Abstract
The tissue-specific gene expression of the vitamin K-dependent proteins bone γ-carboxyglutamate-protein (BGP) and matrix γ-carboxyglutamate-protein (MGP) in Atlantic salmon (Salmo salar L.) was investigated. In previous studies, BGP, the most abundant non-collagenous protein of bone, was almost exclusively associated with bone, whereas the non-structural protein MGP has a more widespread tissue distribution. In-situ hybridization of juvenile Atlantic salmon (∼40 g, fresh water) vertebrae demonstrated expression of bgp and mgp mRNA in osteoblasts lining the trabecular bone, whereas no staining was observed in the compact bone. By separating the trabecular and compact bone of both juvenile (∼40 g, fresh water) and adult (∼1000 g, sea water) Atlantic salmon, we observed that the two vertebral bone compartments displayed different levels of bgp, whereas no such differences were seen for mgp. Measurements of the mineral content and Ca/P molar ratio in adult salmon revealed no significant differences between trabecular and compact bone. In conclusion, the osteoblasts covering the salmon vertebrae have unique gene expression patterns and levels of bgp and mgp. Further, the study confirms the presence of mRNA from the vitamin K-dependent proteins BGP and MGP in the vertebrae, fin and gills of Atlantic salmon.
Keywords: Ca/P, fish, in-situ hybridization, mineral content, mineralization, osteoblast, spine, teleost
Introduction
Bone is a specialized connective tissue consisting of mineralized extracellular matrix and bone cells (osteoprogenitor cells, osteoblasts, osteocytes and osteoclasts). Across vertebrates the non-mineralized bone matrix, the osteoid, mainly consists of collagen deposited by osteoblasts. The osteoid is subsequently mineralized, forming hydroxyapatite crystals (Hauschka & Carr, 1982; Huysseune, 2000), which is the major mineral crystal present in mineralized extracellular matrix (Poser & Price, 1979; Hauschka & Carr, 1982). The aquatic environment imposes different challenges upon the organism compared with terrestrial life, resulting in a wide range of combinations of the basic constituents of bone in fish. Both the structure and composition of bone have been studied in several teleost species, and different solutions to varying functional challenges have displayed a wide diversity of cartilages, modes of mineralization and differences in bone cell types and content (Huysseune, 2000). Whereas phylogenetic primitive teleost species, like salmonids, generally have cellular bone with osteocytes embedded in the bone matrix, more advanced teleosts have acellular bones lacking osteocytes (Moss, 1961). In the salmon vertebrae, osteoblastic activity can be found in two different regions: in the compact bone and in a special category of cancellous bone called trabecular bone (Huysseune, 2000). In the growth zones of the teleost vertebrae, osteoblasts can be identified together with osteoprogenitor cells and osteocytes, whereas no osteoclasts have been detected (Witten, 1997). All types of extracellular matrix-producing cells express a combination of proteins, having distinct functions in mineralization and/or deposition of osteoid. Although it is still being discussed, bone proteins such as bone γ-carboxyglutamate-protein (BGP) (synonym osteocalcin) and matrix γ-carboxyglutamate-protein (MGP) seem to be involved in the building of extracellular matrix (Luo et al. 1997), (Lee et al. 2007). BGP and MGP belong to a family of vitamin K-dependent (VKD) proteins that require post-translational modification by carboxylation of protein-bound glutamate residues to γ-carboxyglutamate (Gla) residues (Suttie, 1992; Ferland, 1998) to become functional. BGP is the most abundant non-collagenous protein in bone and was originally isolated from bovine bone (Price et al. 1976). Available results in several vertebrate species indicate that the bgp expression is tissue specific and restricted to bone, dentin (Price, 1985; Ducy et al. 1996; Pinto et al. 2001; Viegas et al. 2002) and scales (Nishimoto et al. 1992). BGP is synthesized by osteoblasts and odontoblasts (Dimuzio et al. 1983). Although Ducy et al. (1996) showed that BGP did not have a major impact on bone mineralization, Boskey et al. (1998) later found evidence that BGP may function as a regulator of bone maturation. MGP, originally purified from mammalian bone (Price & Williamson, 1985), is synthesized by osteoblasts but, in contrast to BGP, it is also synthesized by a wide variety of other cells, like vascular smooth muscle cells and chondrocytes. Studies on the mgp knockout mouse have shown that lack of MGP leads to calcification of arteries and cartilage, indicating that MGP is a negative regulator of calcification, facilitating normal bone growth and development (Luo et al. 1997).
High frequencies of bone deformities are a major concern in salmon aquaculture, causing concern for animal welfare. Although bone growth has been well studied in teleost species (Huysseune, 2000; Witten et al. 2000; Roy et al. 2002), the molecular mechanisms behind vertebral bone growth and mineralization in salmon have been relatively little studied (Wargelius et al. 2005, 2009). Studies on VKD proteins and their corresponding genes could be a useful tool to increase the knowledge about proteins involved in bone growth and mineralization in Atlantic salmon (Salmo salar L.). Thus, bgp and mgp mRNA were identified in both juvenile and adult Atlantic salmon, and the spatial tissue distribution and gene expression level in vertebrae and several other tissues were investigated.
Materials and methods
Tissue sampling
To estimate the spatial (in-situ hybridization) and gene expression [quantitative polymerase chain reaction (qPCR), n = 3 fish per stage and tissue] level of mgp and bgp in somatic tissues (eyes, gills, heart, liver, skeletal muscle, vertebrae, pectoral fin and skin), juvenile [(fresh water (FW)] Atlantic salmon with a mean weight of ∼40 g were used. In addition, vertebrae from juvenile (∼40 g, FW, n = 3) and adult (∼1000 g, sea water, n = 3) salmon were microdissected into trabecular bone and compact bone for analyses of the bgp and mgp expressional level, whereas mineral content was measured only in adult salmon (n = 3). All fish were collected from the Institute of Marine Research facilities at Matre Aquaculture Research station, Norway (N 60°52′, E 05°35′). The fish were reared in indoor tanks (both FW and sea water) and fed to satiation with a standard commercial diet. They were subjected to a natural photoperiod and ambient water temperature (3–18 °C throughout the year). Water flow was kept at ∼2.5 L min−1 kg−1 fish. All fish were anesthetized with benzocain (2 ml L−1) and killed with a blow to the head. Tissues were carefully dissected and cleaned from surrounding tissue. For gene expression studies, tissues were immediately flash frozen in liquid nitrogen and kept at −80 °C until further analysis. For spatial expression studies, tissues were fixed in freshly made 4% paraformaldehyde (PFA) in 1× phosphate-buffered saline (PBS). For the fine dissection of compact and trabecular bone, clean frozen vertebral columns were cut and the notochordal tissue was scraped away. The ring of compact bone of the vertebrae was cut away with a scalpel, whereas the rest was considered to contain mostly trabecular bone.
