Abstract
Background
Dairy food and calcium have been hypothesized to play roles that differ among individual cancer sites, but the evidence has been limited and inconsistent. Moreover, the effect of dairy food and calcium on cancer in total is unclear.
Methods
Dairy food and calcium in relation to total cancer as well as cancer at individual sites were examined in the NIH-AARP Diet and Health Study. Intakes of dairy food and calcium from foods and supplements were assessed with a food frequency questionnaire. Incident cancer cases were identified through linkage with state cancer registries. Cox proportional hazard model was used to estimate relative risks (RRs) and tow-sided 95% confidence intervals (CI).
Results
During average of 7 years of follow-up, we identified 36,965 and 16,605 cancer cases in men and women, respectively. Calcium intake was not related to total cancer in men, but was non-linearly associated with total cancer in women: risk decreased up to approximately 1,300 mg/day, above which no further risk reduction was observed. In both men and women, dairy food and calcium were inversely associated with cancers of the digestive system (multivariate RR for the highest quintile of total calcium vs. the lowest = 0.84, 95% CI: 0.77–0.92 in men and 0.77, 95% CI: 0.69–0.91 in women). Decreased risk was particularly pronounced with colorectal cancer. Supplemental calcium was also inversely associated with colorectal cancer risk.
Conclusions
Our study suggests that calcium intake is associated with lower risk of total cancer and cancers of the digestive system, especially colorectal cancer.
Because of the benefit of calcium on bone health 1, dietary guidance has emphasized intakes of both calcium and dairy food. For example, the Institute of Medicine recommends 1,200 mg/day of calcium for adults 50 years old and over 1 and the Dietary Guidelines for Americans, 2005 recommends 3 cups/day of fat-free or low-fat dairy food 2.
The role of dairy food and calcium in cancer, however, has engendered considerable controversy due to observations from epidemiologic studies of protective, null, and even positive associations for different organ sites 3–7. Dairy food and calcium have been consistently inversely associated with colorectal cancer 3, whereas few studies have suggested possible inverse associations with lung and breast cancer 4, 5. On the other hand, positive associations have been reported for prostate and ovarian cancer 6, 7.
Because of limited evidence on the relation of dairy food and calcium to cancers at individual sites, especially cancers with a relatively low incidence, it has been difficult to assess the overall effect of dairy food and calcium on total cancer. Therefore, we examined in a large cohort of men and women whether intakes of dairy food and calcium were associated with risk of total cancer as well as cancer at multiple individual sites.
METHODS
Study population
The NIH-AARP Diet and Health Study, a collaboration between the National Institutes of Health (NIH) and AARP (formerly known as the American Association of Retired Persons), mailed a questionnaire to AARP members 50–71 years old and residing in one of six U.S. states (California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania) and two metropolitan areas (Atlanta, Georgia, and Detroit, Michigan) in 1995–96 8. Among 567,169 participants who returned questionnaires, we excluded individuals who provided duplicate questionnaires (n=179), requested to be withdrawn (n=6), had moved out of the study area or died before baseline (n=582), indicated they were proxies for the intended respondents (n=15,760), had any prevalent cancer except non-melanoma skin cancer at baseline (n=51,193), and had reported end stage renal disease at baseline (n=997). In addition, we excluded individuals who reported extreme intakes (greater than twice the interquartile ranges above the 75th percentile or below the 25th percentile of sex-specific log transformed intake) of total energy (n=4,417) and calcium from foods (n=1,225). After these exclusions, the analytic cohort consisted of 293,907 men and 198,903 women. For analyses of ovarian and endometrial cancer, we excluded women who reported, respectively, a history of oophorectomy (n=43,536) and hysterectomy (n= 81,025) at baseline. The study was approved by the National Cancer Institute (NCI) Special Studies Institutional Review Board.
Dietary and risk factor assessment
At baseline in 1995–96, dietary intake was assessed with a self-administered 124 item food frequency questionnaire (FFQ), an earlier grid-based version of the Diet History Questionnaire developed at NCI 9. Participants were asked to report their usual frequency of intake and portion size over the last 12 months, using 10 predefined frequency categories ranging from ‘never’ to ‘6+ times per day’ for beverages, from ‘never’ to ‘2+ times per day’ for solid foods, and 3 categories of portion size. The food items, portion sizes, and nutrient database were constructed using the U.S. Department of Agriculture (USDA)’s 1994–96 Continuing Survey of Food Intake by Individuals (CSFII) 10, 11. The FFQ also inquired about frequency and types of multivitamins and frequency and dose of individual calcium supplements including Tums. Calcium intake was estimated from foods only (dietary calcium) and from foods and supplements combined (total calcium). Dietary calcium intake was adjusted for total energy intake using the residual method 12.
Dairy food and its serving sizes was defined by the Pyramid Servings Database corresponding to the 1994–1996 CSFII, which utilizes a recipe file to disaggregate food mixtures into their component ingredients and assigns them to food groups. One serving of dairy food was defined according to standard portion sizes developed by USDA, such as 1 cup of milk or yogurt, 1.5 oz of natural cheese, or 2 oz of processed cheese 13. We did not consider butter a dairy food because it has not generally been included in dairy food in previous studies and was not a major contributor to calcium intake.
The FFQ used in the study was calibrated against two non-consecutive 24-hour dietary recalls in 1,953 AARP participants 14. The energy-adjusted correlation coefficient of dietary calcium intake between a FFQ and the reference method was 0.63 in men and 0.64 in women.
In the baseline questionnaire, we also asked about demographic characteristics, current body weight and height, medical history, family history of cancer, and lifestyle factors including frequency of vigorous physical activity that lasted at least 20 minutes, smoking status, time since quitting smoking, and smoking dose. In a subsequent questionnaire mailed in 1996–97, we inquired information on detailed medical history including medication use and prostate cancer screening using a prostate specific antigen (PSA) test.
Cancer ascertainment
During follow-up from 1995 to 2003, cancer cases were identified through probabilistic linkage with cancer registry database from the original eight state and three additional states (Arizona, Nevada, and Texas). We have expanded cancer ascertainment areas to follow participants who moved to those states. The cancer registries are certified by the North American Association of Central Cancer Registries as being ≥90% complete within two years of cancer occurrence. Our case ascertainment method has been described in a previous study, which demonstrated that approximately 90% of cancers were identified through the registries 15. Vital status was ascertained through annual linkage of the cohort to the Social Security Administration Death Master File (SSADMF) in the U.S., follow-up searches of the National Death Index Plus for participants who matched to the SSADMF, cancer registry linkage, questionnaire responses, and responses to other mailings.
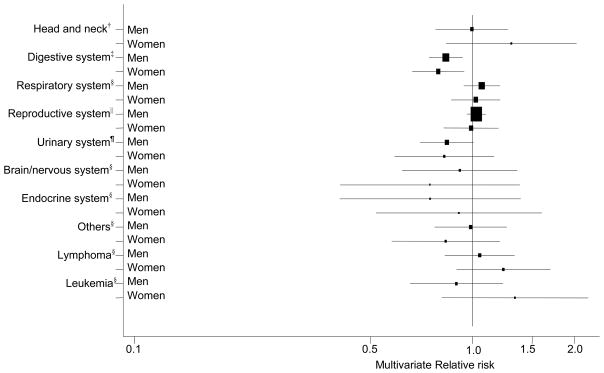
Incident cancer cases were invasive and comprised only the first malignancy diagnosed during the follow-up period if multiple cancers were diagnosed in the same participant. We defined cancers using the Surveillance Epidemiology and End Results (SEER) incidence site recode and the International Classification of Diseases for Oncology code (3rd ed.): head and neck (C000-C009, C019-C119, C129-C140, C142-C148, C300-C301, C310-C329, C339-C349, C381-C384, C388, C390, C398, and C399), esophagus (C150-C159), stomach (C160-C169), colorectal (C180-C189, C199, C209, and C260), liver (C220 and C221), pancreas (C250-C259), lung (C340-C349), breast (C500-C509), ovarian (C569), endometrial (C540-C549, and C559), prostate (C619), bladder (C670-C679), kidney (C649 and C659), thyroid (C739), and brain (C710-C719) cancer. Non-Hodgkin lymphoma (NHL), leukemia, and myeloma were also defined by SEER definition. Total cancer included those cancers listed above, skin cancer excluding basal/squamous, other miscellaneous cancers, and unspecified cancers. We also grouped cancers by anatomical system; head and neck, digestive system, respiratory system (excluding larynx), reproductive system, urinary system, brain/other nervous system, endocrine system, and others. The definition of cancer in each anatomical system is provided in a footnote in figure 2.
Figure 2. Multivariate relative risk* and 95% confidence intervals of cancers by anatomical system** comparing the highest quintile of total calcium intake vs. the lowest.
* Adjusted for race/ethnicity, education, marital status, BMI, family history of cancer, vigorous physical activity, alcohol and intakes of red meat and total energy + additional variables listed in each footnote. The squares and horizontal lines correspond with the multivariate RR and 95% CI.
† Adjusted for variables listed in the asterisked footnote + smoking status, time since quitting smoking, smoking dose, intake of fruit and vegetables, and menopausal hormone therapy (MHT) use in women.
‡ Adjusted for variables listed in the asterisked footnote + smoking status, time since quitting smoking, smoking dose, MHT use in women, antacid use, intakes of fruit and vegetables, whole grains, and folate
§ Adjusted for variables listed in the asterisked footnote + smoking status, time since quitting smoking, smoking dose, and MHT use in women.
|| Adjusted for variables listed in the asterisked footnote + smoking, personal history of diabetes, PSA test, and intakes of tomatoes, alpha-linolenic acid, and selenium in men. For women, the model was adjusted for variables listed in the asterisked footnote + smoking, MHT use, parity, oral contraceptive use, personal history of oophorectomy and hysterectomy, and intake of fat.
¶ Adjusted for variables listed in the asterisked footnote + smoking status, time since quitting smoking, smoking dose, personal history of diabetes and hypertension, parity, MHT and oral contraceptive use in women, and intakes of fruit and vegetables, total beverages, and protein.
