Abstract
Cardiac transplantation remains the best treatment in advanced heart failure patients with a high risk of death. However, an inadequate supply of donor hearts decreases the likelihood of transplantation for many patients. Ventricular assist devices (VAD) are being increasingly used as a bridge to transplant in patients who may not survive long enough to receive a heart. This expansion in VAD use has been associated with increasing rates of allosensitization in cardiac transplant candidates. Anti-HLA antibodies can be detected prior to transplantation using different techniques. Complement-dependent lymphocytotoxicity assays are widely used to measure the panel reactive antibody (PRA), and for crossmatch purposes. Newer assays using solid phase flow techniques feature improved specificity and offer detailed information concerning antibody specificities, which may lead to improvements in donor-recipient matching. Allosensitization prolongs the wait time for transplantation and increases the risk of post-transplant complications and death; therefore, decreasing anti-HLA antibodies in sensitized transplant candidates is of vital importance. Plasmapheresis, intravenous immunoglobulin (IVIG), and rituximab have been used to decrease the PRA prior to transplantation with varying degrees of success. The most significant post-transplant complications seen in allosensitized recipients are antibody-mediated rejection (AMR) and cardiac allograft vasculopathy (CAV). AMR often manifests with severe allograft dysfunction and hemodynamic compromise. The underlying pathophysiology is not fully understood, but appears to involve complement-mediated activation of endothelial cells resulting in ischemic injury. The treatment of AMR in cardiac recipients is largely empirical, and includes high-dose corticosteroids, plasmapheresis, IVIG and rituximab. Cardiac allograft vasculopathy (CAV) is characterized by diffuse concentric stenosis of allograft coronary arteries due to intimal expansion. Its pathophysiology is unclear, but may involve chronic complement-mediated endothelial injury. Sirolimus and everolimus can delay the progression of CAV. In some non-sensitized cardiac transplant recipients, the de novo formation of anti-HLA antibodies after transplantation may increase the likelihood of adverse clinical outcomes. Serial post-transplant PRAs may be advisable in patients at high risk of de novo allosensitization.
Index words: Histocompatibility, graft rejection/therapy, HLA antigens/immunology, isoantibodies/blood, heart transplantation, heart-assist devices, adult
1. Background
Cardiac transplantation has evolved over the last several decades to become the best available therapy in select patients with advanced heart failure with a high probability of death. The evolution in the field has been propelled by the development of newer, more effective immunosuppressive agents that decrease the likelihood of acute cellular rejection and increase post-transplant survival, while having modest effects on the incidence of infection and malignancy after transplantation. However, in spite of encouraging progress, the availability of donor hearts remains rate-limiting in the provision of transplantation to those in need1. An inadequate number of available hearts means longer wait list times for many transplant candidates, with a potential for higher wait list mortality for the sickest patients.
Recognizing the limitations of the donor pool, pioneer cardiothoracic surgeons in the late 1960s ushered in an alternative for cardiac transplant candidates who would not live long enough to obtain a new heart. This technology involved mechanical circulatory support with a total artificial heart or ventricular assist devices (VADs). Mechanical circulatory support as a bridge to transplantation was introduced in 1969 when the first total artificial heart was implanted as a bridge to transplantation. Initially, the technology had major disadvantages that limited its widespread applicability but, over the last 40 years, tremendous progress has been achieved. In the mid-1990s wearable implantable VADs began to be used widely as a bridge to transplant2. By the end of the last decade, the mechanical performance and clinical benefits of VADs had noticeably outweighed their drawbacks. With broader utilization of VADs, higher rates of allosensitization were increasingly recognized in supported transplant candidates3–5, complicating the ability to obtain an appropriate donor organ.
In view of the inadequate supply of donor hearts, and the growing prevalence of heart failure in developed countries, it is expected that the number of patients with advanced heart failure requiring bridging to transplantation with VADs will increase. Recently published data show that the mean survival of UNOS status 2 patients on the cardiac transplant waiting list has improved since 1990 and currently matches mean post-transplant survival at 1 year. This observation suggests that the risk-benefit ratio may not favor transplantation in patients listed under status 26. In the coming years, primarily those patients who are eligible for status 1 will be likely to receive a heart transplant. Currently, the status 1 category on the heart transplant wait list is largely populated by VAD-supported patients, and this phenomenon is expected to grow in the future. Understanding this trend in cardiac “transplantability” is fundamental in recognizing the increasing challenge that allosensitization represents for the ever-growing number of cardiac transplant candidates that are bridged to transplant with VADs. Pre- and post-transplant allosensitization have been associated with outcomes that impact allograft survival negatively; therefore, effective strategies to prevent and decrease allosensitization in this population are necessary.
This review will focus on the clinical aspects of allosensitized cardiac transplant recipients. We will discuss methods for determining allosensitization, risk factors for allosensitization, the impact of allosensitization pre- and post-cardiac transplant, and available strategies to decrease sensitization in patients awaiting heart transplantation and to treat antibody-mediated rejection following transplantation.
2. Detection of anti-HLA antibodies
Histocompatibility testing identifies appropriate donor-recipient pairs to achieve successful transplantation. Pre-transplant crossmatching identifies recipient serum antibodies that react with donor antigens, a condition that defines the concept of allosensitization. It is critically important to determine whether these antibodies may increase the risk of post-transplant adverse outcomes, as is the case with anti-HLA immunoglobulins7.
Screening for allosensitization through the detection of anti-HLA antibodies is at the core of compatible donor selection in solid organ transplantation. One of major the limitations of our current understanding of histocompatibility testing is the lack of complete knowledge regarding which antibody specificities are likely to increase the risk of post-transplant complications. The limited specificity of certain crossmatch techniques has confounded this issue further.
Anti-HLA antibodies are directed to donor major histocompatibility complex (MHC) class I and II HLA antigens that are expressed on allograft endothelial cells. The risk of early graft failure after transplant is higher in the presence of a positive crossmatch with donor HLA antigens, due to circulating recipient anti-donor antibodies. To detect allosensitization, transplant candidates undergo testing that exposes HLA antigens from random individuals to the recipient’s serum through a variety of different techniques, collectively referred to as a panel-reactive antibody (PRA) test8, 9. The rationale for PRA testing in cardiac transplant candidates comes from prior experience in kidney transplantation, showing an inverse relationship between PRA level and allograft survival10–12. Most early studies identified HLA class I and II antibodies with complement-dependent lymphocytotoxic techniques, however progress over the years has made other analytical methods available including flow cytometry and solid-phase flow methods. Over the last four decades, technological progress has led to the introduction of different methods for antibody detection13. Table 1 includes a summary of characteristics of currently used antibody detection techniques in recipients awaiting heart transplantation.
Table 1.
Currently used anti-HLA antibody detection techniques in candidates awaiting heart transplantation.
| Characteristic | Cell-based techiques | Solid-phase techniques |
|---|---|---|
| Sensitivity | CDC < CDC + DTT | ELISA < Flow |
| HLA antigens | Natural configuration on cell surface, unable to detect specificities | Isolated proteins bound to artifical surface, may detect specificities if single antigens are used |
| False-positive reactions | Non-HLA specific antibodies | Reactions with cryptic epitopes on denatured HLA molecules |
| False-negative reactions | Antibody levels below detection threshold | Loss of epitope expression on isolated molecules |
CDC (Complement-dependent lymphocytotoxicity assay); DTT (dithiothreitol); ELISA (enzyme-linked immunosorbent assay). (From Zeevi A, Girnita A, Duquesnoy R. Immunol Res 2006;36:255–264, with permission).
2.1. Clinical relevance of detected antibodies
The histocompatibility testing techniques used vary among centers. The complement-dependent lymphocytotoxicity assay continues to be broadly used in all cardiac transplant candidates as an initial screening method to rule out an elevated PRA and as a rapid crossmatch technique. Considering the limited specificity of this technique, patients who have a PRA ≥ 10% undergo further testing with more specific methods, typically flow cytometry or antibody detection using single antigen beads in a Luminex® template. These tests can identify high risk Class I or Class II anti-HLA antibodies, and commonly offer perspective regarding the likelihood that a particular candidate will receive a new heart within a reasonable timeframe. Unacceptable donor antigens can also be identified, making possible the use of virtual crossmatching in some cases14.
Complement-dependent lymphocytotoxicity has been studied specifically in the context of heart transplantation outcomes. In the early 1990s, Lavee and colleagues described how a CDC PRA ≥ 10% was associated with an increased incidence of acute cellular rejection and cardiac allograft vasculopathy in the early post-transplant period8. Smith and colleagues also showed that heart transplant candidates transplanted against a positive crossmatch had drastically reduced allograft survival during the first year when compared with patients with a negative crossmatch15. In a retrospective study of heart transplant recipients, Kobashigawa and colleagues found that transplant candidates with a PRA ≥ 11% detected by CDC had higher post-transplant mortality when compared to those with a lower PRA. Eighty-eight percent of deaths in allosensitized patients occurred within 3 months after transplant and were mostly due to immune-related causes (allograft rejection and cardiac allograft vasculopathy)16.
While flow PRA results correlate well with post-transplant clinical outcomes17, data on the clinical use of newer solid-phase assays such as antibody detection using single antigen beads in a Luminex® template to estimate the likelihood of antibody-mediated rejection (AMR), cardiac allograft vasculopathy (CAV), or allograft survival are lacking. In addition, contemporary solid-phase techniques based on the Luminex® template use recombinant HLA antigens which may not be identical in shape to the HLA antigens found on donor cell surfaces. This fact may raise questions concerning the validity of the information obtained. It is also clear that besides offering information on known specificities, more specific techniques offer a wealth of data concerning less known antibodies, which have uncertain clinical significance. While transplant centers that use contemporary antibody-detection technologies rely on their results to make clinical decisions, evidence-based data on the clinical utility and prognostic significance of newer methods for histocompatibility testing are limited.
