Abstract
The progeny of embryonic stem (ES) cells may eventually be used to replace damaged tissues in transplantation, yet their immunogenicity remains ill-defined. The major histocompatibility complex (MHC) is a determinant of immunogenicity in transplantation. Herein, we show differences in MHC expression between mouse ES cells and ES cell derived insulin producing cell clusters (IPCCs), including a relatively higher expression of MHC Class I in IPCCs and a faster, more dramatic induction of MHC Class I in IPCCs following challenge with interferon-γ (IFN-γ). MHC Class II was induced on IPCCs, but not ES cells, after exposure to IFN-γ. Transplantation of syngeneic or allogeneic IPCCs was insufficient to trigger up-regulation of MHC class I within three days after transplantation. These data highlight differences in MHC expression between ES cells and a fully differentiated ES cell derived tissue and suggest how the progeny of ES cells may be susceptible to rejection after transplantation.
Keywords: ES cells, immunogenicity, pancreatic
The progeny of embryonic stem (ES) cells may eventually be used to provide an alternative or supplementary source of tissue for cell replacement therapy in diseases that lead to organ degeneration or failure, such as Type 1 diabetes (1, 2). However, the use of such ES-cell derived tissues in transplantation will, in part, rely on an appreciation of how the host immune system will respond when these tissues are transplanted, an issue that hitherto remains ill-defined (1, 3).
The principal system responsible for the recognition of transplanted allogeneic tissues by the immune system is the major histocompatibility complex (MHC) (4, 5). The MHC encodes a series of highly polymorphic genes (6) and produces two classes of cell surface glycoproteins, referred to as class I and class II MHC molecules. MHC molecules present antigen in the form of peptides to T cells (7). Recognition of these MHC-peptide complexes is a requisite step in the instigation of an adaptive immune response (7).
While we and others have tackled the issue of expression of MHC molecules in undifferentiated ES cells (hereafter ES cells) and partially differentiated embryoid bodies (EBs) (8-11), MHC characterisation of fully differentiated ES cell derived tissue has been lacking. We therefore compared MHC expression in ES cells and ES cell derived insulin-producing cell clusters (IPCCs) during differentiation and after challenge with the inflammatory mediator IFN-γ in vitro and in vivo following transplantation.
MHC expression was initially assessed in two ES cell lines, ESF122 (H2k) and ESF134 (H2b). Undifferentiated ESF122 and ESF134 cell lines were devoid of class I and class II MHC expression, as assessed by flow cytometry (Figure 1A). In sharp contrast, splenocytes isolated from C57BL/6 mice strongly expressed class I and class II MHC molecules (data not shown). MHC Class I expression in ESF 122 was also undetectable when gauged by immunohistochemistry (IHC) on cytospin preparations (Figure 1B). These data indicate that ES cells fail to appreciably express either class I or class II MHC molecules.
Figure 1. Expression of MHC molecules in undifferentiated ES cells under homeostasis and following treatment with IFN-γ in vitro.
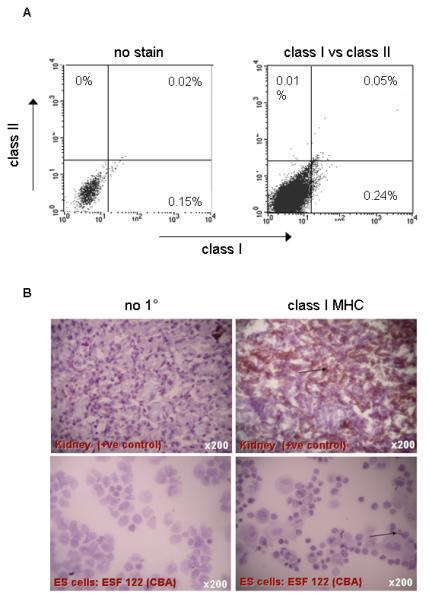
A: No MHC class I and II expression above background was observed, as judged by flow cytometry of single cell suspensions of ESF 122 (H2k) or ESF134 (H2b) ES cells. Data are representative of n=3 experiments. In contrast, splenocytes isolated from C57BL/6 mice served as positive controls for MHC staining and strongly expressed class I and class II MHC molecules (data not shown).