** Cancers in each anatomical system are: digestive system (C150-C189, C199, C209-C212, C218, C220-C221, C239-C260, C268-C269, C480-C482, C488), respiratory system excluding larynx (C300-C301, C310-C319, C339-C349, C381-C384, C388, C390, C398, C399), reproductive system (men: C600-C609, C619-C639; women: C510-C519, C529-C549, C559, C569-C589), urinary system (C649, C659, C669-C689), brain/other nervous system (C700-C729), and endocrine system (C739-C759, C379).
Statistical analysis
We estimated relative risks (RRs) and two-sided 95% confidence intervals (CI) with the Cox proportional hazards model 16 using the SAS PROC PHREG procedure 17. We calculated person-years of follow-up time from the date of the baseline questionnaire until the date of a cancer diagnosis, death, move-out of the registry areas, or end of follow-up (Dec. 31, 2003), whichever occurred first. We confirmed that the proportional hazard assumption was met for the main exposures and covariates by including interaction terms with time and using the Wald chi-square procedure to test whether all coefficients equaled 0. For dairy food analyses, we used a nutrient density model, in which daily intake was expressed as number of pyramid servings per 1,000 kcal of total energy intake. The RRs were estimated according to sex-specific quintiles of dairy food and dietary and total calcium intakes. Supplemental calcium intake was categorized into four groups, 0, >0–<400, 400–<1,000, and ≥1,000 mg/day. We performed a test for linear trend across quintiles/categories of intake by using the median value in each quintile/category.
In multivariate models, we adjusted for potential risk factors pertinent to individual cancers and listed covariates for site-specific cancers in footnotes in tables. Potential risk factors examined were race/ethnicity, education, marital status, body mass index (BMI), family history of cancer, vigorous physical activity, smoking status, time since quitting smoking, smoking dose, personal history of hypertension and diabetes, antacid use, consumption of alcohol and total beverages, intakes of fruits and vegetables, red meat, whole grains, tomatoes, total fat, protein, folate, alpha-linoleic acid, selenium and total energy, PSA test in men, and hormone replacement therapy use and its duration, parity, oral contraceptive use, age at menopause, and history of oophorectomy and hysterectomy in women. Dietary and supplemental calcium intakes were mutually adjusted in the multivariate models.
We assigned missing responses in most of covariates (missingness <4%) to their respective reference group after checking that cancer risk in individuals with such missing values did not statistically significantly differ from that of individuals in the reference group using a Wald-statistics. We, however, created an indicator variable for missing responses for smoking, antacid use, personal history of hypertension, and PSA test because individuals with missing response in those variables showed significantly different risk of cancer compared to their respective reference group. In sensitivity analyses, we analyzed the data using an indicator variable for missing responses in each covariate 18, 19 and also excluding participants with missing responses in any covariate 20 and found that the results from both methods were similar to those of the main analyses.
Whether the associations for dairy food and calcium intakes differed by sex were examined by including a cross-product term of sex and intake of dairy food or calcium and no significant interaction was observed.
We also tested whether dairy food and total calcium intakes were log-linearly associated with risk of total cancer by comparing a non-parametric regression curve obtained using restricted cubic splines with the linear model 21. The likelihood ratio test and visual inspection of the restricted cubic splines were used. The number and location of the knots were identified through a stepwise selection process.
RESULTS
During 3,383,377 person-years of follow-up, we identified 36,965 cancer cases in men and 16,605 cancer cases in women. The 10th – 90th percentiles of dietary and total calcium and dairy food intake were 478–1,247 mg/day, 526–1,530 mg/day, and 0.3–2.9 servings/day, respectively, in men and 409–1,101 mg/day, 494–1,881 mg/day, and 0.3–2.7 servings/day, respectively, in women. Dairy food was positively correlated with dietary calcium (r = 0.83 in men and 0.82 in women) and total calcium (r = 0.68 in men and 0.47 in women), and dietary calcium was also positively correlated with total calcium (r =0.83 in men and 0.60 in women). The prevalence of calcium containing multivitamin use was 49% in men and 57% in women, and the prevalence of individual calcium supplement use (≥4 times/week) was 14% in men and 41% in women.
Compared to participants in the lowest quintile of dairy food or total calcium intake, participants in the highest quintile were more likely to be non-Hispanic White, to be college educated, to be physically active, and to be current hormone replacement therapy users among women, but they were less likely to smoke cigarettes and to drink alcohol (Table 1). Women in the highest quintile of total calcium intake had a lower BMI than those in the lowest quintile.
Table 1.
Selected characteristics of study participants by categories of dairy food and total calcium intakes
| Dairy foods |
Total calcium* |
|||||||
|---|---|---|---|---|---|---|---|---|
| Men |
Women |
Men |
Women |
|||||
| Quintile | Quintile | Quintile | Quintile | Quintile | Quintile | Quintile | Quintile | |
| 1 | 5 | 1 | 5 | 1 | 5 | 1 | 5 | |
| Dairy food† (serving/1,000 kcal) | 0.2 | 1.4 | 0.2 | 1.6 | 0.3 | 1.4 | 0.3 | 1.2 |
| Dietary calcium† (mg/day) | 496 | 1325 | 428 | 1157 | 491 | 1239 | 451 | 925 |
| Supplemental calcium† (mg/day) | 141 | 149 | 356 | 388 | 20 | 398 | 29 | 1037 |
| White, non-Hispanic (%) | 87 | 94 | 82 | 93 | 90 | 94 | 84 | 94 |
| College and postcollege (%) | 40 | 45 | 27 | 32 | 39 | 48 | 23 | 37 |
| Married (%) | 83 | 84 | 45 | 41 | 84 | 84 | 45 | 45 |
| Body mass index (kg/m2)† | 27.1 | 27.3 | 26.8 | 26.7 | 27.3 | 27.1 | 27.5 | 25.8 |
| Family history of any cancer (%) | 46 | 47 | 50 | 51 | 47 | 47 | 50 | 52 |
| Current smokers (%) | 13 | 10 | 19 | 12 | 15 | 8 | 21 | 9 |
| >20 cigarettes/day among ever smokers (%) | 50 | 47 | 31 | 28 | 50 | 48 | 32 | 31 |
| Quit smoking ≥10 years ago among former smokers (%) | 70 | 72 | 57 | 63 | 69 | 73 | 55 | 66 |
| Physical activity, ≥5 times/week (%) | 21 | 22 | 15 | 18 | 17 | 25 | 11 | 22 |
| Personal history of diabetes (%) | 8 | 13 | 7 | 9 | 7 | 12 | 7 | 5 |
| Personal history of hypertension (%) | 42 | 40 | 41 | 40 | 39 | 36 | 41 | 33 |
| Antacid use (%) | 22 | 33 | 19 | 27 | 30 | 32 | 25 | 34 |
| Current menopausal hormone therapy use (%) | - | - | 41 | 45 | - | - | 35 | 54 |
| Multivitamin use (%) | 49 | 54 | 54 | 64 | 26 | 71 | 30 | 79 |
| Alcohol (g/day)† | 31 | 9 | 9 | 4 | 32 | 10 | 9 | 5 |
| Fruit and vegetables (servings/1,000 kcal)† | 3.7 | 3.4 | 4.7 | 4.1 | 3.1 | 3.8 | 3.9 | 4.8 |
| Red meat (g/1,000 kcal)† | 41 | 32 | 32 | 24 | 45 | 30 | 37 | 23 |
| Whole grains (servings/1,000 kcal)† | 0.6 | 0.7 | 0.6 | 0.7 | 0.5 | 0.8 | 0.6 | 0.7 |
| Total fat (% of energy)† | 30 | 29 | 31 | 27 | 31 | 28 | 33 | 27 |
| Total folate (ug/day)† | 606 | 672 | 573 | 629 | 455 | 794 | 415 | 751 |
| Total energy (kcal/day)† | 2065 | 1959 | 1542 | 1545 | 2044 | 2035 | 1594 | 1538 |
Calcium from diet and supplements combined
Mean value
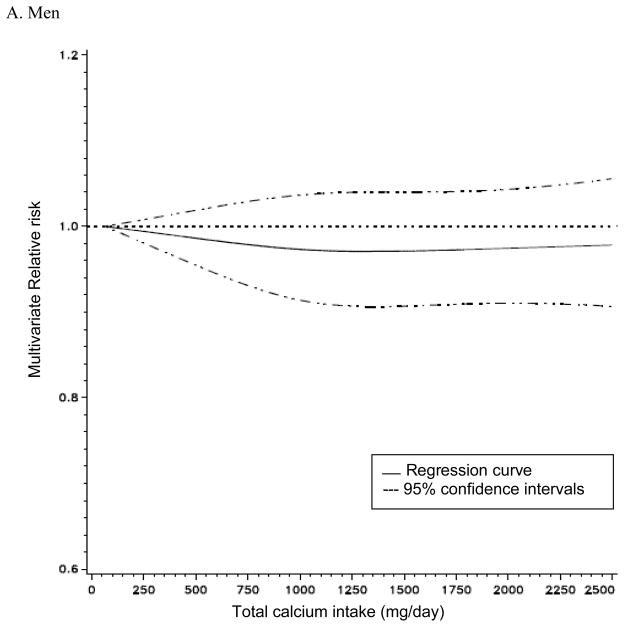
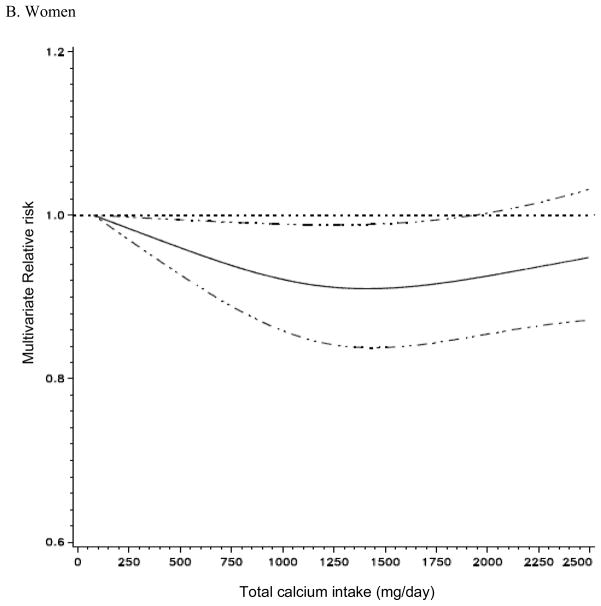
Total calcium intake was not associated with risk of total cancer incidence in men, but it was non-linearly associated with the risk of total cancer incidence in women (p for non-linearity = 0.05, Figure 1). In non-parametric regression analyses, the RRs for total cancer incidence in women decreased up to approximately 1,300 mg/day of total calcium intake and no further risk reduction was observed above 1,300 mg/day. The association between total calcium intake and risk of total cancer did not differ by cancer stages or cancer grades (data not shown). In a secondary analysis excluding non-aggressive prostate cancer that contributed to a large proportion of total cancer cases, we observed a weak inverse association between total calcium and total cancer incidence in men. With the lowest quintile of total calcium intake as reference, the multivariate RRs for total cancer incidence in the second through the highest quintile were 1.00, 0.98, 0.99, and 0.96 (95% CI: 0.92–1.02, p trend=0.16) in men.