Post-transplant outcomes in VAD recipients are also affected by allosensitization as evidenced by slightly higher rates of allograft rejection. However, overall allograft survival seems to be unaffected18, 19. Recent small studies of heart transplant candidates supported with VADs have offered limited evidence into the relevance of pre-transplant allosensitization in this specific group in the contemporary era. Schmid and colleagues followed 41 patients bridged with VADs and found that post-transplant survival and the incidence of allograft rejection was comparable to controls without VAD support20. Pamboukian and colleagues studied 98 patients with and without VAD support and reported on their rates of allosensitization and post-transplant outcomes. Even though VAD patients had a higher likelihood of having a PRA ≥ 10% (19% vs. 2%), this was not associated with higher rates of allograft rejection or vasculopathy. Post-transplant survival was unaffected in VAD-supported patients21. Other studies have found similar rates of allosensitization in VAD recipients, however this factor does not seem to affect the likelihood of unfavorable immune outcomes or allograft survival22, 23.
The apparent lack of impact of allosensitization on post-transplant outcomes in VAD recipients may be related to more aggressive immunosuppression used in this group. Because of their higher PRAs, VAD-supported heart transplant candidates are more likely to receive desensitization therapies prior to transplant, and allograft rejection episodes may be treated more aggressively. Post-transplant survival rate in patients supported with VADs is similar to that of non-supported patients, yet the causes of death are different. Up to 75% of post-transplant mortality in VAD-supported heart recipients is related to infectious complications, perhaps related to the more aggressive immunosuppression they receive, or direct effects of some VADs on the immune system (see below), whereas rejection may account for 20%. Non-supported transplant recipients commonly die from rejection (38%), ischemic complications (31%), and respiratory failure (23%)24.
3. Mechanisms of allosensitization
3.1. Allosensitization by exposure to foreign antigens
Commonly recognized risk factors for allosensitization in all transplant candidates include previous allografts, blood product transfusions, and pregnancy5. As explained above, the use of VADs as a bridge to transplantation has also been recognized as a risk factor for allosensitization resulting in an elevated PRA25, 26. The most frequent cause of allosensitization before cardiac transplantation is previous blood transfusions. With the increasing number of older patients who have undergone previous cardiac surgery being listed for transplantation, the number of sensitized candidates is likely to increase. Although cardiac retransplantation represents less than 3% of all cardiac transplants1, patients with prior allografts are also more likely to be sensitized. Finally, women with a history of pregnancy may have become sensitized to paternal antigens27, 28.
3.2. Ventricular assist devices as risk factors for allosensitization in cardiac transplant candidates
Ventricular assist devices consist of a combination of non-biological and bioprosthetic materials, in continuous contact with circulating blood (Fig. 1). Prior to VAD introduction, it was known that common inert materials trigger host responses that include inflammation, fibrosis, and coagulation, and, not unexpectedly, similar responses were seen in VAD recipients9, 29. These responses contribute to the pathogenesis of complications seen in VAD recipients, such as thromboembolism and systemic infection. The incidence of thromboembolism has decreased significantly in VADs that feature a textured interior surface that promotes the growth of a neo-intimal layer. However, the development of a neo-intima is associated with the deposition of cytokine-releasing macrophages and activated helper T-lymphocytes on the VAD surface9. These helper T-lymphocytes show a heightened level of activation when compared with those of controls with advanced heart failure. Their activation profile is marked by enhanced spontaneous proliferation after interleukin-2 (IL-2) stimulation, but contrasted by a susceptibility to premature cell death, as evidenced by surface expression of CD95, a marker of activation-induced apoptosis. In addition to a susceptibility to early cell death, T-lymphocytes of VAD recipients exhibit impaired proliferative responses to T-cell receptor (TCR)-mediated activation30. The combination of these observations may result in an impairment of cellular immunity in VAD recipients, with vulnerability to systemic Candida infections30. Activated T-lymphocytes in VAD patients selectively express Th2-type cytokines, such as IL-4 and transformation growth factor β(TGF-β). It has been postulated that an excessive load of circulating apoptotic waste and the predominant expression of Th2-type cytokines is responsible for B-lymphocyte hyperreactivity, evolution into plasma cells, and auto-antibody production in VAD recipients. These patients show a three to four-fold frequency of anti-HLA class I and II IgG levels (Fig. 2), and also significantly higher levels of anti-phospholipid antibody, when compared to controls with advanced heart failure9. While anti-HLA IgM antibodies that may cause a positive crossmatch are also produced under these circumstances, there is no definite association between their presence and deleterious effects after cardiac transplantation15, 31 (Table 2).
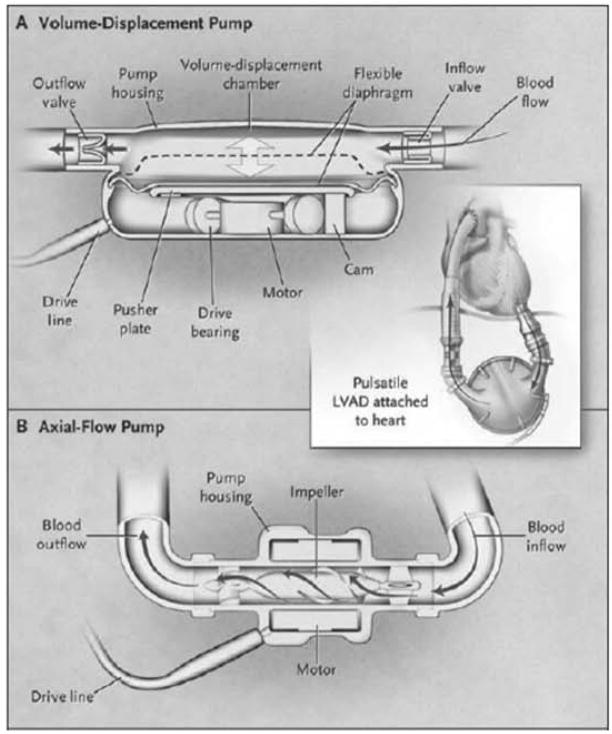
Figure 1.
Mechanisms of action of ventricular assist devices. The volume displacement VAD (A) consists of a chamber or sac that fills and empties cyclically. A percutaneous driveline provides electrical power to a motor that moves a pusher plate up and down repeatedly, compressing the volume chamber and resulting in pulsatile blood flow. The backward regurgitation of blood flow is prevented by inflow and outflow valves. Some of these VADs feature a textured lining that promotes neo-intimal proliferation, and may be responsible for immune activation and allosensitization. The axial flow VAD (B) is smaller in size, and features a helical rotor that drives blood flow from the left ventricle into the ascending aorta. A percutaneous driveline provides electrical power to a motor that makes the impeller spin while levitating inside the pump with the use of electromagnetism. This mechanism provides continuous, non-pulsatile blood flow. (From Baughman KL, Jarcho JA. N Engl J Med 2007;357:846–849, with permission. Copyright ©2007 Massachussetts Medical Society. All rights reserved).
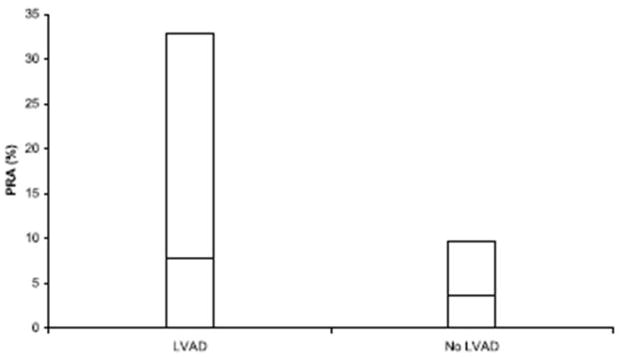
Figure 2.
PRA levels in VAD-supported versus non-supported cardiac transplant candidates. Patients who underwent VAD support before transplantation demonstrate an increase in 90th percentile (taller bar) and mean (the horizontal line within each bar) PRA levels when compared to non-VAD controls (p<0.0001) (From Joyce DL, Southard RE, Torre-Amione G, et al. J Heart Lung Transplant 2005;24:2054–2059, with permission).
Table 2.
Elements with presumed involvement in the immune system dysfunction seen in LVAD recipients.
| Cells | |
| Activated macrophages/monocytes(CD14+, CD68+, NFκB+) | Found on LVAD surface; stimulate T-lymphocyte activation via IL-2 receptor pathways |
| CD95(Fas)+ T-lymphocytes | Found in circulation, on LVAD surface; in heightened activation state, prone to apoptosis, poor response to TCR-mediated activation |
| Hyperreactive B-lymphocytes | Found in circulation; release anti-HLA Class I and II IgG, anti-phospolipid antibody |
| Cytokines | |
| IL-2 | Promotes T-cell activation, down-regulated by selective loss of Th1 T-cells |
| IFN-γ | Promotes T-cell activation, down-regulated by selective loss of Th1 T-cells |
| IL-10 | Stimulates B-cell hyperreactivity |
| sCD40L | Stimulates B-cell hyperreactivity |
IL (interleukin); IFN (interferon); sCD40L (soluble CD40 ligand), TCR (T-cell receptor).
Polyclonal expansion of B-lymphocytes and their subsequent hyperreactivity may also be associated with elevated levels of CD40 ligand (CD40L) derived from inappropriately activated T-lymphocytes. CD40 is a member of the TNF receptor superfamily that is expressed in B-lymphocytes and has an important role in stimulatory pathways resulting in B-cell survival and proliferation. Its ligand, CD40L, is expressed by activated T-lymphocytes. Its circulating form in serum has been found to be biologically active in terms of B-lymphocyte activation and is predictive of autoantibody formation and autoimmune disease activity32. In a study of 111 patients supported with textured pulsatile LVADs as bridge to transplantation, Schuster and colleagues showed that increased serum levels of CD40L are associated with clinical allosensitization detected with a complement-dependent lymphocytotoxicity assay in VAD recipients32(Fig. 3).