RNA extraction, DNase treatment and cDNA synthesis (250 ng)
Total RNA was extracted and isolated from the different tissues using FastPrep and TRI reagent® (Sigma-Aldrich Norway AS, Oslo, Norway) according to the manufacturer’s instructions. After the elimination of genomic DNA by RQ DNase I (Promega GmbH, Manheim, Germany) treatment, the samples were stored at −80 °C until further analysis. The quantity and quality of the isolated RNA were assessed by a NanoDrop® spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Only samples with a 260/280-nm absorbance ratio of 1.8–2.0 were approved. The RNA integrity was evaluated by an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA) using an RNA 6000 Nano LabChip® kit (Agilent Technologies) and only samples showing no sign of RNA degradation were used. First strand cDNA was reverse transcribed from 250 ng RNA using a Reverse Transcription Core kit (RT-RTCK-05; Eurogenetec, Seraing, Belgium).
Molecular cloning of full-length salmon bgp and mgp
cDNA from salmon vertebrae was produced using a SMART PCR cDNA synthesis kit (Clontech; Takara Bio Company, Mountain View, CA, USA). For amplification of bgp and mgp, the PCR was performed on vertebral cDNA using the sequences listed in Table 1. PCR products of the expected size were visualized by 2% agarose gel electrophoresis and ethidium bromide staining, excised from the gel, and eluted from the agarose slice using the QIAquick Gel Extraction Kit (Invitrogen Life Technologies, Carlsbad, CA, USA) following the manufacturer’s instructions. The resulting DNA fragments were cloned into the pCR-4 Topo vector (Invitrogen Life Technologies). Final identification was achieved by standard DNA sequence analysis at the Sequencing Facility at the University of Bergen (Bergen, Norway). Full-length salmon mgp (Accession no. AY182239) and partially cloned bgp (Accession no. EG856879) were used to pick up clones for in-situ hybridization (NCBI GenBank). The search for bgp sequence identity between Atlantic salmon and other fish species was performed at ExPASy BLAST2 using the ncbi blastp2.2.17 program. For the partially cloned bgp, 3′-rapid amplification of cDNA ends (RACE) was performed using the forward primer 5′-GCTGGGATCATTGCTGCCTACACC-3′ and a 308-bp sequence was obtained.
Table 1.
Primers and probes for Atlantic salmon (S. salar L.) used for the real-time PCR study and the cloning of constructs with mgp or bgp, which were used for the production of probes for in-situ hybridization.
| Used for | Gene | Sequence |
|---|---|---|
| Real-time PCR | bgp | Forward: 5′-TTGCAGACTTGTCCCTGACTCA-3′ |
| Reverse: 5′-TGTCCATCATATTCTCACACCAGTAGT-3′ | ||
| Probe: 5′-TGGAGAGTCTGAGGGAGGTGTGTGAGCTTA-3′ | ||
| mgp | Forward: 5′-GAAAGCACAGAATCCTTTGAAGATGT-3′ | |
| Reverse: 5′-GTGGACTCTGTGGGTTGATGAA-3′ | ||
| Probe: 5′-TTTGTCAGTCCATACCGAGCCAAC-3′ | ||
| ef1α | Forward: 5′-CCCCTCCAGGACGTTTACAAA-3′ | |
| Reverse: 5′-CACACGGCCCACAGGTACA-3′ | ||
| Probe: 5′-ATCGGTGGTATTGGA-3′ | ||
| In-situ | bgp | Forward: 5′-GGGACTGGTGAGAGAGACAAG-3′ |
| Reverse: 5′-GAAGTTGGTTTGTGGAGTGGG-3′ | ||
| mgp | Forward: 5′-CTTCTTAGGACCAAGAGACC-3′ | |
| Reverse: 5′-GGAGGTGAGTGTGAAGCTAA-3′ |
Real-time polymerase chain reaction
The primer sequences for the control gene elongation factor alpha (ef1α) were derived from Olsvik et al. (2005). Primers and probes for the amplification and detection of bgp and mgp, in addition to ef1α, were designed using the Primer Express 2.0 software (Applied Biosystems, Foster City, CA, USA) and are listed in Table 1. Real-time PCR was carried out on an ABI 7700 (Applied Biosystems, Oslo, Norway) system, with the following thermal cycling conditions: 50 °C for 2 min followed by 98 °C for 10 min and then 40 cycles of 95 °C for 15 s followed by 60 °C for 1 min. The samples were run in triplicate in a 96-well PCR plate. No-template controls for each gene were run on each PCR plate. To determine the efficiency of targets (bgp and mgp) in relation to reference (ef1α), we used the standard curve method and a validation experiment described in ABI User Bulletin no. 2 (ABI 7700 sequence detection system). In the validation experiment, 250, 125, 62.5 and 31.25 ng of RNA were used for cDNA synthesis and the slope of log input amount of RNA vs. delta cycle threshold (Ct) was 0.02 for bgp/ef1α and 0.06 for mgp/ef1α, which is < 0.1. This demonstrates that the efficiency between target and reference is approximately equal. The relative expression level was calculated using the comparative Ct method (ABI User Bulletin no. 2, ABI 7700 sequence detection system).