B: MHC class I expression was also undetectable by immunohistochemistry. Cytospin preparations of ESF 122 (H2k) ES cells were stained for class I MHC. Photographs were taken with a Nikon Coolpix camera attached to a Zeiss Axiovert light microscope. Control staining with no primary antibody (no 1°) is shown in the left panel. Kidney served as positive control (see arrow) and ES cells were devoid of MHC Class I expression (see arrow).
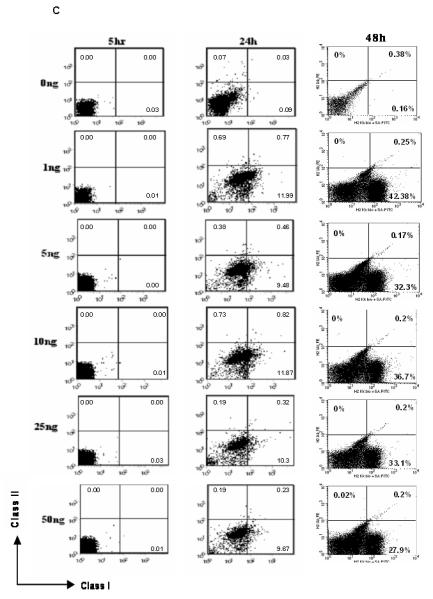
C: ESF 122 ES cells were stimulated in vitro with varying doses of IFN-γ over several time points to determine if IFN-γ treatment can alter MHC protein expression. After the allotted time, cells were stained with class I and class II MHC antibodies before acquisition on a flow cytometer. Gates were determined using unstained, isotype and no primary antibody controls. Data are representative of n=3 experiments per time point.
Transplantation of ES cell derivatives may elicit the production of inflammatory cytokines, such as interferon-γ (IFN-γ), that can cause an up-regulation of MHC expression (12). To model this, MHC expression was assessed in ES cells following IFN-γ stimulation in vitro. ES cells expressed the IFN-γ receptor (IFN-γR)-β chain and this expression was accentuated following challenge with IFN-γ (data not shown). While 48 hours of stimulation with IFN-γ in vitro markedly augmented the expression of class I MHC proteins on ES cells (Figure 1C), class II MHC expression remained low (Figure 1C). These data show that expression of class I, but not class II MHC, can be induced in ES cells after challenge with IFN-γ.
We next tested the hypothesis that ES cells and fully differentiated ES cell derivatives may exhibit differing MHC molecule expression. Having previously modified and compared methods for the differentiation of ES cells to IPCCs (2), we generated IPCCs from ESF122 using the modified Blyszczuk protocol and assessed the differentiation status of IPCCs in the penultimate (immature) and final week (mature) of the differentiation program. As expected, expression of the undifferentiated ES cell surface marker, SSEA-1, was negligible in both IPCC populations, verifying the fully differentiated status of IPCCs (data not shown). Like ES cells (Figure 1A and B), neither class I nor class II MHC expression was detected in immature IPCCs (Figure 2A). Compared with ES cells (Figure 1B), class I MHC expression in mature IPCCs was clearly detectable, albeit at a low level (Figure 2B). However, in a similar vein to ES cells (Figure 1B), MHC class II expression remained negligible in mature IPCCs (Figure 2B). Thus, contrasting with ES cells, IPCCs express relatively higher levels of class I MHC but not class II MHC.
Figure 2. MHC molecule expression during IPCC development and following treatment with IFN-γ in vitro.