Figure 3.
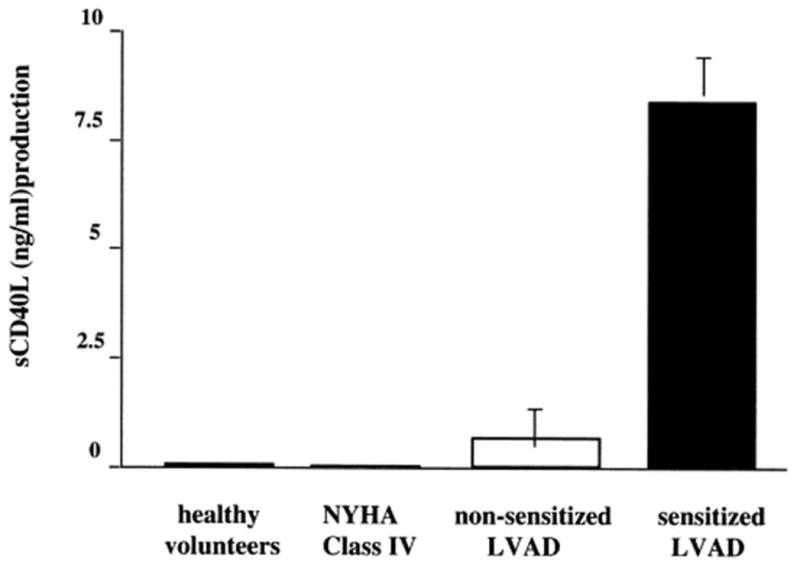
Increased levels of soluble CD40 ligand (CD40L) in the circulation of allosensitized left ventricular assist device (LVAD) recipients. Sensitized LVAD recipients had over eight-fold higher levels of serum CD40L when compared with either non-sensitized LVAD recipients, heart failure controls, or healthy volunteers. Results are expressed as the mean ± SEM of experiments using sera from 12 sensitized LVAD recipients, 10 non-sensitized LVAD recipients, 8 NYHA class IV heart failure controls, and 6 healthy volunteers. (From Schuster M, Kocher A, John R, et al. Human Immunology 2002;63:211–220, with permission).
Perioperative platelet transfusions also result in the development of anti-HLA class I antibodies in VAD recipients4, 33. Red blood cell transfusions appear to have a less significant impact on the level of circulating anti-HLA antibodies, especially when leukocyte-reduced products are used34, 35. Anti-HLA class II antibody levels do not seem to be affected by blood product transfusions. HLA-DR3 may be associated with a higher likelihood of developing anti-HLA class II antibodies in VAD recipients9.
There is some evidence to suggest that the degree of sensitization may vary between different VAD types, being lower for devices without a textured interior surface and axial flow devices, due to a smaller area of contact between the device and bloodstream, with a lesser degree of immune activation36, 37. Some early data with axial flow devices(MicroMed DeBakey® LVAD) showed elevated production of C5a and IL-6 during the first 12 weeks after implantation, when compared to patients that received a non-textured pulsatile device (Novacor® LVAD). This finding created concern for the possibility of increased B-cell activation and subsequent sensitization mediated by IL-638. However, investigators in that study did not look at anti-HLA antibodies or post-transplant outcomes in those patients.
More recent data have shown that the initial immune system abnormalities noted after the implantation of axial flow pumps, including CD95-mediated T lymphocyte activation and apoptosis, seem to resolve after 7 weeks39. This improvement in immune activation is thought to account for the lower degree of sensitization more commonly seen in patients with axial flow devices. A small study in patients supported with an axial flow pump who had no anti-HLA antibodies prior to implantation, found that there were no detectable levels of anti-HLA Class I or Class II IgG after a mean follow up period of 87 days post-implantation. Furthermore, acute cellular rejection episodes and post-transplant survival within the first year were comparable to published statistics in patients without mechanical circulatory support40.
4. Management of allosensitized cardiac transplant candidates
The management of allosensitized cardiac transplant candidates presents steep challenges for transplant cardiologists and surgeons. The differences in specificity of different antibody-detection techniques, the uncertainty about which antibody specificities are relevant, and the incomplete understanding of B-cell immunity in allotransplantation make solid progress in this area difficult. There is limited available literature on strategies to treat allosensitization in cardiac transplant candidates, and much of the current therapeutic practices are derived from experience with the transplantation of other solid organs. It is clear, however, that allosensitized patients experience delays to transplantation due the relatively limited acceptable donor pool imposed by their anti-HLA antibody specificities9, 35 (Fig. 4).
Figure 4.
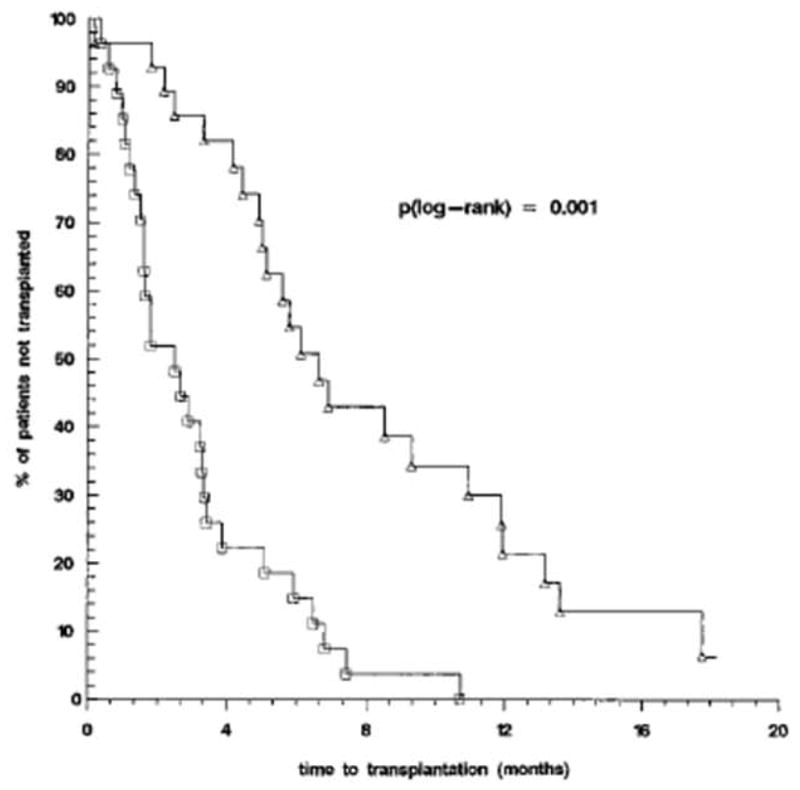
Effect of IgG anti-HLA Class I antibodies on waiting time to cardiac transplantation. In 37 allosensitized VAD recipients (△), presence of anti-HLA Class I IgG increased waiting time to cardiac transplantation compared with 18 non-sensitized VAD recipients (□)(p<0.001). (From John R, Lietz K, Burke E, et al. Circulation 1999;100(19 Suppl):II229-II235, with permission).
The initial step in managing allosensitized transplant candidates is to avoid further exposure to foreign human antigens by minimizing the transfusion of blood products as much as possible. Once a cardiac transplant candidate has become sensitized, traditionally indicated by a PRA ≥ 10%, the time required to wait for a donor that is crossmatch-negative may be prohibitive. Therefore, measures to decrease the likelihood of having a positive prospective crossmatch, which could result in transplantation delays and a higher chance of dying on the wait list, should be considered.
In patients ill enough to require VAD support, decreasing the levels of circulating alloantibodies is of particular importance. Contemporary VADs have limited durability, and continuously expose patients to serious complications including systemic infection, hemolysis, and thromboembolism. Some of these complications can jeopardize the candidate’s eligibility for transplantation, and also can profoundly affect quality of life and survival. Until further improvements are made to mechanical circulatory support devices, the goal at most centers is to transplant VAD recipients as expeditiously as possible, following an initial period of postoperative recovery, to allow improvement in end-organ function, and physical rehabilitation. High degrees of allosensitization pose a great threat for this patient subset, and broader regional sharing for status 1A and 1B heart recipients makes crossmatching challenging.
4.1. Methods to decrease levels of allosensitization
4.1.1. Plasmapheresis
Mechanical removal of circulating antibodies with plasmapheresis has been used in highly allosensitized cardiac transplant candidates to decrease the likelihood of allograft rejection. It can be implemented in patients with a high PRA, either en route to the operating room on the day they will receive their transplant or while on the wait list to decrease their PRA and increase the likelihood of finding a negative crossmatch donor. It has been often combined with varying doses of intravenous immunoglobulin (IVIG). There is data that support the preoperative use of plasmapheresis and IVIG, showing post-transplant outcomes similar to those of non-allosensitized patients. In a study by Pisani et al., 16 out of 118 cardiac transplant candidates were found to have a PRA ≥ 10%, with a high rate of positive crossmatch. All sensitized patients underwent plasmapheresis in combination with IVIG immediately prior to transplantation. The frequency of rejection and allograft survival was similar between sensitized patients who underwent plasmapheresis and IVIG when compared to non-sensitized controls41. Similar results have been reported by other investigators with small case series42, 43.
