Abstract
The human glucocorticoid receptor (GR) gene produces C-terminal GRβ and GRα isoforms through alternative use of specific exons 9β and α, respectively. We explored the transcriptional activity of GRβ on endogenous genes by developing HeLa cells stably expressing EGFP-GRβ or EGFP. Microarray analyses revealed that GRβ had intrinsic gene-specific transcriptional activity, regulating mRNA expression of a large number of genes negatively or positively. Majority of GRβ-responsive genes was distinct from those modulated by GRα, while GRβ and GRα mutually modulated each other’s transcriptional activity in a subpopulation of genes. We did not observe in HCT116 cells nuclear translocation of GRβ and activation of this receptor by RU 486, a synthetic steroid previously reported to bind GRβ and to induce nuclear translocation. Our results indicate that GRβ has intrinsic, GRα-independent, gene-specific transcriptional activity, in addition to its previously reported dominant negative effect on GRα-induced transactivation of GRE-driven promoters.
Keywords: GR gene splicing variant, gene expression, nuclear translocation, RU 486
INTRODUCTION
Glucocorticoids are steroid hormones secreted by the adrenal glands, important for maintenance of basal and stress-related homeostasis [1, 2]. At pharmacologic doses, glucocorticoids are used as potent immunosuppressive and anti-inflammatory agents in the management of many inflammatory, allergic, autoimmune and lymphoproliferative diseases [2]. The actions of glucocorticoids are mediated by multiple isoforms of an intracellular receptor protein, the glucocorticoid receptor (GR), which belongs to the steroid/sterol/thyroid/retinoid/orphan nuclear receptor superfamily, functioning as a hormone-activated transcription factor that regulates the expression of a large number of glucocorticoid-responsive genes [3].
The human GR gene generates two C-terminal receptor isoforms, termed GRα and GRβ, by alternative use of exons 9 α and β, respectively, each an array of 8 N-terminal isoforms through use of 8 different active translation initiation sites [3–5]. GRα isoforms, are activated by glucocorticoid ligands, are ubiquitously expressed in almost all human tissues and organs and mediate most of the known actions of these hormones [3]. In the absence of ligand, GRα isoform molecules reside in the cytoplasm as part of a large multiprotein complex with several heat shock proteins (hsps) [1, 3]. Upon hormone binding, GRα isoforms undergo a conformational change, which results in dissociation from the hsps, unmasking or activation of their nuclear localization signals (NLSs), and translocation into the nucleus [1, 3]. Ligand-activated GRα isoforms homo- or hetero-dimerize among isoforms, binds onto GREs in the regulatory regions of glucocorticoid-responsive genes, attract several so-called coactivators and chromatin-remodeling factors, and influence transcription [1]. Alternatively GR isoforms interact with other transcription factors, such as the nuclear factor of κB and the activator protein 1, and influence the transcription of genes responsive to these factors.
GRβ isoforms are also ubiquitously expressed in most tissues [6]. GRβ are identical to GRα albeit with a different C-terminal region, having 15 nonhomologous amino acids at their C-terminal end [4]. Thus, GRβ isoforms share the same N-terminal (NTD) and DNA-binding (DBD) domains with GRα, but have a unique “ligand-binding” domain (LBD). GRβ are unable to bind known glucocorticoids, are located mainly in the nucleus, and generally fail to activate the transcription of glucocorticoid-responsive genes [7]. GRβ function as dominant negative isoforms of GRα-induced transactivation of GRE-containing, glucocorticoid-responsive promoters and function as a natural inhibitor of glucocorticoid actions [6–8]. The ability of GRβ to antagonize and moderate GRα effects suggests that GRβ may play a role in the regulation of target cell sensitivity to glucocorticoids [6, 9]. The expression of GRβ is induced by cytokines, and an increase of the GRβ/GRα ratio has been reported in patients with asthma, rheumatoid arthritis and chronic lymphocytic leukemia [6, 9]. GRβ was recently reported to weakly bind the synthetic glucocorticoid antagonist RU 486 from among 57 glucocorticoid-related natural and synthetic steroids tested, and to translocate slowly from the cytoplasm into the nucleus in response to this steroid, independently of GRα [10]. Furthermore, GRβ was shown to have an intrinsic transcriptional activity that RU 486 modulated further [10].
