Abstract
The role of estrogen in bone resorption has been specifically related to the effect of estrogen on the signalling pathway that inhibits the formation of osteoclasts. However, osteoclast apoptosis and a significant reduction in the number of these cells have been observed in the alveolar bone of female rats treated with estradiol. In the present study, the expression of estrogen receptor β (ERβ) in the cells of alveolar bone was evaluated in estradiol-treated and -untreated female rats. In order to test the possible direct action of estrogen on osteoclasts, the relationship between apoptosis and ERβ expression in these cells was also analysed. The animals received estradiol for 14 days and the alveolar bone fragments were embedded in paraffin for the quantification of tartrate-resistant acid phosphatase-positive osteoclasts. The expression of ERβ and apoptosis in the osteoclasts were evaluated by ERβ immunohistochemistry and Terminal deoxynucleotidyl transferase-mediated dUTP Nick-End Labelling (TUNEL) methods, respectively. To confirm osteoclast death by apoptosis, these cells were analysed under transmission electron microscopy. Some osteoclasts from estradiol-treated animals were found to be undergoing apoptosis and the number of tartrate-resistant acid phosphatase-positive osteoclasts was significantly reduced. ERβ immunolabelling was observed in the cytoplasm and nuclei of active osteoblasts, osteocytes and osteoclasts in both groups, suggesting a direct participation of estrogen on alveolar bone cells. However, following estradiol treatment, a strong ERβ immunolabelling was often observed in the TUNEL-positive osteoclasts. Therefore, these results indicate that, in addition to the other signalling pathway, the reduction of alveolar bone resorption is also related to a direct action of estrogen on osteoclasts, promoting apoptosis in these cells, via ERβ.
Keywords: alveolar bone, apoptosis, bone cells, estrogen receptor β, osteoclast
Introduction
Bone is a mineralized tissue that undergoes continuous remodelling by the coordinated action of osteoblasts, osteocytes and osteoclasts, which maintain the bone tissue homeostasis. This process is controlled by local and systemic factors that regulate the proliferation, differentiation, activity and survival of bone cells. Estrogen plays an important role in the maintenance of bone homeostasis and is widely used to inhibit bone resorption in menopausal women (Rickard et al. 1999; Xing & Boyce, 2005). It is generally accepted that estrogen participates in the production of growing factors and cytokines, which are important mediators for osteoclast formation, activity and survival (Riggs, 2000; Phan et al. 2004). Estrogen stimulates the release of transforming growth factor beta, which inhibits osteoclast activity (Hughes & Boyce, 1997). This steroidal hormone also down-regulates interleukin-1, interleukin-6, tumoral necrosis factor alpha, macrophage colony-stimulating factor and prostaglandin-E2, which in turn exert important roles in the osteoclast differentiation (Riggs, 2000; Phan et al. 2004). Estrogen also stimulates the secretion of osteoprotegerin (OPG), a soluble decoy receptor produced by osteoblasts that binds to the receptor activator of nuclear factor-kappa B ligand (RANKL) and suppresses the activation of receptor activator of nuclear factor-kappa B (RANK) in the osteoclast precursors. Therefore, estrogen may also interfere in the RANK–RANKL–OPG signalling pathway and then inhibits osteoclast formation and bone resorption (Riggs, 2000; Hofbauer & Heufelder, 2001; Väänänen, 2005). All of these studies have provided strong evidence of the indirect effects of estrogen on bone resorption. However, some authors have suggested that estrogen reduces bone resorption by acting directly on osteoclasts (Kameda et al. 1997; Parikka et al. 2001). In-vivo (Hughes et al. 1996; Faloni et al. 2007) and in-vitro (Kameda et al. 1997; Stern, 2007) studies have demonstrated that estrogen induces osteoclast apoptosis and this process of cell death has been related, at least in part, to the reduction of the osteoclast number in the alveolar bone of female rats treated with estradiol (Faloni et al. 2007).
The biological effect of estrogen on target cells, including bone cells, is mediated by two estrogen receptors (ERs) – ERα and ERβ (Vidal et al. 1999; Bord et al. 2001; Braidman et al. 2001; Nilsson et al. 2001; Sørensen et al. 2006). However, the role of estrogen on bone resorption has not yet been clarified. There is evidence in vivo that both receptors are expressed in bone cells, including osteoclasts (Bord et al. 2001), suggesting a direct action of estrogen on these cells. However, in-vitro analyses have demonstrated that mature osteoclasts express only ERβ. With regard to the fact that preosteoclasts are positive for both receptors (ERα and ERβ), it has been suggested that estrogen directly attenuates osteoclastogenesis but has no effect on resorption by mature osteoclasts (Sørensen et al. 2006).
Most studies on the effect of estrogen on bone cells are focused on in-vitro and/or in-vivo analyses of cells from long bones (Bord et al. 2001; Piva et al. 2005; Sørensen et al. 2006). However, in-vivo evidence that confirms a direct effect of estrogen on alveolar bone osteoclasts is scarce in the literature. The alveolar bone of young rats exhibits rapid and intense remodelling to accommodate the growing and eruption of teeth. Therefore, this tissue is a suitable in-vivo model to investigate the hormonal action on bone cells (Faloni et al. 2007). Recently, osteoclast apoptosis and a significant reduction in the number of these cells were observed in the alveolar bone of female rats treated with estradiol for 7 days (Faloni et al. 2007). In the present study, we proposed to evaluate the expression of ERβ in the alveolar bone cells of untreated and female rats treated with estradiol for 14 days. In order to test the possible direct action of estrogen on bone resorption, via osteoclasts, the number of osteoclasts and the double-labelling ERβ/Terminal deoxynucleotidyl transferase-mediated dUTP Nick-End Labelling (TUNEL) were also analysed.
