Abstract
Skeletal muscles have a heterogeneous fiber type composition, which reflects their functional demand. The daily muscle use and the percentage of slow-type fibers have been shown to be positively correlated in skeletal muscles of larger animals but for smaller animals there is no information. The examination of this relationship in adult rats was the purpose of this study. We hypothesized a positive relationship between the percentage of fatigue-resistant fibers in each muscle and its total duration of use per day. Fourteen Wistar strain male rats (410–450 g) were used. A radio-telemetric device was implanted to record muscle activity continuously from the superficial masseter, deep masseter, anterior belly of digastric and anterior temporalis muscles. The degree of daily muscle use was quantified by the total duration of muscle activity per day (duty time) exceeding specified levels of the peak activity (2, 5, 20 and 50%). The fiber type composition of the muscles was examined by the myosin heavy chain content of the fibers by means of immunohistochemical staining. At lower activity levels (exceeding 2 and 5% of the peak activity), the duty time of the anterior belly of digastric muscle was significantly (P < 0.01) longer than those of the other muscles. The anterior belly of digastric muscle also contained the highest percentage of slow-type fibers (type I fiber and hybrid fiber co-expressing myosin heavy chain I + IIA) (ca. 11%; P < 0.05). By regression analysis for all four muscles, an inter-muscular comparison showed a positive relationship between the duty time (exceeding 50% of the peak activity) and the percentage of type IIX fibers (P < 0.05), which demonstrate intermediate physiological properties relative to type IIA and IIB fibers. For the jaw muscles of adult male rats, the variations of fiber type composition and muscle use suggest that the muscle containing the largest amounts of slow-type fibers (the anterior belly of digastric muscle) is mainly involved in low-amplitude activities and that the amount of type IIX fibers is positively related to the generation of large muscle forces, validating our hypothesis.
Keywords: electromyogram, jaw muscle, myosin heavy chain, telemetry
Introduction
Skeletal muscles have a heterogeneous fiber type composition consisting of fibers with different physiological properties, such as contraction velocity and fatigability (Schiaffino & Reggiani, 1996). Slow-contracting fibers are fatigue-resistant and are identified as type I fibers, whereas the fast-contracting, fatigable fibers are categorized as type II fibers. These characteristics are mainly related to the expression of various myosin heavy chain (MyHC) isoforms (Pette & Staron, 2000; Cobos et al. 2001). MyHC-I is found in the slow-type fibers. MyHC-IIA, MyHC-IIX and MyHC-IIB can be found in the fast-type fibers, in which IIB fibers show the fastest contraction velocities and extreme fatigability, whereas the IIA fibers show some fatigue-resistance but not to the same level as observed in slow-type fibers.
Skeletal muscle fibers adjust their phenotypic properties in response to altered functional demands (Pette & Staron, 2000). Long-term electrical stimulation of skeletal muscle has been demonstrated to cause an increase in the percentage of slow-type fatigue-resistant fibers (Kernell et al. 1987). Inactivity of the muscle is followed by an increase in fast-type fibers (Templeton et al. 1988). The percentage of slow-type fibers has been shown to be positively correlated to the daily muscle use in leg muscles of human (Monster et al. 1978) and cat (Kernell & Hensbergen, 1998), and in rabbit jaw muscles (van Wessel et al. 2005). Their results suggest the existence of such a relationship in skeletal muscles of larger animals but for smaller animals there is no such information. This relationship between daily muscle use and fiber type composition could be different in small animals as their muscles have to meet different requirements than in larger animals in both activity duration and intensity. It is known, for instance, that skeletal muscles in smaller animals in general contain less slow-type fibers compared with larger animals (Tuxen & Kirkeby, 1990). It also remains undecided to what extent the intensity of daily muscle use affects fiber type composition.
Therefore, the aim of this study was to examine the relationship between the fiber type composition of the rat jaw muscles and their daily muscle activity at specific levels of intensity. A radio-telemetric device was used to record biopotentials determined as an electromyogram (EMG) from which the total duration of muscle activity per day was computed. Muscle fibers were characterized by their content of MyHC isoforms, as identified with monoclonal antibodies (Bredman et al. 1990). We hypothesized a positive relationship between the percentage of fatigue-resistant fibers in each muscle and its total duration of use per day.