4.1.2. Intravenous immunoglobulin
Intravenous immunoglobulin has also been used alone to decrease the level of allosensitization prior to heart transplantation, especially in VAD recipients. In a series of four VAD recipients who developed high levels of allosensitization after device implantation, treatment with IVIG resulted in decreases in their PRA to levels below 10% within 4 months from administration44. John et al. published a head-to-head comparison of IVIG and plasmapheresis in 55 allosensitized VAD recipients prior to transplantation. IVIG resulted in a mean reduction of 33% in anti-HLA Class I reactivity one week after treatment, with minimal side effects. Plasmapheresis achieved similar results, but required longer treatments and was associated with a higher rate of infectious complications (Fig. 5). In patients whose PRA did not respond to low-dose IVIG, higher doses achieved comparable reductions in the degree of allosensitization, with an observed increased incidence of acute renal failure. Notably in this series, treatment with IVIG significantly reduced wait time to transplantation45.
Fig 5.
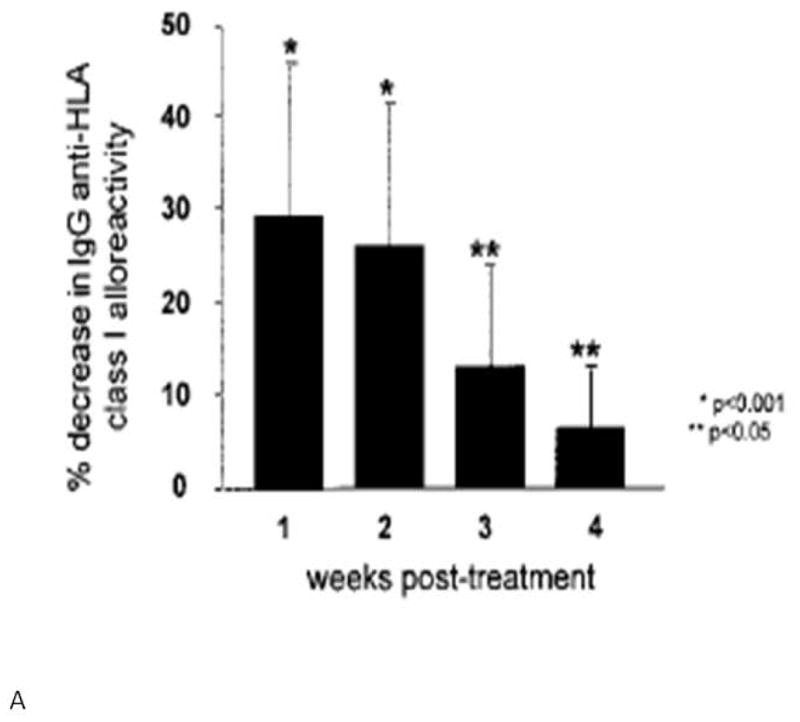
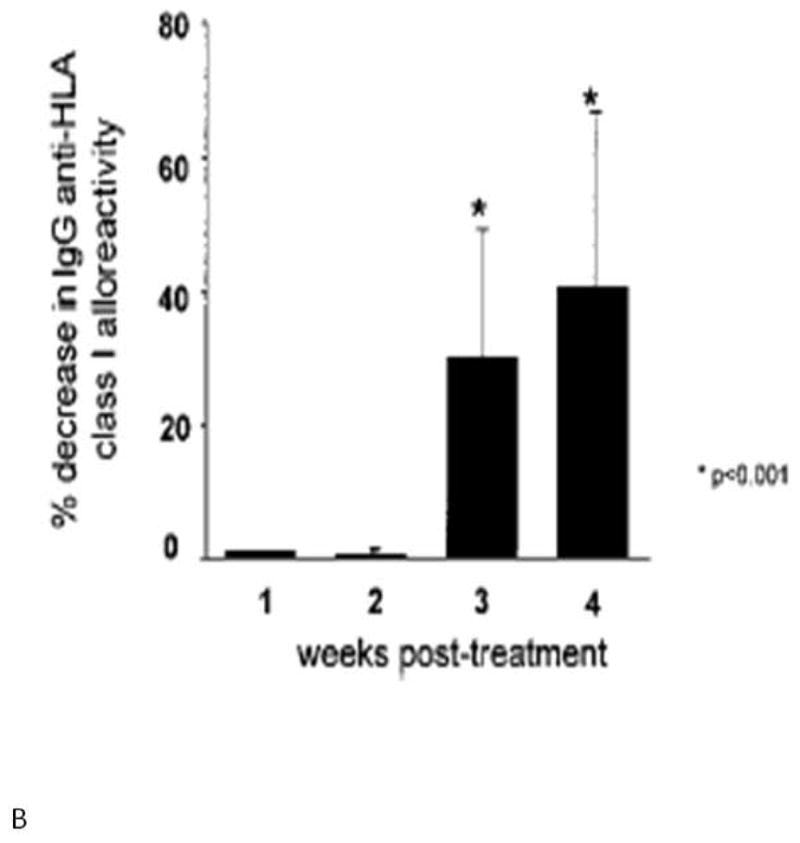
Panel A shows the effect of IVIG therapy on reduction of serum anti-HLA Class I IgG alloreactivity. Maximal reduction in serum alloreactivity occurs within 1 week of IVIG therapy. Panel B shows the effect of plasmapheresis on anti-HLA Class I IgG alloreactivity. Maximal reduction in alloreactivity occurs 4 weeks after treatment. (From John R, Lietz K, Burke E, et al. Circulation 1999;100(19 Suppl):II229-II235, with permission).
4.1.3. Rituximab
In recent years, rituximab, a chimeric monoclonal antibody to CD20, has been used anecdotically to diminish the degree of allosensitization in cardiac transplant candidates. Rituximab depletes B-lymphocytes through complement-dependent cytotoxicity, antibody-dependent cytotoxicity, and induction of apoptosis46. Animal studies in baboons have shown that this agent effectively depletes CD20+ B-lymphocytes, resulting in significant blunting of IgM and IgG responses. Studies in humans have focused mostly on the use of rituximab to treat B-cell lymphomas, however it has also been used to treat autoimmune disorders and in organ transplantation, recognizing its immunomodulatory properties47. A study in allosensitized patients on dialysis awaiting kidney transplantation found that while rituximab was a powerful B-cell ablational agent, some B-cell subpopulations such as CD19+/CD5+ B-cells recovered to baseline levels within 6 months. Other subsets, such as B memory cells may remain suppressed for up to 2 years after treatment48. These findings suggest that alloreactivity may not be fully suppressed after rituximab administration.
Most of the available data in treating allosensitized transplant candidates with rituximab come from the renal transplant literature. Vieira et al. selected 9 highly sensitized kidney transplant candidates to be treated with escalating single doses of rituximab. They achieved significant B-lymphocyte depletion with a moderate decrease in PRA in two patients and the suggestion of loss of antibody specificities in 5. Two patients showed no change in their PRA after rituximab. These effects were associated with infectious complications in some patients49.
Among very few early reports in the heart transplant literature, Balfour et al. reported the use of rituximab in a pediatric transplant candidate who had failed previous treatments with IVIG, plasmapheresis and mycophenolate mofetil. The patient was successfully transplanted after a crossmatch-negative donor was found50.
Unfortunately, published experience with rituximab in allosensitized cardiac transplant candidates is scarce at this time. Further data are needed to determine the usefulness of rituximab in allosensitized transplant candidates.
5. Clinical outcomes in allosensitized cardiac transplant candidates
Pre-transplant allosensitization increases the likelihood of AMR and CAV, and decreases overall allograft survival3, 15, 16, 51–53 (Fig. 6). The relationship between anti-HLA antibodies prior to transplantation and the development of these conditions is well recognized, emphasizing the importance of proper PRA screening, and assignment of appropriate donor-recipient matches.
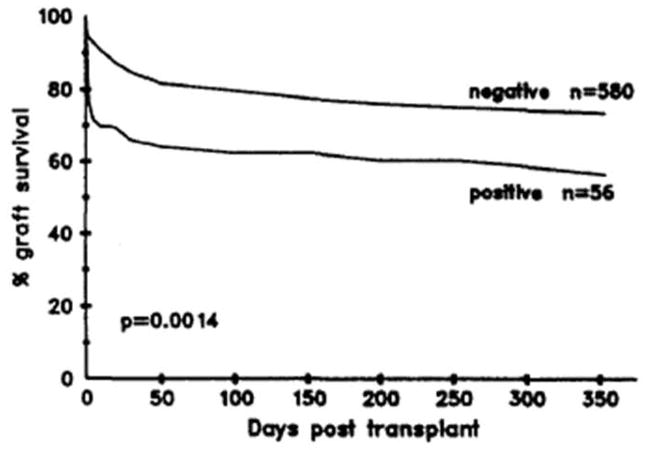
Figure 6.
Survival according to CDC crossmatch results in 636 cardiac transplant recipients between 1982 and 1992. (From Smith JD, Danskine AJ, Laylor RM, et al. Transplant Immunol 1993;1:60–65, with permission).
5.1. Antibody-mediated rejection
Antibody-mediated rejection is not an unusual occurrence54. Institutional experience suggests that the incidence of AMR may be approximately 15%5, however this figure varies depending on the patients studied and the diagnostic methods used. In series where patients were commonly given OKT3, AMR was evident in up to 52% of cases; whereas in series where C4d deposition associated with allograft dysfunction is used as the diagnostic criterion, the reported incidence has been as low as 3%53. Acute AMR typically occurs shortly after transplantation, usually within the first four months, but more commonly during the first 4 weeks after transplantation55. However, it can occasionally present several months or years after transplant. Up to 68% patients who develop AMR early on show evidence of significant allograft dysfunction, in contrast with those in whom AMR presents late, where the frequency of allograft dysfunction is estimated at 13%5, 53.
The pathophysiology of AMR is not fully understood. It is likely the result of antibody-induced, complement-mediated activation of endothelial cells. The process continues with cytokine release and increased endothelial adherence of leukocytes, culminating in allograft ischemic injury56. C4d, an inactive byproduct of complement activation, has been observed in the capillaries of cardiac allografts with AMR, suggesting recent complement activity57.
