Abstract
Lung transplantation is the treatment option for a variety of end stage pulmonary diseases. Post transplant development of antibodies (Abs) against donor HLA and non-HLA antigens have been associated with acute and chronic rejection of transplanted organs. Development of bronchiolitis obliterans syndrome (BOS) following lung transplantation has been correlated with de novo production of anti-donor-HLA Abs. However, only a portion of the patients with BOS demonstrate detectable anti-donor-HLA Abs. Airway epithelium is considered as a major target for lung allograft rejection. In this study we demonstrate that many BOS+ patients (12 of 36) develop Abs reactive to epithelial cell antigen that is distinct from HLA. Further, de novo production of anti-epithelial cell antibody precedes clinical onset of BOS. N-terminal sequencing and BLASTX analysis as well as blocking with K-alpha1 tubulin specific antibody identified the epithelial antigen as K-α1 tubulin. Binding of the de novo produced anti-K-α1 tubulin antibodies to the airway epithelial cells resulted in the increased expression of transcription factors (TCF5 and c-Myc), leading to increased expression of fibrogenic growth factors, activation of cell cycle signaling and fibro-proliferation, the central events in immunopathogenesis of BOS following human lung transplantation.
Keywords: human, lung, transplantation, antibodies
Introduction
The development of bronchiolitis obliterans syndrome (BOS) remains the Achilles heel of human lung transplantation. BOS occurs in nearly all allografts by ten years after transplant and is the main cause of morbidity and mortality following lung transplantation (1). BOS is a fibroproliferative process that involves inflammation and fibrosis of the lamina propria and lumen resulting in progressive decline in pulmonary function and eventual allograft failure (2). Progression of BOS can be slowed but unresponsive to the current immunosuppressive therapies instituted after transplantation (3-6).
Many studies in the literature have proposed that the development of BOS involves a final common pathway of alloimmune injury, with subsequent release of immunologic mediators and production of growth factors, leading to luminal occlusion and fibrous scarring of small airways (7-9). Several studies have shown that the airway epithelial cells (AECs) are the main target for the immunologic insult during the pathogenesis of allograft rejection (10-15). In vitro studies from our laboratory (16, 17) and others (18) have demonstrated that activation of epithelial cells results in the production of growth factors including transforming growth factor (TGF)-β, epidermal growth factor (EGF), basic fibroblast growth factor (bFGF) and endothelin (ET)-1. Exposure to these growth factors results in the activation and proliferation of fibroblasts and smooth muscle cells. More significantly, in vivo studies have revealed a temporal relationship between elevated levels of growth factors and significant fibroblast migration and proliferation within the small airways (19, 20).
Previous studies from our laboratory (21-23) and others (24-27) have implicated that the development of anti-donor HLA Abs after lung transplantation predispose patients to the development of chronic rejection. These studies demonstrated that binding of anti-HLA class I Abs stimulated the proliferation of epithelial, endothelial and smooth muscle cells (16,17) However, there are many incidences of BOS in patients where Abs to mismatched donor HLA can't be readily demonstrated suggesting a role for Abs to non-HLA antigens in the pathogenesis of BOS. The importance of non-HLA Abs in acute as well as chronic rejection has been previously studied in liver, renal and cardiac allografts (28-30). In this report, we demonstrate that Abs that recognizes the K-α-1 tubulin expressed on epithelial surface can be defined in human lung transplant recipients undergoing BOS. These Abs bind to AECs and specific ligation results in increased expression of fibrogenic growth factors, activation of cell cycle signaling and fibro-proliferation. Therefore, we propose a pathogenic role for Abs to K-α1 tubulin in the immunopathogenesis of BOS following human lung transplantation.
Materials and Methods
Human subjects
Patients who underwent lung transplantation at the Washington University Medical Center/Barnes-Jewish Hospital were enrolled in this study after obtaining informed consent in accordance with a protocol approved by the Institutional Review Board. The mean age of transplantation was 52.0 ± 8.1 and the male to female ratio was 1:1. The end stage pulmonary pathologies were chronic obstructive pulmonary disease, AT1 deficiency, cystic fibrosis, and IPF. Most of the transplants were bilateral. The standard immunosuppression protocol consisted of cyclosporine, azathioprine and prednisone. After BOS was diagnosed, the immunotherapy protocol was modified to FK506 (tacrolimus), mycophenolate mofetil and prednisone.
The diagnosis of BOS was made according to ISHLT standard criteria (23). A patient was diagnosed with BOS (BOS+) if either of the following criteria were satisfied: their forced expiratory volume in one second (FEV1) was measured at less than 80% of the baseline established in their stable post-operative period or there was histologic evidence of bronchiolitis obliterans. A patient was considered free of BOS (BOS-) if their FEV1 remained above the 80% level and their pulmonary histology remained negative for bronchiolitis obliterans. Serum samples were collected from the patients on the day of their transplant prior to surgery and then in post-transplant months 12, 24, 26, 48. Further samples were collected at varying follow-ups depending on the patient's clinical status. All serum samples were processed in our laboratory on their day of collection and then stored at -70°C until further use.
Complement-Dependent Cytotoxicity Assays
The anti-HLA reactivity was determined on an HLA reference panel consisting of T and B-lymphocytes from 50 unrelated individuals of known HLA specificity (panel-reactive Abs [PRA]). Briefly, isolated lymphocytes were incubated in the presence of undiluted patient serum (diluted 1/1) for 40 min at 22°C. Then, both three-wash Amos-modified and antiglobulin-augmented complement-dependent cytotoxicity assays (CDC) assays were performed according to the National Institutes of Health standard protocols as previously described (22). Patients were considered positive for PRA, if there was cytotoxicity against 2% or more of the cells in the panel of lymphocytes in any of the tested sera.
FLOW PRA Assay
The FLOW PRA assay was carried out according to the manufacturer's instructions (One Lambda Inc. Los Angeles, CA, USA). For class I and II FLOW PRA assays, positivity with a single bead produced a clear and distinct peak of fluorescence that contained approximately 2.4% and 2.9% of the total events collected respectively (i.e., 1/24th and 1/29 of the bead pool). The cutoff was determined based on the average percent binding of 20 different normal human sera obtained from healthy volunteers. Therefore, results were recorded as positive when >=2.4% of class I and 2.9% of class II beads exhibited a distinct fluorescence peak that was above the fluorescence exhibited by the negative control.