Figure 1. Non-parametric regression curve* for the association between total calcium intake and risk of all cancers.
* A Men: the model adjusted for race/ethnicity, education, marital status, body mass index, family history of cancer, vigorous physical activity, smoking status, time since quitting smoking, smoking dose, antacid use, personal history of diabetes and hypertension, PSA test, alcohol, and intakes of fat, red meat, fruit and vegetables, whole grains, protein, beverages, folate, tomatoes, alpha-linolenic acid, selenium and total energy. B. Women: the model adjusted for race/ethnicity, education, marital status, body mass index, family history of cancer, vigorous physical activity, smoking status, time since quitting smoking, smoking dose, antacid use, age at menopause, parity, oral contraceptive use, HRT use, duration of hormone replacement therapy use, personal history of diabetes, hypertension, oophorectomy and hysterectomy, alcohol, and intakes of fat, red meat, fruit and vegetables, whole grains, total beverages, protein, folate and total energy.
Dairy food and dietary and supplemental calcium were not related to total cancer incidence in men, whereas dietary calcium, but not dairy food or supplemental calcium, was inversely related to total cancer incidence in women (Table 2 and 3). When cancers diagnosed during the first 2 years of follow-up were excluded, the association with total cancer became slightly stronger for dietary calcium in men (multivariate RR in the highest quintile vs. the lowest (RRQ5 vs. Q1) = 0.96, 95% CI: 0.92–1.00, p trend=0.05, n=27,720 cases) and for dairy food in women (multivariate RRQ5 vs. Q1 = 0.93, 95% CI: 0.88–0.99, p trend=0.01, n=12,395 cases).
Table 2.
Multivariate relative risk* and 95% confidence intervals of cancer for categories of dairy food and dietary, supplemental and total calcium intakes in men
| Categories† |
p trend | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| All cancers‡ (n=36,965§) | ||||||
| Dairy foods | 1.00 | 1.02 (0.98–1.05) | 1.02 (0.99–1.06) | 1.04 (1.01–1.08) | 1.03 (0.99–1.07) | 0.19 |
| Dietary calcium | 1.00 | 1.00 (0.96–1.03) | 0.99 (0.96–1.03) | 1.01 (0.97–1.05) | 1.00 (0.96–1.05) | 0.76 |
| Supplemental calcium | 1.00 | 0.97 (0.94–1.00) | 0.97 (0.93–1.01) | 0.96 (0.90–1.03) | - | 0.18 |
| Total calcium | 1.00 | 0.99 (0.96–1.03) | 0.99 (0.96–1.03) | 0.95 (0.96–1.03) | 0.99 (0.95–1.03) | 0.74 |
| Head and neck cancer|| (n=1,038) | ||||||
| Dairy foods | 1.00 | 0.98 (0.82–1.18) | 0.89 (0.74–1.08) | 0.87 (0.72–1.06) | 0.84 (0.69–1.02) | 0.05 |
| Dietary calcium | 1.00 | 0.93 (0.78–1.12) | 0.84 (0.69–1.02) | 0.92 (0.76–1.12) | 0.78 (0.64–0.96) | 0.03 |
| Supplemental calcium | 1.00 | 1.21 (1.06–1.38) | 1.22 (0.98–1.52) | 1.18 (0.81–1.73) | - | 0.07 |
| Total calcium | 1.00 | 1.00 (0.83–1.20) | 0.90 (0.74–1.10) | 0.99 (0.82–1.21) | 0.99 (0.81–1.21) | 0.99 |
| Esophageal cancer¶ (n=468) | ||||||
| Dairy foods | 1.00 | 1.11 (0.85–1.46) | 1.07 (0.82–1.41) | 0.75 (0.55–1.02) | 0.70 (0.51–0.96) | 0.002 |
| Dietary calcium | 1.00 | 0.91 (0.70–1.19) | 0.72 (0.54–0.97) | 0.75 (0.56–1.00) | 0.66 (0.49–0.90) | 0.01 |
| Supplemental calcium | 1.00 | 0.94 (0.77–1.15) | 1.21 (0.89–1.63) | 1.32 (0.80–2.17) | - | 0.14 |
| Total calcium | 1.00 | 0.98 (0.74–1.29) | 0.84 (0.63–1.13) | 0.89 (0.67–1.19) | 0.84 (0.62–1.13) | 0.23 |
| Stomach cancer** (n=512) | ||||||
| Dairy foods | 1.00 | 1.03 (0.80–1.34) | 0.95 (0.73–1.24) | 0.72 (0.54–0.96) | 0.80 (0.61–1.06) | 0.03 |
| Dietary calcium | 1.00 | 1.13 (0.87–1.47) | 0.95 (0.72–1.25) | 0.93 (0.70–1.24) | 0.87 (0.65–1.16) | 0.15 |
| Supplemental calcium | 1.00 | 1.12 (0.92–1.35) | 0.99 (0.72–1.36) | 1.51 (0.95–2.39) | - | 0.17 |
| Total calcium | 1.00 | 1.07 (0.81–1.41) | 1.13 (0.86–1.48) | 1.04 (0.79–1.38) | 0.96 (0.72–1.29) | 0.58 |
| Colorectal cancer†† (n=3,463) | ||||||
| Dairy foods | 1.00 | 0.91 (0.82–1.01) | 0.83 (0.75–0.93) | 0.89 (0.80–0.99) | 0.85 (0.76–0.94) | 0.01 |
| Dietary calcium | 1.00 | 0.86 (0.77–0.95) | 0.83 (0.74–0.92) | 0.85 (0.76–0.95) | 0.84 (0.75–0.94) | 0.03 |
| Supplemental calcium | 1.00 | 0.96 (0.88–1.05) | 0.91(0.79–1.04) | 0.74 (0.58–0.94) | - | 0.01 |
| Total calcium | 1.00 | 0.89 (0.80–0.98) | 0.83 (0.75–0.93) | 0.87 (0.78–0.97) | 0.79 (0.70–0.89) | 0.001 |
| Liver cancer‡‡ (n=311) | ||||||
| Dairy foods | 1.00 | 1.02 (0.72–1.45) | 1.05 (0.74–1.50) | 1.07 (0.75–1.52) | 1.04 (0.72–1.48) | 0.84 |
| Dietary calcium | 1.00 | 0.84 (0.59–1.19) | 0.97 (0.69–1.38) | 0.87 (0.61–1.26) | 0.99 (0.69–1.42) | 0.86 |
| Supplemental calcium | 1.00 | 0.92 (0.72–1.17) | 0.78 (0.50–1.20) | 1.03 (0.53–2.03) | - | 0.54 |
| Total calcium | 1.00 | 0.75 (0.52–1.08) | 1.20 (0.87–1.67) | 0.83 (0.58–1.20) | 0.88 (0.61–1.27) | 0.61 |
| Pancreatic cancer§§ (n=717) | ||||||
| Dairy foods | 1.00 | 0.96 (0.76–1.20) | 1.01 (0.80–1.27) | 1.00 (0.79–1.26) | 0.82 (0.64–1.05) | 0.13 |
| Dietary calcium | 1.00 | 0.94 (0.75–1.18) | 1.04 (0.83–1.31) | 0.92 (0.72–1.16) | 0.82 (0.64–1.06) | 0.11 |
| Supplemental calcium | 1.00 | 0.95 (0.81–1.11) | 0.90 (0.69–1.19) | 1.17 (0.77–1.77) | - | 0.90 |
| Total calcium | 1.00 | 0.93 (0.74–1.16) | 0.90 (0.72–1.14) | 0.98 (0.78–1.23) | 0.87 (0.68–1.11) | 0.39 |
| Lung cancer§§ (n=4,287) | ||||||
| Dairy foods | 1.00 | 1.04 (0.94–1.14) | 1.01 (0.92–1.11) | 1.04 (0.95–1.15) | 1.05 (0.95–1.16) | 0.36 |
| Dietary calcium | 1.00 | 0.97 (0.89–1.07) | 1.02 (0.93–1.13) | 1.04 (0.95–1.15) | 1.00 (0.91–1.11) | 0.68 |
| Supplemental calcium | 1.00 | 0.97 (0.91–1.03) | 1.03 (0.92–1.15) | 1.04 (0.86–1.27) | - | 0.64 |
| Total calcium | 1.00 | 1.05 (0.96–1.15) | 1.03 (0.94–1.13) | 1.03 (0.94–1.13) | 1.03 (0.93–1.13) | 0.76 |
| Prostate cancer|||| (n=17,189) | ||||||
| Dairy foods | 1.00 | 1.02 (0.97–1.07) | 1.05 (1.00–1.10) | 1.06 (1.01–1.12) | 1.06 (1.01–1.12) | 0.01 |
| Dietary calcium | 1.00 | 1.01 (0.96–1.06) | 1.00 (0.95–1.05) | 1.03 (0.98–1.08) | 1.04 (0.98–1.09) | 0.14 |
| Supplemental calcium | 1.00 | 0.99 (0.95–1.02) | 0.97 (0.92–1.03) | 0.96 (0.88–1.05) | - | 0.21 |
| Total calcium | 1.00 | 0.99 (0.95–1.04) | 1.01 (0.96–1.06) | 1.00 (0.95–1.05) | 1.03 (0.98–1.08) | 0.21 |
| Bladder cancer¶¶ (n=1,417) | ||||||
| Dairy foods | 1.00 | 1.02 (0.86–1.19) | 0.94 (0.80–1.11) | 0.85 (0.72–1.01) | 0.86 (0.72–1.02) | 0.03 |