Materials and methods
Experimental animals
Fourteen-week-old Wistar strain male rats, weighing from 410 to 450 g, were randomly divided into two groups. In one group (n = 8), a telemetric device was implanted in the shoulder area for the recording of jaw muscle activities. Each animal was housed individually in a cage (45 × 22 × 18 cm) and fed with pellets and water ad libitum. Day–night rhythm was ensured by automatic dimmed lighting (08:00–20:00 hours). Except for the daily care and regular physical examination, they were left undisturbed to minimize any external influence. After the recording period, the animals were killed with an overdose of sodium pentobarbital (300 mg/kg Nembutal; Dinabott, Osaka, Japan) for the verification of the electrode locations. In a separate second group (n = 6), the jaw muscles were dissected for the immunohistochemical determination of their fiber type composition. Damaging the muscle tissue after removal of the electrodes was therefore avoided. The protocol of the experiment was approved by the Animal Care and Use Committee at the Hiroshima University.
Telemetric system
The current system for recording EMGs has been described in previous studies (Langenbach et al. 2004; van Wessel et al. 2005; Kawai et al. 2007). Briefly, implantable three-channel transmitters for biopotential recording (F50-EEE, 45 × 17 × 10 mm, 14 g; Data Sciences International, St Paul, MN, USA) were used to record muscle activity. For the implantation of this device, the animal was anesthetized with intra-abdominal injections of sodium pentobarbital at a dose of 50 mg/kg body weight. The transmitter was placed under the skin of the shoulder area and the three pairs of bipolar electrodes were subcutaneously led to an incision in the right submandibular region. From here, the bipolar electrodes were inserted into the center of the right superficial masseter, deep masseter, anterior belly of digastric or anterior temporalis muscle (n = 6 for each muscle; three muscles in each of the eight animals) and sutured at the muscle surface to prevent them from dislodging. The distance between the two tips of the bipolar electrodes (each consisting of a double helix, diameter 0.45 mm) was 1–2 mm and the effective electrode tip length was 7 mm. These procedures were completed under sterile conditions. An antibiotic (100 mg/kg phosphomycin disodium salt; Sigma-Aldrich Co., St Louis, MO, USA) was administered 3 days preceding and 2 days following surgery. An analgesic (5 μg/kg buprenorphine, Lepetan; Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) was provided immediately after surgery. Muscle activities were continuously recorded for 1 week, starting at 1 week after surgery.
In the device, the biopotentials were filtered (first-order low-pass filter, 158 Hz) and sampled (250 Hz) on the input of each channel. The transmitted data were then collected by a receiver (RPC-1, Data Sciences International) placed under the cage. The signals were stored on a PC hard disk, using the Dataquest A.R.T. data-acquisition system (Data Sciences International).
Muscle activity analysis
The method of analysis was similar to that performed previously (van Wessel et al. 2005; Kawai et al. 2007). Briefly, the muscle activities of a 24-h period (day 3) were analyzed using Spike2 software (Cambridge Electronic Design, Cambridge, UK). After motion artifacts had been removed (5-Hz high-pass filter), the signal was rectified, averaged and downsampled (20-ms window, i.e. five samples). To eliminate possible artifacts, 0.001% of the samples (i.e. 43 samples) with the largest amplitudes were excluded. The peak EMG was defined as the largest of the remaining samples. Daily muscle use was characterized by means of the total duration of muscle activity (duty time), determined for muscle activities exceeding 2, 5, 20 and 50% of the day’s peak activity. A burst was defined as a series of consecutive samples exceeding the aforementioned activity levels (van Wessel et al. 2005; Kawai et al. 2007). Note that the duty time for activations exceeding a certain level included the duty times for activations exceeding all higher levels. Duty time exceeding the 2% level was assumed to represent the overall muscle use including all levels and types of muscle activities. Muscle activity exceeding 50% of the peak EMG was considered representative of the most forceful muscle use.
Immunohistochemical analysis
Right jaw muscles were isolated, rapidly frozen in liquid nitrogen-cooled isopentane and stored at −80 °C until further processing. For each of the four muscle portions (superficial and deep masseter, anterior belly of digastric, and anterior temporalis muscles), the muscles were brought to −20 °C and serial transverse sections (10 μm) were made with a cryomicrotome. They were obtained from the belly of the muscles perpendicular to the main direction of the muscle fibers.