Materials and methods
Ten 22-day-old female Holtzman rats (Rattus norvegicus albinus) were maintained in a room with controlled temperature (23 ± 2 °C) and standard lighting conditions (12-h light/dark cycle) with food and water ad libitum. National guidelines for laboratory animal care were followed in this study, which was authorized by the Ethical Committee for Animal Research of the São Paulo Federal University, Brazil (UNIFESP/EPM).
The animals were divided into two groups, estrogen (EG) and sham (SG), containing five animals each. The rats from the EG received intramuscular injections of 0.125 mg 100 g−1 body weight of estrogen (estradiol hexahydrobenzoate, Benzoginoestril®, Sanofi Aventis, Brazil), diluted in corn oil, for 14 days. The rats of the SG received the same dosage of corn oil as used as vehicle for estrogen dilution. At 24 h after the last injection, the rats from the EG and SG were killed with chloral hydrate (600 mg kg−1 body weight). Fragments of the maxilla containing alveolar bone surrounding the first molars were removed and immediately immersed in the fixative solutions.
Light microscopy
Fragments of the maxilla were fixed in 4% formaldehyde (prepared from paraformaldehyde), buffered at pH 7.2 with 0.1 m sodium phosphate, for 48 h. After decalcification for 45 days in 7% EDTA solution containing 0.5% formaldehyde, in 0.1 m sodium phosphate buffer at pH 7.2, the specimens were dehydrated and embedded in paraffin. The sections (6 μm thick) were stained with haematoxylin and eosin, and submitted to the tartrate-resistant acid phosphatase (TRAP) reaction. Sections adhered to silanized slides were submitted to the TUNEL method, immunohistochemistry for the detection of ERβ and ERβ/TUNEL combined methods.
TRAP
The TRAP method was used as an osteoclast marker (Boabaid et al. 2001; Faloni et al. 2007; Blumer et al. 2008). Deparaffinized sections were immersed in medium prepared by dissolving 8 mg of naphthol AS-BI (Sigma Chemical Company, St Louis, MO, USA) in 500 μL of N–N-dimethylformamide (Sigma Chemical Company) followed by the addition of 50 mL of 0.2 m sodium acetate buffer (pH 5.0) containing 70 mg of Fast Red Salt (Sigma Chemical Company). Sodium tartrate dihydrate (50 mm) was added and the medium was filtered. After incubation at 37 °C, the sections were washed in distilled water and counterstained with haematoxylin.
Number of TRAP-positive osteoclasts per millimetre of alveolar bone surface
The number of osteoclasts in the alveolar bone surface was quantified in the sections submitted to the TRAP reaction. Three non-serial sections of the alveolar bone surface surrounding the upper first molar from each animal were used, totalling around 18 500 μm of bone surface per animal. The shortest distance between the sections was 60 μm.
The linear surface of alveolar bone (in mm) was measured by using an image analysis system (Image-Pro Express 6.0, Olympus) at × 4. Multinucleated TRAP-positive osteoclasts on the alveolar bone surface were counted using a light microscope (Carl Zeiss, Inc., Jena, Germany) at × 400.
Statistical analysis
The differences between the groups were statistically analysed by the SigmaStat 2.0 software (Jandel Scientific, Sausalito, CA, USA), the Mann–Whitney test was applied and the significance level accepted was P ≤ 0.05.
Immunohistochemistry for ERβ
For antigen retrieval, deparaffinized sections were immersed in 0.001 m sodium citrate buffer, pH 6.0, and maintained at 90–94 °C in a microwave oven for 30 min (Sasso-Cerri et al. 2005). After a cooling-off period, the endogenous peroxidase was blocked with 3% hydrogen peroxide for 20 min. The slides were washed in 0.05 m Tris–HCl-buffered saline (TBS) at pH 7.2 and treated with 2% bovine serum albumin for 20 min at room temperature (23 ± 2 °C). Subsequently, the sections were incubated with the primary rabbit antibody anti-rat ERβ (Upstate Cell Signalling Solutions, Lake Placid, NY, USA), diluted 1 : 200 in TBS, for 16 h in a humidified chamber at 4 °C. After washings in TBS, the immunoreaction was detected by a Vectastain Kit (Vector Laboratories, Inc., Burlingame, CA, USA). Sections were incubated at room temperature with biotinylated anti-rabbit IgG for 30 min and, after washings in TBS, were incubated with avidin-biotin-peroxidase complex for 30 min. Peroxidase activity was revealed by 0.06% 3,3′-diaminobenzedine (Sigma-Aldrich, Chemie, Germany) in TBS; some sections were counterstained with Carazzi’s haematoxylin. As negative controls, the immunohistochemical reaction was performed replacing the primary antibody by bovine serum albumin. Sections of uterus were used as positive controls for the ERβ immunohistochemical reaction.
TUNEL method
The TUNEL method for the detection of DNA breaks (Gavrieli et al. 1992) was performed by using the ApopTag® Plus Peroxidase In Situ Apoptosis Detection Kit (Chemicon International, Chemicula, CA, USA). The protocol was performed as previously described (Cerri et al. 2000; Cerri, 2005). The reaction was revealed by using a Vip Substrate Kit (Vector Laboratories, Inc.) and the sections were counterstained with methyl green (Merck, Germany). Sections of mammary gland provided by the manufacturer of the kit were used as positive controls. Negative controls were incubated in a Terminal deoxynucleotidyl transferase-free enzyme solution.