| Dietary calcium | 1.00 | 1.14 (0.97–1.34) | 0.99 (0.84–1.17) | 0.90 (0.75–1.07) | 0.94 (0.78–1.12) | 0.10 |
| Supplemental calcium | 1.00 | 0.90 (0.80–1.01) | 0.99 (0.83–1.20) | 0.84 (0.59–1.18) | - | 0.34 |
| Total calcium | 1.00 | 0.96 (0.82–1.13) | 0.82 (0.69–0.97) | 0.87 (0.73–1.03) | 0.87 (0.73–1.03) | 0.11 |
| Kidney cancer*** (n=991) | ||||||
| Dairy foods | 1.00 | 1.06 (0.86–1.30) | 1.09 (0.89–1.34) | 1.12 (0.91–1.38) | 1.08 (0.87–1.35) | 0.54 |
| Dietary calcium | 1.00 | 0.93 (0.76–1.14) | 0.93 (0.75–1.14) | 0.97 (0.78–1.20) | 0.98 (0.78–1.24) | 0.86 |
| Supplemental calcium | 1.00 | 0.89 (0.77–1.02) | 0.86 (0.68–1.09) | 0.90 (0.61–1.34) | - | 0.22 |
| Total calcium | 1.00 | 0.90 (0.74–1.10) | 0.93 (0.76–1.14) | 0.94 (0.76–1.15) | 0.80 (0.64–1.01) | 0.10 |
| Thyroid cancer‡‡ (n=170) | ||||||
| Dairy foods | 1.00 | 1.02 (0.60–1.72) | 1.48 (0.91–2.42) | 1.59 (0.98–2.57) | 0.78 (0.45–1.37) | 0.41 |
| Dietary calcium | 1.00 | 1.53 (0.90–2.62) | 1.67 (0.98–2.84) | 1.71 (1.00–2.91) | 1.19 (0.67–2.12) | 0.98 |
| Supplemental calcium | 1.00 | 0.85 (0.61–1.17) | 0.92 (0.54–1.57) | 0.70 (0.26–1.91) | - | 0.44 |
| Total calcium | 1.00 | 0.95 (0.58–1.56) | 1.07 (0.66–1.74) | 1.15 (0.71–1.87) | 0.79 (0.46–1.34) | 0.43 |
| Brain cancer‡‡ (n=390) | ||||||
| Dairy foods | 1.00 | 0.82 (0.59–1.14) | 0.89 (0.64–1.22) | 1.01 (0.74–1.37) | 0.83 (0.60–1.14) | 0.52 |
| Dietary calcium | 1.00 | 0.81 (0.58–1.13) | 1.02 (0.74–1.39) | 0.77 (0.55–1.08) | 0.91 (0.65–1.26) | 0.65 |
| Supplemental calcium | 1.00 | 1.18 (0.95–1.46) | 1.31 (0.94–1.83) | 1.11 (0.62–1.99) | - | 0.25 |
| Total calcium | 1.00 | 0.79 (0.57–1.10) | 0.82 (0.59–1.13) | 0.91 (0.66–1.25) | 0.89 (0.65–1.23) | 0.98 |
| Non-Hodgkin Lymphoma‡‡ (n=1,267) | ||||||
| Dairy foods | 1.00 | 0.92 (0.76–1.10) | 1.09 (0.91–1.30) | 1.06 (0.89–1.27) | 1.02 (0.85–1.22) | 0.56 |
| Dietary calcium | 1.00 | 0.97 (0.81–1.17) | 1.01 (0.84–1.21) | 1.11 (0.93–1.33) | 1.00 (0.83–1.20) | 0.76 |
| Supplemental calcium | 1.00 | 0.84 (0.74–0.95) | 0.87 (0.71–1.07) | 0.99 (0.72–1.36) | - | 0.33 |
| Total calcium | 1.00 | 1.03 (0.86–1.24) | 1.15 (0.96–1.38) | 0.95 (0.79–1.14) | 1.02 (0.85–1.23) | 0.77 |
| Leukemia‡‡ (n=757) | ||||||
| Dairy foods | 1.00 | 1.16 (0.92–1.46) | 0.93 (0.73–1.19) | 1.16 (0.92–1.47) | 1.11 (0.88–1.41) | 0.41 |
| Dietary calcium | 1.00 | 0.90 (0.72–1.13) | 0.82 (0.65–1.04) | 0.95 (0.76–1.19) | 0.87 (0.69–1.11) | 0.49 |
| Supplemental calcium | 1.00 | 1.17 (1.01–1.36) | 0.96 (0.73–1.25) | 0.93 (0.59–1.47) | - | 0.78 |
| Total calcium | 1.00 | 0.93 (0.74–1.17) | 0.95 (0.75–1.19) | 0.96 (0.76–1.21) | 0.88 (0.69–1.21) | 0.38 |
| Myeloma‡‡ (n=369) | ||||||
| Dairy foods | 1.00 | 1.06 (0.75–1.49) | 1.38 (1.00–1.92) | 1.15 (0.81–1.61) | 1.14 (0.81–1.61) | 0.63 |
| Dietary calcium | 1.00 | 1.08 (0.77–1.51) | 1.07 (0.76–1.50) | 1.27 (0.91–1.77) | 1.05 (0.74–1.50) | 0.71 |
| Supplemental calcium | 1.00 | 0.92 (0.74–1.14) | 0.69 (0.46–1.04) | 0.60 (0.28–1.27) | - | 0.04 |
| Total calcium | 1.00 | 1.03 (0.74–1.43) | 1.07 (0.77–1.48) | 0.91 (0.65–1.28) | 0.92 (0.65–1.30) | 0.42 |
Adjusted for race/ethnicity (non-Hispanic White, non-Hispanic Black, and others), education (less than high school, high school graduate, some college, and college graduate/postgraduate), marital status (married, not-married), body mass index (BMI, continuous), family history of cancer (yes, no), vigorous physical activity (never/rarely, ≤3 times/month, 1–2, 3–4, and ≥5 time/week), alcohol consumption (0, <5, 5–<15, 15–<30, and ≥30 g/day), and intakes of red meat (quintiles) and total energy (continuous) and additional variables listed in each footnote below.
Dairy food and dietary and total calcium intakes were categorized according to quintiles of the distribution. The medians for category 1–5 were 0.2, 0.4, 0.6, 0.8, and 1.4 servings/1000kcal/day for dairy food; 478, 616, 739, 899 and 1247 mg/day for dietary calcium; and 526, 498, 857, 1073, and 1530 mg/day for total calcium. Supplemental calcium intake was categorized into four groups, 0, >0–<400, 400–<1,000, and ≥1,000 mg/day. Dietary and supplemental calcium intakes were mutually adjusted in the multivariate models.
Adjusted for variables listed in the asterisked footnote + smoking status (never, former, and current), time since quitting smoking (never, stopped ≥10 years ago, stopped 5–9 years ago, stopped 1–4 years ago, stopped <1 year ago, and currently smoking), smoking dose (0, 1–10, 11–20, 21–30, 31–40, 41–50, 51–60, >60 cigarettes/day), antacid use (yes, no), personal history of diabetes (yes, no) and hypertension (yes, no), PSA test (yes, no), and intakes of fat (quintiles), fruit and vegetables (quintiles), whole grains (quintiles), protein (quintiles), total beverages (quintiles), folate (quintiles), tomatoes (quintiles), alpha-linolenic acid (quintiles), and selenium (quintiles).
Number of cases
Adjusted for variables listed in the asterisked footnote + smoking status, time since quitting smoking, smoking dose, and intakes of fruit and vegetables.
Adjusted for variables listed in the asterisked footnote + smoking status, time since quitting smoking, smoking dose, antacid use, and intake of fruit and vegetables.
Adjusted for variables listed in the asterisked footnote + smoking (never, ≤20 cigarettes/day in the past, >20 cigarettes/day in the past, currently ≤20 cigarettes/day, and currently >20 cigarettes/day), and antacid use.
Adjusted for variables listed in the asterisked footnote + smoking, and intakes of fruit and vegetables, whole grains, and folate.
Adjusted for variables listed in the asterisked footnote + smoking.
Adjusted for variables listed in the asterisked footnote +smoking status, time since quitting smoking, and smoking dose.
Adjusted for variables listed in the asterisked footnote + smoking, personal history of diabetes, PSA test, and intakes of tomatoes, alpha-linolenic acid, and selenium.
Adjusted for variables listed in the asterisked footnote + smoking status, time since quitting smoking, smoking dose, and intakes of fruit and vegetables, and total beverages.
Adjusted for variables listed in the asterisked footnote + smoking, personal history of diabetes and hypertension, and intake of protein.
Table 3.