After overnight fixation at −20 °C in a mixture of methanol:acetone:acetic acid:water (35:35:5:25) (Wessels et al. 1991), five consecutive sections were incubated with monoclonal antibodies raised against purified myosin (antibody 219-1D1 recognized MyHC-I, antibody 333-7H1 recognized MyHC-IIA, antibody 332-3D4 recognized MyHC-IIA and MyHC-IIX, antibody 340-3B5 recognized all fast MyHC isoforms, and antibody 249-5A4 recognized MyHC-cardiac α myosin) (Sant’Ana Pereira et al. 1995; Korfage et al. 2001). The indirect unconjugated immunoperoxidase technique (peroxidase antiperoxidase complex technique) was applied to detect the specific binding of the different antibodies and nickel-diaminobenzidine was used to visualize the staining (Hancock, 1986).
Evaluation of the fiber type distribution was performed at similar locations of the electrode insertions in the muscle activity analysis group. This selection was carefully executed because a heterogeneity in the fiber type distribution exists in the jaw muscles (Cobos et al. 2001; Sano et al. 2007). At each sample area, pictures were taken using a digital camera attached to a microscope. On average, each sample area consisted of about 200 fibers. Fibers were classified by means of the five consecutive incubated sections. All fibers expressing MyHC-I and/or MyHC-cardiac α (pure or hybrid) were considered as slow-type fibers. Fibers expressing only pure myosin heavy chain MyHC-IIA, MyHC-IIB, MyHC-IIX or a combination of these fast MyHCs were considered as fast-type fibers. In hybrid fibers, fibers expressing MyHC-I and/or MyHC-cardiac α in combination with MyHC-IIA were considered as slow-type fibers.
Statistics
anova was used to detect significant differences in the daily duty time and fiber type composition. Where the anova was significant, a Bonferroni/Dunn procedure was used as a post-hoc test. Regression analysis was performed to investigate the relationship between the muscle activity and fiber type composition. In all tests, a P-value < 0.05 was considered statistically significant.
Results
The duty times were longest for activities exceeding 2% of the peak EMG (i.e. overall daily muscle use) and declined rapidly in all muscles for higher threshold activity levels (Table 1). The duty time of the anterior temporalis muscle was the shortest except for activities exceeding 2% of the peak EMG, for which the superficial masseter muscle showed the shortest duty time. The duty time of the anterior belly of digastric muscle was, at lower activity levels (exceeding 2 and 5% of the peak EMG, respectively, 32.8 and 15.8%), significantly longer (P < 0.01) than those of the other three muscles. At the highest activity levels (exceeding 50% of the peak EMG) no significant differences in duty time were detected.
Table 1.
Means and SD of the duty time of rat jaw muscles at various activity levels.
| Duty time (%) |
||
|---|---|---|
| Mean | SD | |
| 2% peak activity | ||
| Superficial masseter | 13.64 | 5.23 |
| Deep masseter | 19.98 | 8.20 |
| Digastric | 32.78** | 7.83 |
| Temporalis | 15.17 | 4.73 |
| 5% peak activity | ||
| Superficial masseter | 5.59 | 1.99 |
| Deep masseter | 6.63 | 2.29 |
| Digastric | 15.83** | 4.67 |
| Temporalis | 4.46 | 1.06 |
| 20% peak activity | ||
| Superficial masseter | 0.85 | 0.45 |
| Deep masseter | 0.78 | 0.29 |
| Digastric | 1.44* | 0.53 |
| Temporalis | 0.50 | 0.11 |
| 50% peak activity | ||
| Superficial masseter | 0.06 | 0.04 |
| Deep masseter | 0.06 | 0.04 |
| Digastric | 0.05 | 0.02 |
| Temporalis | 0.04 | 0.02 |
Significant differences (*P < 0.05; **P < 0.01) with all other muscles (n = 6).