In-situ hybridization
Vertebrae from juvenile (n = 3 fish, FW) salmon were collected and fixed in freshly made 4% paraformaldehyde/1×PBS overnight. After fixation, vertebrae were washed for 4 × 15 min in 1×PBS followed by decalcification for 8 weeks at 4 °C in a solution containing 10% EDTA/0.5 m Tris–HCl, pH 7.5. After decalcification, specimens were washed for 4 × 15 min in 1×PBS before incubating overnight in a solution containing 25% sucrose/1×PBS/25% Tissue-tek® (Sakura Finetek Norway AS, Oslo, Norway). Samples were frozen in 100% Tissue tek® and kept at −80 °C until sectioning. Frozen tissue samples were sectioned on a Leica CM 1900 (Leica Microsystems GmbH, Wetzlar, Germany) cryostat. Serial longitudinal sections and cross-sections (10 μm) of the different tissues were mounted onto SuperFrost®+ glass slides and air dried at room temperature for at least 1 h before being frozen and kept at −80 °C until further analysis. For in-situ hybridization of bgp, mgp and collagen type I alpha 2, 648-bp (Accession no. FJ172977), 802-bp (Accession no. AY182239) and 618-bp (Accession no. CA064459) probes were used, respectively (primers used for the constructs are listed in Table 1). All sequences covered the open reading frame for each gene. For the production of anti-sense and sense probes, T3 and T7 polymerase was used according to the manufacturer’s recommendations (Roche, Basel, Switzerland). Probes were used at a concentration of 1000 ng mL−1 in the hybridization solution. A total of four slides per tissue were thawed and air dried for a minimum of 3 h at room temperature. All sections were rehydrated and rinsed in 2× saline sodium citrate buffer (SSC), before proteinase K treatment (2 mg mL−1 proteinase K) for 10 min. Tissues were then post-fixed in 4% paraformaldehyde in 1×PBS before acetic anhydride treatment. Sections were hybridized overnight at 60 °C in a solution containing 50% formamide, dextran sulphate, 20×SSC, 50×Denhardt’s, tRNA, S. salar sperm DNA, 10% SDS and diethylpyro-carbonate (DEPC)-treated water. After hybridization, sections were washed, kept in 50% formamide for 30 min (60 °C) and treated with RNase A (0.02 mg mL−1) for 30 min at 37 °C. Slides were incubated at 4 °C with anti-digoxigenin fragment antigen binding (FAB) fragments conjugated with alkaline phosphatase (1 : 2000; Roche Diagnostics, Mannheim, Germany) overnight. The next day sections were incubated in the dark for 5–6 h in chromogene substrate containing nitroblue tetrazolium (NBT) and X-phosphate disodium salt (BCIP). Pictures were taken with Q Capture Pro 5.1 (QImaging, Tucson, AZ, USA), using Adobe Photoshop CS2 (Adobe Systems Inc., San Jose, CA, USA).
Total mineral content and Ca/P analysis
Vertebrae (n= 3 fish, 10 vertebrae from each fish in each sample) were carefully dissected, separating trabecular and compact bone, for the measurement of both total mineral content and Ca/P molar ratio (adult salmon only). For mineral content estimation, the two bone types were defatted in hexane baths, dried overnight at 100 °C and then incinerated for 13.5 h in a muffle furnace (115 °C for 0.5 h, 540 °C for 5 h and 750 °C for 8 h). Mineral content was calculated using: mineral content (%) = (mineral weight/dry weight) × 100. Analysis of elemental Ca and P were performed on ashed trabecular and compact bone by Analytica AB (Luleå, Sweden), where the samples were acid digested and analyzed by inductively coupled plasma atomic emission spectroscopy (ICP-AES) according to the Environmental Protection Agency (EPA) method 200.7.
Statistical analysis
Data were statistically evaluated using an unpaired t-test with Welch’s correction. Differences were considered significant at P < 0.05. Data analyses were performed using GraphPad Prism 5.0 (GraphPad, La Jolla, CA, USA).
Results
A complete cDNA clone that encoded for the Atlantic salmon protein BGP was isolated by PCR. The 648-bp sequence for salmon bgp coded for a protein of 101 amino acid residues from the first possible translation site at nucleotide 91 (Fig. 1) (Accession no. FJ172977). The predicted protein shares 72, 65, 63, 62 and 54% sequence identity with BGP proteins identified in common carp (Cyprinus carpio), gilthead sea bream (Sparus aurata), European sea bass (Dicentrarchus labrax), zebrafish (Danio rerio) and sole (Solea senegalesis), respectively. The characteristic Gla domain found in BGP and other members of the VKD family of proteins was located at amino acids 56–97 (Fig. 1, gray marking) that shares 96, 72, 81, 75, 75 and 72% sequence identity with rainbow trout (Oncorhynchus mykiss), common carp, gilthead sea bream, European sea bass, zebrafish and sole, respectively.
Fig. 1.
Atlantic salmon (S. salar L.) BGP gene sequence (Accession no. FJ172977). Nucleotides are numbered in the left margin and the amino acid sequence is shown under the coding sequence and numbered in the right margin. The Gla-domain of the amino acid sequence is marked in gray.