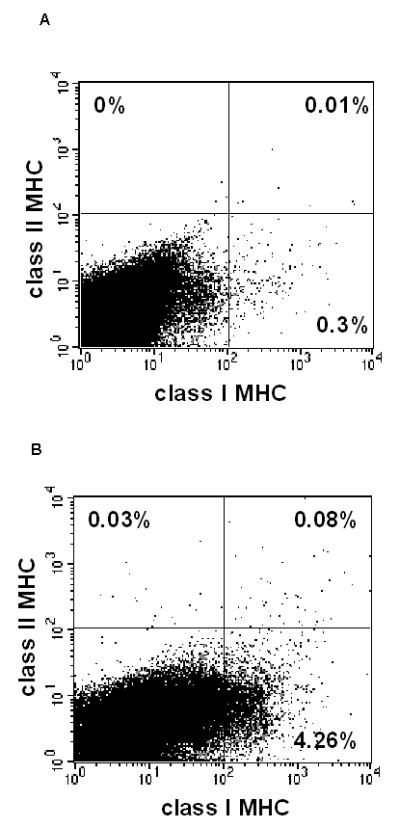
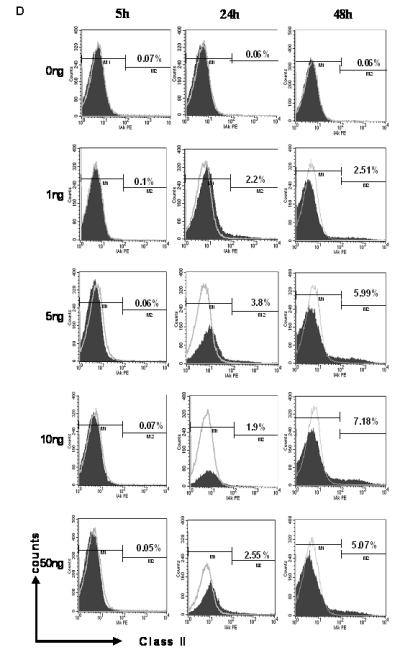
We generated IPCCs from ESF122 ES cells using the modified Blyszczuk protocol (see reference 2) and assessed the differentiation status of IPCCs in the penultimate (immature) and final week (mature) of the differentiation program. As expected, expression of the undifferentiated ES cell surface marker, SSEA-1, was negligible in both IPCC populations, verifying the fully differentiated status of IPCCs (data not shown)
Immature IPCCs were stained for class I MHC and analysed by flow cytometry (A). This was repeated for mature IPCCs (B). MHC Class I expression increases to an average of approx. 4% cells in mature IPCCs, as assessed by flow cytometry. Gates for flow cytometry were set using unstained, isotype and no primary antibody controls. Data are representative of n=3 experiments.
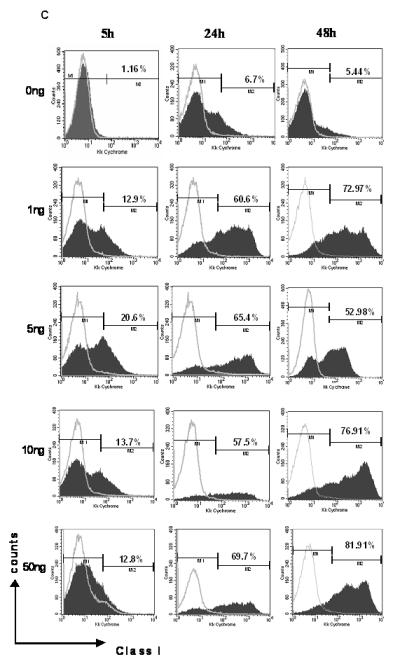
C: IPCCs generated using ESF 122 ES cells were assayed for expression of class I MHC molecules by flow cytometry. The IPCCs were stimulated in vitro with varying doses of IFN-γ over several time points to determine if the IFN-γ treatment would alter the IPCC expression of class I MHC. After the allotted time, the IPCCs were harvested and stained with the corresponding antibodies before acquiring the data on a flow cytometer. Gates were set using unstained, isotype (grey line) and no primary antibody controls. Representative flow cytometry dot plots from n=3 independent differentiation experiments are displayed.