In this study, we examined the transcriptional activity of GRβ by using cDNA microarrays. We found that this isoform had intrinsic transcriptional activity on a distinct host of genes, most of which were not regulated by the synthetic glucocorticoid dexamethasone. Although previously reported, RU 486 did not change the subcellular location of GRβ or its transcriptional activity on a GRE-containing promoter.
MATERIALS AND METHODS
Plasmids
pCR3.1-CMV/Intron-EGFP, which expresses the enhanced green fluorescence protein (EGFP) under the control of the Cytomegalovirus (CMV) enhancer and the chicken β-actin promoter, was constructed by subcloning the DNA fragment, which contains the CMV enhancer/chicken β-actin promoter, its intron and EGFP cDNA obtained from pCX-EGFP (a gift from Dr. M. Okabe, University of Osaka, Japan) into pCR3.1 (Invitrogen, Carlsbad, CA), which contains a neomycin-resistant cassette. pCR3.1-CMV/Intron-EGFP-GRβ and -GRα were further constructed by subcloning the human GRβ and GRα cDNAs, excised, respectively, from pF25-hGRβ and -hGRα [11] into pCR3.1-CMV/Intron-EGFP. pRShGRα and pRShGRβ, which express the human GRα and β, respectively, and pRSVerbA−1, which contains the thyroid hormone receptor cDNA in an inverse orientation and was used as a negative control for the above two plasmids, and pMMTV-luc, which expresses the firefly luciferase under the control of the glucocorticoid-responsive mouse mammary tumor virus promoter, were reported previously [8]. pGL4.73-hRLuc/SV40, which expresses the renilla luciferase under the control of the SV40 promoter, was purchased from Promega Corp. (Madison, WI).
Generation of HeLa cells stably expressing EGFP-GRβ
HeLa cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 100 U/mL of penicillin and 100 μg/mL of streptomycin. They were transfected with pCR3.1-CMV/Intron-EGFP or pCR3.1-CMV/Intron-EGFP-GRβ with FuGENE (Roche Applied Science, Indianapolis, IN). After 2 days of transfection, they were cultured in the presence of neomycin sulfate (Invitrogen). The cell colonies that grew in the presence of neomycin were subsequently harvested. The limiting dilution was further performed for expression-positive cells to establish the stable HeLa cell clone expressing EGFP or EGFP-GRβ that was originated from a single cell.
Microarray Analyses
HeLa cells stably expressing EGFP or EGFP-GRβ were cultured in the medium containing dextrane/charcoal-treated FBS (Hyclone, Logan, UT) and antibiotics, and incubated in the absence or presence of 10−6 M dexamethasone. After 8 hours of culture, their total RNAs were purified. The total RNAs obtained were labeled with the 3DNA Array 9000 Expression Array Detection kit (Genisphere Inc., Hatfield, PA).
The human 12K cDNA microarrays were constructed as previously described [12]. Using these microarrays, we performed two comparisons: (1) HeLa cells expressing EGFP (HeLa/EGFP) vs. HeLa cells expressing EGFP-GRβ (HeLa/EGFP-GRβ) and (2) HeLa/EGFP cultured in the absence of dexamethasone vs. those cells cultured in the presence of this glucocorticoid. The array chips were scanned for obtaining hybridized images with the ScanArray Express microarray scanner (Perkin Elmer Life Sciences, Inc., Boston, MA). After LOWESS normalization, one sample Student t test was applied to the three gene expression values (from total of 3 hybridizations, one forward and one reverse hybridization for each of the comparisons) of each gene and a reliable gene list was generated by setting the less stringent p value cutoff as 0.05. Final candidate genes were obtained by further applying z test with genes located outside one standard deviation (fold changes are around 2 or higher).
Western blot analyses
HeLa/EGFP and HeLa/EGFP-GRβ were lysed as previously described [13], and the cell lysates were run on SDS-PAGE gels. Separated proteins were further blotted on the nitrocellulose membranes. Expression of EGFP and EGFP-GRβ were probed with anti-GRα, -hGRβ and/or -GFP antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and were subsequently visualized with the chemiluminescence reaction.