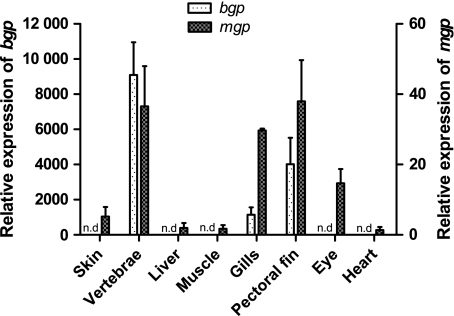
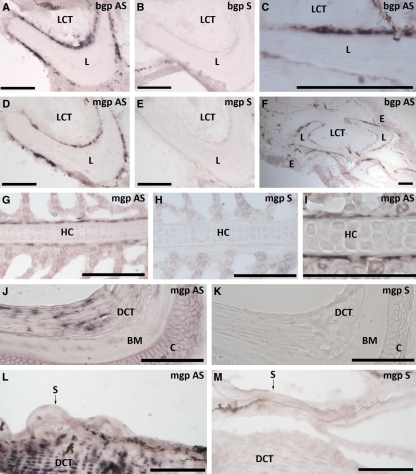
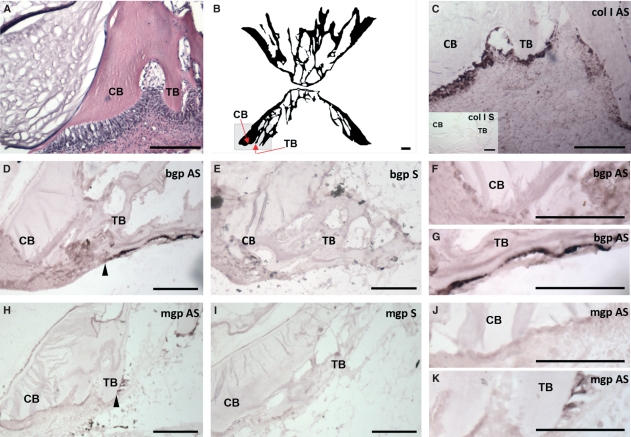
Of the different tissues analyzed for gene expression level in juvenile Atlantic salmon, only vertebrae, gills and pectoral fin showed the presence of bgp (Fig. 2). The gene was highly expressed in vertebrae compared with the two other tissues, with the lowest relative expression in the gills. In-situ hybridization was performed on sections of vertebrae, gills and pectoral fin to identify cells expressing the bgp gene (Figs 3 and 4). In transverse sections of the pectoral fin, expression of bgp was found in the osteoblasts lining the paired hemitrichia forming lepidotrichia (Fig. 3A,C,F). Signal for the corresponding mRNA was also observed in the osteoblasts lining the trabecular bone of the vertebrae (Fig. 4D,G). The osteoblasts lining the compact bone of the vertebrae did not show expression of bgp (Fig. 4D,F). For all tissues, no background signal was found using the sense probe for the gene (Figs 3B and 4E). To verify that gene expression could be detected in osteoblasts lining the compact bone, in-situ hybridization with collagen type I alpha 2 was performed and there was strong expression in osteoblasts of both the compact and trabecular bone (Fig. 4C).
Fig. 2.
Relative gene expression of bgp (left y-axis) and mgp (right y-axis) in different tissues of juvenile Atlantic salmon (S. salar L). The x-axis presents the tissues used, whereas the two y-axes show the relative abundance of transcripts in relation to the reference gene (ef1α). n.d, not detectable. Data are given as mean + SE, n = 3.
Fig. 3.
Tissue-specific gene expression of bgp AS and mgp antisense (AS) in selected tissues of juvenile Atlantic salmon (S. salar L.). In the pectoral fin, expression was located in the osteoblasts lining the paired hemitrichia forming the lepidotrichia for both bgp (A,C,F; transverse section) and mgp (D; transverse section). In the gill (G,I; sagittal section), mgp expression was located in cells lining the cellular hyaline cartilage (zellknorpel) of the gill filament. Expression of mgp was also found in the dense connective tissue of the eyes (cornea) (J; transverse section) and in the dense connective tissue of the dermis of the skin (L; transverse section). For all tissues, no background signal was found using the sense probe [bgp/mgp sense (S)] for the genes (B,E,H,K,M). Scale bar: 100 μm. LCT, loose connective tissue; L, lepidothrichia; E, epidermis; HC, hyaline cartilage; DCT, dense connective tissue; BM, Bowman′s membrane; C, cornea, S, scale.
Fig. 4.
Morphology and gene expression of bgp antisense (AS) and mgp AS in sections of juvenile Atlantic salmon (S. salar L.) vertebrae (sagittal sections). (A) Vertebrae morphology (with a hematoxylin-eosin-stained section) with the compact bone (CB) and trabecular bone (TB) marked. The gray area in (B) shows the area from which the other pictures are taken. (C) Gene expression of collagen type I alpha 2 (col I) in both CB and TB. For both bgp (D,F,G) and mgp (H,J,K), their corresponding mRNA was only expressed in the osteoblasts lining the TB of the vertebrae. The transition zone between osteoblasts expressing and not expressing bgp/mgp is marked with an arrowhead. No background signal was found using the sense probe [bgp/mgp sense (S)] for the genes (E,I). Scale bars: 100 μm.
To identify the expressional level of mgp in different tissues of juvenile Atlantic salmon, real-time PCR was performed on skin, vertebrae, liver, skeletal muscle, gills, pectoral fin, eyes and heart. Amplification showed the presence of salmon mgp in all of the investigated tissues, with the highest relative expression in vertebrae, gills, fin and eye, whereas lower levels of mgp were found in skin, liver, skeletal muscle and heart (Fig. 2). Tissue-specific expression was investigated in the heart, liver, gill, fin, eye and vertebrae. No tissue-specific expression could be detected in the heart and liver but in the other tissues measured expression was associated with chondrogenic and osteogenic cells. As for bgp, mgp was found in the osteoblasts lining the paired hemitrichia forming lepidotrichia in the pectoral fin (transverse sections, Fig. 3D). In gill sections, mgp was expressed in a thin layer of cells lining the cellular hyaline cartilage (zellknorpel) of the gill filaments (sagittal sections, Fig. 3G, I). Expression of mgp was also found in the dense connective tissue of the cornea (transverse section of the eye, Fig. 3J) and in the dense connective tissue (dermis) of the skin (transverse sections, Fig. 3L). In the vertebrae mgp was found in the osteoblasts lining the trabecular bone only, whereas no expression was found in the osteoblasts lining the compact bone (Fig. 4H,J,K). The control hybridization with sense salmon mgp showed no positive signal for any of the tissues (Figs 3E,H,K,M and 4I).