Double labelling: ERβ immunohistochemistry and TUNEL reaction
In some sections, immunohistochemistry for ERβ detection was carried out and, subsequently, the same sections were submitted to the TUNEL method for detection of cell death. The sections were then counterstained with methyl green, dehydrated and mounted.
Transmission electron microscopy
Specimens containing alveolar bone of the first molars were fixed for 16 h in a mixture of 4% glutaraldehyde and 4% formaldehyde (freshly prepared from paraformaldehyde) buffered at pH 7.2 with 0.1 m sodium cacodylate. After decalcification for 45 days in a 7% solution of EDTA, the specimens were postfixed in cacodylate-buffered 1% osmium tetroxide for 1.5 h. The specimens were then immersed in 2% aqueous uranyl acetate for 2 h, dehydrated in graded concentrations of ethanol, treated with propylene oxide and then embedded in Araldite.
Semithin sections stained by 1% toluidine blue were examined in a light microscope and suitable regions were carefully selected for trimming of the blocks. Ultrathin sections were collected onto grids and stained in alcoholic 1% uranyl acetate and in lead citrate solution and examined in a Philips CM 100 transmission electron microscope.
Results
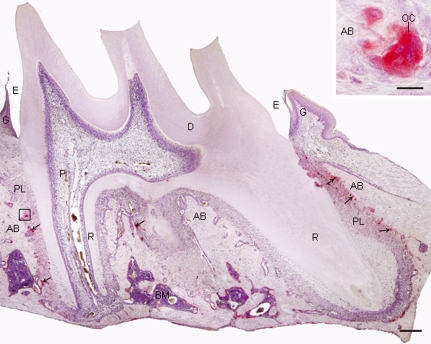
Examination of the alveolar bone from the upper first molar of young rats (36 days old) revealed several multinucleated osteoclasts of variable shapes apposed to resorption bone surfaces; these osteoclasts exhibited conspicuous TRAP activity (Fig. 1). In comparison to the SG, an apparent decrease in the number of TRAP-positive osteoclasts was observed in the alveolar bone surface of the EG. The quantitative analysis revealed that the number of TRAP-positive osteoclasts per mm of alveolar bone surface decreased in all animals of the EG, resulting in a decrease of 52% in the EG compared with the SG. The difference between the groups was statistically significant (Table 1).
Fig. 1.
Light micrograph of a sagittal section of the first upper molar submitted to the TRAP reaction and counterstained with haematoxylin. The alveolar bone (AB) surrounding the molar roots (R) exhibits TRAP-positive osteoclasts, in red (arrows). Inset: high magnification of the outlined area; strong TRAP activity is observed in the cytoplasm of the osteoclast (OC). PL, periodontal ligament; D, dentine; P, dental pulp; E, enamel space; G, gingiva; BM, bone marrow. Bar: 200 μm; 15 μm (inset).
Table 1.
Number of TRAP-positive osteoclasts per mm of alveolar bone linear surface in the animals from the SG and EG.
| Animals | SG | EG* |
|---|---|---|
| 1 | 2.69 | 1.88 |
| 2 | 4.36 | 2.01 |
| 3 | 2.33 | 0.84 |
| 4 | 3.27 | 1.46 |
| 5 | 2.58 | 1.06 |
| Mean ± SD | 3.0 ± 0.81 | 1.45 ± 0.50 |
SD: Standard deviation.
Statistically significant (P ≤ 0.05).
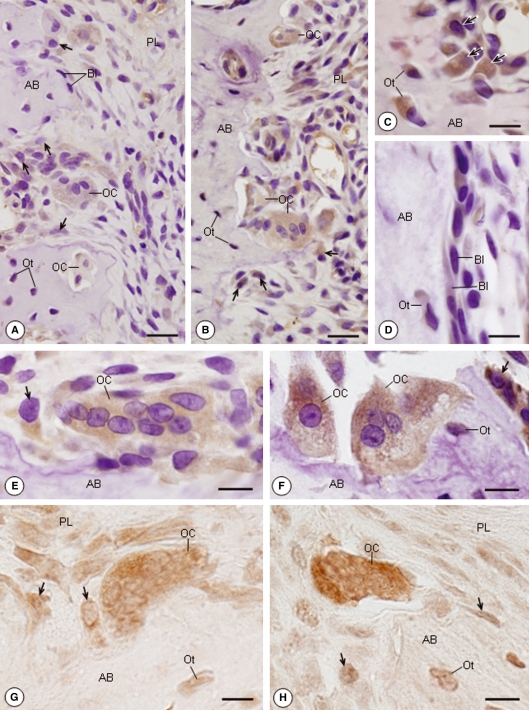
Sections of alveolar bone from the EG and SG submitted to immunohistochemistry for ERβ detection exhibited positive immunolabelling in the cytoplasm of the bone cells, characterized by a brown/yellow stain (Fig. 2A–F). A strong immunoreaction was often found in the cytoplasm of large osteoblasts apposed to the matrix bone in formation (Fig. 2A,C,E). However, the quiescent osteoblasts (bone-lining cells) carpeting the bone surface were weakly immunolabelled or negative on immunohistochemistry (Fig. 2A,D). ERβ-positive immunolabelling was also observed in the cytoplasm of osteocytes (Fig. 2C,D). The cytoplasm of osteoclasts located in the bone excavations was positive on ERβ immunohistochemistry in both groups. However, in comparison to the SG, an enhanced positive immunolabelling was evident in the osteoclasts of the EG (Fig. 2E–H). When haematoxylin counterstaining was omitted, a slight immunolabelling for ERβ was also observed in the nuclei of osteoblasts, osteocytes and osteoclasts in the SG and EG (Fig. 2G,H).
