur (Williams & Newell, 2005). Studies on cadavers that enable an analysis of whole human muscle have shown that the size of the muscle fibres and the distribution of the muscle fibre types vary (Lexell et al. 1983; Lexell & Taylor, 1989). Differences can be found with regard to the superficial or deeper parts as well as the proximal or distal parts of the muscle (Nygaard & Sanchez, 1982; Lexell et al. 1983; Mahon et al. 1984). Moreover, differences can be found with regard to the cranial or caudal parts or even the ventral or dorsal parts of the muscle (Korfage & Van Eijden, 1999). In our study we wanted to explore the differences in the fibre type composition of the psoas major muscle between its cranial and caudal portion. Lindman et al. (1990) discovered marked differences in the distribution as well as in the cross-sectional fibre area with regard to the superior, middle and inferior part of the trapezius muscle. Korfage & Van Eijden (1999), however, studied the fibre type composition of the temporalis muscle and described differences in the distribution and CSAs of muscle fibres in the anterior, middle and posterior portions of the muscle. In summary, different muscles with different functional demands and engagements of a particular part of the muscle showed different immunohistochemical fibre type compositions (Lindman et al. 1990; Korfage & Van Eijden, 1999). In our study, we demonstrated that the fibre type composition of the left and right psoas major muscle changed from the cranial towards the caudal part of its origin. The percentage of type I fibres in both muscles had a decreasing trend, whereas the percentage of type II fibres had an increasing trend. In the left psoas major muscle at the level of the third lumbar vertebra and in the right psoas major muscle at the level of the fourth lumbar vertebra, the percentage of type IIA muscle fibres was significantly higher than the percentage of type I fibres (Fig. 2). According to its function, the conclusion is that the caudal part of the psoas major muscle could have a dynamic role. Moreover, the percentage of type IIX muscle fibres increased from the cranial towards the caudal part of the psoas major muscle origin, which also supports the previous conclusion. However, at the level of the first and second lumbar vertebra we found no significant difference between the percentage of type I and IIA muscle fibres, which refers to the static role of the cranial part of the psoas major muscle (Fig. 2).
Conclusions
The psoas major muscle is composed of type I, IIA and IIX muscle fibres. It is mainly composed of type IIA fibres (59.28%) compared with type I fibres (40.72%), which indicates its important dynamic function as the main flexor of the hip joint and, in addition, the abductor and supinator of the leg in the hip joint. Type I fibres of the psoas major muscle have the largest CSA (2571.12 μm2) and the percentage of type I fibres is high, which indicates its postural function as the stabilizer of the lumbar spine, sacroiliac and hip joints as well as the stabilizer of lumbar lordosis. The fibre type composition of the psoas major muscle changes at the levels of its origin from the first to the fourth lumbar vertebra, which suggests a primarily postural role of the cranial part, whereas the caudal part of the muscle has a dynamic role.
Author contributions
All authors contributed equally to the design of the article, analysis and interpretation of data as well as critical revision and final approval of the article.
References
- Andersen JL, Schiaffino S. Mismatch between myosin heavy chain mRNA and protein distribution in human skeletal muscle fibers. Am J Physiol. 1997;272:1881–1889. doi: 10.1152/ajpcell.1997.272.6.C1881. [DOI] [PubMed] [Google Scholar]
- Bogduk N, Pearcy M, Hadfield G. Anatomy and biomechanics of psoas major. Clin Biomech. 1992;7:109–119. doi: 10.1016/0268-0033(92)90024-X. [DOI] [PubMed] [Google Scholar]
- Boyd-Clark LC, Briggs CA, Galea MP. Comparative histochemical composition of muscle fibres in a pre- and a postvertebral muscle of the cervical spine. J Anat. 2001;199:709–716. doi: 10.1046/j.1469-7580.2001.19960709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havenith MG, Visser R, Schrijvers-van Schendel JM, et al. Muscle fiber typing in routinely processed skeletal muscle with monoclonal antibodies. Histochemistry. 1990;93:497–499. doi: 10.1007/BF00266407. [DOI] [PubMed] [Google Scholar]
- Hilber K, Galler S, Gohlsch B, et al. Kinetic properties of myosin heavy chain isoforms in single fibers from human skeletal muscle. FEBS Lett. 1999;455:267–270. doi: 10.1016/s0014-5793(99)00903-5. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Polgar J, Weightman D, et al. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci. 1973;18:111–129. doi: 10.1016/0022-510x(73)90023-3. [DOI] [PubMed] [Google Scholar]
- Kimura T. Composition of psoas major muscle fibers compared among humans, orangutans and monkeys. Z Morphol Anthropol. 2002;83:305–314. [PubMed] [Google Scholar]
- Korfage JAM, Van Eijden TMGJ. Regional differences in fibre type composition in human temporalis muscle. J Anat. 1999;194:355–362. doi: 10.1046/j.1469-7580.1999.19430355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexell J, Taylor CC. Variability in muscle fibre areas in whole human quadriceps muscle: how to reduce sampling errors in biopsy techniques. Clin Physiol. 1989;9:333–343. doi: 10.1111/j.1475-097x.1989.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Lexell J, Henriksson-Larsén K, Sjöström M. Distribution of different fibre types in human skeletal muscles. 2. A study of cross-sections of whole m. vastus lateralis. Acta Physiol Scand. 1983;117:115–122. doi: 10.1111/j.1748-1716.1983.tb07185.x. [DOI] [PubMed] [Google Scholar]