Multivariate relative risk* and 95% confidence intervals of cancer for categories of dairy food and dietary, supplemental and total calcium intakes in women
| Categories† |
p trend | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| All cancers‡ (n=16,605§) | ||||||
| Dairy foods | 1.00 | 0.98 (0.94–1.03) | 0.95 (0.90–1.00) | 0.97 (0.92–1.02) | 0.95 (0.90–1.00) | 0.10 |
| Dietary calcium | 1.00 | 0.96 (0.91–1.00) | 0.95 (0.90–1.00) | 0.95 (0.90–1.01) | 0.93 (0.87–0.99) | 0.04 |
| Supplemental calcium | 1.00 | 0.97 (0.92–1.01) | 0.95 (0.91–1.00) | 0.96 (0.91–1.02) | 0.28 | |
| Total calcium | 1.00 | 0.98 (0.93–1.03) | 0.94 (0.89–0.99) | 0.93 (0.88–0.98) | 0.96 (0.91–1.02) | 0.23 |
| Head and neck cancer|| (n=325) | ||||||
| Dairy foods | 1.00 | 0.75 (0.53–1.05) | 0.90 (0.64–1.25) | 1.02 (0.73–1.41) | 0.89 (0.63–1.27) | 0.99 |
| Dietary calcium | 1.00 | 0.92 (0.66–1.28) | 1.09 (0.79–1.52) | 0.98 (0.69–1.40) | 1.03 (0.72–1.47) | 0.80 |
| Supplemental calcium | 1.00 | 1.16 (0.88–1.52) | 1.22 (0.90–1.66) | 1.05 (0.72–1.53) | 0.75 | |
| Total calcium | 1.00 | 1.33 (0.97–1.83) | 0.92 (0.64–1.31) | 1.02 (0.71–1.46) | 1.30 (0.91–1.86) | 0.42 |
| Esophageal cancer¶ (n=79) | ||||||
| Dairy foods | 1.00 | 0.82 (0.42–1.60) | 1.21 (0.65–2.25) | 0.85 (0.42–1.72) | 0.64 (0.29–1.44) | 0.31 |
| Dietary calcium | 1.00 | 0.83 (0.44–1.57) | 1.03 (0.55–1.94) | 0.68 (0.32–1.42) | 0.60 (0.27–1.34) | 0.18 |
| Supplemental calcium | 1.00 | 1.31 (0.75–2.28) | 1.35 (0.73–2.48) | 0.79 (0.35–1.81) | 0.61 | |
| Total calcium | 1.00 | 1.47 (0.80–2.71) | 1.14 (0.59–2.22) | 0.56 (0.24–1.31) | 0.83 (0.38–1.81) | 0.18 |
| Stomach cancer** (n=143) | ||||||
| Dairy foods | 1.00 | 0.61 (0.37–1.01) | 0.49 (0.28–0.84) | 0.74 (0.46–1.19) | 0.58 (0.35–0.97) | 0.16 |
| Dietary calcium | 1.00 | 0.95 (0.58–1.56) | 0.50 (0.28–0.91) | 0.87 (0.52–1.46) | 0.84 (0.50–1.41) | 0.65 |
| Supplemental calcium | 1.00 | 0.81 (0.53–1.23) | 1.15 (0.75–1.76) | 0.54 (0.29–1.01) | 0.20 | |
| Total calcium | 1.00 | 0.90 (0.54–1.50) | 1.37 (0.85–2.20) | 0.55 (0.30–1.01) | 0.71 (0.40–1.25) | 0.08 |
| Colorectal cancer†† (n=1,635) | ||||||
| Dairy foods | 1.00 | 0.84 (0.73–0.98) | 0.86 (0.74–0.99 | 0.78 (0.67–0.91) | 0.72 (0.61–0.84) | <0.001 |
| Dietary calcium | 1.00 | 0.86 (0.74–1.00) | 0.81 (0.70–0.95) | 0.74 (0.63–0.87) | 0.70 (0.59–0.82) | <0.001 |
| Supplemental calcium | 1.00 | 1.02 (0.89–1.17) | 0.86 (0.73–1.00) | 0.86 (0.72–1.02) | 0.02 | |
| Total calcium | 1.00 | 0.87 (0.75–1.01) | 0.83 (0.71–0.97) | 0.71 (0.60–0.84) | 0.72 (0.61–0.86) | 0.001 |
| Liver cancer‡‡ (n=86) | ||||||
| Dairy foods | 1.00 | 0.91 (0.42–1.99) | 1.83 (0.93–3.58) | 1.35 (0.66–2.77) | 1.58 (0.78–3.20) | 0.17 |
| Dietary calcium | 1.00 | 0.48 (0.21–1.11) | 1.14 (0.58–2.21) | 1.32 (0.68–2.55) | 1.45 (0.75–2.82) | 0.05 |
| Supplemental calcium | 1.00 | 0.50 (0.29–0.86) | 0.61 (0.34–1.08) | 0.42 (0.19–0.91) | 0.04 | |
| Total calcium | 1.00 | 1.38 (0.73–2.61) | 1.10 (0.55–2.17) | 1.05 (0.52–2.13) | 0.96 (0.46–2.03) | 0.60 |
| Pancreatic cancer§§ (n=384) | ||||||
| Dairy foods | 1.00 | 1.19 (0.86–1.66) | 1.24 (0.89–1.72) | 1.34 (0.96–1.85) | 1.12 (0.80–1.58) | 0.64 |
| Dietary calcium | 1.00 | 1.01 (0.74–1.38) | 0.98 (0.71–1.35) | 1.05 (0.77–1.45) | 0.85 (0.60–1.20) | 0.36 |
| Supplemental calcium | 1.00 | 0.89 (0.69–1.15) | 1.02 (0.77–1.33) | 0.79 (0.57–1.11) | 0.34 | |
| Total calcium | 1.00 | 1.03 (0.75–1.40) | 0.93 (0.67–1.28) | 0.97 (0.71–1.34) | 0.88 (0.63–1.24) | 0.40 |
| Lung cancer§§ (n=2,457) | ||||||
| Dairy foods | 1.00 | 0.91 (0.81–1.03) | 0.86 (0.76–0.97) | 0.89 (0.79–1.01) | 0.92 (0.81–1.04) | 0.27 |
| Dietary calcium | 1.00 | 0.88 (0.78–0.99) | 0.87 (0.76–0.98) | 0.91 (0.80–1.03) | 0.93 (0.81–1.05) | 0.48 |
| Supplemental calcium | 1.00 | 1.04 (0.94–1.14) | 1.03 (0.92–1.16) | 1.04 (0.92–1.19) | 0.58 | |
| Total calcium | 1.00 | 0.96 (0.85–1.08) | 0.91 (0.81–1.03) | 0.94 (0.83–1.07) | 1.00 (0.88–1.14) | 0.95 |
| Breast cancer|||| (n=5,856) | ||||||
| Dairy foods | 1.00 | 0.98 (0.90–1.06) | 0.97 (0.89–1.05) | 0.94 (0.87–1.03) | 0.96 (0.88–1.04) | 0.28 |
| Dietary calcium | 1.00 | 0.96 (0.89–1.04) | 0.95 (0.87–1.03) | 0.97 (0.89–1.05) | 0.94 (0.86–1.03) | 0.28 |
| Supplemental calcium | 1.00 | 0.95 (0.89–1.02) | 0.97 (0.90–1.04) | 0.98 (0.91–1.06) | 0.91 | |
| Total calcium | 1.00 | 0.96 (0.88–1.04) | 0.95 (0.87–1.03) | 0.94 (0.86–1.02) | 0.98 (0.90–1.07) | 0.84 |
| Ovarian cancer¶¶ (n=515) | ||||||
| Dairy foods | 1.00 | 1.04 (0.78–1.38) | 0.94 (0.70–1.25) | 1.26 (0.96–1.65) | 1.03 (0.77–1.37) | 0.60 |
| Dietary calcium | 1.00 | 1.04 (0.78–1.39) | 1.05 (0.79–1.40) | 1.25 (0.94–1.65) | 1.02 (0.75–1.37) | 0.77 |
| Supplemental calcium | 1.00 | 0.88 (0.70–1.10) | 0.99 (0.78–1.26) | 0.97 (0.74–1.27) | 0.85 | |
| Total calcium | 1.00 | 1.04 (0.78–1.38) | 0.99 (0.74–1.33) | 1.05 (0.79–1.41) | 1.14 (0.85–1.52) | 0.34 |
| Endometrial cancer*** (n=1,171) | ||||||
| Dairy foods | 1.00 | 1.19 (0.98–1.45) | 1.17 (0.96–1.42) | 1.22 (1.01–1.48) | 1.17 (0.97–1.42) | 0.27 |
| Dietary calcium | 1.00 | 1.09 (0.90–1.32) | 1.26 (1.04–1.52) | 1.06 (0.87–1.29) | 1.20 (0.99–1.46) | 0.18 |
| Supplemental calcium | 1.00 | 0.96 (0.83–1.11) | 0.92 (0.78–1.08) | 0.92 (0.77–1.11) | 0.35 | |
| Total calcium | 1.00 | 1.11 (0.92–1.33) | 1.01 (0.84–1.22) | 1.10 (0.91–1.32) | 0.99 (0.81–1.20) | 0.66 |
| Bladder cancer††† (n=264) | ||||||
| Dairy foods | 1.00 | 1.27 (0.86–1.88) | 1.24 (0.83–1.85) | 1.25 (0.83–1.88) | 1.45 (0.97–2.18) | 0.12 |
| Dietary calcium | 1.00 | 1.03 (0.70–1.52) | 1.08 (0.73–1.60) | 1.02 (0.68–1.54) | 1.23 (0.82–1.84) | 0.33 |
| Supplemental calcium | 1.00 | 1.17 (0.87–1.58) | 0.91 (0.63–1.29) | 1.03 (0.69–1.53) | 0.70 | |
| Total calcium | 1.00 | 0.97 (0.67–1.41) | 0.94 (0.64–1.37) | 0.91 (0.61–1.35) | 0.97 (0.65–1.46) | 0.85 |
| Kidney cancer‡‡‡ (n=367) | ||||||
| Dairy foods | 1.00 | 1.02 (0.72–1.45) | 1.11 (0.78–1.57) | 1.06 (0.75–1.51) | 1.13 (0.79–1.63) | 0.49 |
| Dietary calcium | 1.00 | 0.84 (0.59–1.19) | 1.13 (0.81–1.59) | 0.77 (0.53–1.12) | 1.02 (0.70–1.48) | 0.87 |
| Supplemental calcium | 1.00 | 0.93 (0.73–1.20) | 0.79 (0.59–1.06) | 0.79 (0.57–1.12) | 0.12 | |
| Total calcium | 1.00 | 0.87 (0.63–1.21) | 0.81 (0.58–1.13) | 0.73 (0.51–1.03) | 0.79 (0.55–1.13) | 0.21 |
| Thyroid cancer‡‡ (n=199) | ||||||
| Dairy foods | 1.00 | 0.93 (0.59–1.46) | 0.91 (0.58–1.44) | 0.90 (0.57–1.41) | 1.04 (0.67–1.62) | 0.74 |
| Dietary calcium | 1.00 | 1.06 (0.69–1.64) | 0.73 (0.45–1.18) | 0.89 (0.57–1.41) | 1.01 (0.64–1.58) | 0.98 |
| Supplemental calcium | 1.00 | 1.16 (0.81–1.66) | 1.15 (0.78–1.71) | 1.07 (0.68–1.68) | 0.87 | |
| Total calcium | 1.00 | 0.98 (0.63–1.54) | 1.07 (0.69–1.67) | 0.88 (0.55–1.41) | 1.04 (0.65–1.65) | 0.98 |
| Brain cancer‡‡ (n=160) | ||||||
| Dairy foods | 1.00 | 0.83 (0.53–1.29) | 0.45 (0.26–0.77) | 0.61 (0.37–0.99) | 0.77 (0.49–1.23) | 0.38 |
| Dietary calcium | 1.00 | 0.89 (0.56–1.39) | 0.56 (0.34–0.94) | 0.65 (0.39–1.06) | 0.70 (0.43–1.15) | 0.14 |
| Supplemental calcium | 1.00 | 1.03 (0.69–1.53) | 1.21 (0.80–1.85) | 0.84 (0.49–1.41) | 0.67 | |
| Total calcium | 1.00 | 0.94 (0.59–1.50) | 0.70 (0.42–1.16) | 0.79 (0.48–1.29) | 0.72 (0.43–1.21) | 0.20 |
| Non-Hodgkin Lymphoma‡‡ (n=660) | ||||||
| Dairy foods | 1.00 | 1.41 (1.10–1.80) | 1.06 (0.82–1.37) | 1.17 (0.91–1.51) | 1.00 (0.77–1.30) | 0.26 |
| Dietary calcium | 1.00 | 1.41 (1.11–1.81) | 1.15 (0.89–1.49) | 1.06 (0.81–1.38) | 1.13 (0.87–1.47) | 0.69 |
| Supplemental calcium | 1.00 | 0.82 (0.67–1.00) | 0.95 (0.77–1.18) | 1.25 (0.99–1.56) | 0.01 | |
| Total calcium | 1.00 | 0.96 (0.75–1.23) | 0.89 (0.69–1.15) | 0.99 (0.77–1.28) | 1.24 (0.97–1.59) | 0.03 |