The criteria to diagnose AMR have not been uniform. Clinically, it presents with hemodynamic compromise in 29–47% of cases, especially when it occurs early post-transplant. The criteria for hemodynamic compromise also vary between centers, but generally include a decrease in left ventricular ejection fraction, unexplained elevation in cardiac filling pressures with a simultaneous decrease in cardiac output, and the need for inotropic support. In addition to clinical signs, pathologic markers of AMR have to be present.
In 2005, ISHLT proposed criteria for the immunopathologic diagnosis of AMR5. In the presence of clinical evidence of allograft dysfunction, the following diagnostic criteria were suggested:
Histologic evidence of acute capillary injury (endothelial swelling or denudation with congestion and macrophage infiltration, with possible neutrophil infiltration and interstitial edema and/or hemorrhage in more severe cases).
Immunopathologic evidence for antibody-mediated injury (tissue immunofluorescence positive for IgM or IgG + complement deposition (C3d, C4d or C1q); or CD68-positive macrophages in endothelium; endovascular fibrin can be seen in more severe cases).
Serologic evidence of donor-specific anti-HLA Class I or Class II antibodies, or other anti-donor antibodies at the time of biopsy.
Even though immunoglobulin (Ig) deposits are part of the proposed diagnostic criteria for AMR, immunofluorescence for endothelial Ig deposits does not correlate well with clinical AMR, or with circulating anti-HLA antibodies58. Recently, immunofluorescence for C4d has shown a high degree of correlation with serum anti-HLA antibodies57. Rodriguez et al. studied 665 consecutive endomyocardial biopsies from 165 cardiac transplant recipients with immunofluorescence for the presence of Ig and complement deposits. The combined detection of C4d and C3d correlated well with acute AMR and clinical evidence of heart failure. The additional detection of Ig and C1q did not improve diagnostic accuracy59. In addition, some early evidence suggests that immunofluorescence for C4d may be helpful in assessing the response to treatment for AMR in cardiac transplant recipients57, 60.
AMR causes very severe episodes of rejection, often with hemodynamic compromise, that may not respond to intensification of immunosuppressive therapy alone. In addition to hemodynamic support with inotropic agents, current therapeutic strategies center on inactivation of circulating antibodies.
5.1.1. Treatment of AMR
Recognizing the importance of antibody production and complement deposition in allograft endothelium as the underlying pathophysiology in AMR, mechanical removal of circulating antibodies with plasmapheresis was one of the first therapies tested on affected patients. In a series of 328 cardiac transplant recipients in the late 1980s, 3.4% of patients were found to have a positive prospective IgG crossmatch. These patients experienced acute AMR much earlier than controls with a negative crossmatch. Moreover, AMR was associated with hemodynamic compromise in 73% of cases. In addition to intensification of immunosuppression, plasmapheresis was used in the patients affected with AMR, with a treatment success rate of 75%55. Other smaller series in Europe and Asia have documented similar experiences61, 62. High-dose intravenous corticosteroids, T-lymphocyte depleting agents such as rabbit anti-thymocyte globulin (r-ATG), and tacrolimus have been used in addition to plasmapheresis, with varying degrees of success. Intravenous immunoglobulin has also been used with to provide further immune modulation in some patients with AMR. More than 90% of patients treated aggressively for AMR recover, but remain with a high risk of recurrence53.
A few case series and case reports have also documented successful treatment of AMR with rituximab-based therapy63–65. Garrett et al. treated 8 patients with pathologic evidence of AMR with weekly doses of rituximab for 4 weeks. All patients recovered their baseline LVEF without associated infectious or other drug-related complications66.
5.2. Cardiac allograft vasculopathy
Allosensitization also contributes significantly to an increased risk of developing CAV52, 56, 59, 67, 68. CAV is a major cause of cardiac allograft failure and decreased survival after the first post-transplantation year67. While it is possible that both T- and B-lymphocyte mediated immunity play a role in its pathogenesis, patients who demonstrate circulating donor-specific anti-HLA antibodies, or an immunohistologic pattern of antibody-mediated rejection early after transplant, are more like to develop CAV52.
CAV causes diffuse concentric stenosis of the coronary arteries, as a result of intimal expansion and adventitial sclerosis. While detailed human studies on the pathogenesis of CAV are lacking, it is believed that the pathologic process is initiated by antibody and complement-mediated injury to endothelial cells, and may be accelerated by common coronary disease risk factors such as hypertension and hyperlipidemia. The antibodies most frequently associated with CAV are those against donor HLA, in particular to Class I antigens, which are richly expressed in human endothelial cells. HLA Class II antigens are constitutively expressed in human endothelium, and their synthesis can be stimulated by pro-inflammatory molecules such as interferon-γ59. Anti-HLA IgG can stimulate the proliferation of endothelial and smooth muscle cells, causing intimal expansion. While lytic levels of complement cause hyperacute rejection, the role of complement in CAV is not well understood. Complement activation can result in the release of tissue growth factors that cause endothelial proliferation, and migration of fibroblasts and smooth muscle cells. In addition to this, sub-lytic doses of the membrane attack complex of complement can induce endothelial production of tissue factor. This phenomenon has been deemed responsible for the pro-coagulant characteristics and intimal fibrin formation seen in coronary arteries with CAV. The presence of fibrin has been independently associated with CAV, allograft failure, and death67, 69.
The diagnosis of CAV is challenging due to the often diffuse nature of the disease. Comparative coronary angiography and intravascular ultrasound (IVUS)70 are widely used to demonstrate progressive narrowing of allograft coronary arteries (Fig. 7). Serial dobutamine stress echocardiography is also used to detect clinically significant CAV, and some data suggest that it may have prognostic value comparable to IVUS and coronary angiography in predicting the occurrence of cardiac events71.
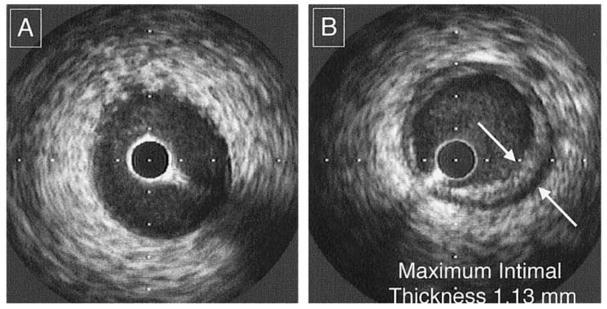
Figure 7.
Intravascular ultrasound example of a de novo lesion of transplant vasculopathy. No lesions are shown at baseline examination (A). When the same site is identified on follow-up examination, significant intimal thickening is seen at that site (B). This lesion is defined as a de novo lesion of transplant vasculopathy. Note that this lesion is circumferential and non-eccentric. (From Kapadia SR, Nissen SE, Tuzcu EM. Curr Opin Cardiol 1999;14:140–150, with permission).
The therapeutic options for CAV are limited. In contrast with native coronary artery disease, percutaneous coronary interventions are not commonly possible due to the diffuse nature of stenotic process. Stents have been used occasionally for palliative purposes in patients with symptomatic ischemia. Coronary artery bypass grafts are of limited applicability for similar reasons. Retransplantation may be an option for select candidates with severe multivessel CAV with evidence of allograft dysfunction. However, patients must be chosen carefully to allow acceptable outcomes of retransplantation to be achieved72.
Contemporary immunosuppressive agents such as sirolimus and everolimus have demonstrated benefits in delaying the onset of clinically-evident CAV73, 74. When CAV is detected with angiography, intensification of immunosuppression with these agents is an acceptable choice to slow the progression of CAV75.
6. The importance of de novo anti-HLA antibodies after cardiac transplantation
In spite of advances in immunosuppressive therapy, the incidence of acute AMR and CAV continues to limit long-term outcomes54, 76. A study by Rose et al. demonstrated that the presence of anti-HLA antibodies post-cardiac transplant results in lower survival when compared to patients who are antibody-free. At 5 years post-transplant, survival in the antibody-negative group was 90%, compared to 53% in the antibody-positive group. Acute rejection and infection, partly related to augmented immunosuppression to treat rejection episodes, were the leading causes of death in antibody-positive patients. Circulating anti-HLA antibodies were also associated with higher rates of CAV77. These findings have been corroborated by subsequent studies12, 78, 79.
Anti-HLA antibodies can develop in patients who were not allosensitized before transplantation. This suggests that the transplanted heart may release alloantigens responsible for neo-sensitization of the recipient. Tambur et al. showed that up to 35% of non-allosensitized cardiac allograft recipients can develop anti-HLA antibodies within the first year post-transplant. Antibodies against HLA Class I antigens were more commonly present than anti-HLA Class II antibodies, with PRAs ranging widely from 10% to almost 80% for both classes. HLA-A mismatch, female gender, and longer ischemic time were identified as risk factors for de novo sensitization after transplantation. Anti-HLA Class I and II antibodies showed strong associations with the incidence of early acute cellular rejection. In addition, de novo Class II antibodies were associated with more severe CAV and higher mortality due to allograft failure (Fig. 8). Of all anti-HLA Class I antibodies, 41% represented donor-specific antibodies. Donor-specific Class II antibodies were uncommon (<10%). The relative infrequency of donor-specific antibodies may suggest that most are bound to allograft antigens, therefore being underrepresented in the serum80.
Figure 8.