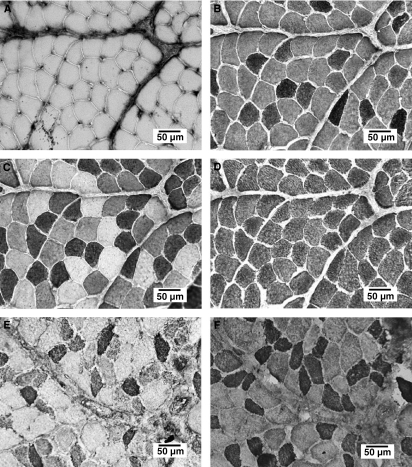
The rat jaw muscles all contained large numbers of fast-type fibers (Table 2) ranging from 88.6% (anterior belly of digastric muscle) to 99.9% (superficial masseter muscle). Representative examples of the fiber type composition in the superficial masseter and anterior belly of digastric muscles are shown in Fig. 1, portraying four consecutive sections incubated with different antibodies against MyHC isoform. There were no type I fibers present in the superficial masseter muscle (Fig. 1A), whereas all fibers contained fast MyHC isoforms (Fig. 1D). In contrast, the anterior belly of digastric muscle contained relatively many type I (Fig. 1E) and type IIA (Fig. 1F) fibers and the percentage of slow-type fibers was the highest (11.3%; P < 0.05). MyHC-cardiac α was not detected in any of the examined jaw muscles.
Table 2.
The fiber type distribution in jaw muscles.
| Slow-type fibers |
Fast-type fibers |
|||
|---|---|---|---|---|
| Mean (%) | SD | Mean (%) | SD | |
| Superficial masseter | 0 | 0 | 99.9 | 0.2 |
| Deep masseter | 2.6 | 1.41 | 97.4 | 1.51 |
| Digastric | 11.3* | 4.78 | 88.6** | 4.78 |
| Temporalis | 5.8 | 3.38 | 93.9 | 3.08 |
Significant differences (*P < 0.05; **P < 0.01) between all muscles (n = 6).
Fig. 1.
Immunohistochemical sections showing the fiber type composition in the superficial masseter (A–D) and anterior belly of digastric (E,F) muscles. The consecutive sections were incubated with different antibodies against MyHC-I (A,E), MyHC-IIA (B,F), MyHC-IIA and MyHC-IIX (C), and all fast MyHC (MyHC-IIA, MyHC-IIX and MyHC-IIB) (D). Note that there were no type I fibers in the superficial masseter muscle.
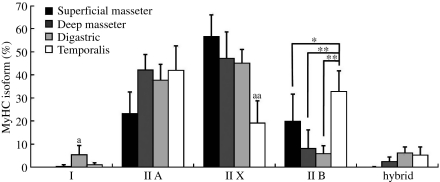
In all rat jaw muscles except for the anterior temporalis muscle, the majority of fibers were of type IIX (Fig. 2) and their fiber type compositions did not significantly differ from each other although some inter-muscular differences were visible. Compared with the other muscles the anterior temporalis muscle contained a relatively high percentage of type IIA fibers (42.1%), the lowest percentage of type IIX fibers (19.1%; P < 0.01) and the highest percentage of type IIB fibers (32.8%; P < 0.05 to the superficial masseter muscle, P < 0.01 to the deep masseter and anterior belly of digastric muscles).
Fig. 2.
Total proportion of fibers (mean + SD) expressing a given MyHC isoform in jaw muscles. Significant differences between muscles: *P < 0.05 and **P < 0.01. The percentage of the MyHC isoform was significantly the highest (a: P < 0.05) and lowest (aa: P < 0.01) in four muscles.
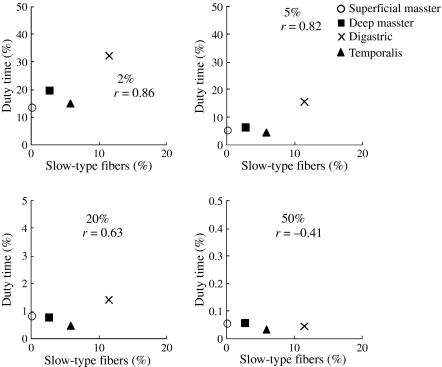
Figure 3 shows for each of the muscles the relationship between their percentage of slow-type fibers and duty time for activities exceeding 2, 5, 20 and 50% of the peak EMG. An inter-muscular comparison (Table 3) showed a positive, but not significant, relationship between the duty time at the lower activity levels (exceeding 2 and 5% of the peak EMG) and the percentage of slow-type fibers (r < 0.86). The correlation of inter-muscular differences in the percentage of type IIA fibers with the accompanying duty times showed similar (not significant) tendencies as seen for the slow-type fibers. For the type IIX fibers, a significant positive correlation was found only for activities exceeding the 50% level (P < 0.05). For the type IIB fibers this correlation showed a consistent negative (not significant) tendency.