| Leukemia‡‡ (n=280) | ||||||
| Dairy foods | 1.00 | 1.08 (0.73–1.60) | 1.07 (0.72–1.59) | 1.39 (0.95–2.02) | 1.20 (0.81–1.77) | 0.26 |
| Dietary calcium | 1.00 | 1.10 (0.75–1.61) | 0.96 (0.64–1.43) | 1.35 (0.93–1.96) | 1.13 (0.76–1.67) | 0.41 |
| Supplemental calcium | 1.00 | 0.81 (0.60–1.09) | 0.71 (0.50–1.00) | 1.12 (0.79–1.57) | 0.54 | |
| Total calcium | 1.00 | 0.92 (0.63–1.35) | 1.00 (0.68–1.46) | 0.92 (0.62–1.36) | 1.25 (0.86–1.83) | 0.17 |
| Myeloma‡‡ (n=175) | ||||||
| Dairy foods | 1.00 | 1.24 (0.76–2.01) | 1.33 (0.84–2.15) | 1.18 (0.72–1.95) | 1.24 (0.75–2.03) | 0.61 |
| Dietary calcium | 1.00 | 1.72 (1.03–2.86) | 1.71 (1.02–2.87) | 1.71 (1.01–2.88) | 1.43 (0.83–2.49) | 0.55 |
| Supplemental calcium | 1.00 | 0.84 (0.57–1.24) | 1.33 (0.91–1.95) | 0.64 (0.37–1.11) | 0.47 | |
| Total calcium | 1.00 | 1.33 (0.79–2.24) | 1.54 (0.93–2.57) | 1.91 (1.16–3.15) | 1.36 (0.79–2.36) | 0.25 |
Adjusted for race/ethnicity (non-Hispanic White, non-Hispanic Black, and others), education (less than high school, high school graduate, some college, and college graduate/postgraduate), marital status (married, not-married), body mass index (BMI, continuous), family history of cancer (yes, no), vigorous physical activity (never/rarely, ≤3 times/month, 1–2, 3–4, and ≥5 time/week), menopausal hormone therapy use (never, past, and current), alcohol consumption (0, <5, 5–<15, 15–<30, and ≥30 g/day), and intakes of red meat (quintiles) and total energy (continuous) and additional variables race/ethnicity, education, marital status, BMI, family history of cancer, vigorous physical activity,
Dairy food and dietary and total calcium intakes were categorized according to quintiles of the distribution. The medians for category 1–5 were 0.2, 0.4, 0.7, 1.0 and 1.6 servings/1000kcal/day for dairy food; 409, 532, 648, 798 and 1101 mg/day for dietary calcium; and 494, 717, 969, 1296, and 1881 mg/day for total calcium. Supplemental calcium intake was categorized into four groups, 0, >0–<400, 400–<1,000, and ≥1,000 mg/day. Dietary and supplemental calcium intakes were mutually adjusted in the multivariate models.
Adjusted for variables listed in the asterisked footnote + smoking status (never, former, and current), time since quitting smoking (never, stopped ≥10 years ago, stopped 5–9 years ago, stopped 1–4 years ago, stopped <1 years ago, and currently smoking), smoking dose (0, 1–10, 11–20, 21–30, 31–40, 41–50, 51–60, >60 cigarettes/day), antacid use (yes, no), age at menopause(<50, 50–54, and ≥55 years old), parity (nulliparous, ≤2, and ≥3 children), oral contraceptive use (never, <5, and ≥5 years), duration of hormone replacement therapy use (never, <10 and ≥10 years), personal history of diabetes (yes, no), hypertension (yes, no), oophorectomy (yes, no) and hysterectomy (yes, no), and intakes of fat (quintiles), fruit and vegetables (quintiles), whole grains (quintiles), total beverages (quintiles), protein (quintiles), and folate (quintiles).
Number of cases
Adjusted for variables listed in the asterisked footnote + smoking status, time since quitting smoking, smoking dose, and intake of fruit and vegetables
Adjusted for variables listed in the asterisked footnote + smoking status, time since quitting smoking, smoking dose, antacid use, and intake of fruit and vegetables
Adjusted for variables listed in the asterisked footnote + smoking (never, ≤20 cigarettes/day in the past, >20 cigarettes/day in the past, currently≤20 cigarettes/day, and currently >20 cigarettes/day), and antacid use
Adjusted for variables listed in the asterisked footnote + smoking, intakes of fruit and vegetables, whole grains, and folate.
Adjusted for variables listed in the asterisked footnote + smoking
Adjusted for variables listed in the asterisked footnote + smoking status, time since quitting smoking, and smoking dose
Adjusted for variables listed in the asterisked footnote + smoking, combined age at first birth and number of children, age at menopause, and intake of fat.
Adjusted for variables listed in the asterisked footnote + smoking, parity, oral contraceptive use, and duration of hormone replacement use.
Adjusted for variables listed in the asterisked footnote + smoking, parity, and oral contraceptive use.
Adjusted for variables listed in the asterisked footnote + smoking status, time since quitting smoking and smoking dose, parity, oral contraceptive use, and intakes of fruit and vegetables, and total beverages.
Adjusted for variables listed in the asterisked footnote + smoking, parity, oral contraceptive use, personal history of diabetes and hypertension, and intake of protein.
Death from total cancer and death from cardiovascular disease (CVD) in relation to dairy food and calcium intakes were examined. Dairy food and dietary, supplemental and total calcium intakes were not associated with total cancer mortality in both men (n=8,787 death) and women (n=4,479 death): the multivariate RRQ5 vs. Q1 for dairy food and total calcium were 1.05 (95% CI: 0.97–1.14) and 1.02 (95%CI: 0.94–1.11), respectively, in men and 0.93 (95% CI: 0.84–1.04) and 0.97 (95% CI:0.87–1.08), respectively, in women.
Total calcium intake was related to a 16% lower risk of cancers of the digestive system in men (multivariate RRQ5 vs. Q1 = 0.84, 95% CI: 0.77–0.92) and a 23% lower risk in women (multivariate RRQ5 vs. Q1 = 0.77, 95% CI: 0.69–0.91, Figure 2). Similar inverse association with cancers of the digestive system was observed for dairy food intake in both men and women (data not shown). Total calcium and dairy food intakes were not associated with cancer in any other anatomical systems.
For specific cancers in men, dairy food was inversely associated with cancers of head and neck, esophagus, stomach, colorectum, and bladder, whereas it was positively associated with prostate cancer (Table 2). Dietary and supplemental calcium were also significantly inversely associated with colorectal cancer. Total calcium was significantly related to a lower risk of colorectal cancer and showed a weak inverse association with kidney cancer. On the other hand, total calcium was not related to prostate cancer.
In women, dairy food was significantly inversely associated with colorectal cancer and showed a weak inverse association with stomach cancer (Table 3). Dietary, supplemental, and total calcium were also inversely related to colorectal cancer. Supplemental calcium was also inversely associated with liver cancer, while total calcium was related to an increased risk of NHL. Dairy food, dietary, supplemental, and total calcium were not related to breast, ovarian, and endometrial cancer.
When we examined the associations among men and women combined, we found that the results for esophageal, stomach, liver, pancreatic, and brain cancer were similar to those for men alone. We also observed that total calcium was now significantly inversely associated with kidney cancer (the multivariate RRQ5 vs.Q1 = 0.77, 95% CI: 0.63–0.94, p trend=0.01).
DISCUSSION
In this large prospective cohort study, we found that total calcium was nonlinearly associated with total cancer incidence in women: risk decreased up to approximately 1,300 mg/day, after which no further risk reduction was observed. The associations with dairy food and calcium differed among individual cancer sites. Dairy food and calcium were inversely associated with cancers of the digestive system in both men and women, especially with colorectal cancer. Supplemental calcium was also inversely associated with colorectal cancer. Calcium intake was not related to breast or prostate cancer.