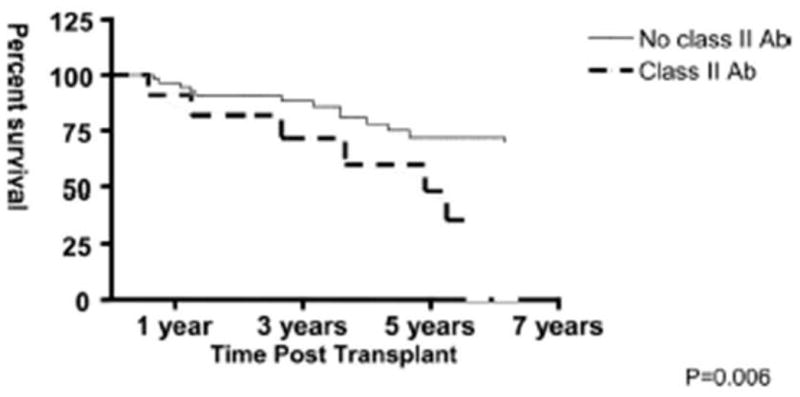
Survival curve comparing patients that exhibit de novo anti-HLA Class II antibodies vs. patients without anti-HLA Class II antibodies. Patients who produce Class II antibodies have significantly worse survival (p=0.006). (From Tambur AR, Pamboukian SV, Constanzo MR, et al. Transplantation 2005;80:1019–1025, with permission).
While contemporary data in cardiac transplant recipients show that de novo anti-HLA antibodies may have a major impact on post-transplant outcomes, the available evidence is not enough to recommend serial PRA screening in all cardiac allograft recipients. It may be advisable to conduct serial PRAs during the first year post-transplant in patients with features that will place them in a high risk category for post-transplant allosensitization. Serial PRAs in this subset could potentially signal the need for more aggressive immunosuppression to prevent acute rejection episodes and delay allograft failure, however the clinical utility and cost-effectiveness of such an approach remain in question.
7. Conclusions
Cardiac transplantation has become the best available therapy in select patients with advanced heart failure with a high probability of death. However, an inadequate number of available hearts remains rate-limiting in the provision of transplantation to those in need, leading to longer wait list times for many transplant candidates, with a potential for higher wait list mortality.
In view of the limited donor pool, mechanical circulatory support with VADs was introduced as a bridge to transplant. Unfortunately, broad use of VADs has been associated with higher rates of allosensitization, causing delays to transplantation due to difficulty in finding a crossmatch-negative donor. In addition to VAD-related sensitization, cardiac transplant candidates are frequently exposed to other sources of sensitization such as blood product transfusions.
The consequences of pre-transplant allosensitization on clinical outcomes after transplantation can be serious. Antibody-mediated rejection and cardiac allograft vasculopathy are particularly prominent and can result in graft failure and decreased survival. The pathophysiology of these conditions is not fully understood; therefore, available treatments are based on expert opinion and anecdotal clinical data. For this reason, significant emphasis is placed in preventing and decreasing allosensitization prior to transplantation.
Current strategies to decrease allosensitization focus on the direct removal of circulating antibodies with plasmapheresis, inactivation of antibodies with IVIG, and B-lymphocyte depletion with rituximab. These approaches have also been used to treat antibody-mediated rejection after transplantation with encouraging results. However, most treatments are based on theoretical assumptions and scarce clinical data, therefore their true efficacy is unknown.
In the future, current strategies to avoid allosensitization will be complemented by improvements in VAD design that will decrease the likelihood of immune activation. Newer generation devices may result in lower degrees of allosensitization by reducing the surface area in direct contact with the bloodstream, limiting the use of textured surfaces, and utilizing less immunogenic biocompatible materials.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Taylor DO, Edwards LB, Boucek MM, Trulock EP, Aurora P, Christie J, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult heart transplant report--2007. J Heart Lung Transplant. 2007;26:769–81. doi: 10.1016/j.healun.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Frazier OH, Kirklin JK, editors. Mechanical circulatory support. Oxford, England: Elsevier; 2006. [Google Scholar]
- 3.Gonzalez-Stawinski GV, Atik FA, McCarthy PM, Roselli EE, Hoercher K, Navia JL, et al. Early and late rejection and HLA sensitization at the time of heart transplantation in patients bridged with left ventricular assist devices. Transplant Proc. 2005;37:1349–51. doi: 10.1016/j.transproceed.2004.12.111. [DOI] [PubMed] [Google Scholar]
- 4.Massad MG, Cook DJ, Schmitt SK, Smedira NG, McCarthy JF, Vargo RL, et al. Factors influencing HLA sensitization in implantable LVAD recipients. Ann Thorac Surg. 1997;64:1120–5. doi: 10.1016/s0003-4975(97)00807-2. [DOI] [PubMed] [Google Scholar]
- 5.Reed EF, Demetris AJ, Hammond E, Itescu S, Kobashigawa JA, Reinsmoen NL, et al. Acute antibody-mediated rejection of cardiac transplants. J Heart Lung Transplant. 2006;25:153–9. doi: 10.1016/j.healun.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Lietz K, Miller LW. Improved survival of patients with end-stage heart failure listed for heart transplantation: analysis of organ procurement and transplantation network/U.S. United Network of Organ Sharing data, 1990 to 2005. J Am Coll Cardiol. 2007;50:1282–90. doi: 10.1016/j.jacc.2007.04.099. [DOI] [PubMed] [Google Scholar]
- 7.Kerman RH. Understanding the sensitized patient. Heart Fail Clin. 2007;3:1–9. doi: 10.1016/j.hfc.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Lavee J, Kormos RL, Duquesnoy RJ, Zerbe TR, Armitage JM, Vanek M, et al. Influence of panel-reactive antibody and lymphocytotoxic crossmatch on survival after heart transplantation. J Heart Lung Transplant. 1991;10:921–9. discussion 29–30. [PubMed] [Google Scholar]
- 9.Itescu S, Schuster M, Burke E, Ankersmit J, Kocher A, Deng M, et al. Immunobiologic consequences of assist devices. Cardiol Clin. 2003;21:119–33. ix–x. doi: 10.1016/s0733-8651(02)00135-2. [DOI] [PubMed] [Google Scholar]
- 10.Terasaki P, Mickey MR, Iwaki Y, Cicciarelli J, Cecka M, Cook D, et al. Long-term survival of kidney grafts. Transplant Proc. 1989;21:615–7. [PubMed] [Google Scholar]
- 11.Opelz G. Correlation of HLA matching with kidney graft survival in patients with or without cyclosporine treatment. Transplantation. 1985;40:240–3. doi: 10.1097/00007890-198509000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Barr ML, Cohen DJ, Benvenisty AI, Hardy M, Reemtsma K, Rose EA, et al. Effect of anti-HLA antibodies on the long-term survival of heart and kidney allografts. Transplant Proc. 1993;25:262–4. [PubMed] [Google Scholar]
- 13.Saidman SL. Histocompatibility testing for highly sensitized transplant candidates. Transplant Proc. 2007;39:673–5. doi: 10.1016/j.transproceed.2007.01.053. [DOI] [PubMed] [Google Scholar]
- 14.Appel JZ, 3rd, Hartwig MG, Cantu E, 3rd, Palmer SM, Reinsmoen NL, Davis RD. Role of flow cytometry to define unacceptable HLA antigens in lung transplant recipients with HLA-specific antibodies. Transplantation. 2006;81:1049–57. doi: 10.1097/01.tp.0000204046.89396.c5. [DOI] [PubMed] [Google Scholar]
- 15.Smith JD, Danskine AJ, Laylor RM, Rose ML, Yacoub MH. The effect of panel reactive antibodies and the donor specific crossmatch on graft survival after heart and heart-lung transplantation. Transpl Immunol. 1993;1:60–5. doi: 10.1016/0966-3274(93)90060-l. [DOI] [PubMed] [Google Scholar]
- 16.Kobashigawa JA, Sabad A, Drinkwater D, Cogert GA, Moriguchi JD, Kawata N, et al. Pretransplant panel reactive-antibody screens. Are they truly a marker for poor outcome after cardiac transplantation? Circulation. 1996;94:II294–7. [PubMed] [Google Scholar]
- 17.Tambur AR, Bray RA, Takemoto SK, Mancini M, Costanzo MR, Kobashigawa JA, et al. Flow cytometric detection of HLA-specific antibodies as a predictor of heart allograft rejection. Transplantation. 2000;70:1055–9. doi: 10.1097/00007890-200010150-00011. [DOI] [PubMed] [Google Scholar]
- 18.DeNofrio D, Rho R, Morales FJ, Kamoun M, Kearns J, Dorozinsky C, et al. Detection of anti-HLA antibody by flow cytometry in patients with a left ventricular assist device is associated with early rejection following heart transplantation. Transplantation. 2000;69:814–8. doi: 10.1097/00007890-200003150-00024. [DOI] [PubMed] [Google Scholar]
- 19.John R, Lietz K, Schuster M, Naka Y, Rao V, Mancini DM, et al. Immunologic sensitization in recipients of left ventricular assist devices. J Thorac Cardiovasc Surg. 2003;125:578–91. doi: 10.1067/mtc.2003.30. [DOI] [PubMed] [Google Scholar]
- 20.Schmid C, Welp H, Klotz S, Baba HA, Wilhelm MJ, Scheld HH. Outcome of patients surviving to heart transplantation after being mechanically bridged for more than 100 days. J Heart Lung Transplant. 2003;22:1054–8. doi: 10.1016/s1053-2498(02)01179-8. [DOI] [PubMed] [Google Scholar]