Cell lines
The AEC line was developed from lung biopsies, immortalized by transfection with the pRSV-Tag plasmid, and cultured in small airway growth medium (SAGM, Cambrex Bio Science, Walkerville, MD). Bronchial epithelial cells (NHBE) were obtained from ATCC and cultured in bronchial epithelial growth medium (BEGM, Cambrex Bio Science, Walkerville, MD). Endothelial cells were obtained from Cambrex and were cultured in endothelial growth medium (EGM) (Cambrex Bio Science, Walkerville, MD). Kidney cell lines (KCLs) were developed from the renal cortex of cadaveric kidneys, immortalized by transfection with the pRSV-Tag plasmid, and cultured in RPMI-1640 medium (Sigma, St. Louis, MO) supplemented with 15% fetal bovine serum, 2mM l-glutamine, 25mM HPES buffer, 1mM sodium pyruvate, 100 U/ml penicillin, and 0.1 mg/ml streptomycin. Peripheral blood lymphocytes (PBLs) were isolated by Ficoll gradient centrifugation from healthy donor voluntary blood samples and used for testing on the same day as isolation. All other cell lines were frozen at -70°C until use. Upon thaw, all cell lines were maintained in sterile 5% CO2 incubation in respective growth media at 37°C.
Fluorescence activated cell sorter (FACS) analysis
Tracheal AECs, endothelial cells, KCLs and PBLs (1×106 cells) were exposed to 100 μl of selected BOS+ and BOS- patient serum samples, panel reactive human serum (positive control), normal healthy volunteer serum (negative control) or no serum (negative control). After washing, the cells were stained with fluorescein isothiocyanate-conjugated goat anti-human IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). Cells were analyzed using a FACScan flow cytometer (BD Biosciences, San Diego, CA). The data analysis was performed using the CellQuest software (BD Biosciences).
Western blot analysis
Crude extracts from the epithelial, endothelial and peripheral blood mononuclear cells were extracted by resuspending 10 million cells in M-PER protein extraction reagent (Pierce, Rockford, IL). The whole cell lysates were then separated under reducing conditions using 4-12% gradient Bis Tris gel (Invitrogen, Carlsbad, CA) electrophoresis. The proteins were transferred to a nitrocellulose membrane and blocked with Tris-buffered saline (TBS) with 5% skim milk protein overnight. After washing one time with TBS, the nitrocellulose membrane was either cut into 0.5 cm strips or left intact. Each strip was immuno blotted with a different patient serum (BOS+ or BOS-) or serum from a normal volunteer (negative control) (all at 1:1500) overnight. The serum was washed from the membranes which were then incubated for one hour with horse radish peroxidase-conjugated goat anti-human IgG (1:10,000) (Jackson Immuno Research Laboratories, West Grove, PA). The immunoreactivity was detected by using the SuperSignal West Pico Chemiluminescent Substrate western blot detection system (Pierce, Rockford, IL).
K-α1 tubulin was purchased from Abnova Corporation (Taiwan, ROC) and was reconstituted according the manufacturer's instructions and then frozen at -70°C until use. Fifty micrograms of the protein was run under reducing conditions through a 4-12% gradient Bis Tris gel (Invitrogen, Carlsbad, CA). The protein was transferred, blocked and washed as above. The nitrocellulose membrane was then cut into eight equal slices. These membrane slices were incubated overnight with mouse anti- K-α1 tubulin monoclonal antibody (1:100), normal human serum (1:1500), 3 BOS+ sera and 3 BOS- sera (1:1500). The membranes were washed after incubation. Horse radish peroxidase-conjugated goat anti-mouse IgG secondary antibody (1:10,000) (Jackson ImmunoResearch Laboratories, West Grove, PA) was applied to the K-α1 tubulin antibody while with horse radish peroxidase-conjugated goat anti-human IgG (1:1000) (Jackson ImmunoResearch Laboratories, West Grove, PA) was added to the other samples for one hour. Detection was achieved as above.
Luminex
AECs were cultured in 6-well plates in SAGM to 80% confluency. The growth media was then removed and the cells were maintained in a starvation media (small airway basic medium (Cambrex Bio Science, Walkerville, MD)) for 24 hours. After this time period, the starvation medium was removed and three BOS+ and three BOS- patients' sera were individually added to each well (1000 μl). After one hour of incubation at 37°C, 1000 ml of starvation media was added to the sera in each of the wells, cultured for 24 hr. the entire well volume was then collected and frozen at -70°C until use.
Growth factor four-plex combined with cytokine 20-plex (VEGF, G-CSF, bFGF, PDGF-BB + FGF basic, GM-CSF, IFN-γ, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p40/p70, IL-13, IL-17, IP-10, KC, MCP-1, MIG, MIP-1α, TNF-α, and VEGF basic) analysis kits were obtained from Biosource International (Camarillo, CA). Millipore multiscreen 96 well filter plates (Bedford, MA) were used for all multiplex growth factor kits. Assays were run in triplicate using 50 μl of sample according to the manufacturer's protocol. Data was collected using the Luminex-100 system Version 1.7, a dual laser flow analyzer (Luminex, Austin, TX). Data analysis was performed using the MasterPlex QT 1.0 system (MiraiBio, Alameda, CA). A five-parameter regression formula was used to calculate the sample concentrations from the standard curves. All 96 samples were analyzed with the LINCOplex kit (Linco Research Inc.).
Gene array
Expression profiles of intracellular signal genes in the isolated AECs were analyzed using the Signal Transduction PathwayFinder gene array (Super Array, Frederick, MD) as per manufacturers' recommendation. Briefly, total RNA was extracted from 10 × 106 using Trizol reagent. The RNA was reverse-transcribed and radiolabeled using AmpoLabeling-LPR Kit (Super Array Biosciences, Frederick, MD). The radiolabeled probes were hybridized with GEArray Q series Signal Transduction PathwayFinder gene array (Super Array Biosciences, Frederick, MD) overnight at 60°C. Membranes were washed twice with 2× SSC/1% SDS and twice with 0.2× SSC/0.5% SDS and then exposed to x-ray film. After overnight exposure at −70° C, the film was developed. The data obtained from the membranes was analyzed using the Gene Array Analyzer Software (Super Array Biosciences, Frederick, MD). The data were normalized using GAPDH and data represented as relative expression (Gene of interest/GAPDH).
Protein isolation and sequencing
With the identical protocol used in the western blotting above, the AECs were prepared and the proteins separated under reducing conditions. The proteins were then transferred to a PVDF membrane. When the transfer was complete, the membrane was stained with Coomassie blue stain for 1 minute. The membrane was destained overnight. The band at 48 kDa was sharply excised and dried. The sample was then sent to ProSeq Protein Microsequencing (Boxford, MA) for sequence determination. In brief, phenylisothiocyanate reacts with the amino acid residue at the amino terminus under basic conditions to form a phenylthiocarbamoyl derivative. Tri fluoroacetic acid then cleaves off the first amino acid as its anilinothialinone derivative and leaves the new amino terminus for the next degradation cycle. The ATZ amino acid is then removed by extraction with N-butyl chloride and converted to a phenylthiohydantoin derivative with 25% TFA/water. The PTH-amino acid is transferred to a reverse-phase C-18 column for detection at 270nm. This chromatogram provides standard retention times of the amino acids for comparison with a standard chromatogram. The HPLC chromatograms are collected using a computer data analysis system. This process is repeated sequentially to provide the N-terminal sequence of the protein/peptide.