Fig. 2.
Light micrographs of portions of alveolar bone (AB) of rats from the SG (A,E,G) and EG (B–D,F,H) submitted to immunohistochemistry for detection of ERβ. (A,B) Osteoblasts (arrows) and osteoclasts (OCs) exhibit a positive immunoreaction in their cytoplasm (brown/yellow colour). Bl, Bone-lining cells; Ot, osteocytes; PL, periodontal ligament. Bar: 30 μm. (C) In regions where bone formation is evident, large osteoblasts (arrows) apposed to the bone surface and Ots show strongly immunolabelled cytoplasm. Bar: 12 μm. (D) Bls, apparently inactive osteoblasts, exhibit weak or negative ERβ immunolabelling. Immunoreaction is observed in the Ot. Bar: 10 μm. (E,F) Multinucleated OCs adjacent to the bone surface exhibit immunopositive cytoplasm; a strong immunostaining is observed in the EG (F) in comparison to the SG (E). Immunopositive osteoblasts (arrows) and Ot. Bar: 10 μm. (G,H) Immunohistochemistry reaction without counterstaining with haematoxylin. The nuclei of osteoblasts (arrows), Ots and OCs are also positive to ERβ immunolabeling. In the OCs, a conspicuous immunostaining is observed in the cytoplasm in contrast to the nuclei. Note an enhanced immunolabelling in the OC cytoplasm of the EG (H) in comparison to the SG (G). Bar: 7 μm (G); 8 μm (H).
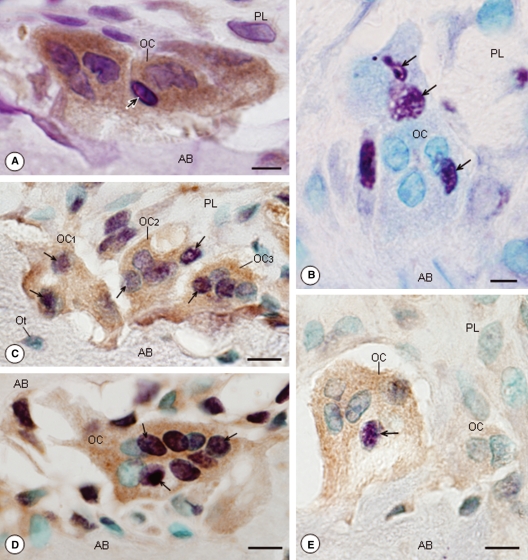
In the EG, some osteoclasts with conspicuous immunoreaction in their cytoplasm exhibited condensed chromatin strongly stained by haematoxylin, typical of apoptosis (Fig. 3A). Osteoclasts with TUNEL-positive nuclei, stained in purple, were found in this group (Fig. 3B). After the double labelling, osteoclasts that were strongly ERβ-immunopositive in the EG showed all or almost all nuclei positive in the TUNEL method (Fig. 3C–E). However, in the SG, no TUNEL labelling was found in the ERβ-immunopositive osteoclasts (data not illustrated).
Fig. 3.
Light micrographs of portions of alveolar bone (AB) of rats from the EG. (A) A portion of AB submitted to immunohistochemistry for ERβ detection and counterstained with haematoxylin. The osteoclast (OC), with enhanced ERβ immunolabelling in the cytoplasm (brown/yellow colour), shows a nucleus with condensed chromatin strongly stained by haematoxylin (arrow). PL, periodontal ligament. Bar: 6 μm. (B) A portion of AB submitted to the TUNEL method (purple colour) and counterstained with methyl green. The OC adjacent to the bone surface exhibits some TUNEL-positive nuclei (arrows). Bar: 5 μm. (C,D,E) Portions of AB submitted to the combined methods [immunohistochemistry for ERβ detection (brown/yellow colour) and TUNEL method (purple colour)] and counterstained with methyl green. The OCs are strongly stained by ERβ immunolabelling (brown/yellow colour) and show TUNEL-positive nuclei (purple colour; arrows). In (C), all nuclei (OC1 and OC2) or almost all nuclei (OC3) are TUNEL-positive; in (D), most nuclei are strongly TUNEL-positive (arrows). Bar: 10 μm (C); 9 μm (D); 9 μm (E). Ot, osteocytes.
The uterine sections that were used as a positive control for the immunohistochemistry showed ERβ-positive immunolabelling in the epithelium, endometrial glands and myometrium. Positive immunolabelling was not found in the alveolar bone sections that were used as a negative control. The mammary gland sections that were used as a positive control for the TUNEL method showed numerous TUNEL-positive cells, whereas in the alveolar bone sections that were used as a negative control, no TUNEL-positive structures were found (data not illustrated).