- 21.Pamboukian SV, Costanzo MR, Dunlap S, Rayburn B, Westfall AO, You ZY, et al. Relationship between bridging with ventricular assist device on rejection after heart transplantation. J Heart Lung Transplant. 2005;24:310–5. doi: 10.1016/j.healun.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Stawinski GV, Cook DJ, Chang AS, Atik F, Navia JL, Banbury M, et al. Early and midterm risk of coronary allograft vasculopathy in patients bridged to orthotopic heart transplantation with ventricular assist devices. Transplantation. 2005;79:1175–9. doi: 10.1097/00007890-200505150-00041. [DOI] [PubMed] [Google Scholar]
- 23.Pagani FD, Dyke DB, Wright S, Cody R, Aaronson KD. Development of anti-major histocompatibility complex class I or II antibodies following left ventricular assist device implantation: effects on subsequent allograft rejection and survival. J Heart Lung Transplant. 2001;20:646–53. doi: 10.1016/s1053-2498(01)00232-7. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Stawinski GV, Cook DJ, Chang AS, Banbury MK, Navia JL, Hoercher K, et al. Ventricular assist devices and aggressive immunosuppression: looking beyond overall survival. J Heart Lung Transplant. 2006;25:613–8. doi: 10.1016/j.healun.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Erren M, Schluter B, Fobker M, Plenz G, Baba H, Willeke P, et al. Immunologic effects of implantation of left ventricular assist devices. Transplant Proc. 2001;33:1965–8. doi: 10.1016/s0041-1345(00)02756-1. [DOI] [PubMed] [Google Scholar]
- 26.McKenna DH, Jr, Eastlund T, Segall M, Noreen HJ, Park S. HLA alloimmunization in patients requiring ventricular assist device support. J Heart Lung Transplant. 2002;21:1218–24. doi: 10.1016/s1053-2498(02)00448-5. [DOI] [PubMed] [Google Scholar]
- 27.Miller LW, Phelan DL, Noedel N, McBride LR, Pennington DG. Multiparous women: is routine antibody screening enough in cardiac transplantation? Transplant Proc. 1991;23:1135–6. [PubMed] [Google Scholar]
- 28.Tait BD, Dandie WJ, Griffiths AP, Esmore DS, Snell GI, Bergin P, et al. Covert presensitization to HLA antigens in parous heart and lung transplant recipients may predispose to early allograft rejection. Transplant Proc. 1995;27:2143–4. [PubMed] [Google Scholar]
- 29.Spanier T, Oz M, Levin H, Weinberg A, Stamatis K, Stern D, et al. Activation of coagulation and fibrinolytic pathways in patients with left ventricular assist devices. J Thorac Cardiovasc Surg. 1996;112:1090–7. doi: 10.1016/S0022-5223(96)70111-3. [DOI] [PubMed] [Google Scholar]
- 30.Ankersmit HJ, Tugulea S, Spanier T, Weinberg AD, Artrip JH, Burke EM, et al. Activation-induced T-cell death and immune dysfunction after implantation of left-ventricular assist device. Lancet. 1999;354:550–5. doi: 10.1016/s0140-6736(98)10359-8. [DOI] [PubMed] [Google Scholar]
- 31.Scheinin SA, Radovancevic B, Kimball P, Duncan JM, Van Buren CT, Frazier OH, et al. Effect of IgM-positive crossmatches on survival in heart transplant recipients. Tex Heart Inst J. 1995;22:67–71. [PMC free article] [PubMed] [Google Scholar]
- 32.Schuster M, Kocher A, John R, Hoffman M, Ankersmit J, Lietz K, et al. B-cell activation and allosensitization after left ventricular assist device implantation is due to T-cell activation and CD40 ligand expression. Hum Immunol. 2002;63:211–20. doi: 10.1016/s0198-8859(01)00380-9. [DOI] [PubMed] [Google Scholar]
- 33.Moazami N, Itescu S, Williams MR, Argenziano M, Weinberg A, Oz MC. Platelet transfusions are associated with the development of anti-major histocompatibility complex class I antibodies in patients with left ventricular assist support. J Heart Lung Transplant. 1998;17:876–80. [PubMed] [Google Scholar]
- 34.van Marwijk Kooy M, van Prooijen HC, Moes M, Bosma-Stants I, Akkerman JW. Use of leukocyte-depleted platelet concentrates for the prevention of refractoriness and primary HLA alloimmunization: a prospective, randomized trial. Blood. 1991;77:201–5. [PubMed] [Google Scholar]
- 35.Drakos SG, Stringham JC, Long JW, Gilbert EM, Fuller TC, Campbell BK, et al. Prevalence and risks of allosensitization in HeartMate left ventricular assist device recipients: the impact of leukofiltered cellular blood product transfusions. J Thorac Cardiovasc Surg. 2007;133:1612–9. doi: 10.1016/j.jtcvs.2006.11.062. [DOI] [PubMed] [Google Scholar]
- 36.Joyce DL, Southard RE, Torre-Amione G, Noon GP, Land GA, Loebe M. Impact of left ventricular assist device (LVAD)-mediated humoral sensitization on post-transplant outcomes. J Heart Lung Transplant. 2005;24:2054–9. doi: 10.1016/j.healun.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 37.Baran DA, Gass AL, Galin ID, Zucker MJ, Arroyo LH, Goldstein DJ, et al. Lack of sensitization and equivalent post-transplant outcomes with the Novacor left ventricular assist device. J Heart Lung Transplant. 2005;24:1886–90. doi: 10.1016/j.healun.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Loebe M, Koster A, Sanger S, Potapov EV, Kuppe H, Noon GP, et al. Inflammatory response after implantation of a left ventricular assist device: comparison between the axial flow MicroMed DeBakey VAD and the pulsatile Novacor device. Asaio J. 2001;47:272–4. doi: 10.1097/00002480-200105000-00023. [DOI] [PubMed] [Google Scholar]
- 39.Ankersmit HJ, Wieselthaler G, Moser B, Gerlitz S, Roth G, Boltz-Nitulescu G, et al. Transitory immunologic response after implantation of the DeBakey VAD continuous-axial-flow pump. J Thorac Cardiovasc Surg. 2002;123:557–61. doi: 10.1067/mtc.2002.120011. [DOI] [PubMed] [Google Scholar]
- 40.Grinda JM, Bricourt MO, Amrein C, Salvi S, Guillemain R, Francois A, et al. Human leukocyte antigen sensitization in ventricular assist device recipients: a lesser risk with the DeBakey axial pump. Ann Thorac Surg. 2005;80:945–8. doi: 10.1016/j.athoracsur.2005.03.096. [DOI] [PubMed] [Google Scholar]
- 41.Pisani BA, Mullen GM, Malinowska K, Lawless CE, Mendez J, Silver MA, et al. Plasmapheresis with intravenous immunoglobulin G is effective in patients with elevated panel reactive antibody prior to cardiac transplantation. J Heart Lung Transplant. 1999;18:701–6. doi: 10.1016/s1053-2498(99)00022-4. [DOI] [PubMed] [Google Scholar]
- 42.Larson DF, Elkund DK, Arabia F, Copeland JG. Plasmapheresis during cardiopulmonary bypass: a proposed treatment for presensitized cardiac transplantation patients. J Extra Corpor Technol. 1999;31:177–83. [PubMed] [Google Scholar]
- 43.Leech SH, Lopez-Cepero M, LeFor WM, DiChiara L, Weston M, Furukawa S, et al. Management of the sensitized cardiac recipient: the use of plasmapheresis and intravenous immunoglobulin. Clin Transplant. 2006;20:476–84. doi: 10.1111/j.1399-0012.2006.00509.x. [DOI] [PubMed] [Google Scholar]
- 44.Dowling RD, Jones JW, Carroll MS, Gray LA., Jr Use of intravenous immunoglobulin in sensitized LVAD recipients. Transplant Proc. 1998;30:1110–1. doi: 10.1016/s0041-1345(98)00172-9. [DOI] [PubMed] [Google Scholar]
- 45.John R, Lietz K, Burke E, Ankersmit J, Mancini D, Suciu-Foca N, et al. Intravenous immunoglobulin reduces anti-HLA alloreactivity and shortens waiting time to cardiac transplantation in highly sensitized left ventricular assist device recipients. Circulation. 1999;100:II229–35. doi: 10.1161/01.cir.100.suppl_2.ii-229. [DOI] [PubMed] [Google Scholar]
- 46.Smith MR. Rituximab (monoclonal anti-CD20 antibody): mechanisms of action and resistance. Oncogene. 2003;22:7359–68. doi: 10.1038/sj.onc.1206939. [DOI] [PubMed] [Google Scholar]
- 47.Becker YT, Samaniego-Picota M, Sollinger HW. The emerging role of rituximab in organ transplantation. Transpl Int. 2006;19:621–8. doi: 10.1111/j.1432-2277.2006.00345.x. [DOI] [PubMed] [Google Scholar]
- 48.Sidner RA, Book BK, Agarwal A, Bearden CM, Vieira CA, Pescovitz MD. In vivo human B-cell subset recovery after in vivo depletion with rituximab, anti-human CD20 monoclonal antibody. Hum Antibodies. 2004;13:55–62. [PubMed] [Google Scholar]
- 49.Vieira CA, Agarwal A, Book BK, Sidner RA, Bearden CM, Gebel HM, et al. Rituximab for reduction of anti-HLA antibodies in patients awaiting renal transplantation: 1. Safety, pharmacodynamics, and pharmacokinetics Transplantation. 2004;77:542–8. doi: 10.1097/01.tp.0000112934.12622.2b. [DOI] [PubMed] [Google Scholar]
- 50.Balfour IC, Fiore A, Graff RJ, Knutsen AP. Use of rituximab to decrease panel-reactive antibodies. J Heart Lung Transplant. 2005;24:628–30. doi: 10.1016/j.healun.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 51.Loh E, Bergin JD, Couper GS, Mudge GH., Jr Role of panel-reactive antibody cross-reactivity in predicting survival after orthotopic heart transplantation. J Heart Lung Transplant. 1994;13:194–201. [PubMed] [Google Scholar]