- Lindman R, Eriksson A, Thornell LE. Fiber type composition of the human male trapezius muscle: enzyme-histochemical characteristics. Am J Anat. 1990;189:236–244. doi: 10.1002/aja.1001890306. [DOI] [PubMed] [Google Scholar]
- Mahon M, Toman A, Willan PL, et al. Variability of histochemical and morphometric data from needle biopsy specimens of human quadriceps femoris muscle. J Neurol Sci. 1984;63:85–100. doi: 10.1016/0022-510x(84)90111-4. [DOI] [PubMed] [Google Scholar]
- Mannion AF, Dumas GA, Cooper RG, et al. Muscle fibre size and type distribution in thoracic and lumbar regions of erector spinae in healthy subjects without low back pain: normal values and sex differences. J Anat. 1997;190:505–513. doi: 10.1046/j.1469-7580.1997.19040505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marieb EN. Human Anatomy and Physiology. Redwood City, CA: The Benjamin/Cummings Publishing Company, Inc; 1989. Muscles and muscle tissue; pp. 240–265. [Google Scholar]
- Nikolic M, Malnar-Dragojevic D, Bobinac D, et al. Age-related skeletal muscle atrophy in humans: an immunohistochemical and morphometric study. Coll Antropol. 2001;25:545–553. [PubMed] [Google Scholar]
- Nygaard E, Sanchez J. Intramuscular variation of fiber types in the brachial biceps and the lateral vastus muscles of elderly men: how representative is a small biopsy sample? Anat Rec. 1982;203:451–459. doi: 10.1002/ar.1092030404. [DOI] [PubMed] [Google Scholar]
- Parkkola R, Alanen A, Kalimo H, et al. MR relaxation times and fiber type predominance of the psoas and multifidus muscle. An autopsy study. Acta Radiol. 1993;24:16–19. [PubMed] [Google Scholar]
- Penning L. Psoas muscle and lumbar spine stability: a concept uniting existing controversies. Eur Spine J. 2000;9:577–585. doi: 10.1007/s005860000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning L. Spine stabilization by psoas muscle during walking and running. Eur Spine J. 2002;11:89–90. doi: 10.1007/s005860100336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pette D, Staron RS. Mammalian skeletal muscle fiber type transitions. Int Rev Cytol. 1997;170:143–223. doi: 10.1016/s0074-7696(08)61622-8. [DOI] [PubMed] [Google Scholar]
- Pette D, Peuker H, Staron RS. The impact of biochemical methods for single muscle fibre analysis. Acta Physiol Scand. 1999;166:261–277. doi: 10.1046/j.1365-201x.1999.00568.x. [DOI] [PubMed] [Google Scholar]
- Santaguida PL, McGill MS. The psoas major muscle: a three-dimensional geometric study. J Biomech. 1995;28:339–345. doi: 10.1016/0021-9290(94)00064-b. [DOI] [PubMed] [Google Scholar]
- Sciotte JJ, Morris TJ. Skeletal muscle function and fibre types: the relationship between occlusal function and the phenotype of jaw-closing muscles in human. J Orthod. 2000;27:15–30. doi: 10.1093/ortho/27.1.15. [DOI] [PubMed] [Google Scholar]
- Scott W, Stevens J, Binder-Macleod SA. Human skeletal muscle fiber type classifications. Phys Ther. 2001;81:1810–1816. [PubMed] [Google Scholar]
- Skyrme AD, Cahill DJ, Marsh HP, et al. Psoas major and its controversial rotational action. Clin Anat. 1999;12:264–265. doi: 10.1002/(SICI)1098-2353(1999)12:4<264::AID-CA4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Smerdu V, Karsch-Mizrachi I, Campione M, et al. Type IIx myosin heavy chain transcripts are expressed in type IIb fibers of human skeletal muscle. Am J Physiol. 1994;267:1723–1728. doi: 10.1152/ajpcell.1994.267.6.C1723. [DOI] [PubMed] [Google Scholar]
- Srinivasan RC, Lungren MP, Langenderfer JE, et al. Fiber type composition and maximum shortening velocity of muscles crossing the human shoulder. Clin Anat. 2007;20:144–149. doi: 10.1002/ca.20349. [DOI] [PubMed] [Google Scholar]
- Staron RS. Human skeletal muscle fiber types: delineation, development, and distribution. Can J Appl Physiol. 1997;22:307–327. doi: 10.1139/h97-020. [DOI] [PubMed] [Google Scholar]
- Terzis G, Georgiadis G, Vassiliadou E, et al. Relationship between shot put performance and triceps brachii fiber type composition and power production. Eur J Appl Physiol. 2003;90:10–15. doi: 10.1007/s00421-003-0847-x. [DOI] [PubMed] [Google Scholar]
- Trappe S, Creer A, Slivka D, et al. Single muscle fiber function with concurrent exercise or nutrition countermeasures during 60 days of bed rest in women. J Appl Physiol. 2007;103:1242–1250. doi: 10.1152/japplphysiol.00560.2007. [DOI] [PubMed] [Google Scholar]
- Walther DS. Applied Kinesiology. I. Pueblo: Systems DC; 1981. Pelvis and thigh muscles; pp. 302–305. [Google Scholar]
- Williams A, Newell RLM. Pelvic girdle, gluteal region and hip joint. In: Standring S, editor. Gray’s Anatomy. 39th edn. Churchill Livingstone, Edinburgh: Elsevier; 2005. pp. 1444–1446. [Google Scholar]
- Yoshio M, Murakami G, Sato T, et al. The function of the psoas major muscle: passive kinetics and morphological studies using donated cadavers. J Orthop Sci. 2002;7:199–207. doi: 10.1007/s007760200034. [DOI] [PubMed] [Google Scholar]
- Zheng A, Rahkila P, Vuori J, et al. Quantification of carbonic anhydrase III and myoglobin in different fiber types of human psoas muscle. Histochemistry. 1992;97:77–81. doi: 10.1007/BF00271284. [DOI] [PubMed] [Google Scholar]