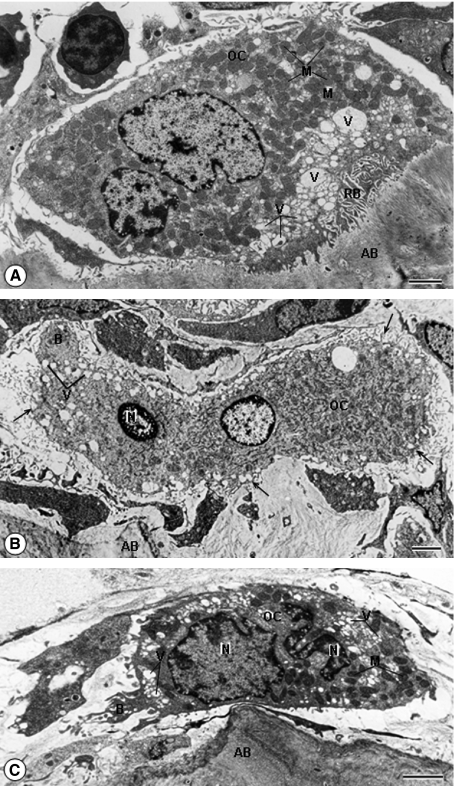
The ultrastructural examination revealed, in the SG, several osteoclasts exhibiting numerous mitochondria and vacuoles next to the ruffled border, apposed to the bone surface (Fig. 4A). Multinucleated osteoclasts exhibiting a convoluted nucleus with masses of condensed chromatin were found in the alveolar bone of the EG. These altered osteclasts, apparently shrunken, exhibited numerous vacuoles and mitochondria; cytoplasmic projections and bleb-like structures protruding from the irregularly outlined surface were also observed in these cells. Usually, a ruffled border and clear zone were not observed in the altered osteoclasts (Fig. 4B,C).
Fig. 4.
Electron micrographs of portions of alveolar bone (AB) of rats from the SG (A) and EG (B,C). (A) A typical osteoclast (OC), exhibiting numerous mitochondria (M) and vacuoles (V) next to ruffled border (RB), is apposed to the bone surface. Bar: 2 μm. (B) A multinucleated osteoclast (OC) located next to the bone surface (AB) shows a nucleus (N) with condensed peripheral chromatin. The OC cytoplasm, with organelles apparently intact, shows cytoplasmic projections (arrows) and a bleb-like structure (B) protruding from the irregularly outlined surface. Bar: 2.5 μm. (C) In the OC, the electron-opaque cytoplasm is apparently shrunken and the convoluted nuclei show masses of condensed chromatin (N). The cytoplasm, containing several vacuoles (V) and mitochondria (M), exhibits a bleb-like structure protruding from the osteoclast surface (B). Bar: 2 μm.
Discussion
In the present study, ERβ was immunohistochemically detected in the alveolar bone cells of young female rats, suggesting that estrogen may interfere directly in alveolar bone homeostasis, including bone resorption. ERs are transcription factors that are expressed in different tissues and cell types. It has been suggested that estrogens diffuse in and out of cells but are retained in target cell nuclei by ER protein (Brzozowski et al. 1997). Although the nuclei were positive on immunolabelling, enhanced immunostaining was also observed in the cytoplasm of bone cells. Considering that ERs are translocated into the nucleus after ligand binding, it has been suggested that immunoreactivity in the cytoplasm may therefore represent non-ligand-associated receptors (Jensen & DeSombre, 1973; Vidal et al. 1999). Once bound by estrogens, ER undergoes a conformational change, allowing the receptor to interact with chromatin and to modulate the expression of target genes (Brzozowski et al. 1997). However, ERβ has also been localized in the mitochondria (McEwen et al. 2001; Cammarata et al. 2004; Chen et al. 2004, 2005; Yang et al. 2004; Levin, 2005; Yager & Chen, 2007), suggesting that this organelle is an important target for the action of estrogen (Simpkins et al. 2008).
The pattern of ERβ immunolabelling has been demonstrated to differ significantly in the cortical and cancellous bone. In cortical bone, osteoblasts and osteocytes show weak positivity for ERβ compared with cancellous bone (Bord et al. 2001). The immunohistochemistry for ERβ revealed a strong immunolabelling in the large osteoblasts (active cells) adjacent to the forming alveolar bone surface, whereas quiescent osteoblasts (bone-lining cells) exhibited weak or negative immunoreaction. Thus, it is possible that the variations in the intensity of the immunolabelling of ER subtypes may not be due only to differences in the type of bone (cortical or cancellous) but could be related to the cellular activity. Previous studies have shown that estrogen exerts a direct action on osteoblasts, promoting an anti-apoptotic effect (Kousteni et al. 2002; Xing & Boyce, 2005) and stimulating bone formation (Liu & Howard, 1991; Chow et al. 1992; Michael et al. 2005). Furthermore, ERβ may play an active part in the osteogenic differentiation of periodontal ligament cells (Tang et al. 2008). Therefore, it is conceivable that estrogen–ERβ binding may play a role in the control of osteoblast activity in the alveolar bone.
Although the osteocytes are enclosed inside lacunae, these cells exhibit several cytoplasmic processes that communicate with neighbouring osteocytes and osteoblasts (Blumer et al. 2008), and thereby participate in the maintenance of the balance between bone formation and resorption. It has been demonstrated that estrogen modulates the life span of osteocytes as estrogen deficiency, as osteoporosis, leads to osteocyte apoptosis in both humans and mice (Tomkinson et al. 1997; Kousteni et al. 2002). Our results are in agreement with previous findings (Manolagas et al. 2002) and reinforce the direct role of estrogen on these cells.