- 52.Taylor DO, Yowell RL, Kfoury AG, Hammond EH, Renlund DG. Allograft coronary artery disease: clinical correlations with circulating anti-HLA antibodies and the immunohistopathologic pattern of vascular rejection. J Heart Lung Transplant. 2000;19:518–21. doi: 10.1016/s1053-2498(00)00095-4. [DOI] [PubMed] [Google Scholar]
- 53.Uber WE, Self SE, Van Bakel AB, Pereira NL. Acute antibody-mediated rejection following heart transplantation. Am J Transplant. 2007;7:2064–74. doi: 10.1111/j.1600-6143.2007.01900.x. [DOI] [PubMed] [Google Scholar]
- 54.Lones MA, Czer LS, Trento A, Harasty D, Miller JM, Fishbein MC. Clinical-pathologic features of humoral rejection in cardiac allografts: a study in 81 consecutive patients. J Heart Lung Transplant. 1995;14:151–62. [PubMed] [Google Scholar]
- 55.Ratkovec RM, Hammond EH, O’Connell JB, Bristow MR, DeWitt CW, Richenbacher WE, et al. Outcome of cardiac transplant recipients with a positive donor-specific crossmatch--preliminary results with plasmapheresis. Transplantation. 1992;54:651–5. doi: 10.1097/00007890-199210000-00017. [DOI] [PubMed] [Google Scholar]
- 56.Michaels PJ, Espejo ML, Kobashigawa J, Alejos JC, Burch C, Takemoto S, et al. Humoral rejection in cardiac transplantation: risk factors, hemodynamic consequences and relationship to transplant coronary artery disease. J Heart Lung Transplant. 2003;22:58–69. doi: 10.1016/s1053-2498(02)00472-2. [DOI] [PubMed] [Google Scholar]
- 57.Smith RN, Brousaides N, Grazette L, Saidman S, Semigran M, Disalvo T, et al. C4d deposition in cardiac allografts correlates with alloantibody. J Heart Lung Transplant. 2005;24:1202–10. doi: 10.1016/j.healun.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 58.Cherry R, Nielsen H, Reed E, Reemtsma K, Suciu-Foca N, Marboe CC. Vascular (humoral) rejection in human cardiac allograft biopsies: relation to circulating anti-HLA antibodies. J Heart Lung Transplant. 1992;11:24–9. discussion 30. [PubMed] [Google Scholar]
- 59.Rodriguez ER, Skojec DV, Tan CD, Zachary AA, Kasper EK, Conte JV, et al. Antibody-mediated rejection in human cardiac allografts: evaluation of immunoglobulins and complement activation products C4d and C3d as markers. Am J Transplant. 2005;5:2778–85. doi: 10.1111/j.1600-6143.2005.01074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crespo-Leiro MG, Veiga-Barreiro A, Domenech N, Paniagua MJ, Pinon P, Gonzalez-Cuesta M, et al. Humoral heart rejection (severe allograft dysfunction with no signs of cellular rejection or ischemia): incidence, management, and the value of C4d for diagnosis. Am J Transplant. 2005;5:2560–4. doi: 10.1111/j.1600-6143.2005.01039.x. [DOI] [PubMed] [Google Scholar]
- 61.Grauhan O, Knosalla C, Ewert R, Hummel M, Loebe M, Weng YG, et al. Plasmapheresis and cyclophosphamide in the treatment of humoral rejection after heart transplantation. J Heart Lung Transplant. 2001;20:316–21. doi: 10.1016/s1053-2498(00)00211-4. [DOI] [PubMed] [Google Scholar]
- 62.Wang SS, Chou NK, Ko WJ, Chi NH, Hung SC, Hsu RB, et al. Effect of plasmapheresis for acute humoral rejection after heart transplantation. Transplant Proc. 2006;38:3692–4. doi: 10.1016/j.transproceed.2006.10.060. [DOI] [PubMed] [Google Scholar]
- 63.Aranda JM, Jr, Scornik JC, Normann SJ, Lottenberg R, Schofield RS, Pauly DF, et al. Anti-CD20 monoclonal antibody (rituximab) therapy for acute cardiac humoral rejection: a case report. Transplantation. 2002;73:907–10. doi: 10.1097/00007890-200203270-00013. [DOI] [PubMed] [Google Scholar]
- 64.Baran DA, Lubitz S, Alvi S, Fallon JT, Kaplan S, Galin I, et al. Refractory humoral cardiac allograft rejection successfully treated with a single dose of rituximab. Transplant Proc. 2004;36:3164–6. doi: 10.1016/j.transproceed.2004.10.087. [DOI] [PubMed] [Google Scholar]
- 65.Kaczmarek I, Deutsch MA, Sadoni S, Brenner P, Schmauss D, Daebritz SH, et al. Successful management of antibody-mediated cardiac allograft rejection with combined immunoadsorption and anti-CD20 monoclonal antibody treatment: case report and literature review. J Heart Lung Transplant. 2007;26:511–5. doi: 10.1016/j.healun.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 66.Garrett HE, Jr, Duvall-Seaman D, Helsley B, Groshart K. Treatment of vascular rejection with rituximab in cardiac transplantation. J Heart Lung Transplant. 2005;24:1337–42. doi: 10.1016/j.healun.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 67.Soleimani B, Lechler RI, Hornick PI, George AJ. Role of alloantibodies in the pathogenesis of graft arteriosclerosis in cardiac transplantation. Am J Transplant. 2006;6:1781–5. doi: 10.1111/j.1600-6143.2006.01401.x. [DOI] [PubMed] [Google Scholar]
- 68.Wehner J, Morrell CN, Reynolds T, Rodriguez ER, Baldwin WM., 3rd Antibody and complement in transplant vasculopathy. Circ Res. 2007;100:191–203. doi: 10.1161/01.RES.0000255032.33661.88. [DOI] [PubMed] [Google Scholar]
- 69.Labarrere CA, Pitts D, Halbrook H, Faulk WP. Tissue plasminogen activator, plasminogen activator inhibitor-1, and fibrin as indexes of clinical course in cardiac allograft recipients. An immunocytochemical study. Circulation. 1994;89:1599–608. doi: 10.1161/01.cir.89.4.1599. [DOI] [PubMed] [Google Scholar]
- 70.Mehra MR, Ventura HO, Stapleton DD, Smart FW, Collins TC, Ramee SR. Presence of severe intimal thickening by intravascular ultrasonography predicts cardiac events in cardiac allograft vasculopathy. J Heart Lung Transplant. 1995;14:632–9. [PubMed] [Google Scholar]
- 71.Spes CH, Klauss V, Mudra H, Schnaack SD, Tammen AR, Rieber J, et al. Diagnostic and prognostic value of serial dobutamine stress echocardiography for noninvasive assessment of cardiac allograft vasculopathy: a comparison with coronary angiography and intravascular ultrasound. Circulation. 1999;100:509–15. doi: 10.1161/01.cir.100.5.509. [DOI] [PubMed] [Google Scholar]
- 72.Johnson MR, Aaronson KD, Canter CE, Kirklin JK, Mancini D, Mehra MR, et al. Heart retransplantation. Am J Transplant. 2007;7:2075–81. doi: 10.1111/j.1600-6143.2007.01902.x. [DOI] [PubMed] [Google Scholar]
- 73.Eisen HJ, Tuzcu EM, Dorent R, Kobashigawa J, Mancini D, Valantine-von Kaeppler HA, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med. 2003;349:847–58. doi: 10.1056/NEJMoa022171. [DOI] [PubMed] [Google Scholar]
- 74.Keogh A, Richardson M, Ruygrok P, Spratt P, Galbraith A, O’Driscoll G, et al. Sirolimus in de novo heart transplant recipients reduces acute rejection and prevents coronary artery disease at 2 years: a randomized clinical trial. Circulation. 2004;110:2694–700. doi: 10.1161/01.CIR.0000136812.90177.94. [DOI] [PubMed] [Google Scholar]
- 75.Mancini D, Pinney S, Burkhoff D, LaManca J, Itescu S, Burke E, et al. Use of rapamycin slows progression of cardiac transplantation vasculopathy. Circulation. 2003;108:48–53. doi: 10.1161/01.CIR.0000070421.38604.2B. [DOI] [PubMed] [Google Scholar]
- 76.Leech SH, Mather PJ, Eisen HJ, Pina IL, Margulies KB, Bove AA, et al. Donor-specific HLA antibodies after transplantation are associated with deterioration in cardiac function. Clin Transplant. 1996;10:639–45. [PubMed] [Google Scholar]
- 77.Rose EA, Smith CR, Petrossian GA, Barr ML, Reemtsma K. Humoral immune responses after cardiac transplantation: correlation with fatal rejection and graft atherosclerosis. Surgery. 1989;106:203–7. discussion 07–8. [PubMed] [Google Scholar]
- 78.Reed EF, Hong B, Ho E, Harris PE, Weinberger J, Suciu-Foca N. Monitoring of soluble HLA alloantigens and anti-HLA antibodies identifies heart allograft recipients at risk of transplant-associated coronary artery disease. Transplantation. 1996;61:566–72. doi: 10.1097/00007890-199602270-00009. [DOI] [PubMed] [Google Scholar]
- 79.Leprince P, Fretz C, Dorent R, Boudifa A, Jourdan J, Youssoub JJ, et al. Posttransplantation cytotoxic immunoglobulin G is associated with a high rate of acute allograft dysfunctions in heart transplant recipients. Am Heart J. 1999;138:586–92. doi: 10.1016/s0002-8703(99)70164-x. [DOI] [PubMed] [Google Scholar]
- 80.Tambur AR, Pamboukian SV, Costanzo MR, Herrera ND, Dunlap S, Montpetit M, et al. The presence of HLA-directed antibodies after heart transplantation is associated with poor allograft outcome. Transplantation. 2005;80:1019–25. doi: 10.1097/01.tp.0000180564.14050.49. [DOI] [PubMed] [Google Scholar]