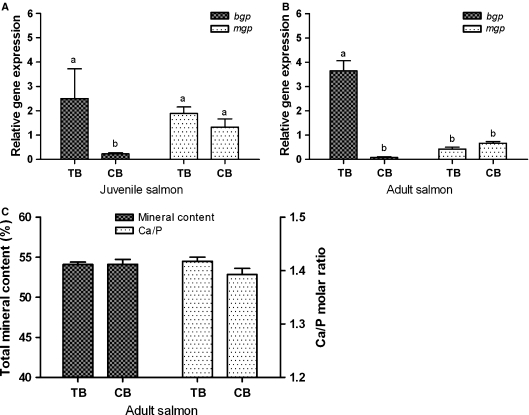
We wanted to measure if the differential spatial expression in trabecular and compact bone was reflected in the relative gene expression of mgp and bgp in the same vertebral compartments. Samples from trabecular and compact bone revealed a significantly higher expression of bgp in trabecular bone in comparison to compact bone in both juvenile and adult fish (Fig.5A, P = 0.01; Fig. 5B, P = 0.01). No expressional differences were detected between the two tissues for mgp in juvenile and adult fish. When comparing gene expression levels between the two bone compartments, bgp showed a significantly higher expression than mgp in trabecular bone (P = 0.02) in adult fish, whereas in juvenile fish the gene expression level of trabecular bone bgp was not significantly different to that of mgp in trabecular and compact bone. Further, to estimate if the expressional differences between the bone types affected the mineral content of the bone types, the percentage mineral content [(ash weight/dry weight) × 100] and Ca/P ratio were both measured in microdissected bone from the two bone compartments in adult Atlantic salmon. The measurements of mineral content revealed no significant differences between trabecular and compact bone, with means of 54.13 ± 0.45 and 54.15 ± 1.05%, respectively (Fig. 5C). Results showed that trabecular bone had mean Ca and P contents of 369 ± 42 and 201 ± 41 g kg−1, whereas compact bone Ca and P contents were 353 ± 17 and 196 ± 7.9 g kg−1, respectively. The mean Ca/P molar ratio of the trabecular bone (1.42 ± 0.01) was not significantly different (P = 0.17) from the Ca/P molar ratio of the compact bone (1.39 ± 0.02).
Fig. 5.
Gene expression, mineral content and Ca/P molar ratio in compact bone (CB) and trabecular bone (TB) in salmon. Relative gene expression of bgp and mgp in TB and CB bone of juvenile (A) and adult (B) Atlantic salmon (S. salar L.) vertebrae. The x-axis presents the two different bone compartments, whereas the y-axis shows the relative abundance of transcripts in relation to the reference gene (ef1α). Data are given as mean + SE (n = 3, significant differences are marked). (C) Total mineral content (%, left y-axis) and Ca/P molar ratio (right y-axis) for TB and CB (x-axis) of adult salmon vertebrae. Data are shown as mean ± SE (n= 3). The letters a and b demarcate significant differences between bars (P < 0.05).
Discussion
Bone Gla-protein is the most abundant non-collagenous bone protein in most teleosts and is present in teleost species at levels similar to those found in mammalian bone (Nishimoto et al. 2003). Comparison of the BGP proteins from fish, amphibians, chicken, mouse, rat and human indicates that its overall structure has remained relatively preserved from its presumed origin in bony fish (Pinto et al. 2001; Viegas et al. 2002) and within the teleosts there are conserved features in the BGP gene, such as the Gla domain (Nishimoto et al. 2003). Laizé et al. (2006) showed that both zebrafish and rainbow trout have two BGP isoforms, denoted OC1 (short isoform) and OC2 (long isoform), whereas in most bony fish only one of the two isoforms appears to be present. The identified salmon bgp from the present study displays a similar structure to the previously identified BGP in other teleosts and bgp in salmon is likely to be a homologue to OC1, as it lacks the sequence part that codes for the acidic prodomain found in OC2. However, as the OC2 isoform exists in other salmonid fish it is likely that the gene also exist in Atlantic salmon.
In vertebrates, bgp expression is only found in bony and dental tissue (Price, 1985; Nishimoto et al. 1992; Ducy et al. 1996; Pinto et al. 2001; Viegas et al. 2002). In accordance with these results bgp expression was found in the osteoblasts lining the lepidotrichia and the trabecular bone of the vertebrae but not in vertebral compact bone, skin, liver, skeletal muscle, heart and eye. No expression of bgp was found in the gill filaments. To our knowledge, no studies exist reporting the absence of bgp expression in salmon vertebral osteoblasts. However, a recent study of salmon teeth reported that bgp is not expressed in osteoblasts that deposit the attachment bone or that are involved in remodeling of the oral surface of the dentary bone. In contrast, it is highly expressed in the osteoblasts along the aboral surface of the dentary bone (Huysseune et al. 2008). During development in mammals, BGP first appears at the onset of mineralization and an increase in protein synthesis occurs concomitant with hydroxyapatite deposition during skeletal growth (Lian et al. 1982). BGP acts as an inhibitor of bone formation and is required to stimulate bone mineral maturation (Boskey et al. 1998). However, augmented BGP levels indicate active mineralization and this increase in BGP level may be involved in the whole animal’s adjustment to an active mineralization. The observed difference in the expression of bgp in different compartments of the vertebrae raises the possibility that lineage progression from progenitor to mature osteoblasts may follow different pathways (Candeliere et al. 2001; Nordvik et al. 2005).
Novel functions of BGP are discussed, and recent reports suggest that the secretion of BGP into the circulation might have an endocrine function where bgp affects the visceral adiposity of the animal (Lee et al. 2007). If BGP is a source of endocrine signals in salmon, the detected expression in the trabecular and lepidotrichial osteoblasts indicates that these cells may exert some endocrinological function during mineralization, whereas the compact bone tissue does not. However, the lack of bgp expression in the compact bone does not have to demarcate that the gene has never been expressed in this tissue. It has previously been reported that the BGP content in mineralized tissues in another teleost, the meagre (Argyrosomus regius), seems to be dependent not only on the origin of the tissue but also on the developmental stage (Simes et al. 2001, 2004). The differences in the gene expression level of bgp between trabecular and compact bone were persistent from juvenile to adult Atlantic salmon. It is thus likely that the lack of expression in the compact bone is persistent through both life stages. However, we did not measure bgp expression during development of the vertebrae and it is possible that bgp can be expressed in compact bone during the fry stage. Another possible explanation is that the predicted bgp isoform OC2, present in zebrafish and rainbow trout (Laizé et al. 2006), also exists in Atlantic salmon and might be expressed in the compact bone, thereby exerting functional redundancy for bgp in this tissue.