The reduction of bone resorption by estrogen has been related to multiple effects on bone cells and their precursors (Väänänen, 2005). Several studies have demonstrated that estrogen acts on the osteoblastic lineage as an indirect pathway to inhibit bone resorption. Estrogen binds to osteoblastic cells and suppresses the production of several cytokines, such as interleukin-1, tumour necrosis factor, interleukin-6 and macrophage colony-stimulating factor (Rickard et al. 1999; Phan et al. 2004; Michael et al. 2005). These cytokines interfere in the osteoclastogenesis as well as in the activity and survival of osteoclasts, acting indirectly on bone resorption (Riggs, 2000; Phan et al. 2004). Moreover, it has been demonstrated that estrogen interferes in the RANK–RANKL interaction, which plays an important role in osteoclastogenesis (Hofbauer & Heufelder, 2001). Estrogen stimulates the secretion of OPG, a glycoprotein produced by osteoblasts, which acts as a decoy receptor for RANKL and thereafter inhibits osteoclast formation (Hofbauer & Heufelder, 2001; Phan et al. 2004; Väänänen, 2005). Thus, it is well established that estrogen may act indirectly on bone resorption, via osteoblasts and mesenchymal cells, by different pathways (Riggs, 2000; Phan et al. 2004). However, in-vivo studies showing evidence of the direct role of estrogen on the alveolar bone osteoclasts are scarce in the literature. In the present study, ERβ-positive osteoclasts were observed in untreated and estrogen-treated female rats, indicating that alveolar bone osteoclasts are target cells for estrogen. This conclusion is reinforced by the fact that an enhanced immunolabelling was found in the osteoclasts of estrogen-treated rats. High concentrations of a determined hormone are associated with an increased number of receptors, which accentuate the hormonal cell sensibility (Rhoades & Tanner, 1995). The increased expression (up-regulation) of ERβ in the prostate has been demonstrated in newborn male rats after treatment with synthetic estrogen (diethylstilbestrol) (Khurana et al. 2000). Thus, our immunohistochemical findings provide clear evidence that alveolar bone osteoclasts can be directly controlled by estrogen.
A significant decrease in the number of osteoclasts was found in the estradiol-treated female rats. These cells were TUNEL-positive and showed typical characteristics of apoptosis, such as shrinkage, nuclei with peripheral condensed chromatin and bleb-like projections in the cytoplasmic surface (Cerri et al. 2000; Boabaid et al. 2001; Cerri, 2005; Cerri & Katchburian, 2005; Sasso-Cerri et al. 2006; Bran et al. 2008). Moreover, the clear zone and ruffled border, structures closely associated with resorptive activity, were not observed in the apoptotic osteoclasts, as described by other authors (Ito et al. 2001; Faloni et al. 2007). In female rats treated with estrogen for 7 days, a reduction in the number of osteoclasts associated with the presence of apoptotic osteoclasts has suggested that estrogen reduces alveolar bone resorption via osteoclast apoptosis (Faloni et al. 2007). The results of the present study provide evidence that estrogen participates in the osteoclast life span as osteoclasts showing apoptotic features or TUNEL-positive osteoclasts showed strong ERβ immunolabelling. According to Tirado et al. (2004) and recent findings obtained in our laboratory, there is a direct parallelism between the overexpression of ERβ and apoptosis in germ cells, similar to what was observed in the present study. However, the way in which estradiol induces apoptosis is not understood. It has been suggested that estrogen could modulate mitochondrial function via ERβ (Cammarata et al. 2004; Chen et al. 2004, 2005; Yang et al. 2004; Levin, 2005; Yager & Chen, 2007; Simpkins et al. 2008). In germ cells, the induction of germ cell apoptosis by estrogen also occurs via mitochondria, in addition to extrinsic pathways (Mishra & Shaha, 2005). It is known that the release of cytochrome c by mitochondria promotes the activation of caspases, a family of proteolytic enzymes that induces apoptosis (Gogvadze & Zhivotovsky, 2007; Bran et al. 2008). Therefore, a possible induction of osteoclast apoptosis by estrogen, via mitochondria, should be further investigated.
In conclusion, the results provide clear evidence that alveolar bone cells can be directly controlled by estrogen and reinforce the idea that the reduction of alveolar bone resorption caused by estrogen resulted, at least in part, from osteoclast apoptosis. The parallelism between ERβ overexpression and apoptosis suggests that estrogen participates in the control of osteoclast life span via ERs. Future studies are necessary to elucidate the intracellular pathway by which estrogen, via ER, induces osteoclast apoptosis.
Acknowledgments
The authors wish to thank Mr Nivalde Basso (Biosciences Institute, UNESP-Botucatu, São Paulo, Brazil) for his assistance with the transmission electron microscope. This research was supported by FUNDUNESP and CNPq (Brazil).