Confocal microscopy analyses
HeLa/EGFP-GRβ and HCT116 cells were transfected and/or treated with the indicated plasmids/compounds. They were first fixed with 4% paraformaldehyde and subsequently mounted on glass slides with the Vectashield with DAPI (4,6-diamidino-2-phenylindole) (Vector Laboratories, Inc., Burlingame, CA) or directly examined under the microscope. Emitted signals were recorded with the Zeiss LSM510 confocal microscope (Carl Zeiss, Thornwood, New York, NY) at the NICHD Microscopy & Imaging Core (Eunice Kennedy Shriver National Institute of Child Health and Human Development) with the assistance of Dr. Vincent Schram.
SYBR Green-based real-time PCR
Total RNAs obtained from HeLa/EGFP and HeLa/EGFP-GRβ treated with or without dexamethasone, and those from HCT116 cells transfected with the GR-expressing plasmids using the Nucleofector system (Amaxa GmbH, Cologne, Germany), were reverse transcribed, and the real-time PCR reaction was performed in triplicate in a 7500 Real-time PCR system (Applied Biosystems, Foster City, CA), as previously described [13]. Primer pairs used for the reactions are shown in Supplementary Table 1. Obtained Ct (threshold cycle) values of each gene were normalized for those of the acidic ribosomal phosphoprotein P0 (RPLP0) and their relative mRNA expression was demonstrated as fold induction over the baseline. The dissociation curves of used primer pairs showed a single peak and samples after PCR reactions had a single expected DNA band in an agarose gel analysis.
Reporter assays
HCT116 cells were transfected with the GR-expressing plasmids indicated together with pMMTV-luc and pGL4.73-hRLuc/SV40, as previously described [13]. After 24 hours of transfection, they were cultured with 10−6 M of dexamethasone or 10−10, 10−8 or 10−6 M of RU 486 for an additional 24 hours. The firefly and renilla luciferase assays were performed by using their detection kits (Promega Corp.).
Statistical Analyses
Statistical analysis used for our microarray samples was explained above. Unpaired Student t tests with a two-tailed p value were employed for all other statistical analyses.
RESULTS
Establishment of HeLa cells stably expressing EGFP-GRβ
To examine the transcriptional effects of GRβ on endogenous genes, we created a HeLa cell clone, which stably expressed EGFP-GRβ (HeLa/EGFP-GRβ) along with its control cell line that expressed just EGFP (HeLa/EGFP). Western blot analysis of these cells confirmed that the former line expressed EGFP-GRβ, while the latter expressed EGFP (Supplementary Figure 1 A, middle two gels). Both cell lines expressed endogenous GRα and GRβ, which were respectively detected with their specific antibodies (Supplementary Figure 1A, top and bottom gels). EGFP-GRβ was mainly expressed in the nucleus of HeLa/EGFP-GRβ cells as determined by confocal microscopy, while some of this receptor fusion molecule was also seen in the cytoplasm (Supplementary Figure 1 B).
GRβ has distinct transcriptional effects
We examined the influence of GRβ expression or dexamethasone treatment on the transcriptional activity of endogenous genes using cDNA microarrays. HeLa/EGFP-GRβ and HeLa/EGFP were cultured in medium containing dextran/charcoal-treated FBS, and HeLa/EGFP were further incubated in the absence or presence of dexamethasone over 8 hours. Total RNAs from HeLa/EGFP and HeLa/EGFP-GRβ cultured in the absence of dexamethasone were used for examining the impact of GRβ on gene expression, while those from HeLa/EGFP cultured in the absence and presence of dexamethasone were used to evaluate the effect of this glucocorticoid via GRα. These analyses revealed that GRβ modulated mRNA expression of 299 genes, while dexamethasone and, hence, activation of GRα, influenced mRNA expression of 279 genes (Figure 1). Highly regulated genes by GRβ and dexamethasone are shown in Supplementary Tables 2 and 3, respectively. Only 5 genes were regulated both by GRβ and dexamethasone (Figure 1), and are shown in Supplementary Table 4.
Figure 1. Dexamethasone and GRβ have distinct transcriptional effects in HeLa cells with very little overlap (Venn diagram).