Fig. 3.
Relationship between the mean percentage of slow-type fibers and mean duty time for activations exceeding 2, 5, 20 and 50% of the peak EMG.
Table 3.
Correlation coefficients between the percentage of slow and each fast type (typeIIA, typeIIX, and typeIIB) fibers and muscle activity at various activity levels. Significant differences (*p < 0.05) between all muscles (n = 6).
| Duty time |
||||
|---|---|---|---|---|
| 2% | 5% | 20% | 50% | |
| Slow-type fibers | 0.86 | 0.82 | 0.63 | −0.41 |
| Fast-type fibers | ||||
| typeIIA | 0.32 | 0.10 | −0.15 | −0.50 |
| typeIIX | 0.10 | 0.25 | 0.50 | 0.93* |
| typeIIB | −0.73 | −0.72 | −0.78 | −0.60 |
Discussion
In this study, the daily muscle activity and muscle fiber type compositions of four different rat jaw muscles were investigated by radio-telemetry and immunohistochemistry. The variations in fiber type composition and muscle use suggest that the muscle showing the longest duty time also contains the largest amount of slow-type fibers, and that the amount of type IIX fibers is positively related to the generation of large muscle forces.
Rats were divided into two different groups, one for muscle activity and one for immunohistochemical analysis, in order to minimize the influence of the device for muscle activity recording on the jaw muscle tissue for the histological analysis. The analysis of the fiber type distribution just around the insertion location of the electrodes can be difficult because of the tousled character of the fiber alignment as a result of the removal of the electrodes. Moreover, previous studies in the rabbit have shown that the fiber type distribution around the implanted electrodes is similar to that in untreated muscles and that, within muscles, individual variations in fiber type compositions and duty time are not correlated (van Wessel et al. 2005). Lastly, previous studies in the jaw muscles demonstrated that the variation in fiber type distribution between muscle regions can be very significant (Sano et al. 2007). Therefore, care was taken to perform the evaluation of the fiber type distribution of each muscle at similar locations of electrode insertion in muscles. The decision on the electrode location was made with particular care in the anterior belly of digastric muscle because a clear heterogeneity of the fiber distribution exists in this muscle. In the anterior belly of digastric muscle, type I fibers can be detected only at the central area, whereas the peripheral area contains a larger proportion of type IIB fibers (Cobos et al. 2001; Sano et al. 2007). The electrode location was in the central area of the muscle that contained type I fibers.
It was clear that the muscle containing the largest amount (P < 0.05) of slow-type fibers (anterior belly of digastric muscle) also showed the longest duty time (P < 0.01) for low-amplitude activities. The percentage of type IIX fibers, the most abundant fiber type in the rat jaw muscles, was significantly correlated (P < 0.05) with the daily muscle activity at the high activity level. Because type IIX fibers are fatigable, this fiber type would predominantly be used in infrequent powerful contractions of the muscles. These results confirmed previous studies in leg and trunk muscles, describing that muscles containing a large amount of slow-type fibers are more active than those composed largely of fast-type fibers (Monster et al. 1978; Kernell & Hensbergen, 1998). In these studies muscle activity was evaluated as the total amount of muscle activity per day, regardless of a threshold. This probably corresponds to the muscle activity exceeding 2% level that represents overall muscle use in this study.