References
- Blumer MJF, Longato S, Fritsch H. Localization of tartrate-resistant acid phosphatase (TRAP), membrane type-1 matrix metalloproteinase (MT1-MMP) and macrophages during early endochondral bone formation. J Anat. 2008;213:431–441. doi: 10.1111/j.1469-7580.2008.00958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boabaid F, Cerri PS, Katchburian E. Apoptotic bone cells may be engulfed by osteoclasts during alveolar bone resorption in young rats. Tissue Cell. 2001;33:318–325. doi: 10.1054/tice.2001.0179. [DOI] [PubMed] [Google Scholar]
- Bord S, Horner A, Beavan S, et al. Estrogen receptors alpha and beta are differentially expressed in developing human bone. J Clin Endocrinol Metab. 2001;86:2309–2314. doi: 10.1210/jcem.86.5.7513. [DOI] [PubMed] [Google Scholar]
- Braidman IP, Hainey L, Batra G, et al. Localization of estrogen receptor beta protein expression in adult human bone. J Bone Miner Res. 2001;16:214–220. doi: 10.1359/jbmr.2001.16.2.214. [DOI] [PubMed] [Google Scholar]
- Bran GM, Stern-Straeter J, Hörmann K, et al. Apoptosis in bone for tissue engineering. Arch Med Res. 2008;39:467–482. doi: 10.1016/j.arcmed.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Brzozowski AM, Pike AC, Dauter Z, et al. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389:753–758. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- Cammarata PR, Chu S, Moor A, et al. Subcellular distribution of native estrogen receptor alpha and beta subtypes in cultured human lens epithelial cells. Exp Eye Res. 2004;78:861–871. doi: 10.1016/j.exer.2003.09.027. [DOI] [PubMed] [Google Scholar]
- Cerri PS. Osteoblasts engulf apoptotic bodies during alveolar bone formation in the rat maxilla. Anat Rec A Discov Mol Cell Evol Biol. 2005;286:833–840. doi: 10.1002/ar.a.20220. [DOI] [PubMed] [Google Scholar]
- Cerri PS, Katchburian E. Apoptosis in the epithelial cells of the rests of Malassez of the periodontium of rat molars. J Periodontal Res. 2005;40:365–372. doi: 10.1111/j.1600-0765.2005.00810.x. [DOI] [PubMed] [Google Scholar]
- Cerri PS, Freymüller E, Katchburian E. Apoptosis in the early developing periodontium of rat molars. Anat Rec. 2000;258:136–144. doi: 10.1002/(SICI)1097-0185(20000201)258:2<136::AID-AR3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Chen JQ, Delannoy M, Cooke C, et al. Mitochondrial localization of ERalpha and ERbeta in human MCF7 cells. Am J Physiol Endocrinol Metab. 2004;286:E1011–E1022. doi: 10.1152/ajpendo.00508.2003. [DOI] [PubMed] [Google Scholar]
- Chen JQ, Yager JD, Russo J. Regulation of mitochondrial respiratory chain structure and function by estrogens/estrogen receptors and potential physiological/pathophysiological implications. Biochim Biophys Acta. 2005;1746:1–17. doi: 10.1016/j.bbamcr.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Chow JW, Lean JM, Chambers TJ. 17 beta-estradiol stimulates cancellous bone formation in female rats. Endocrinology. 1992;130:3025–3032. doi: 10.1210/endo.130.5.1572310. [DOI] [PubMed] [Google Scholar]
- Faloni APS, Sasso-Cerri E, Katchburian E, et al. Decrease in the number and apoptosis of alveolar bone osteoclasts in estrogen-treated rats. J Periodontal Res. 2007;42:193–201. doi: 10.1111/j.1600-0765.2006.00932.x. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogvadze V, Zhivotovsky B. Alteration of mitochondrial function and cell sensitization to death. J Bioenerg Biomembr. 2007;39:23–30. doi: 10.1007/s10863-006-9054-x. [DOI] [PubMed] [Google Scholar]
- Hofbauer LC, Heufelder AE. Role of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin in bone cell biology. J Mol Med. 2001;9:243–253. doi: 10.1007/s001090100226. [DOI] [PubMed] [Google Scholar]
- Hughes DE, Boyce BF. Apoptosis in bone physiology and disease. J Clin Pathol. 1997;50:132–137. doi: 10.1136/mp.50.3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DE, Dai A, Tiffe JC, et al. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-B. Nat Med. 1996;2:1132–1135. doi: 10.1038/nm1096-1132. [DOI] [PubMed] [Google Scholar]
- Ito M, Amizuka N, Nakajima T, et al. Bisphosphonate acts on osteoclasts independent of ruffled borders in osteosclerotic (oc/oc) mice. Bone. 2001;28:609–616. doi: 10.1016/s8756-3282(01)00429-x. [DOI] [PubMed] [Google Scholar]
- Jensen EV, DeSombre ER. Estrogen-receptor interaction. Science. 1973;182:126–134. doi: 10.1126/science.182.4108.126. [DOI] [PubMed] [Google Scholar]
- Kameda T, Mano H, Yuasa T, et al. Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J Exp Med. 1997;186:489–495. doi: 10.1084/jem.186.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana S, Ranmal S, Ben-Jonathan N. Exposure of newborn male and female rats to environmental estrogens: delayed and sustained hyperprolactinemia and alterations in estrogen receptor expression. Endocrinology. 2000;141:4512–4517. doi: 10.1210/endo.141.12.7823. [DOI] [PubMed] [Google Scholar]
- Kousteni S, Chen JR, Bellido T, et al. Reversal of bone loss in mice by nongenotropic signalling of sex steroids. Science. 2002;298:843–846. doi: 10.1126/science.1074935. [DOI] [PubMed] [Google Scholar]
- Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Howard GA. Bone-cell changes in estrogen-induced bone-mass, increase in mice: dissociation of osteoclasts from bone surfaces. Anat Rec. 1991;229:240–250. doi: 10.1002/ar.1092290211. [DOI] [PubMed] [Google Scholar]