Results
Sera from BOS+ lung transplant recipients develop Abs specifically reactive to AECs
In lung allografts epithelium has been shown to be the primary target of immune responses. Abs has been postulated to play a crucial role in chronic rejection of allografts. 36 BOS+ lung transplant recipients with no detectable anti-HLA Abs by Flow PRA, cytotoxicity or ELISA were tested by flow cytometry against a panel of 30 PBMCs covering most of the common HLA allelic antigens in the population studied (see Table 1). None of the 36 sera recognized any of the PBMCs tested indicating the absence of any anti-HLA Abs in those sera. In order to test for the presence of Abs that recognize epithelial cells, we analyzed immunoreactivity of sera from these 36 BOS+, 36 BOS- lung transplant recipients and 10 normal volunteers by flow cytometry against a panel of epithelial cells. None of these sera had detectable anti-HLA Abs by cytotoxicity, ELISA as well as Flow PRA methods. However, 12 of the 36 BOS+ lung transplant recipients had Abs which specifically bound to epithelial cells (Table 1). In contrast, none of the sera from BOS negative patients (36 patients) or normal donors (10 volunteers) reacted to the panel of epithelial cells tested. In order to test the specificity of the Abs, we analyzed the immunoreactivity of the positive sera against endothelial cells, Kidney tubular epithelial cells (KCLs) and PBMCs by flow cytometry. The list of the cell lines tested, their origin and HLA phenotypes are summarized in table 2. FACS analysis of the sera from BOS positive patients demonstrated a significant binding of the Abs to the AECs (Fig 1A). However no binding was observed with the endothelial cells (1B), KCL (1C) or the peripheral blood mononuclear cells (1D) tested suggesting tissue restricted specificity of the Abs. These results demonstrate that a portion of lung transplant recipients develop Abs specific for AECs which is distinct from Abs to MHC alloantigens.
Table 1. Sera From BOS+ Lung Transplant Recipients Reacts Specifically To Airway Epithelial Cells.
| Target cells | ||||
|---|---|---|---|---|
| Sera Source | Normal peripheral Blood Lymphocytes* | Human Airway epithelial cells | Human Endothelial cells | Human kidney tubular epithelial cells |
| Post-lung transplant BOS+ | Negative (0/36) | Positive (12/36) | Negative (0/36) | Negative (0/36) |
| Post-lung transplant BOS- | Negative (0/36) | Negative (0/36) | Negative (0/36) | Negative (0/36) |
| Pre-lung transplant sera | Negative (0/12) | Negative (0/12) | Negative (0/12) | Negative (0/12) |
| Normal human sera | Negative (0/12) | Negative (0/12) | Negative (0/12) | Negative (0/12) |
- (number positive / number tested)
Table 2. Airway Epithelial Cell Specificity Of The Antibodies In BOS+ Sera.
| Epithelial cells | Kidney epithelial cells |
Endothelial cells# |
Lymphocytes* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient No. | KBU866 | KCC266 | LAV430 | LAB728 | KJE206 | KJQ253 | KIW8 10 | KGH664 | KJX739 | LGH367 | HEK293 | ||
| A2, 29;B7,27, DR3,14 |
A3,-; B65,-, DR13,15 |
A2,32; B44,61; DR13,15 |
A1,2; B8,60; Dr3,13 |
A3,-; B7,39; DR8,15 |
A68,-; B44,51; DR7,13 |
A1,2; B8,60; DR13,17 |
A1,26; B8,39; DR4,16 |
A11,28; B44,53; DR8,11 |
A3,28;,break/>B7,2; DR3,15 |
A2,3; B7,- |
|||
| 1 | + | + | + | + | + | + | + | + | + | + | - | -(0/3) | - (0/36) |
| 2 | + | + | + | + | + | + | + | + | + | + | - | -(0/3) | - (0/36) |
| 3 | + | + | + | + | + | + | + | + | + | + | - | -(0/3) | - (0/36) |
| 4 | + | + | + | + | + | + | + | + | + | + | - | -(0/3) | - (0/36) |
| 5 | + | + | + | + | + | + | + | + | + | + | - | -(0/3) | - (0/36) |
| 6 | + | + | + | + | + | + | + | + | + | + | - | -(0/3) | - (0/36) |
| 7 | + | + | + | + | + | + | + | + | + | + | - | -(0/3) | - (0/36) |
| 8 | + | + | + | + | + | + | + | + | + | + | - | -(0/3) | - (0/36) |
| 9 | + | + | + | + | + | + | + | + | + | + | - | -(0/3) | - (0/36) |
| 10 | + | + | + | + | + | + | + | + | + | + | - | -(0/3) | - (0/36) |
| 11 | + | + | + | + | + | + | + | + | + | + | - | -(0/3) | - (0/36) |
| 12 | + | + | + | + | + | + | + | + | + | + | - | -(0/3) | - (0/36) |
- cell panel containing all HLA-type of the recipients
- EC-1: A2,-; B18,44; DR11,12 EC-2:A1,68; B7,15; DR11,15 EC-3: A3,23; B44,49; DR11,12
Figure 1. BOS+ patient serum with positive FACS on AEC line.

Sera from BOS+ and BOS-patients with no anti-HLA reactivity were tested against epithelial cells, endothelial cells, PBMCs and macrophages by flow cytometry. A: epithelial cells, B: Endothelial cells, C: PBMCs and D: KCL. A representative BOS+ sera with epithelial reactivity is overlaid on a BOS- sera.
Binding of Abs to AECs results in increased expression of fibrogenic growth factors
In order to determine whether the ligation of cell surface molecule on the AECs by the Abs developed in BOS+ lung transplant recipients, we incubated AEC with Abs following which growth factor production by the AEC was determined by luminex assay. It has been demonstrated that increased expression of growth factors can accelerate the fibro-proliferation cascade which is considered to be one of the major pathways towards the induction of chronic rejection in transplanted allografts. In order to study the expression of growth factors subsequent to binding of anti-epithelial Abs, we exposed epithelial cells to BOS+ sera with and without anti-epithelial reactivity for 1 hr at 37 C. Expression of the growth factors in the culture supernatant were quantitated after 24 hr. of culture using a 4-plex growth factor luminex assay. Binding of the anti-epithelial antibody to the epithelial cells, as shown in Fig.2, resulted in a significant increase in the expression of VEGF (6 fold, 13 vs 79ng/ml, p<0.05), HB-EGF (2 fold 27 vs 56 ng/ml, p<0.05) and TGF-β (2.5 fold, 12 vs 32 ng/ml, p<0.05) compared to the expression levels observed on exposure to sera with no epithelial specific reactivity. These results indicate that binding of the anti-epithelial antibody to the epithelial cells results in increased production of growth factors that activate the fibro-proliferation cascade a critical event in BOS development in lung transplant recipients. Therefore, these Abs may play a role in the pathogenesis of BOS following lung transplantation.