According to the size principle (Henneman et al. 1965), the smallest motor units, typically consisting of slow-type and fatigue-resistant fibers, are the first to be recruited. With more powerful contractions the larger motor units, consisting of fast-type and fatigable fibers, will also be activated. Hence, the slow-type motor units are active during longer periods of time than the fast-type motor units (Hennig & Lømo, 1985). On this basis we would expect muscles with a higher percentage of slow-type fibers to have a dominant role in long-lasting but low-intensity posture contractions. The results in this study can largely be accounted for by this size principle. Similar results were shown for ankle muscles in the cat and rhesus monkey, respectively (Hensbergen & Kernell, 1997; Hodgson et al. 2001). A recent study in the rabbit (van Wessel et al. 2005) showed that the amount of slow-type fibers in the jaw muscles correlated only to their duty time exceeding high activity levels (exceeding 20% of the peak EMG). However, the developmental changes in the fiber type composition were not associated with accompanying variations in their duty times at any level (Langenbach et al. 2008).
Summed over the entire day, the jaw muscles are involved in low-amplitude activities during significant amounts of time (superficial masseter muscle, over 3 h; anterior belly of digastric muscle, ca. 8 h), whereas activities exceeding 50% of the peak EMG have a total duration of only 30–60 s. On the basis of that, muscle activities exceeding the 50% level reflect muscle use during specific functions of the masticatory system, whereas muscle activities exceeding the 2% level were assumed to represent the overall muscle activity including low-intensity activities during rest periods and posture.
Muscles adapt to functional demands by changing their phenotype (Pette & Staron, 2000). The muscle subjected to electrical activation of the motor nerve (chronic stimulation) became slower, more fatigue-resistant and weaker (Kernell et al. 1987). Therefore, although the fiber type composition of skeletal muscles may be largely decided by genetic factors in each animal, the fiber type composition will adapt to the existing functional demands. In our study, duty time of the anterior belly of digastric muscle was longest at a low activity level and this muscle contained the largest amount of slow-type fibers. The anterior belly of digastric muscle is a jaw-opening muscle that does not need a powerful contractile force (Kawai et al. 2007). This muscle may be important to stabilize both the jaw and hyoid, and the muscle is involved in swallowing and other behaviors in which the hyoid or tongue is required (Cobos et al. 2001).
The anterior temporalis muscle showed a relatively short duty time, inconsistent with its percentage of slow-type fibers. This implies that the amount of muscle use is not the only determinant of the percentage of the slow-type fibers. Kernell & Hensbergen (1998) reported that, in comparisons between cat and human with regard to the mean duty time and percentage of type I fibers of leg muscles, duty times of human were very similar to those of cats but muscles contained a much larger percentage of type I fibers in human than in cat. This discrepancy was explained by the animal size. Smaller animals have, in general, fewer slow-type fibers than larger animals (Bredman et al. 1990; Cobos et al. 2001), corresponding with the function of muscles requiring faster contraction velocities in small animals compared with larger animals.
Acknowledgments
The authors gratefully thank Prof. Theo M.G.J. van Eijden, who unfortunately died on February 28, 2007, for his constructive comments on this study. We are also grateful to Jan Harm Koolstra for his constructive criticism on the manuscript and Leo van Ruijven for technical advice. This research was supported by a grant (no. 19890138) for Science Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- Bredman JJ, Weijs WA, Moorman AFM, et al. Histochemical and functional fibre typing of the rabbit masseter muscle. J Anat. 1990;168:31–47. [PMC free article] [PubMed] [Google Scholar]