The cellularity of vertebral bone varies, and in some teleost lineages the vertebrae are devoid of osteocytes (Moss, 1961). In salmon, the osteocyte number is 10 times as high in the trabecular bone compared with the compact bone (Nordvik et al. 2005). Results from the present study show that the compact bone contains almost no osteocytes, whereas the trabecular bone does (see Fig. 4A). In mammals, osteocytes may signal through their canaliculi to the surrounding osteoblasts (Hall, 2005). It is possible that the vertebral trabecular osteoblasts must constantly receive information from the osteocytes to grow and function, whereas the osteoblasts of the compact bone lack such signalling, and thus grow and function in response to other cues. This theory is not supported in carp, which contain similar amounts of BGP in bone with both relatively low (as in compact bone) and relatively high (as in trabecular bone) cellularity (Nishimoto et al. 2003). However, that study did not compare the different bone compartments of the vertebrae but compared vertebral projections, dorsal spines, rib bone and operculum. Nishimoto et al. (2003) also reported that BGP levels are high in mineralized tissues that are not used as an available reservoir for calcium. As most fishes can mobilize calcium from the scales (Flik et al. 1986), many fish species display a high content of BGP in their bones and a low content in their scales. Apparently, a bony tissue with a low level of BGP might demineralize more easily than a bony tissue that is high in BGP. However, our results do not support the compact bone demineralizing as the mineral content was the same in both the trabecular and compact bone (Fig. 5B).
Results from the present study indicate that the osteoblasts from trabecular and compact bone in salmon display different molecular signatures. This is supported by an earlier study of rat calvaria bone where bgp was expressed in the trabecular surface of growing bone but not in the osteogenic front (Candeliere et al. 2001). In the same study it was shown that only a few osteoblast markers were expressed by all osteoblasts irrespective of their position, whereas markers like bgp were differentially expressed in subpopulations of osteoblasts. Vertebral osteoblast cultures of teleosts also display lineage-specific functional properties (Pombinho et al. 2004), possibly reflecting a different compartmental origin within the vertebrae. In Atlantic salmon, trabecular and compact bone osteoblasts deposit bone with different structure and cellularity, and the presence of at least two osteoblast populations in vertebrae is suggested (Nordvik et al. 2005). As demonstrated by the in-situ hybridization in the present study, the expression of bgp differs between trabecular and compact bone in Atlantic salmon and is reflected in the regional differences in bone microarchitecture. However, the nature of the signals leading to diversity in osteoblast gene expression profiles remains unknown (Candeliere et al. 2001).
Matrix Gla-protein is expressed in bone, cartilage and soft tissues like heart, kidney and arteries in fish, amphibians, birds and mammals (Price et al. 1987, 1998; Knapen et al. 2000; Cancela et al. 2001; Sponk et al. 2001; Simes et al. 2003, 2004). In the present study, we were able to detect spatial mgp expression in salmon trabecular bone osteoblasts, osteoblasts lining the lepidotrichia, cells lining the cellular hyaline cartilage of the gill filament and in the dense connective tissue of the cornea and dermis. In the investigated soft tissues (liver, skeletal muscle and heart) the detected gene expression level was relatively low, and perhaps not high enough to be detected spatially with in-situ hybridization. The finding of low mgp expression in soft tissues is in agreement with previous findings in mammals (Price et al. 1987; Shanahan et al. 1999), amphibians (Cancela et al. 2001) and fish (Simes et al. 2003). In-situ hybridization in mouse and gilthead sea bream has shown that mgp mRNA production is consistently more abundant in the periphery of the zone of resting and proliferative chondrocytes than in the central area (Luo et al. 1997; Pinto et al. 2003). In comparison, we detected strong expression of mgp in cells lining the cellular hyaline cartilage of the salmon gill filament. Further studies are needed to determine the nature of these cells. Spatial expression of mgp could not be detected in the osteoblasts lining the compact bone of juvenile salmon (∼40 g) vertebrae but was found in the osteoblasts of the trabecular bone, whereas the gene expression level between compact and trabecular bone in both juvenile (∼40 g) and adult (∼1000 g) salmon revealed no expressional differences between the two bone types. As for BGP, it has been shown that the MGP level in teleost bone may be dependent on the developmental stage of the fish (Simes et al. 2004). The difference between the gene expression levels of mgp and the detected spatial expression in compact bone is not conclusive between samples. A possible explanation for this is that the gene expression transition zone between mgp-positive and bgp-positive cells is not located at the same point. Another reason for the differential result using the two techniques could be that there are continuous variable mgp synthesis rates in the vertebral bone as detected during development in another teleost species (Simes et al. 2004).
In the present study we investigated whether the biochemical properties of the compact and trabecular bone differed due to the differential expression of bgp and mgp in the bone compartments of the vertebrae. Neither the mineral content nor the Ca/P molar ratio was significantly affected. It is shown that lack of mgp leads to over-calcification in mouse (Luo et al. 1997), which is not the case for salmon compact bone in the present study. It is possible that the lack of mgp in compact bone is temporal and associated with temporal changes in expression, as it was observed lack of mgp expression in the samples taken for in-situ hybridization, whereas no expressional differences were observed measuring expressional levels in microdissected samples. It is also possible that the difference in gene expression level is not as pronounced between tissues for mgp as it is for bgp. Therefore, it was also possible to detect an expressional difference for bgp in the smaller vertebrae even though the microdissection was much more challenging in the juvenile fish. Another interesting observation is that lack of bgp and temporal lack of mgp in neighbouring osteoblasts do not seem to affect the mineral content of the bone.