Figure 2. Binding of anti-epithelial Abs to AECs results in increased expression of fibrogenic growth factors.
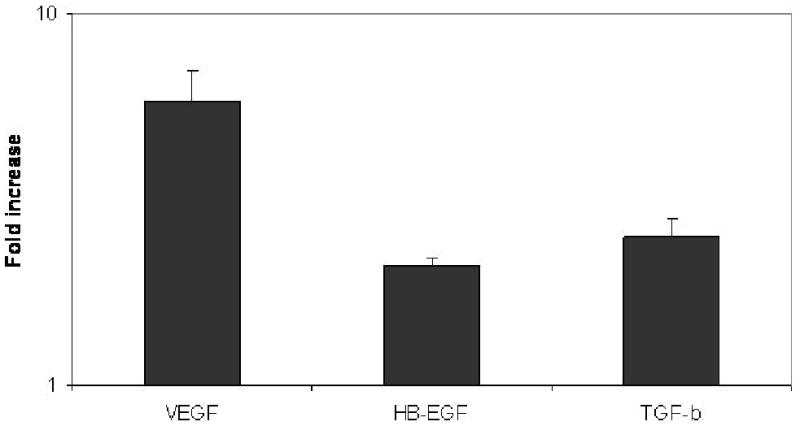
Airway epithelial cells were incubated with sera from BOS+ patients with and without epithelial reactivity for 1 hr and cultured 24 hr. at 37 C. Growth factors secreted in the culture supernatant were analyzed by growth factor luminex assay (Biosource International). A significant increase in the expression of VEGF (6-fold), HB-EGF (2-fold), and TGF-β (2.5 fold) in culture supernatants of AECs exposed to BOS+ sera compared to sera with no epithelial reactivity. The bars represent fold increase observed in means from 3 different experiments.
Antibody binding to AECs also increases the expression of PKC, transcription factors and stress proteins
In order to study the intra-cellular signaling events following binding of the anti-epithelial antibody to the epithelial cells we exposed AECs to BOS+ sera with and without anti-epithelial reactivity for 30 mins. Expression profile of the various signaling cascade intermediates were analyzed by using a Pathway finder gene array (Superarray Inc., Bethesda, MD). Gene array analysis presented in Fig. 3 demonstrated a 4-fold increase in the expression of heat shock proteins (HSPs) 27 (0.75 vs 3.1) and 90 (0.67 vs 2.95) as well as phosphokinase C (0.9 vs 4.0) after binding of the anti-epithelial Abs to the epithelial cells. There was also a > two fold increase in production of transcription factors TCF5 (0.74 vs 1.98) and c-Myc (1.02 vs 2.17) which are involved in inflammatory response, cell cycle progression, apoptosis and cellular transformation (Fig. 3). These results demonstrate that binding of the anti-epithelial antibody results in the activation of PKC and stress pathways which could result in increased expression of growth factors.
Figure 3. Gene array analysis.

Airway epithelial cells were incubated with sera from BOS+ patients with and without epithelial reactivity for 30 mins on ice. Expression profile of the signaling intermediates in stress signaling, calcium and cytoskeleton signaling were analyzed on a Signaling transduction Pathway finder gene array. The bars represent fold increase observed in means from 3 different experiments Gene array analysis demonstrated a 4-fold increase in the expression of heat shock proteins (HSPs) 27 and 90 as well as phosphokinase C (PKC) after binding of the anti-epithelial Abs to the epithelial cells. There was also significant increase in production of transcription factors TCF5 and c-Myc (2 fold).
K-α1 tubulin is the target antigen recognized by BOS+ sera
In order to identify the antigen recognized by BOS+ sera we performed western blot analysis against the crude antigenic extract of epithelial cells. Western blot analysis demonstrated that the BOS+ sera recognized a polypeptide of 48kDa. No immunoreactivity was observed with the BOS- sera or the normal human sera (Fig. 4). In order to identify the epithelial antigen we electroeluted the 48kDa polypeptide from the crude airway epithelial cell extract and sequenced the polypeptide. The sequence of the epithelial antigen was MRECISIHVGQAGVQIXNA. BLAST analysis of the sequence demonstrated a 100% homology to the K-α1 tubulin protein. These results indicate that the epithelial cell target antigen recognized by the BOS+ sera is K-α1 tubulin.
Figure 4. Temporal development of anti-AEC antibody detected with western blot.

Total protein extracts from AECs were electrophoresed on a 4-12% SDS-PAGE and transferred on to nitrocellulose membranes. Sera collected from lung transplant recipients at varying time intervals were used to probe the membranes (1:1500 dilutions). The figure presented represents epithelial reactivity observed in one of the BOS+ patient with epithelial reactivity. No reactivity was observed immediately post-transplant immediately post-transplant. But, presence of antibody was detected starting at 11 months after transplant and remained constant up to and even after BOS development.
Kinetics of anti-epithelial antibody development
To investigate the temporal development of the non-HLA anti-AEC Abs relative to lung allograft transplantation, serial serum samples collected on the day of transplant to time of BOS diagnosis were analyzed by western blot using purified K-α1 tubulin. A representative result is given in figure 5. As seen in Fig 5, serum from the day of transplant and three months following transplant did not bind to the AECs (Data not shown) and did not recognize the 48Kda antigen of AEC by western blot. However, the several other post transplant serum samples beginning from post-transplant months 11, 14, 16, 17, 19, 20, 21 and 23 showed recognition of the 48 kDa protein of the AEC. It is of significance that clinical diagnosis of BOS was only made 20 months post-transplantation whereas reactivity to AEC antigen was seen beginning 11th month. These results indicate that the development of anti-epithelial antibody precedes the development of BOS in a subset of lung transplant recipients.
Figure 5. Molecular characterization of epithelial antigen by western blot analysis.

Total protein extracts from AECs were electrophoresed on a 4-12% SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were probed with BOS+ sera and BOS-sera(1:1500 dilution). Membrane bound Abs were detected with HRP-conjugated goat anti-human antibody and developed with chemiluminescent substrate. BOS+ sera with epithelial reactivity recognized a 48 kDa polypeptide. Membrane strips represent one of the BOS+ sera with epithelial reactivity (A) and one from BOS- patient(B).