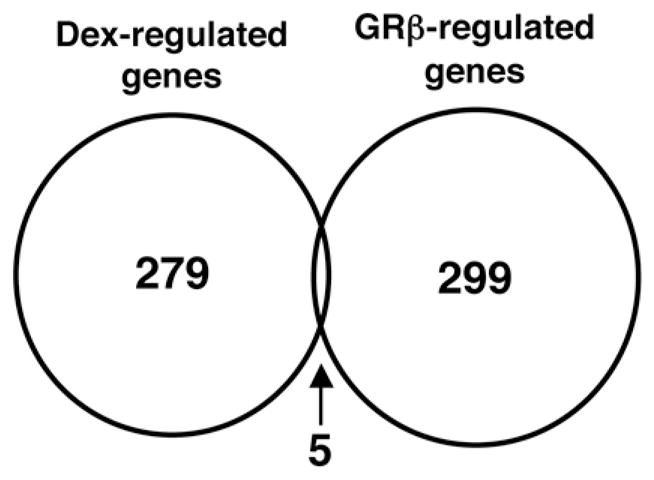
Dexamethasone-regulated genes were obtained by comparing gene expression profiles of HeLa/EGFP in the absence and presence of 10−6M of dexamethasone for 8 hours, while GRβ-regulated genes were identified by comparing gene expression profiles of HeLa/EGFP and HeLa/EGFP-GRβ cells.
GRβ and GRα mutually influence each other’s transcriptional activity
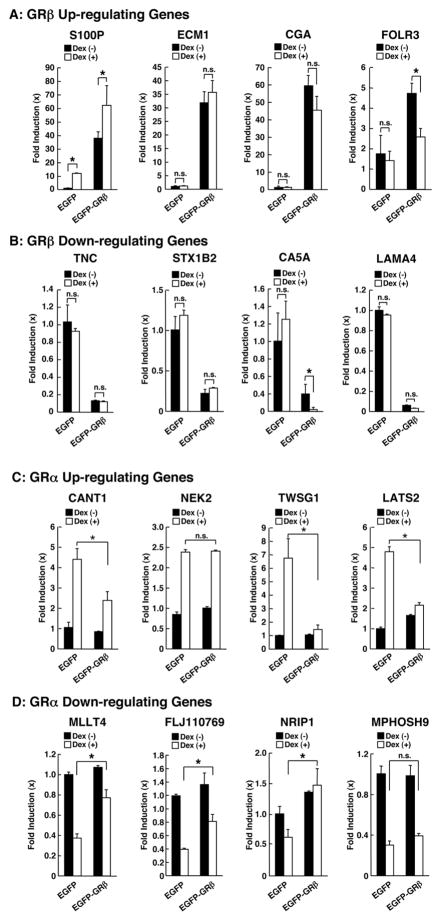
Since GRβ is known to suppress the transcriptional activity of GRα in transient transfection-based reporter assays employing several GRE-containing promoters, we examined the mutual influence of GRβ expression and dexamethasone treatment on mRNA expression of several endogenous genes identified in our microarray analyses (Figure 2). Among the genes strongly up-regulated by GRβ tested, mRNA expression of S100P was further potentiated by dexamethasone, while that of folate receptor 3 (FOLR3) was further suppressed by this compound. Dexamethasone did not influence mRNA expression of the extracellular matrix protein (ECM1) and the glycoprotein hormone α peptide (CGA) (Figure 2 A). mRNA expression of the carbonic anhydrase VA (CA5A), which was strongly down-regulated by GRβ, was further suppressed by dexamethasone, whereas that of tenascin C (TNC), syntaxin 1B (STX1B) and laminin α4 (LAMA4) was not influenced by this steroid (Figure 2 B). GRβ further suppressed dexamethasone-stimulated mRNA expression of calcium activated nucleotidase 1 (CANT1), twisted gastrulation homolog 1 (TWSG1) and large tumor suppressor homolog 2 (LATS2), while it failed to do so on mRNA expression of NIMA (never in mitosis gene A)-related kinase 2 (NEK2) (Figure 2 C). GRβ attenuated dexamethasone-mediated suppression of myeloid/lymphoid or mixed-lineage leukemia 4 (MLLT4), FLJ10769 and nuclear receptor interacting protein 1 (NRIP1) mRNA expression, but not that of M-phase phosphoprotein 9 (MPHOSH9) (Figure 2 D). These results indicate that GRα modulates positively or negatively the transcriptional activity of GRβ in a gene-specific fashion. Similarly, GRβ suppressed GRα-induced transactivation in a gene-specific fashion, whereas it reduced the suppressive effect of GRα on mRNA expression of genes regulated negatively by glucocorticoids.