Concluding remarks
The diverse range of functions for VKD proteins implicates a broad biological impact of vitamin K (Berkner, 2008). Although the exact role of BGP and MGP remains unknown, they have calcium-binding properties that may be important in the regulation of bone growth (Dimuzio et al. 1983; Boskey et al. 1998). In the present study, bgp and mgp mRNA were expressed in vertebrae, gills and pectoral fin in Atlantic salmon, and a differential spatial gene expression of bgp and mgp was detected in osteoblasts lining the trabecular and compact bone of the vertebrae. Further possible reasons for these expressional differences have been described, including developmental stage and endocrine function. The presence of the two VKD proteins BGP and MGP in these tissues suggests the involvement of vitamin K in bone metabolism of Atlantic salmon.
Acknowledgments
This project is part of a strategic institute program called Roles of Fat Soluble Vitamins in Bone Development and Mineral Metabolism, funded by The Research Council of Norway (project no. 153472). We would also like to thank Dr P.G. Fjelldal for providing the tissues for gene expressional level determination and E.-J. Lock and Professor R. Waagbø for helpful comments on the manuscript. Last, but not least, we would like to thank Professor H. Kryvi and Professor S. Grotmol for help with describing the anatomical structures.
References
- Berkner KL. Vitamin K-dependent carboxylation. Vitam Horm. 2008;78:131–156. doi: 10.1016/S0083-6729(07)00007-6. [DOI] [PubMed] [Google Scholar]
- Boskey A, Gadaleta S, Gundberg C, et al. Fourier transform infrared microspectroscopic analysis of bones of osteocalcin-deficient mice provides insight into the function of osteocalcin. Bone. 1998;23:187–196. doi: 10.1016/s8756-3282(98)00092-1. [DOI] [PubMed] [Google Scholar]
- Cancela M, Ohresser M, Reia J, et al. Matrix Gla protein in Xenopus laevis: molecular cloning, tissue distribution, and evolutionary considerations. J Bone Miner Res. 2001;16:1611–1621. doi: 10.1359/jbmr.2001.16.9.1611. [DOI] [PubMed] [Google Scholar]
- Candeliere GA, Liu F, Aubin JE. Individual osteoblasts in the developing calvaria express different gene repertoires. Bone. 2001;28:351–361. doi: 10.1016/s8756-3282(01)00410-0. [DOI] [PubMed] [Google Scholar]
- Dimuzio MT, Bhown M, Butler WT. The biosynthesis of dentine γ-carboxyglutamic acid-containing protein by rat incisor odontoblasts in organ culture. Biochem J. 1983;216:249–257. doi: 10.1042/bj2160249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Desbois C, Boyce B, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–452. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- Ferland G. The vitamin K-dependent proteins: an update. Nutr Rev. 1998;56:223–230. doi: 10.1111/j.1753-4887.1998.tb01753.x. [DOI] [PubMed] [Google Scholar]
- Flik G, Fenwick JC, Kolar Z, et al. Effects of low ambient calcium levels on whole-body Ca2 +flux rates and internal calcium pools in the freshwater cichlid teleost, Oreochromis mossambicus. J Exp Biol. 1986;120:249–264. [Google Scholar]
- Hall BK. Chaps 1, 2, 12, 15 and 24. In: Hall BK, editor. Bones and Cartilage: Developmental and Evolutionary Skeletal Biology. London, UK: Elsevier Academic Press; 2005. [Google Scholar]
- Hauschka P, Carr S. Calcium-dependent alpha-helical structure in osteocalcin. Biochemistry. 1982;21:2538–2547. doi: 10.1021/bi00539a038. [DOI] [PubMed] [Google Scholar]
- Huysseune A. Skeletal system. In: Ostrander GK, editor. The Laboratory Fish. London, UK: Academic Press; 2000. [Google Scholar]
- Huysseune A, Takle H, Soenens M, et al. Unique and shared gene expression patterns in Atlantic salmon (Salmo salar) tooth development. Dev Genes Evol. 2008;218:427–437. doi: 10.1007/s00427-008-0237-9. [DOI] [PubMed] [Google Scholar]
- Knapen M, Hellemons-Boode B, Langeberg-Ledeboer M, et al. Effect of oral anticoagulant treatment on markers for calcium and bone metabolism. Haemostasis. 2000;30:290–297. doi: 10.1159/000054146. [DOI] [PubMed] [Google Scholar]
- Laizé V, Viegas CSB, Price PA, et al. Identification of an osteocalcin isoform in fish with a large acidic prodomain. J Biol Chem. 2006;281:15037–15043. doi: 10.1074/jbc.M600373200. [DOI] [PubMed] [Google Scholar]
- Lee N, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian JB, Roufosse AH, Reit B, et al. Concentration of osteocalcin and phosphoprotein as a function of mineral content and age in cortical bone. Calcif Tissue Int. 1982;34:82–87. [PubMed] [Google Scholar]
- Luo G, Ducy P, Mckee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix Gla protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- Moss ML. Osteogenesis of acellular teleost fish bone. Am J Anat. 1961;108:99. [Google Scholar]
- Nishimoto SK, Araki N, Robinson FD, et al. Discovery of bone γ-carboxyglutamic acid protein in mineralized scales. The abundance and structure of Lepomis macrochirus bone γ-carboxyglutamic acid. J Biol Chem. 1992;267:11600–11605. [PubMed] [Google Scholar]
- Nishimoto SK, Waite JH, Nishimoto M, et al. Structure, activity, and distribution of fish osteocalcin. J Biol Chem. 2003;278:11843–11848. doi: 10.1074/jbc.M211449200. [DOI] [PubMed] [Google Scholar]