Recognition of purified K-α1 tubulin protein by BOS+ sera
In order to further confirm the identity of epithelial antigen recognized as the BOS+ sera as K-α1 tubulin, we performed western blot analysis using a purified recombinant K-α1 tubulin protein. Western blot analysis demonstrated that the purified K-α1 tubulin protein was recognized by the BOS+ sera with epithelial reactivity (figure 6 A). However no reactivity was observed against the purified K-α1 tubulin using sera from BOS+ patients with no reactivity to AECs, sera from BOS- patients and normal human sera. (Fig. 6A). In order to further confirm this observation two strips of K-α1 tubulin were blocked with mouse anti-K-α1 tubulin antibody or control antibody and probed with BOS+ sera with epithelial reactivity. As seen in Fig 6B, blocking with K-α1 tubulin specific antibody abolished the immunoreactivity of the BOS+ sera. These observations further confirmed that the post transplant sera from lung transplant recipients with epithelial specific are indeed recognizing K-α1 tubulin.
Figure 6. The epithelial antigen recognized by BOS+ serum reactivity is K-α1 tubulin protein.
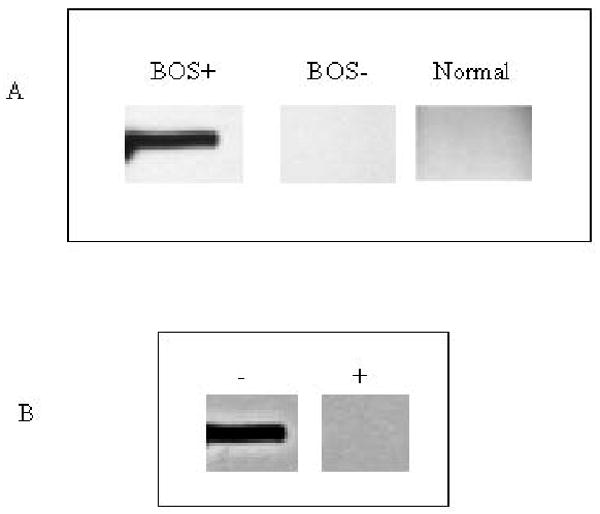
Purified recombinant K-α1 tubulin protein was electrophoresed and transferred onto NCP and probed with BOS+ sera and BOS- sera. The figure shows a representative BOS+ serum with epithelial reactivity binding to the purified K-α1 tubulin protein whereas no reactivity was observed with BOS- sera (A). In order to further confirm, two strips with the purified recombinant K-α1 tubulin protein were blocked with anti-K-α1 tubulin antibody (+) or control antibody (-) and probed with BOS sera with AEC reactivity. Blocking with specific antibody resulted in abolition of BOS sera reactivity with epithelial cells (B).
Sera from BOS+ lung transplant recipients with detectable anti-HLA Abs also recognize K-α1 tubulin
Development of anti-HLA Abs to the mismatched donor HLA antigens has been shown to correlate with the development of BOS in human lung transplant recipients. However reactivity of the sera containing anti-HLA Abs to other antigens encoded by non-MHC have been difficult to analyze due to several reasons including low titer and lack of purified proteins in question. In order to test the presence anti-K-α1 tubulin Abs in the sera from BOS+ sera from lung transplant recipients with ant-HLA antibody we performed western blot analysis with purified k-α1 tubulin protein. Western blot analysis with purified K-α1 tubulin protein demonstrated that 5 of 10 BOS+ sera with anti-HLA Abs also recognized the K-α1 tubulin protein. Therefore, approximately 50% of lung transplant recipients with anti-HLA Abs also produce Abs to the autoantigen K α1 tubulin.
Discussion
Recent studies have suggested a prominent role for Abs developed during the post transplant period to both mismatched donor HLA antigens as well as non-HLA antigens in allograft rejection (31-34). Ability to detect complement deposition and activation using C4d staining have increased the awareness of humoral immune mechanism in the pathogenesis of both acute as well as chronic rejection (35-38). Development of Abs against the donor mismatched HLA antigens has been shown to correlate with the development of chronic rejection in transplant recipients and the development of antibody has been shown to precede clinical evidence of chronic rejection (21, 23, 25, 27, 39-41). Studies from our laboratory (23, 39) and others (24, 33, 42) have demonstrated that post transplant development of anti-HLA Abs correlate with development of BOS following human lung transplantation. However, a substantial proportion of the transplant recipients despite the absence of development of any detectable Abs against donor mismatched HLA antigens undergo chronic rejection and in many of these cases antibody as well as complement deposition has been observed in their grafts (43-46). In the case of kidney and liver allografts, an increasing number of studies have emphasized the clinical importance of Abs against non-HLA antigens(16, 28-30)in the pathogenesis of rejection. In cardiac transplant, autoAbs against the vimentin molecule has been shown to cause accelerated onset of allograft vasculopathy (47, 48). In kidney allografts, Dragun and associates identified that the non-HLA Abs directed to angiotensin II Type 1 receptor was the target of which results in refractory vascular rejection of the graft (29).
In this study we have identified post transplant development of Abs against the K-α1 tubulin in lung transplant recipients with clinical evidence of BOS. This agrees with the emerging data that tissue remodeling following transplantation can expose cryptic self antigens which can become target of immune recognition leading to rejection (49-52). Recent studies by Wilkes and Burlingham have demonstrated that immune responses against collagen V, an auto antigen, can induce obliterative airway disease in rats following lung transplantation (49, 53) and may also play a pathogenic role in BOS following human lung transplantation (52). Our studies have shown that collagen V reactive cells develop during post lung transplantation in humans and they are regulated by IL-10 producing T regulatory cells (39). However, with time, T regulatory cell population are depleted leading to increased allo as well as autoreactivity against the transplanted organ leading to BOS following human lung transplantation (39).