Figure 2. Dexamethasone and GRβ mutually influence each other’s transcriptional activity in HeLa cells.
HeLa/EGFP and HeLa/EGFP-GRβ cells were cultured in the absence or presence of 10−6M of dexamethasone. Influences of dexamethasone on the genes up- or down-regulated by GRβ is shown in panels A and B, respectively, while those of GRβ on the genes up- or down-regulated by dexamethasone are demonstrated in panels C and D, respectively.
*: p<0.01, n.s.: not significant.
GRα and GRβ influence S100P mRNA expression in HCT116 cells
Since GRα and GRβ regulated independently and cooperatively mRNA expression of S100P in HeLa cells, we examined their effects in a different cell line, HCT116 cells, which do not endogenously express these two GR isoforms [13]. S100P is a small calcium-binding protein of the S100 EF-hand-containing family of proteins and alterations in its expression have been associated with the progression of the metastases of several malignant tumors [14], while some of the S100 family proteins are also induced by various cellular stressors [14]. We successfully transfected over 80% of these cells with the Nucleofector system (data not shown). Dexamethasone stimulated S100P mRNA expression in the presence of GRα, whereas GRβ also stimulated its mRNA expression (Figure 3). When the two isoforms were expressed together, S100P mRNA expression was up-regulated in the absence of dexamethasone, but was not further potentiated by its presence, in contrast to our results obtained in HeLa cells. These findings suggest that the direction of the mutual influence observed between GRα and GRβ on the mRNA expression of the S100P gene may be cell-type specific.
Figure 3. Both GRα and GRβ stimulate S100P mRNA expression, while GRβ antagonizes GRα-induced mRNA expression in HCT116 cells.
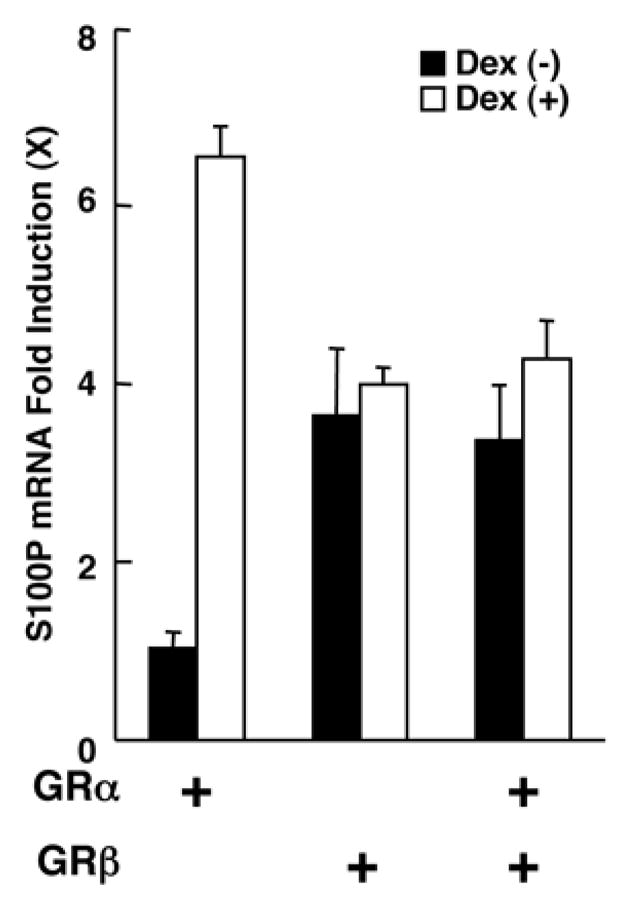
HCT116 cells were transfected with GRα- and/or GRβ-expressing plasmid and were cultured in the absence or presence of 10−6M of dexamethasone.