- Manolagas SC, Kousteni S, Jilka RL. Sex steroids and bone. Recent Prog Horm Res. 2002;57:385–409. doi: 10.1210/rp.57.1.385. [DOI] [PubMed] [Google Scholar]
- McEwen B, Akama K, Alves S, et al. Tracking the estrogen receptor in neurons: implications for estrogen-induced synapse formation. Proc Natl Acad Sci USA. 2001;98:7093–7100. doi: 10.1073/pnas.121146898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael H, Härkönen P, Väänänen HK, et al. Estrogen and testosterone use different cellular pathways to inhibit osteoclastogenesis and bone resorption. J Bone Miner Res. 2005;20:2224–2232. doi: 10.1359/JBMR.050803. [DOI] [PubMed] [Google Scholar]
- Mishra DP, Shaha C. Estrogen-induced spermatogenic cell apoptosis occurs via the mitochondrial pathway. Role of superoxide and nitric oxide. J Biol Chem. 2005;18:6181–6196. doi: 10.1074/jbc.M405970200. [DOI] [PubMed] [Google Scholar]
- Nilsson S, Mäkelä S, Treuter E, et al. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- Parikka V, Lehenkari P, Sassi ML, et al. Estrogen reduces the depth of resorption pits by disturbing the organic bone matrix degradation activity of mature osteoclasts. Endocrinology. 2001;142:5371–5378. doi: 10.1210/endo.142.12.8533. [DOI] [PubMed] [Google Scholar]
- Phan TC, Xu J, Zheng MH. Interaction between osteoblast and osteoclast: impact in bone disease. Histol Histopathol. 2004;19:1325–1344. doi: 10.14670/HH-19.1325. [DOI] [PubMed] [Google Scholar]
- Piva R, Penolazzi L, Lambertini E, et al. Induction of apoptosis of human primary osteoclasts treated with a transcription factor decoy mimicking a promoter region of estrogen receptor alpha. Apoptosis. 2005;10:1079–1094. doi: 10.1007/s10495-005-0618-8. [DOI] [PubMed] [Google Scholar]
- Rhoades RA, Tanner GA. Medical Physiology. Boston: Little, Brown and Company; 1995. p. [Google Scholar]
- Rickard DJ, Subramaniam M, Spelsberg TC. Molecular and cellular mechanisms of estrogen action on the skeleton. J Cell Biochem Suppl. 1999;32/33:123–132. doi: 10.1002/(sici)1097-4644(1999)75:32+<123::aid-jcb15>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Riggs BL. The mechanisms of estrogen regulation of bone resorption. J Clin Invest. 2000;106:1203–1204. doi: 10.1172/JCI11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasso-Cerri E, Freymüller E, Miraglia SM. Testoterone-immunopositive primordial germ cells in the testis of the bullfrog, Rana catesbeiana. J Anat. 2005;206:519–523. doi: 10.1111/j.1469-7580.2005.00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasso-Cerri E, Cerri PS, Freymüller E, et al. Apoptosis during the seasonal spermatogenic cycle of Rana catesbeiana. J Anat. 2006;209:21–29. doi: 10.1111/j.1469-7580.2006.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins JW, Yang SH, Sarkar SN, et al. Estrogen actions on mitochondria-physiological and pathological implications. Mol Cell Endocrinol. 2008;290:51–59. doi: 10.1016/j.mce.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen MG, Henriksen K, Dziegiel MH, et al. Estrogen directly attenuates human osteoclastogenesis, but has no effect on resorption by mature osteoclasts. DNA Cell Biol. 2006;25:475–483. doi: 10.1089/dna.2006.25.475. [DOI] [PubMed] [Google Scholar]
- Stern PH. Antiresorptive agents and osteoclasts apoptosis. J Cell Biochem. 2007;101:1087–1096. doi: 10.1002/jcb.21311. [DOI] [PubMed] [Google Scholar]
- Tang X, Meng H, Han J, et al. Up-regulation of estrogen receptor-B expression during osteogenic differentiation of human periodontal ligament cells. J Periodont Res. 2008;43:311–321. doi: 10.1111/j.1600-0765.2007.01031.x. [DOI] [PubMed] [Google Scholar]
- Tirado OM, Selva DM, Toran N, et al. Increased expression of estrogen receptor beta in pachytene spermatocytes after short-term methoxyacetic acid administration. J Androl. 2004;25:84–94. doi: 10.1002/j.1939-4640.2004.tb02762.x. [DOI] [PubMed] [Google Scholar]
- Tomkinson A, Reeve J, Shaw RW, et al. The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J Clin Endocrinol Metab. 1997;82:3128–3135. doi: 10.1210/jcem.82.9.4200. [DOI] [PubMed] [Google Scholar]
- Väänänen K. Mechanism of osteoclast mediated bone resorption – rationale for the design of new therapeutics. Adv Drug Deliv Rev. 2005;57:959–971. doi: 10.1016/j.addr.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Vidal O, Kindblom LG, Ohlsson C. Expression and localization of estrogen receptor-beta in murine and human bone. J Bone Miner Res. 1999;14:923–929. doi: 10.1359/jbmr.1999.14.6.923. [DOI] [PubMed] [Google Scholar]
- Xing L, Boyce BF. Regulation of apoptosis in osteoclasts and osteoblastic cells. Biochem Biophys Res Commun. 2005;328:709–720. doi: 10.1016/j.bbrc.2004.11.072. [DOI] [PubMed] [Google Scholar]
- Yager JD, Chen JQ. Mitochondrial estrogen receptors – new insights into specific functions. Trends Endocrinol Metab. 2007;18:89–91. doi: 10.1016/j.tem.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Yang SH, Liu R, Perez EJ, et al. Mitochondrial localization of estrogen receptor beta. Proc Natl Acad Sci USA. 2004;101:4130–4135. doi: 10.1073/pnas.0306948101. [DOI] [PMC free article] [PubMed] [Google Scholar]