In lung allografts immune responses targeted against the epithelial cells have been thought to play a crucial role in chronic rejection of the transplanted lungs (8, 11, 16, 54-56). Using primary airway epithelial cell as targets we analyzed the presence of anti-epithelial Abs in a subset of lung transplant recipients with no detectable anti-HLA Abs by both complement dependent assays as well as solid phase assays (ELISA and Flow cytometry) for the detection of Abs against HLA. Our results indicate that a subset of BOS+, anti-HLA antibody-negative patients status post lung transplantation (33%) develop Abs against a cell surface antigen expressed on AECs. Moreover we also observed that 5 of the 10 BOS+ patients with anti-HLA Abs also recognized the antigen expressed on the AECs. Therefore, a large proportion of lung transplant recipients with BOS (37%) develop Abs reactive to AECs. Epithelial specificity of the sera from the lung transplant recipients with anti-epithelial reactivity is clearly evident since these sera did not react against peripheral blood mononuclear cells covering most of the well defined HLA specificities and also was non-reactive to endothelial cells and tubular epithelial cells of kidney origin. This study complements other earlier reports which has demonstrated that lung transplant recipients with pre-existing anti-epithelial Abs have an increased rate of lung allograft rejection in 3 months compared to controls (57) and approximately 32% of lung transplant recipients with BOS develop Abs specific for AECs (57). In addition, anti-epithelial cell Abs have been suggested to play an important role in the rejection of both human cardiac and renal transplants (58-61). In this study, we demonstrated that development of the anti-epithelial antibody preceded the clinical diagnosis of BOS by approximately 9 to 12 months. The observation that anti-epithelial antibody precedes the development of BOS is in concurrence with the observations that anti-HLA antibody development also precedes the clinical onset of BOS (21). These findings provide credence to the view that antibody may play a pathogenic role and immune monitoring during the post transplant period may define human lung transplant recipients who are at higher risk for the development of BOS. Further, it is likely that early identification of this cohort of patients may allow change in immunosuppression which is directed to humoral immunity against the allograft.
BOS is associated histologically with epithelial injury, bronchocentric mononuclear inflammation, fibrosis and proliferation of the lamina propria and obliteration of small airways (62). Gene array and luminex assay results provided in this paper indicated a 4-fold upregulation of heat shock proteins (HSPs) 27 and 90 and constituents of the calcium homeostasis pathway and a 2-fold upregulation in transcription factors TCF5 and cMyc. There was also a 2,3, and 6-fold increase in HB-EGF, TGF-β, and VEGF production by AECs, respectively, after exposure to the epithelial cell specific sera from BOS+ lung transplant recipients. These results strongly suggest that the binding of the antibody to K- α 1 tubulin to AEC activates a PKC-driven calcium maintenance pathway that is regulated by HSPs 27 and 90. These pathways can culminate in increased cellular mitosis, proliferation and growth factor production (Figure 8). It is of significance that TGF-β, PDGF and ET-1 have been found to be significantly elevated in human lung transplant recipient with chronic rejection and VEGF have been shown to be elevated in rat allograft models of rejection (1, 63-65). TCF5 or CCAAT/enhancer binding protein (C/EBP), beta is bZIP transcriptions factor that can homodimerize and bind to DNA regulatory regions. TCF5 has been shown to play an important role in the regulation of immune and inflammatory response genes. It has been shown to bind to the IL-1 response element in the IL-6 gene, as well as to regulatory regions of several acute-phase and cytokine genes (66). Earlier studies in the literature have also demonstrated a novel role for C/EBP-beta in interleukin-1beta-induced connective tissue disease (67). TCF5 has also been shown to bind the promoter and upstream element of collagen gene and stimulates its production (68). Taken together it is reasonable to postulate that ligation of AEC K- α1 tubulin by specific Abs can result in growth factor production, stimulation of collagen production as well as cytokine gene upregulation which are considered as central events in the immunopathogenesis of BOS.
Since a large proportion of lung transplant recipients with BOS reacted to AECs and it was evident that the reactivity was not directed to mismatched donor HLA antigens. Therefore, we set out to determine biochemical and molecular nature of the antigen defined using Abs specific for AEC. Western blot analysis of the crude airway epithelial cell extracts with patient sera demonstrating epithelial reactivity identified a ∼48 kDa protein. Further sequencing and BLAST analysis of the eluted ∼48kDa protein showed a 100% homology to the K-α1 tubulin protein. Thus, for the first time, we were able to define the biochemical and molecular nature of the AEC antigen defined by the sera from post lung transplantation. Western blot analysis of purified K-α1 tubulin protein with sera from patients with anti-epithelial antibody further confirmed that the identified protein is K-α1 tubulin. K-α1 tubulin is a ∼50 kDa protein in its usual poly glycosylated, post-translational form. It is found in cells as one of six different isotypes. Its usual cellular functions include GTP binding, GTPase activity, maintaining cellular structure in the form of microtubules, and microtubule-based intracellular movement. Most of these known functions would indicate that K-α1 tubulin is not prone to act at the cell surface. However, earlier studies have identified autoAbs against K-α1 tubulin in small cell lung cancer and breast cancer(69), relapsing polychondritis (70) and post-cardiac transplant fatal cardiomyopathy (71) indicating that this protein can reach the cell surface and are immunogenic under selected circumstances. This concept has been verified in recent studies demonstrating that the intermediate filament proteins are expressed on the cell surface following activation or apoptosis. The alternate hypothesis is that the indolent inflammatory environment that is present in the post-transplant period may cause an injury-recovery pattern at the level of the airway epithelium. This form of chronic injury may lead to destruction and turnover of the AECs, thereby exposing AEC proteins to the surveillance immune system with resultant antibody production.
In conclusion we have demonstrated that a large proportion (37%) of lung transplant recipients develops anti-K-α1 tubulin Abs during the post transplant period and this is strongly associated with the development of BOS or chronic rejection of the lung allograft. We further demonstrate that binding of the anti-K-α1 tubulin to the epithelial cells resulted in the increased expression of TCF5, a transcription factor involved in the regulation of inflammatory response genes and fibro-proliferation cascade. The increased levels of TCF5 and c-Myc can result in the increased expression of fibrogenic growth factors, HB-EGF, TGF-β, and VEGF, all of which can initiate and sustain the fibro-proliferation cascade, a central event in the immunopathogenesis of BOS following human lung transplantation.
Acknowledgments
This work was supported by NIH HL56543 (TM), Training Grant NIH 5T32AI07163 (TG).