- Nordvik K, Kryvi H, Totland GK, et al. The salmon vertebral body develops through mineralization of two preformed tissues that are encompassed by two layers of bone. J Anat. 2005;206:103–114. doi: 10.1111/j.1469-7580.2005.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsvik P, Lie K, Jordal A-EO, et al. Evaluation of potential reference genes in real-time RT-PCR studies of Atlantic salmon. BMC Mol Biol. 2005;6:21. doi: 10.1186/1471-2199-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto JP, Ohresser MCP, Cancela ML. Cloning of the bone Gla protein gene from the teleost fish Sparus aurata. Evidence for overall conservation in gene organization and bone-specific expression from fish to man. Gene. 2001;270:77–91. doi: 10.1016/s0378-1119(01)00426-7. [DOI] [PubMed] [Google Scholar]
- Pinto J, Conceicao N, Gavaia P, et al. Matrix Gla protein gene expression and protein accumulation colocalize with cartilage distribution during development of the teleost fish Sparus aurata. Bone. 2003;32:201–210. doi: 10.1016/s8756-3282(02)00981-x. [DOI] [PubMed] [Google Scholar]
- Pombinho AR, Laize V, Molha DM, et al. Development of two bone-derived cell lines from the marine teleost Sparus aurata; evidence for extracellular matrix mineralization and cell-type-specific expression of matrix Gla protein and osteocalcin. Cell Tissue Res. 2004;315:393–406. doi: 10.1007/s00441-003-0830-1. [DOI] [PubMed] [Google Scholar]
- Poser JW, Price PA. Method for decarboxylation of gamma-carboxyglutamic acid in proteins: properties of the decarboxylated gamma-carboxyglutamic acid protein from Calf bone. J Biol Chem. 1979;254:431–436. [PubMed] [Google Scholar]
- Price PA. Vitamin K-dependent formation of bone Gla protein (osteocalcin) and its function. Vitam Horm. 1985;42:65–108. doi: 10.1016/s0083-6729(08)60061-8. [DOI] [PubMed] [Google Scholar]
- Price PA, Williamson MK. Primary structure of bovine matrix Gla protein, a new vitamin K-dependent bone protein. J Biol Chem. 1985;260:14971–14975. [PubMed] [Google Scholar]
- Price P, Poser J, Raman N. Primary structure of gamma-carboxyglutamic acid-containing protein from bovine bone. Proc Natl Acad Sci USA. 1976;73:3374–3375. doi: 10.1073/pnas.73.10.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price P, Fraser J, Metz-Virca G. Molecular cloning of matrix Gla protein: implications for substrate recognition by the vitamin K-dependent gamma-carboxylase. Proc Natl Acad Sci USA. 1987;84:8335–8339. doi: 10.1073/pnas.84.23.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price PA, Faus S, Williamson M. Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arterioscler Thromb Vasc Biol. 1998;18:1400–1407. doi: 10.1161/01.atv.18.9.1400. [DOI] [PubMed] [Google Scholar]
- Roy PK, Witten PE, Hall BK, et al. Effects of dietary phosphorus on bone growth and mineralization of vertebrae in haddock (Melanogrammus aeglefinus L.) Fish Physiol Biochem. 2002;27:35–48. [Google Scholar]
- Shanahan CM, Cary NRB, Salisbury JR, et al. Medial localization of mineralization-regulating proteins in association with Mönckeberg’s sclerosis. Circulation. 1999;100:2168–2176. doi: 10.1161/01.cir.100.21.2168. [DOI] [PubMed] [Google Scholar]
- Simes DC, Pinto JP, Gavaia PJ, et al. Characterization of specific antibodies for fish osteocalcin and its usefulness to investigate osteocalcin tissue distribution in lower vertebrates. Bone. 2001;28:S103. [Google Scholar]
- Simes D, Williamson M, Ortiz-Delgado J, et al. Purification of matrix Gla protein from a marine teleost fish, Argyrosomus regius: calcified cartilage and not bone as the primary site of MGP accumulation in fish. J Bone Miner Res. 2003;18:244–259. doi: 10.1359/jbmr.2003.18.2.244. [DOI] [PubMed] [Google Scholar]
- Simes D, Williamson M, Schaff B, et al. Characterization of osteocalcin (BGP) and Matrix Gla Protein (MGP) fish specific antibodies: validation for immunodetection studies in lower vertebrates. Calcif Tissue Int. 2004;74:170–180. doi: 10.1007/s00223-003-0079-4. [DOI] [PubMed] [Google Scholar]
- Sponk HMH, Soute BAM, Schurgers LJ, et al. Matrix Gla protein accumulates at the border of regions of calcification and normal tissue in the media of the arterial vessel wall. Biochem Biophys Res Commun. 2001;289:485–490. doi: 10.1006/bbrc.2001.5996. [DOI] [PubMed] [Google Scholar]
- Suttie JW. Vitamin K and human nutrition. J Am Diet Assoc. 1992;92:585–590. [PubMed] [Google Scholar]
- Viegas C, Pinto JP, Conceicao N, et al. Cloning and characterization of the cDNA and gene encoding Xenopus laevis osteocalcin. Gene. 2002;289:97–107. doi: 10.1016/s0378-1119(02)00480-8. [DOI] [PubMed] [Google Scholar]
- Wargelius A, Fjelldal PG, Benedet S, et al. A peak in gh-receptor expression is associated with growth activation in Atlantic salmon vertebrae, while upregulation of igf-I receptor expression is related to increased bone density. Gen Comp Endocrinol. 2005;142:163–168. doi: 10.1016/j.ygcen.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Wargelius A, Fjelldal PG, Nordgarden U, et al. Continuous light affects mineralization and delays osteoid incorporation in vertebral bone of Atlantic salmon (Salmo salar L.) J Exp Biol. 2009;212:656–661. doi: 10.1242/jeb.024000. [DOI] [PubMed] [Google Scholar]
- Witten PE. Enzyme histochemical characteristics of osteoblasts and mononucleated osteoclasts in a teleost fish with acellular bone (Oreochromis niloticus, Cichlidae) Cell Tissue Res. 1997;287:591–599. doi: 10.1007/s004410050782. [DOI] [PubMed] [Google Scholar]
- Witten PE, Villwock W, Peters N, et al. Bone resorption and bone remodelling in juvenile carp, Cyprinus carpio. J Appl Ichthyol. 2000;16:254–261. [Google Scholar]