Dairy food and calcium have been hypothesized to play roles that differ among individual cancer sites. Dairy food, relatively high in potentially anticarcinogenic nutrients such as calcium, vitamin D, and conjugated linoleic acid have been postulated to protect against the development of colorectal and breast cancer 4, 22. Calcium has been shown to reduce proliferation, stimulate differentiation, and induce apoptosis in cells in gastrointestinal tract and breast 23, 24. In addition, the binding of calcium to bile and fatty acids in gastrointestinal tract has been hypothesized to reduce damage to large bowel mucosa 23. Nevertheless, high calcium intake has been hypothesized to increase the risk of prostate cancer by suppressing 1,25(OH)2 vitamin D and thereby offsetting a potential anti-carcinogenic effect of vitamin D 25. Dairy food has also been found to increase insulin-like growth factor (IGF)-I, a potent mitogen associated with increased risk of prostate cancer 26, 27.
We found that total calcium was non-linearly inversely associated with total cancer in women. In contrast, the Women’s Health Initiative Study, a randomized clinical trial of daily supplementation of 1,000 mg calcium combined with 400 IU vitamin D for an average of seven years, found no effect on total cancer incidence among postmenopausal women (Hazard ratio = 0.98, 95% CI: 0.91–1.05, total cancer cases = 1,634 in treatment group and 1,655 in placebo group)28. We note, however, that in the WHI study, two third of women had at least 800 mg/day of the total calcium at baseline and total number of cancer cases were small.
Consistent with previous studies 3, 22, we found that intakes of dairy food and calcium were significantly inversely associated with risk of colorectal cancer. In a pooled analysis of 10 cohort studies 3, a 15–20% lower risk of colorectal cancer was observed for total calcium (relative risk (RR)= 0.78, 95% CI: 0.69–0.88 for the highest vs. lowest quintile) and milk (RR=0.85, 95% CI: 0.78–0.94 for <70 vs. ≥250 g/day). In addition, randomized clinical trials have found that calcium supplementation reduced recurrence of colorectal adenomas, precursors of colorectal cancer 29, 30.
A limited number of studies, most with case-control designs, have examined dairy food and calcium in relation to head and neck, esophageal, and stomach cancer. Few studies have observed inverse associations of dairy food and calcium with head and neck 31, 32 and esophageal cancer 33–35; other studies have found no associations with cancer in head and neck 36, esophagus 37–41, and stomach 33, 34, 41, 42. Our study is one of the first large prospective cohort studies examining dairy food and calcium in relation to these cancers. We found that dairy food and dietary calcium were inversely associated with cancers of the digestive system in men, while supplemental and total calcium showed suggestive inverse associations in women. Calcium supplement use in men tended to be less prevalent and consistent, and therefore more likely to be measured with error; this may explain why the calcium association in men was reflected in dairy food and dietary calcium intakes. In women, the associations for supplemental and total calcium intakes did not achieve statistical significances, but the numbers of cases for these cancers were small. Overall, these findings suggest that calcium plays a role in preventing cancers of the digestive system. However, we cannot exclude the possibility of confounding by vitamin D found in dairy food and supplements. Vitamin D, tightly related to calcium regulation in the body, has been inversely associated with digestive system cancers in other studies43.
In women, supplemental calcium was inversely associated with liver cancer, but it was positively related to NHL. Given that the association with liver cancer was observed in women only that the number of liver cancer cases was small, this finding is likely due to chance. Few studies 44–47 which investigated the association between dairy food or calcium and NHL suggested that dairy food was associated with an increased risk of NHL. Further investigation of dairy food and calcium in relation to cancers in liver and NHL is warranted.
The associations between dairy food and calcium and breast cancer have been either null or inverse 4, 48–51. Cohort studies which found dairy food and calcium to be inversely associated with breast cancer suggested that the associations may differ by menopausal status 49, 50 or estrogen receptor status of the tumor 51. Our study, conducted mainly among postmenopausal women, found that dairy food and calcium were not associated with breast cancer. Further studies examining the associations by tumor characteristics of breast cancer and by menopausal status might be informative.
Despite the existence of plausible biologic mechanisms, the effects of dairy food and calcium on prostate cancer are unclear. Epidemiologic studies have found null 52–54 as well as positive 7, 55–57 associations for dairy food and calcium. Among studies that observed a positive association between calcium and prostate cancer, a significantly increased risk was generally found only with very high calcium intake (2000 mg/day, compared with approximately <750 mg/day) or in advanced prostate cancer 7, 55. However, our study found that calcium, even at very high intake of calcium (≥2000 vs. 500–<750 mg/day), was not associated with prostate cancer 58.
Some limitations exist in our study. First, we did not examine if associations with dairy food and calcium differed by tumor subtype or tumor aggressiveness of site-specific cancers. It is possible that we missed some associations that may exist only for certain tumor subtypes or aggressive tumors. Second, although we adjusted for all potential risk factors available in our study for site-specific cancers, residual confounding by unknown or unmeasured risk factors may exist for some cancers. Third, in analyses of low incidence cancers, especially cancers in women with small numbers of cases, we had limited statistical power to examine an association and some findings may be due to chance. Lastly, because diet was assessed only once at baseline, this may not reflect long-term usual intake as accurately as repeated measurements of diet during follow-up. Also, we could not examine if dairy food and calcium intakes during earlier life periods or lifelong cumulative intakes are related to cancer risk.
Nevertheless, our study is one of the first cohort studies to examine dairy food and calcium in relation to total cancer as well as low incidence cancers. Moreover, our prospective design avoids the recall and selection biases that can affect results from case-control studies. Our study had high statistical power with more than 53,000 total cancers and at least 100 cases for most individual cancers. Thus substantial effects of dairy food and calcium were unlikely to have been missed. A further strength of our study is that we rigorously controlled for all potential risk factors including both dietary and lifestyle factors for each site-specific cancer.
In conclusion, our findings suggest that calcium intake consistent with current recommendations is associated with a lower risk of total cancer in women and cancers of the digestive system, especially colorectal cancer in both men and women.
Acknowledgments
We are indebted to the participants in the NIH-AARP Diet and Health Study for their outstanding cooperation. Also, we thank cancer registries in Arizona, California, Florida, Georgia, Louisiana, Michigan, New Jersey, Nevada, North Carolina, Pennsylvania, and Texas.
The study was funded by the Intramural Research Program of the National Cancer Institute, National Institutes of Health. The funding source had no role in the design, data collection, analysis, or interpretation of the study or in the decision to submit the manuscript for publication.
References
- 1.Food and nutrition Board. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington DC: National Academy Press; 1997. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Institute of Medicine. [Google Scholar]
- 2.U. S. Department of Health and Human Services and Department of Agriculture. Dietary guidelines for Americans. 2005 http://www.health.gov/dietaryguidelines/dga2005/document/
- 3.Cho E, Smith-Warner SA, Spiegelman D, et al. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. J Natl Cancer Inst. 2004;96:1015–22. doi: 10.1093/jnci/djh185. [DOI] [PubMed] [Google Scholar]
- 4.Moorman PG, Terry PD. Consumption of dairy products and the risk of breast cancer: a review of the literature. Am J Clin Nutr. 2004;80:5–14. doi: 10.1093/ajcn/80.1.5. [DOI] [PubMed] [Google Scholar]
- 5.Breslow RA, Graubard BI, Sinha R, Subar AF. Diet and lung cancer mortality: a 1987 National Health Interview Survey cohort study. Cancer Causes and Control. 2000;11:419–431. doi: 10.1023/a:1008996208313. [DOI] [PubMed] [Google Scholar]
- 6.Larsson SC, Orsini N, Wolk A. Milk, milk products and lactose intake and ovarian cancer risk: a meta-analysis of epidemiological studies. Int J Cancer. 2006;118:431–41. doi: 10.1002/ijc.21305. [DOI] [PubMed] [Google Scholar]
- 7.Giovannucci E, Liu Y, Stampfer MJ, Willett WC. A prospective study of calcium intake and incident and fatal prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:203–10. doi: 10.1158/1055-9965.EPI-05-0586. [DOI] [PubMed] [Google Scholar]
- 8.Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions : the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154:1119–25. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 9.Division of Cancer Control and Population Science. National Institute of Cancer. Diet History Questionnaire. Available from: URL: http://www.riskfactor.cancer.gov/DHQ.