RU 486 did not induce nuclear translocation of GRβ nor did it modulate the transcriptional activity of the MMTV promoter in the presence of this isoform transfected in HCT116 cells
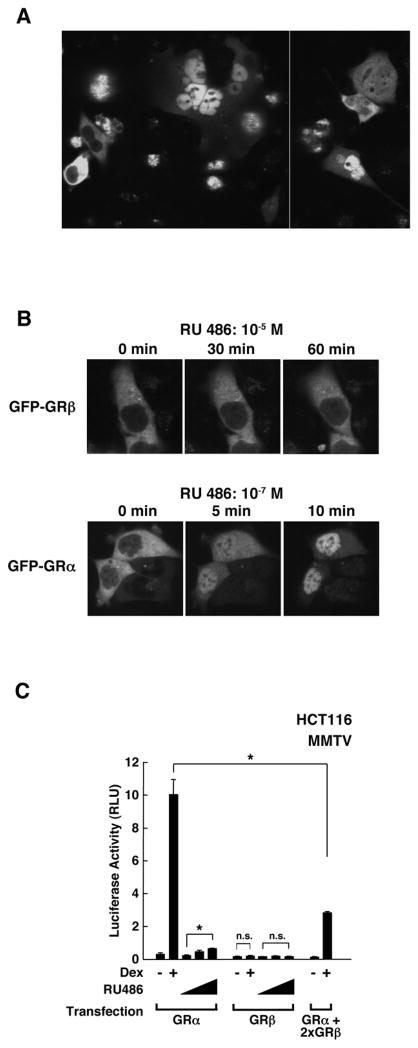
A previous publication demonstrated that RU 486 bound GRβ weakly at its “ligand-binding” pocket and slowly induced its nuclear translocation in COS-7 and U-2 OS cells [10]. Thus, we evaluated RU 486-mediated nuclear translocation of EGFP-GRβ transiently expressed in HCT116 cells (Figure 4). This receptor fusion demonstrated a quite heterogeneous subcellular localization in HCT116 cells cultured in media containing dextran/charcoal-treated FBS; it was located exclusively in the cytoplasm in some cells, while it was sequestered into the nucleus in many other cells, with some other cells demonstrating intermediate subcellular localization (Figure 4 A).
Figure 4. RU 486 does not induce nuclear translocation of GRβ nor does it modulate the transcriptional activity of the MMTV promoter.
A: EGFP-GRβ is heterogeneously localized in the nucleus and cytoplasm of HCT116 cells.
HCT116 cells were transfected with the EGFP-GRβ-expressing plasmid.
B: RU 486 does not induce nuclear translocation of EGFP-GRβ in HCT116 cells.
HCT116 cells were transfected with the EGFP-GRβ- or -GRα-expressing plasmid and nuclear translocation of indicated molecules were monitored after addition of RU 486. Over 10 cells were examined and representative images are shown.
C: GRβ does not influence the transcriptional activity of the MMTV promoter in response to RU 486.
HCT116 cells were transfected with GRα- and/or GRβ-expressing plasmid together with the MMTV-Luc and pGL4.73-hRLuc/SV40, and were cultured in the presence of absence of 10−6M dexamethasone or 10−10, 10−8 and 10−6M of RU 486. Bars represent mean ± S.E. values of firefly luciferase activity normalized for renilla luciferase activity.
*: p<0.01, n.s.: not significant.
In single cells, we time-sequentially monitored the nuclear translocation of EGFP-GRβ and EGFP-GRα, in the presence of 10−5M and 10−7M of RU 486, respectively (Figure 4 B). In cells in which EGFP-GRβ was constitutively localized in the cytoplasm, one hour of incubation with RU 486 caused no nuclear translocation, while cytoplasmic EGFP-GRα readily translocated into the nucleus in response to even 100-times lower concentrations of this compound.
We also examined the transcriptional activity of GRβ on a GRE-containing MMTV promoter in HCT116 cells in the absence and presence of dexamethasone and graded concentrations of RU 486 (Figure 4 C). Dexamethasone strongly stimulated the transcriptional activity of this promoter in the presence of GRα, while RU 486 alone demonstrated a weak stimulatory effect. Both dexamethasone and RU 486 failed to modulate the transcriptional activity of this promoter in the presence of only GRβ. On the other hand, GRβ suppressed dexamethasone-stimulated GRα-mediated transcriptional activity of the MMTV promoter. We therefore conclude that RU 486 does not cause the nuclear translocation of GRβ and does not modulate the transcriptional activity of this isoform on the classic GRE-containing promoter in HCT116 cells.