D: IPCCs generated using ESF 122 ES cells were assayed for expression of class II MHC molecules by flow cytometry. The IPCCs were stimulated in vitro with varying doses of IFN-γ over several time points to determine if the IFN-γ treatment would alter the IPCC expression of class II MHC. After the allotted time, the IPCCs were harvested and stained with the corresponding antibodies before acquisition on a flow cytometer. Gates were set using unstained, isotype (grey line) and no primary antibody controls. Representative flow cytometry dot plots from n=3 independent differentiation experiments. The experiments were repeated in another ES cell line, ESF 134, and similar results were obtained (data not shown).
Next, we assessed the potential of IFN-γ to modulate MHC expression on IPCCs. Contrasting with the data obtained for ES cells (Figure 1C), IFN-γ stimulation of IPCCs caused a more drastic increase in expression of class I MHC protein over time, as judged by flow cytometry (Figure 2C). In a further contrast to ES cells, which increased MHC class I within 24 hours of IFN-γ stimulation (Figure 1C), IPCCs up-regulated MHC Class I more rapidly, within 5 hours post-stimulation (Figure 2C). MHC class II expression was, to a much lesser extent than MHC class I, up-regulated in IPCCs at 48 hours post-treatment with IFN-γ and this up-regulation was maintained after up to 72 hours of IFN-γ exposure (Figure 2D and data not shown). These data diametrically oppose the results obtained following IFN-γ stimulation of ES cells, where MHC Class II expression was not up-regulated after 48 hours of IFN-γ exposure (Figure 1C). Thus, prolonged inflammatory challenge of IPCCs changes MHC molecule expression in vitro. Importantly, following inflammatory challenge with IFN-γ, IPCCs exhibit differing MHC expression characteristics compared to ES cells.
As we have previously shown that IPCCs may be rapidly susceptible to rejection following transplantation (8), we evaluated MHC Class I expression at the incipient stages after transplantation of either syngeneic and allogeneic IPCCs under the kidney capsule. Immunohistochemistry was used to detect MHC Class I expression after transplantation; a lack of markers enabling discrimination of graft versus kidney precluded the use of a potentially more sensitive detection method like flow cytometry. In contrast to the host kidney, which stained strongly for class I MHC by immunohistochemistry, transplanted IPCCs did not up-regulate expression of class I MHC molecules after transplantation into either syngeneic or allogeneic recipient mice (Figure 3A). Interestingly, intra-graft IFN-γ was not detected initially by Q-PCR, but was up-regulated at 3 days following transplantation (Figure 3B and Figure 3C). Thus, class I MHC expression was not up-regulated on IPCCs early after transplantation in spite of the presence of a pro-inflammatory environment.
Figure 3. IPCCs do not up-regulate expression of class I MHC molecules at the incipient stages following transplantation.
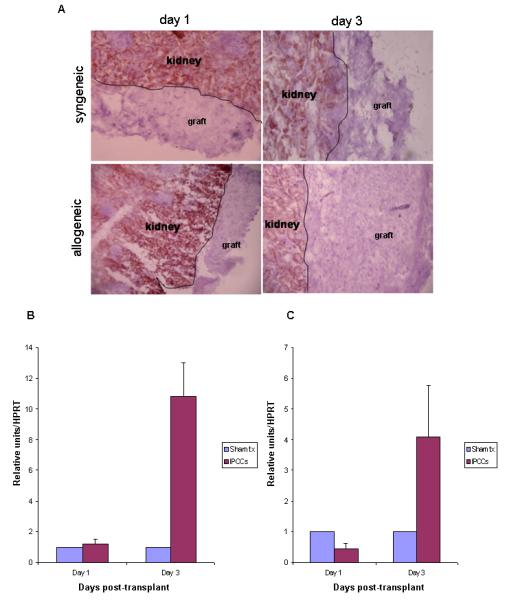
300 ESF 122 derived IPCCs were transplanted under the kidney capsule of syngeneic or allogeneic (C57 BL/10) recipient mice and the graft was removed either 1 day or 3 days later for histological analysis by immunohistochemistry of MHC class I antigen (A) or Q-PCR (B and C). Data for immunohistochemistry are representative of n=3 experiments. Intra-graft mRNA expression for the cytokine IFN-γ was analysed at days 1 and 3 (n=9 mice per time point) after transplantation by Q-PCR. Samples were standardised against HPRT. Data in (B) is from syngeneic IPCCs and (C) from allogeneic IPCCs compared to expression in the kidney of sham transplanted animals which did not receive any IPCCs.