- 10.Subar AF, Midthune D, Kulldorff M, et al. Evaluation of alternative approaches to assign nutrient values to food groups in food frequency questionnaires. Am J Epidemiol. 2000;152:279–86. doi: 10.1093/aje/152.3.279. [DOI] [PubMed] [Google Scholar]
- 11.Tippett KS, Cypel YS. Continuing survey of food intakes by individuals, nationwide food surveys: US Department of Agriculture. Agricultural Research Service; 1997. Design and operation: the continuing survey of food intakes by individuals and diet and health knowledge survey, 1994–96. [Google Scholar]
- 12.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. American Journal of Epidemiology. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 13.U.S Department of Agriculture. The food guide pyramid. Washington, DC: GPO; 1992. p. 30. Home and Garden Bulletin No. 252. [Google Scholar]
- 14.Thompson FE, Kipnis V, Midthune D, et al. Performance of a food-frequency questionnaire in the US NIH-AARP (National Institutes of Health-American Association of Retired Persons) Diet and Health Study. Public Health Nutr. 2007:1–13. doi: 10.1017/S1368980007000419. [DOI] [PubMed] [Google Scholar]
- 15.Michaud DS, Midthune D, Hermansen S, et al. Comparison of cancer registry case ascertainment with SEER estimates and self-reporting in a subset of the NIH-AARP Diet and Health Study. Journal of registry management. 2005;32:70–75. [Google Scholar]
- 16.Cox DR. Regression models and life-tables [with discussion] J Royal Stat Soc (B) 1972;34:187–220. [Google Scholar]
- 17.SAS II. SAS/STAT User’s Guide, Version 8. Cary, NC: SAS Institute Inc; 1999. [Google Scholar]
- 18.Miettinen O. Theoretical epidemiology. New York, NY: Oxford University Press; 1985. [Google Scholar]
- 19.Huberman M, Langholz B. Application of the missing-indicator method in matched case-control studies with incomplete data. Am J Epidemiol. 1999;150:1340–5. doi: 10.1093/oxfordjournals.aje.a009966. [DOI] [PubMed] [Google Scholar]
- 20.Greenland S, Finkle WD. A critical look at methods for handling missing covariates in epidemiologic regression analyses. Am J Epidemiol. 1995;142:1255–64. doi: 10.1093/oxfordjournals.aje.a117592. [DOI] [PubMed] [Google Scholar]
- 21.Durrleman S, Simon R. Flexible regression models with cubic splines. Statistics in Medicine. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 22.Norat T, Riboli E. Dairy products and colorectal cancer. A review of possible mechanisms and epidemiological evidence. Eur J Clin Nutr. 2003;57:1–17. doi: 10.1038/sj.ejcn.1601522. [DOI] [PubMed] [Google Scholar]
- 23.Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer. 2003;3:601–14. doi: 10.1038/nrc1144. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson EA, James KA, Newmark HL, Carroll KK. Effects of dietary fat, calcium, and vitamin D on growth and mammary tumorigenesis induced by 7,12-dimethylbenz(a)anthracene in female Sprague-Dawley rats. Cancer Res. 1989;49:6300–3. [PubMed] [Google Scholar]
- 25.Giovannucci E. Dietary influences of 1,25(OH)2 vitamin D in relation to prostate cancer: a hypothesis. Cancer Causes Control. 1998;9:567–82. doi: 10.1023/a:1008835903714. [DOI] [PubMed] [Google Scholar]
- 26.Chan JM, Stampfer MJ, Giovannucci E, et al. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–6. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 27.Giovannucci E, Pollak M, Liu Y, et al. Nutritional predictors of insulin-like growth factor I and their relationships to cancer in men. Cancer Epidemiol Biomarkers Prev. 2003;12:84–9. [PubMed] [Google Scholar]
- 28.Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–96. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 29.Grau MV, Baron JA, Sandler RS, et al. Prolonged effect of calcium supplementation on risk of colorectal adenomas in a randomized trial. J Natl Cancer Inst. 2007;99:129–36. doi: 10.1093/jnci/djk016. [DOI] [PubMed] [Google Scholar]
- 30.Bonithon-Kopp C, Kronborg O, Giacosa A, Rath U, Faivre J. Calcium and fibre supplementation in prevention of colorectal adenoma recurrence: a randomised intervention trial. European Cancer Prevention Organisation Study Group. Lancet. 2000;356:1300–6. doi: 10.1016/s0140-6736(00)02813-0. [DOI] [PubMed] [Google Scholar]
- 31.Levi F, Pasche C, La Vecchia C, Lucchini F, Franceschi S, Monnier P. Food groups and risk of oral and pharyngeal cancer. Int J Cancer. 1998;77:705–9. doi: 10.1002/(sici)1097-0215(19980831)77:5<705::aid-ijc8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez MJ, Martinez C, Nieto A, et al. Oral and oropharyngeal cancer in Spain: influence of dietary patterns. Eur J Cancer Prev. 2003;12:49–56. doi: 10.1097/00008469-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Chen H, Ward MH, Graubard BI, et al. Dietary patterns and adenocarcinoma of the esophagus and distal stomach. Am J Clin Nutr. 2002;75:137–44. doi: 10.1093/ajcn/75.1.137. [DOI] [PubMed] [Google Scholar]
- 34.Chen H, Tucker KL, Graubard BI, et al. Nutrient intakes and adenocarcinoma of the esophagus and distal stomach. Nutr Cancer. 2002;42:33–40. doi: 10.1207/S15327914NC421_5. [DOI] [PubMed] [Google Scholar]
- 35.Wolfgarten E, Rosendahl U, Nowroth T, et al. Coincidence of nutritional habits and esophageal cancer in Germany. Onkologie. 2001;24:546–51. doi: 10.1159/000055142. [DOI] [PubMed] [Google Scholar]
- 36.Kreimer AR, Randi G, Herrero R, Castellsague X, La Vecchia C, Franceschi S. Diet and body mass, and oral and oropharyngeal squamous cell carcinomas: analysis from the IARC multinational case-control study. Int J Cancer. 2006;118:2293–7. doi: 10.1002/ijc.21577. [DOI] [PubMed] [Google Scholar]
- 37.Brown LM, Swanson CA, Gridley G, et al. Adenocarcinoma of the esophagus: role of obesity and diet. J Natl Cancer Inst. 1995;87:104–9. doi: 10.1093/jnci/87.2.104. [DOI] [PubMed] [Google Scholar]
- 38.Mayne ST, Risch HA, Dubrow R, et al. Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:1055–62. [PubMed] [Google Scholar]
- 39.Brown LM, Swanson CA, Gridley G, et al. Dietary factors and the risk of squamous cell esophageal cancer among black and white men in the United States. Cancer Causes Control. 1998;9:467–74. doi: 10.1023/a:1008861806923. [DOI] [PubMed] [Google Scholar]
- 40.Tzonou A, Lipworth L, Garidou A, et al. Diet and risk of esophageal cancer by histologic type in a low-risk population. Int J Cancer. 1996;68:300–4. doi: 10.1002/(SICI)1097-0215(19961104)68:3<300::AID-IJC6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 41.Zhang ZF, Kurtz RC, Yu GP, et al. Adenocarcinomas of the esophagus and gastric cardia: the role of diet. Nutr Cancer. 1997;27:298–309. doi: 10.1080/01635589709514541. [DOI] [PubMed] [Google Scholar]
- 42.Lissowska J, Gail MH, Pee D, et al. Diet and stomach cancer risk in Warsaw, Poland. Nutr Cancer. 2004;48:149–59. doi: 10.1207/s15327914nc4802_4. [DOI] [PubMed] [Google Scholar]
- 43.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–9. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 44.Chang ET, Smedby KE, Zhang SM, et al. Dietary factors and risk of non-hodgkin lymphoma in men and women. Cancer Epidemiol Biomarkers Prev. 2005;14:512–20. doi: 10.1158/1055-9965.EPI-04-0451. [DOI] [PubMed] [Google Scholar]
- 45.Zheng T, Holford TR, Leaderer B, et al. Diet and nutrient intakes and risk of non-Hodgkin’s lymphoma in Connecticut women. Am J Epidemiol. 2004;159:454–66. doi: 10.1093/aje/kwh067. [DOI] [PubMed] [Google Scholar]
- 46.Tavani A, Pregnolato A, Negri E, et al. Diet and risk of lymphoid neoplasms and soft tissue sarcomas. Nutr Cancer. 1997;27:256–60. doi: 10.1080/01635589709514535. [DOI] [PubMed] [Google Scholar]
- 47.Ward MH, Zahm SH, Weisenburger DD, et al. Dietary factors and non-Hodgkin’s lymphoma in Nebraska (United States) Cancer Causes Control. 1994;5:422–32. doi: 10.1007/BF01694756. [DOI] [PubMed] [Google Scholar]
- 48.Cui Y, Rohan TE. Vitamin D, calcium, and breast cancer risk: a review. Cancer Epidemiol Biomarkers Prev. 2006;15:1427–37. doi: 10.1158/1055-9965.EPI-06-0075. [DOI] [PubMed] [Google Scholar]
- 49.Lin J, Manson JE, Lee IM, Cook NR, Buring JE, Zhang SM. Intakes of calcium and vitamin d and breast cancer risk in women. Arch Intern Med. 2007;167:1050–9. doi: 10.1001/archinte.167.10.1050. [DOI] [PubMed] [Google Scholar]
- 50.Shin MH, Holmes MD, Hankinson SE, Wu K, Colditz GA, Willett WC. Intake of dairy products, calcium, and vitamin d and risk of breast cancer. J Natl Cancer Inst. 2002;94:1301–11. doi: 10.1093/jnci/94.17.1301. [DOI] [PubMed] [Google Scholar]
- 51.McCullough ML, Rodriguez C, Diver WR, et al. Dairy, calcium, and vitamin D intake and postmenopausal breast cancer risk in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:2898–904. doi: 10.1158/1055-9965.EPI-05-0611. [DOI] [PubMed] [Google Scholar]
- 52.Schuurman AG, van den Brandt PA, Dorant E, Goldbohm RA. Animal products, calcium and protein and prostate cancer risk in The Netherlands Cohort Study. Br J Cancer. 1999;80:1107–13. doi: 10.1038/sj.bjc.6690472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koh KA, Sesso HD, Paffenbarger RS, Jr, Lee IM. Dairy products, calcium and prostate cancer risk. Br J Cancer. 2006;95:1582–5. doi: 10.1038/sj.bjc.6603475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rohrmann S, Platz EA, Kavanaugh CJ, Thuita L, Hoffman SC, Helzlsouer KJ. Meat and dairy consumption and subsequent risk of prostate cancer in a US cohort study. Cancer Causes Control. 2007;18:41–50. doi: 10.1007/s10552-006-0082-y. [DOI] [PubMed] [Google Scholar]
- 55.Rodriguez C, McCullough ML, Mondul AM, et al. Calcium, dairy products, and risk of prostate cancer in a prospective cohort of United States men. Cancer Epidemiol Biomarkers Prev. 2003;12:597–603. [PubMed] [Google Scholar]
- 56.Tseng M, Breslow RA, Graubard BI, Ziegler RG. Dairy, calcium, and vitamin D intakes and prostate cancer risk in the National Health and Nutrition Examination Epidemiologic Follow-up Study cohort. Am J Clin Nutr. 2005;81:1147–54. doi: 10.1093/ajcn/81.5.1147. [DOI] [PubMed] [Google Scholar]
- 57.Mitrou PN, Albanes D, Weinstein SJ, et al. A prospective study of dietary calcium, dairy products and prostate cancer risk (Finland) Int J Cancer. 2007 doi: 10.1002/ijc.22553. [DOI] [PubMed] [Google Scholar]
- 58.Park Y, Mitrou PN, Kipnis V, Hollenbeck A, Schatzkin A, Leitzmann MF. Calcium, Dairy Foods, and Risk of Incident and Fatal Prostate Cancer: The NIH-AARP Diet and Health Study. Am J Epidemiol. 2007;166:1270–1279. doi: 10.1093/aje/kwm268. [DOI] [PubMed] [Google Scholar]