DISCUSSION
By using microarrays, we found that GRβ has intrinsic transcriptional activity on many endogenous genes in a HeLa cell line stably expressing EGFP-GRβ. This effect of GRβ was distinct from that of the classic receptor GRα and its direction seemed to be gene-specific. GRβ appears to primarily modulate transcription indirectly through interaction with other transcription factors, co-factors and/or chromatin-associated molecules rather than by binding directly to GREs located in gene promoters. This estimation is based on the fact that the genes whose transcriptional activity was regulated by both GRβ and dexamethasone were few, while the transcriptional activity of a substantial proportion of dexamethasone-regulated genes appeared to be regulated through GRα-GRE interaction on their regulatory regions. Alternatively, GRβ might bind DNA sequences unique to this isoform through its DBD, regulating transcription through hypothetical “GRβ REs”. Since the subdomains of steroid hormone receptors influence each other’s activity [15], the unique GRβ “LBD” might alter the binding specificity of its DBD to DNA and allow it to recognize a set of DNA sequences specific to GRβ and distinct from those of GRα.
GRα and GRβ influenced each other’s transcriptional activity also in a gene-specific fashion. Heterodimerization between these two types of isoforms and/or their indirect interaction through bridging molecules, such as cofactors, chromatin modulators or other transcription factors, may underlie their functional cooperation. Indeed, we previously reported that these C-terminal isoforms interacted with each other on GRα-responsive promoters either directly or through p160 type coactivators [16].
Under our experimental conditions, the glucocorticoid antagonist RU 486 did not influence the subcellular localization of EGFP-GRβ, in contrast to a previous report that demonstrated that RU 486 slowly caused nuclear translocation of GRβ [10]. This report also described that GRβ had 10–20 times lower binding affinity to RU 486 than GRα [10]. In our experiment, we used 100-times higher concentration of RU 486 for GRβ, which could have easily induced its nuclear translocation, but detected no such translocation. Because in HCT116 cells, the nuclear translocation of EGFP-GRα, but not that of EGFP-GRβ, was possible, the difference of our data and those of the earlier report suggest that GRβ might employ a different machinery for its nuclear translocation defective in HCT116 cells. The existence of another yet undescribed subcellular transport system for GRβ is also supported by the fact that GRβ translocated quite slowly in COS-7 and U-2 OS cells, to the tune of days into the nucleus, compared to minutes for GRα, even though the authors used a concentration of RU 486 high enough to induce rapid nuclear translocation of this isoform based on its affinity to this steroid [10]. Alternatively, the authors evaluated the nuclear translocation of GRβ differently; they counted the number of cells that demonstrated specific patterns of GRβ subcellular localization at the different time points rather than monitor the nuclear translocation in the same cells time-sequentially [10]. Subcellular localization of transiently expressed GRβ was quite heterogeneous in HCT116, thus subtle changes in cellular viability, activity and/or functional diversity at the different time points in the presence of the active steroid RU 486 could easily influence its subcellular localization. Further work on the subcellular trafficking of GRβ employing several GRβ mutants, including those defective in NLS and/or nuclear export signals, is necessary to resolve this problem.
Supplementary Material
Acknowledgments
This study was funded by the Intramural Research Program of NICHD, NCCAR and NCI, NIH. Y.A. Su was supported partially by the NIH research contract: NIH-NIDDK-06-925 from the NICHD Intramural Research Programs (263MK611483). We thank Drs. R.M. Evans, G.L. Hager and M. Okabe for providing the plasmids.