In this report, we assessed MHC expression as a harbinger of the immunogenicity of ES cell derived IPCCs and compared it to MHC molecule expression in undifferentiated ES cells. Our data for MHC expression in undifferentiated ES cells are broadly congruous with that found in other studies (8-11) Interestingly, however, we tabled differences in MHC expression between undifferentiated ES cells and a fully differentiated ES cell tissue, IPCCs. These data emphasize the imperative to directly assess the immunogenicity of fully differentiated ES cell derived tissues in transplantation.
What are the implications of this study for the activation of the immune response after transplantation with fully differentiated ES cell tissue? MHC class I expression on IPCCs, even at the relatively low level observed, may be sufficient to activate T cells through either the direct and/or indirect pathways of allorecognition (13). While it seems unlikely that ES cell differentiation to IPCCs would also give rise to immune cells that could serve as immunostimulatory antigen presenting cells capable of stimulating a direct pathway response, this possibility cannot be ruled out. More likely, however, is the presentation of allopeptides derived from MHC molecules expressed by IPCCs. This route of allorecognition could operate for both mismatched major and minor histocompatibility antigen differences between ES cell progeny and the host. For T cell activation in this setting, processed IPCC-derived peptides would be presented by recipient APCs to recipient T cells. IPCCs may be damaged during the transplantation process or may progressively shed antigen enabling recipient APCs to take up donor-derived peptides for presentation to naïve T cells in the draining lymphoid tissue. Thus, even in the event that MHC antigens were fully matched between the ES donor and the host, mismatched minor histocompatibility antigens and/or novel molecules expressed during ES cell differentiation could stimulate an immune response when presented by host antigen presenting cells.
Our data may have other implications for the immunogenicity of ES cell derived tissue in transplantation. Firstly, as suggested by the IFN-γ assay, MHC expression may be profoundly up-regulated by an inflammatory response in ES cell derived tissue; in cases where such an inflammatory response occurs after transplantation, drastic MHC up-regulation may mark these tissues for rejection. Secondly, the lack of or relatively low levels of MHC class I, as was noted on IPCCs during differentiation, may trigger NK cell mediated destruction and this issue warrants further investigation. Auspiciously, several studies have shown that, in spite of a lack of MHC class I expression, NK cells have not deleteriously impacted graft functionality (14-16).
Acknowledgments
1 Funding for this project was provided by the Medical Research Council and BD Preanalytical Systems Europe through a Collaborative Studentship to ASB. Additional support was provided by The Wellcome Trust and European Union. KJW holds a Royal Society Wolfson Research Merit Award.