- Cobos AR, Segade LAG, Fuentes I. Muscle fibre types in the suprahyoid muscles of the rat. J Anat. 2001;198:283–294. doi: 10.1046/j.1469-7580.2001.19830283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock MB. Two-color immunoperoxidase staining: visualization of anatomic relationships between immunoreactive neural elements. Am J Anat. 1986;175:343–352. doi: 10.1002/aja.1001750216. [DOI] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Hennig R, Lømo T. Firing patterns of motor units in normal rats. Nature. 1985;314:164–166. doi: 10.1038/314164a0. [DOI] [PubMed] [Google Scholar]
- Hensbergen E, Kernell D. Daily durations of spontaneous activity in cat’s ankle muscles. Exp Brain Res. 1997;115:325–332. doi: 10.1007/pl00005701. [DOI] [PubMed] [Google Scholar]
- Hodgson JA, Wichayanuparp S, Recktenwald MR, et al. Circadian force and EMG activity in hindlimb muscles of rhesus monkeys. J Neurophysiol. 2001;86:1430–1444. doi: 10.1152/jn.2001.86.3.1430. [DOI] [PubMed] [Google Scholar]
- Kawai N, Tanaka E, Langenbach GEJ, et al. Daily jaw muscle activity in freely moving rats measured with radio-telemetry. Eur J Oral Sci. 2007;115:15–20. doi: 10.1111/j.1600-0722.2007.00424.x. [DOI] [PubMed] [Google Scholar]
- Kernell D, Hensbergen E. Use and fibre type composition in limb muscles of cats. Eur J Morphol. 1998;36:288–292. doi: 10.1076/ejom.36.4.288.5821. [DOI] [PubMed] [Google Scholar]
- Kernell D, Eerbeek O, Verhey BA, et al. Effects of physiological amounts of high- and low-rate chronic stimulation on fast-twitch muscle of the cat hindlimb. I. Speed- and force-related properties. J Neurophysiol. 1987;58:598–613. doi: 10.1152/jn.1987.58.3.598. [DOI] [PubMed] [Google Scholar]
- Korfage JAM, Schueler YT, Brugman P, et al. Differences in myosin heavy-chain composition between human jaw-closing muscles and supra- and infrahyoid muscles. Arch Oral Biol. 2001;46:821–827. doi: 10.1016/s0003-9969(01)00042-5. [DOI] [PubMed] [Google Scholar]
- Langenbach GEJ, van Wessel T, Brugman P, et al. Variation in daily masticatory muscle activity in the rabbit. J Dent Res. 2004;83:55–59. doi: 10.1177/154405910408300111. [DOI] [PubMed] [Google Scholar]
- Langenbach GEJ, van Wessel T, Brugman P, et al. Is fiber-type composition related to daily jaw muscle activity during postnatal development? Cells Tissues Organs. 2008;187:307–315. doi: 10.1159/000112791. [DOI] [PubMed] [Google Scholar]
- Monster AW, Chan H, O’Connor D. Activity patterns of human skeletal muscles: relation to muscle fiber type composition. Science. 1978;200:314–317. doi: 10.1126/science.635587. [DOI] [PubMed] [Google Scholar]
- Pette D, Staron RS. Myosin isoforms, muscle fiber types, and transitions. Microsc Res Tech. 2000;50:500–509. doi: 10.1002/1097-0029(20000915)50:6<500::AID-JEMT7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Sano R, Tanaka E, Korfage JAM, et al. Heterogeneity of fiber characteristics in the rat masseter and digastric muscles. J Anat. 2007;211:464–470. doi: 10.1111/j.1469-7580.2007.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant’Ana Pereira JAA, Wessels A, Nijtmans L, et al. New method for the accurate characterization of single human skeletal muscle fibres demonstrates a relation between mATPase and MyHC expression in pure and hybrid fibre types. J Muscle Res Cell Motil. 1995;16:21–34. doi: 10.1007/BF00125307. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 1996;76:371–409. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- Templeton GH, Sweeney HL, Timson BF, et al. Changes in fiber composition of soleus muscle during rat hindlimb suspension. J Appl Physiol. 1988;65:1191–1195. doi: 10.1152/jappl.1988.65.3.1191. [DOI] [PubMed] [Google Scholar]
- Tuxen A, Kirkeby S. An animal model for human masseter muscle histochemical characterization of mouse, rat, rabbit, cat, dog, pig, and cow masseter muscle. J Oral Maxillofac Surg. 1990;48:1063–1067. doi: 10.1016/0278-2391(90)90290-i. [DOI] [PubMed] [Google Scholar]
- van Wessel T, Langenbach GEJ, van Ruijven LJ, et al. Daily number and lengths of activity bursts in rabbit jaw muscles. Eur J Neurosci. 2005;21:2209–2216. doi: 10.1111/j.1460-9568.2005.04054.x. [DOI] [PubMed] [Google Scholar]
- Wessels A, Vermeulen JL, Virágh S, et al. Spatial distribution of “tissue-specific” antigens in the developing human heart and skeletal muscle. II. An immunohistochemical analysis of myosin heavy chain isoform expression patterns in the embryonic heart. Anat Rec. 1991;229:355–368. doi: 10.1002/ar.1092290309. [DOI] [PubMed] [Google Scholar]