References
- 1.Kino T, Chrousos GP. Glucocorticoid Effect on Gene Expression. In: Steckler T, Kalin NH, Reul JMHM, editors. Handbook on Stress and the Brain. Part 1. Elsevier BV; Amsterdam: 2004. pp. 295–312. [Google Scholar]
- 2.Chrousos GP. Glucocorticoid therapy. In: Felig P, Frohman LA, editors. Endocrinology & Metablism. McGraw-Hill; New York: 2001. pp. 609–632. [Google Scholar]
- 3.Kino T, De Martino MU, Charmandari E, Mirani M, Chrousos GP. Tissue glucocorticoid resistance/hypersensitivity syndromes. J Steroid Biochem Mol Biol. 2003;85:457–467. doi: 10.1016/s0960-0760(03)00218-8. [DOI] [PubMed] [Google Scholar]
- 4.Hollenberg SM, Weinberger C, Ong ES, Cerelli G, Oro A, Lebo R, Thompson EB, Rosenfeld MG, Evans RM. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318:635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu NZ, Cidlowski JA. Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol Cell. 2005;18:331–342. doi: 10.1016/j.molcel.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 6.Lu NZ, Cidlowski JA. The origin and functions of multiple human glucocorticoid receptor isoforms. Ann N Y Acad Sci. 2004;1024:102–123. doi: 10.1196/annals.1321.008. [DOI] [PubMed] [Google Scholar]
- 7.de Castro M, Elliot S, Kino T, Bamberger C, Karl M, Webster E, Chrousos GP. The non-ligand binding β-isoform of the human glucocorticoid receptor (hGRβ): tissue levels, mechanism of action, and potential physiologic role. Mol Med. 1996;2:597–607. [PMC free article] [PubMed] [Google Scholar]
- 8.Bamberger CM, Bamberger AM, de Castro M, Chrousos GP. Glucocorticoid receptor β, a potential endogenous inhibitor of glucocorticoid action in humans. J Clin Invest. 1995;95:2435–2441. doi: 10.1172/JCI117943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vottero A, Chrousos GP. Glucocorticoid Receptor β: View I. Trends Endocrinol Metab. 1999;10:333–338. doi: 10.1016/s1043-2760(99)00179-4. [DOI] [PubMed] [Google Scholar]
- 10.Lewis-Tuffin LJ, Jewell CM, Bienstock RJ, Collins JB, Cidlowski JA. Human glucocorticoid receptor β binds RU-486 and is transcriptionally active. Mol Cell Biol. 2007;27:2266–2282. doi: 10.1128/MCB.01439-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kino T, Stauber RH, Resau JH, Pavlakis GN, Chrousos GP. Pathologic human GR mutant has a transdominant negative effect on the wild-type GR by inhibiting its translocation into the nucleus: importance of the ligand-binding domain for intracellular GR trafficking. J Clin Endocrinol Metab. 2001;86:5600–5608. doi: 10.1210/jcem.86.11.8017. [DOI] [PubMed] [Google Scholar]
- 12.Su YA, Trent JM. Isolation of tumor suppressor genes in melanoma by cDNA microarray. In: Nickoloff BJ, editor. Melanoma Techniques and Protocols: Molecular Diagnosis, Treatment, and Monitoring. Humana Press; Totowa, NJ: 2001. pp. 15–29. [DOI] [PubMed] [Google Scholar]
- 13.Kino T, Ichijo T, Amin ND, Kesavapany S, Wang Y, Kim N, Rao S, Player A, Zheng YL, Garabedian MJ, Kawasaki E, Pant HC, Chrousos GP. Cyclin-dependent kinase 5 differentially regulates the transcriptional activity of the glucocorticoid receptor through phosphorylation: clinical implications for the nervous system response to glucocorticoids and stress. Mol Endocrinol. 2007;21:1552–1568. doi: 10.1210/me.2006-0345. [DOI] [PubMed] [Google Scholar]
- 14.Eckert RL, Broome AM, Ruse M, Robinson N, Ryan D, Lee K. S100 proteins in the epidermis. J Invest Dermatol. 2004;123:23–33. doi: 10.1111/j.0022-202X.2004.22719.x. [DOI] [PubMed] [Google Scholar]
- 15.Kumar R, Thompson EB. Gene regulation by the glucocorticoid receptor: structure:function relationship. J Steroid Biochem Mol Biol. 2005;94:383–394. doi: 10.1016/j.jsbmb.2004.12.046. [DOI] [PubMed] [Google Scholar]
- 16.Charmandari E, Chrousos GP, Ichijo T, Bhattacharyya N, Vottero A, Souvatzoglou E, Kino T. The human glucocorticoid receptor (hGR) β isoform suppresses the transcriptional activity of hGRα by interfering with formation of active coactivator complexes. Mol Endocrinol. 2005;19:52–64. doi: 10.1210/me.2004-0112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.