Abbreviations
- ES cell
Embryonic stem cell
- IPCC
Insulin-producing cell cluster
- MHC
major histocompatibility complex
- IFN-γ
interferon-gamma
References
- 1.Boyd AS, Higashi Y, Wood KJ. Transplanting stem cells: potential targets for immune attack. Modulating the immune response against embryonic stem cell transplantation. Adv Drug Deliv Rev. 2005;57(13):1944. doi: 10.1016/j.addr.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Boyd AS, Wu DC, Higashi Y, Wood KJ. A comparison of protocols used to generate insulin-producing cell clusters from mouse embryonic stem cells. Stem Cells. 2008;26(5):1128. doi: 10.1634/stemcells.2007-0762. [DOI] [PubMed] [Google Scholar]
- 3.Wu DC, Boyd AS, Wood KJ. Embryonic stem cell transplantation: potential applicability in cell replacement therapy and regenerative medicine. Front Biosci. 2007;12:4525. doi: 10.2741/2407. [DOI] [PubMed] [Google Scholar]
- 4.Peugh WN, Superina RA, Wood KJ, Morris PJ. The role of H-2 and non-H-2 antigens and genes in the rejection of murine cardiac allografts. Immunogenetics. 1986;23(1):30. doi: 10.1007/BF00376519. [DOI] [PubMed] [Google Scholar]
- 5.Wood KJ. Oxford Textbook of Medicine: Principles of transplantation immunology. Oxford University Press; 2003. [Google Scholar]
- 6.Beck S, Trowsdale J. The human major histocompatability complex: lessons from the DNA sequence. Annu Rev Genomics Hum Genet. 2000;1:117. doi: 10.1146/annurev.genom.1.1.117. [DOI] [PubMed] [Google Scholar]
- 7.Janeway CA, Jr., Travers P, Walport M, Shlomchik MJ. Immunobiology: The immune system in health and disease. 5 ed Garland Publishing; New York: 2001. [Google Scholar]
- 8.Wu DC, Boyd AS, Wood KJ. Embryonic stem cells and their differentiated derivatives have a fragile immune privilege but still represent novel targets of immune attack. Stem Cells. 2008;26(8):1939. doi: 10.1634/stemcells.2008-0078. [DOI] [PubMed] [Google Scholar]
- 9.Swijnenburg RJ, Schrepfer S, Cao F, et al. In Vivo Imaging of Embryonic Stem Cells Reveals Patterns of Survival and Rejection Following Transplantation. Stem Cells Dev. 2008 doi: 10.1089/scd.2008.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magliocca JF, Held IK, Odorico JS. Undifferentiated murine embryonic stem cells cannot induce portal tolerance but may possess immune privilege secondary to reduced major histocompatibility complex antigen expression. Stem Cells Dev. 2006;15(5):707. doi: 10.1089/scd.2006.15.707. [DOI] [PubMed] [Google Scholar]
- 11.Bonde S, Zavazava N. Immunogenicity and engraftment of mouse embryonic stem cells in allogeneic recipients. Stem Cells. 2006;24(10):2192. doi: 10.1634/stemcells.2006-0022. [DOI] [PubMed] [Google Scholar]
- 12.Bach EA, Szabo SJ, Dighe AS, et al. Ligand-induced autoregulation of IFN-gamma receptor beta chain expression in T helper cell subsets. Science. 1995;270(5239):1215. doi: 10.1126/science.270.5239.1215. [DOI] [PubMed] [Google Scholar]
- 13.Shoskes DA, Wood KJ. Indirect presentation of MHC antigens in transplantation. Immunol Today. 1994;15(1):32. doi: 10.1016/0167-5699(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 14.Hori J, Ng TF, Shatos M, Klassen H, Streilein JW, Young MJ. Neural progenitor cells lack immunogenicity and resist destruction as allografts. Stem Cells. 2003;21(4):405. doi: 10.1634/stemcells.21-4-405. [DOI] [PubMed] [Google Scholar]
- 15.Mammolenti M, Gajavelli S, Tsoulfas P, Levy R. Absence of major histocompatibility complex class I on neural stem cells does not permit natural killer cell killing and prevents recognition by alloreactive cytotoxic T lymphocytes in vitro. Stem Cells. 2004;22(6):1101. doi: 10.1634/stemcells.22-6-1101. [DOI] [PubMed] [Google Scholar]
- 16.Wei J, Blum S, Unger M, et al. Embryonic endothelial progenitor cells armed with a suicide gene target hypoxic lung metastases after intravenous delivery. Cancer Cell. 2004;5(5):477. doi: 10.1016/s1535-6108(04)00116-3. [DOI] [PubMed] [Google Scholar]