References
- 1.Reinsmoen NL, Bolman RM, Savik K, Butters K, Hertz MI. Are multiple immunopathogenetic events occurring during the development of obliterative bronchiolitis and acute rejection? Transplantation. 1993;55:1040–1044. doi: 10.1097/00007890-199305000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Belperio JA, Keane MP, Burdick MD, Lynch JP, 3rd, Xue YY, Li K, Ross DJ, Strieter RM. Critical role for CXCR3 chemokine biology in the pathogenesis of bronchiolitis obliterans syndrome. J Immunol. 2002;169:1037–1049. doi: 10.4049/jimmunol.169.2.1037. [DOI] [PubMed] [Google Scholar]
- 3.Allen MD, Burke CM, McGregor CGA, Baldwin JC, Jamieson SW, Theodore J. Steroid-responsive bronchiolitis after human heart-lung transplantation. J Thorac Cardiovasc Surg. 1986;92:449–451. [PubMed] [Google Scholar]
- 4.Knoop C, Estenne M. Acute and chronic rejection after lung transplantation. Semin Respir Crit Care Med. 2006;27:521–533. doi: 10.1055/s-2006-954609. [DOI] [PubMed] [Google Scholar]
- 5.Wilkes DS, Egan TM, Reynolds HY. Lung transplantation: opportunities for research and clinical advancement. Am J Respir Crit Care Med. 2005;172:944–955. doi: 10.1164/rccm.200501-098WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Judson MA. Clinical aspects of lung transplantation. Clin Chest Med. 1993;14:335–357. [PubMed] [Google Scholar]
- 7.Al-Githmi I, Batawil N, Shigemura N, Hsin M, Lee TW, He GW, Yim A. Bronchiolitis obliterans following lung transplantation. Eur J Cardiothorac Surg. 2006;30:846–851. doi: 10.1016/j.ejcts.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 8.Jaramillo A, Fernandez FG, Kuo EY, Trulock EP, Patterson GA, Mohanakumar T. Immune mechanisms in the pathogenesis of bronchiolitis obliterans syndrome after lung transplantation. Pediatr Transplant. 2005;9:84–93. doi: 10.1111/j.1399-3046.2004.00270.x. [DOI] [PubMed] [Google Scholar]
- 9.Stewart KC, Patterson GA. Current trends in lung transplantation. Am J Transplant. 2001;1:204–210. doi: 10.1034/j.1600-6143.2001.001003204.x. [DOI] [PubMed] [Google Scholar]
- 10.Bernardi P, Delsedime L, Bellis D, Mollo F. Atypical changes of respiratory epithelium after heart-lung transplantation. A case report. Pathol Res Pract. 1989;184:514–518. doi: 10.1016/S0344-0338(89)80144-X. [DOI] [PubMed] [Google Scholar]
- 11.Mauck KA, Hosenpud JD. The bronchial epithelium: a potential allogeneic target for chronic rejection after lung transplantation. J Heart Lung Transplant. 1996;15:709–714. [PubMed] [Google Scholar]
- 12.Spurzem JR, Sacco O, Rossi GA, Beckmann JD, Rennard SI. Regulation of major histocompatibility complex class II gene expression on bovine bronchial epithelial cells. J Lab Clin Med. 1992;120:94–102. [PubMed] [Google Scholar]
- 13.Kubota H, Yagyu K, Otsuka T, Takeshita M, Furuse A. Importance of bronchus-associated lymphoid tissue and major histocompatibility complex class I and class II antigen expression on bronchial epithelium in acute lung allograft rejection and lung infection in rats. Transplant Proc. 1994;26:1856–1858. [PubMed] [Google Scholar]
- 14.Elssner A, Jaumann F, Dobmann S, Behr J, Schwaiblmair M, Reichenspurner H, Furst H, Briegel J, Vogelmeier C. Elevated levels of interleukin-8 and transforming growth factor-beta in bronchoalveolar lavage fluid from patients with bronchiolitis obliterans syndrome: proinflammatory role of bronchial epithelial cells. Munich Lung Transplant Group. Transplantation. 2000;70:362–367. doi: 10.1097/00007890-200007270-00022. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez FG, Jaramillo A, Chen C, Liu DZ, Tung T, Patterson GA, Mohanakumar T. Airway epithelium is the primary target of allograft rejection in murine obliterative airway disease. Am J Transplant. 2004;4:319–325. doi: 10.1111/j.1600-6143.2004.00333.x. [DOI] [PubMed] [Google Scholar]
- 16.Jaramillo A, Naziruddin B, Zhang L, Reznik SI, Smith MA, Aloush AA, Trulock EP, Patterson GA, Mohanakumar T. Activation of human airway epithelial cells by non-HLA Abs developed after lung transplantation: a potential etiological factor for bronchiolitis obliterans syndrome. Transplantation. 2001;71:966–976. doi: 10.1097/00007890-200104150-00023. [DOI] [PubMed] [Google Scholar]
- 17.Jaramillo A. Anti-HLA class I antibody binding to airway epithelial cells induces production of fibrogenic growth factors and apoptotic cell death: a possible mechanism for bronchiolitis obliterans syndrome. Hum Immunol. 2003;64:521–529. doi: 10.1016/s0198-8859(03)00038-7. [DOI] [PubMed] [Google Scholar]
- 18.Langenbach SY, Zheng L, McWilliams T, Levvey B, Orsida B, Bailey M, Williams TJ, Snell GI. Airway vascular changes after lung transplant: potential contribution to the pathophysiology of bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2005;24:1550–1556. doi: 10.1016/j.healun.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Aris RM, Walsh S, Chalermskulrat W, Hathwar V, Neuringer IP. Growth factor upregulation during obliterative bronchiolitis in the mouse model. Am J Respir Crit Care Med. 2002;166:417–422. doi: 10.1164/rccm.2102106. [DOI] [PubMed] [Google Scholar]
- 20.Shimada J, Fushiki S, Tsujimura A, Oka T. Fibroblast growth factor-2 expression is up-regulated after denervation in rat lung tissue. Brain Res Mol Brain Res. 1997;49:295–298. doi: 10.1016/s0169-328x(97)00208-8. [DOI] [PubMed] [Google Scholar]
- 21.Jaramillo A, Smith MA, Phelan D, Sundaresan S, Trulock E, Lynch J, Cooper J, Patterson GA, Mohanakumar T. Temporal relationship between the development of anti-HLA Abs and the development of bronchiolitis obliterans syndrome after lung transplantation. Transplantation Proceedings. 1998 doi: 10.1016/s0041-1345(98)01495-x. [DOI] [PubMed] [Google Scholar]
- 22.Jaramillo A, Smith MA, Phelan D, Sundaresan RS, Trulock EP, Lynch JP, Cooper JD, Patterson GA, Mohanakumar T. Development of ELISA-detected anti-HLA Abs precedes the development of bronchiolitis obliterans syndrome and correlates with progressive decline in pulmonary function after lung transplantation. Transplantation. 1999;67:1155–1161. doi: 10.1097/00007890-199904270-00012. [DOI] [PubMed] [Google Scholar]
- 23.Sundaresan S, Mohanakumar T, Smith MA, Trulock EP, Lynch J, Phelan D, Cooper JD, Patterson GA. HLA-A locus mismatches and development of Abs to HLA after lung transplantation correlate with the development of bronchiolitis obliterans syndrome. Transplantation. 1998;65:648–653. doi: 10.1097/00007890-199803150-00008. [DOI] [PubMed] [Google Scholar]
- 24.Scornik JC, Zander DS, Baz MA, Donnelly WH, Staples ED. Susceptibility of lung transplants to preformed donor-specific HLA Abs as detected by flow cytometry. Transplantation. 1999;68:1542–1546. doi: 10.1097/00007890-199911270-00018. [DOI] [PubMed] [Google Scholar]
- 25.Di Filippo S, Girnita A, Webber SA, Tsao S, Boyle GJ, Miller SA, Gandhi SK, Zeevi A. Impact of ELISA-detected anti-HLA Abs on pediatric cardiac allograft outcome. Hum Immunol. 2005;66:513–518. doi: 10.1016/j.humimm.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Girnita AL, Duquesnoy R, Yousem SA, Iacono AT, Corcoran TE, Buzoianu M, Johnson B, Spichty KJ, Dauber JH, Burckart G, et al. HLA-specific Abs are risk factors for lymphocytic bronchiolitis and chronic lung allograft dysfunction. Am J Transplant. 2005;5:131–138. doi: 10.1111/j.1600-6143.2004.00650.x. [DOI] [PubMed] [Google Scholar]
- 27.Reinsmoen NL, Nelson K, Zeevi A. Anti-HLA antibody analysis and crossmatching in heart and lung transplantation. Transpl Immunol. 2004;13:63–71. doi: 10.1016/j.trim.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Dubel L, Farges O, Sato Y, Bismuth H. Development of anti-tissue Abs in the rat liver transplant model. Transplantation. 1998;65:1135–1137. doi: 10.1097/00007890-199804270-00022. [DOI] [PubMed] [Google Scholar]
- 29.Dragun D. Angiotensin II type 1-receptor activating Abs in renal-allograft rejection. N Engl J Med. 2005;352:558–569. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 30.Behr TM. Detection of humoral rejection in human cardiac allografts by assessing the capillary deposition of complement fragment C4d in endomyocardial biopsies. J Heart Lung Transplant. 1999;18:904–912. doi: 10.1016/s1053-2498(99)00043-1. [DOI] [PubMed] [Google Scholar]
- 31.Iniotaki-Theodoraki A. The role of HLA class I and class II Abs in renal transplantation. Nephrol Dial Transplant. 2001;16 6:150–152. doi: 10.1093/ndt/16.suppl_6.150. [DOI] [PubMed] [Google Scholar]
- 32.Sumitran S. Clinical importance of HLA-specific and non-HLA-specific Abs in allogeneic kidney transplantation. Adv Nephrol Necker Hosp. 2000;30:29–39. [PubMed] [Google Scholar]
- 33.McKenna RM, Takemoto SK, Terasaki PI. Anti-HLA Abs after solid organ transplantation. Transplantation. 2000;69:319–326. doi: 10.1097/00007890-200002150-00001. [DOI] [PubMed] [Google Scholar]
- 34.Moraes JR, Moraes ME, Luo Y, Peters PC, Sagalowsky A, Dawidson I, Lu C, Stastny P. Crossmatch with donor skin: characterization of alloAbs and correlation with early kidney rejection. Transplant Proc. 1991;23:471–472. [PubMed] [Google Scholar]
- 35.Feucht HE. Vascular deposition of complement-split products in kidney allografts with cell-mediated rejection. Clin Exp Immunol. 1991;86:464–470. doi: 10.1111/j.1365-2249.1991.tb02954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collins AB. Complement activation in acute humoral renal allograft rejection: diagnostic significance of C4d deposits in peritubular capillaries. J Am Soc Nephrol. 1999;10:2208–2214. doi: 10.1681/ASN.V10102208. [DOI] [PubMed] [Google Scholar]
- 37.Mauiyyedi S. Chronic humoral rejection: identification of antibody-mediated chronic renal allograft rejection by C4d deposits in peritubular capillaries. J Am Soc Nephrol. 2001;12:574–582. doi: 10.1681/ASN.V123574. [DOI] [PubMed] [Google Scholar]
- 38.Sijpkens YW. Immunologic risk factors and glomerular C4d deposits in chronic transplant glomerulopathy. Kidney Int. 2004;65:2409–2418. doi: 10.1111/j.1523-1755.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- 39.Bharat A, Narayanan K, Street T, Fields RC, Steward N, Aloush A, Meyers B, Schuessler R, Trulock EP, Patterson GA, et al. Early posttransplant inflammation promotes the development of alloimmunity and chronic human lung allograft rejection. Transplantation. 2007;83:150–158. doi: 10.1097/01.tp.0000250579.08042.b6. [DOI] [PubMed] [Google Scholar]
- 40.Hadjiliadis D, Chaparro C, Reinsmoen NL, Gutierrez C, Singer LG, Steele MP, Waddell TK, Davis RD, Hutcheon MA, Palmer SM, et al. Pre-transplant panel reactive antibody in lung transplant recipients is associated with significantly worse post-transplant survival in a multicenter study. J Heart Lung Transplant. 2005;24:S249–254. doi: 10.1016/j.healun.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 41.Girnita AL, McCurry KR, Iacono AT, Duquesnoy R, Corcoran TE, Awad M, Spichty KJ, Yousem SA, Burckart G, Dauber JH, et al. HLA-specific Abs are associated with high-grade and persistent-recurrent lung allograft acute rejection. J Heart Lung Transplant. 2004;23:1135–1141. doi: 10.1016/j.healun.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 42.Schulman LL, Ho EK, Reed EF, McGregor C, Smith CR, Rose EA, Suciu-Foca NM. Immunologic monitoring in lung allograft recipients. Transplantation. 1996;61:252–257. doi: 10.1097/00007890-199601270-00016. [DOI] [PubMed] [Google Scholar]
- 43.Schulman LL, Ho E, Reed EF, McGregor C, Smith CR, Rose EA, Suciu-Foca NM. Immunologic monitoring in lung allograft recipients. Transplantation. 1996;61:252–257. doi: 10.1097/00007890-199601270-00016. [DOI] [PubMed] [Google Scholar]
- 44.Diujvestijn AM, Derhaag JG, van Breda Vriesman PJ. Complement activation by anti-endothelial cell Abs in MHC-mismatched and MHC-matched heart allograft rejection: anti-MHC-, but not anti non-MHC alloAbs are effective in complement activation. Transpl Int. 2000;13:363–371. doi: 10.1007/s001470050715. [DOI] [PubMed] [Google Scholar]
- 45.Derhaag JG, Duijvestijn AM, Damoiseaux JG, van Breda Vriesman PJ. Effects of antibody reactivity to major histocompatibility complex (MHC) and non-MHC alloantigens on graft endothelial cells in heart allograft rejection. Transplantation. 2000;69:1899–1906. doi: 10.1097/00007890-200005150-00027. [DOI] [PubMed] [Google Scholar]
- 46.Joyce S, Mathew JM, Flye MW, Mohanakumar T. A polymorphic human kidney-specific non-MHC alloantigen. Its possible role in tissue-specific allograft immunity. Transplantation. 1992;53:1119–1127. doi: 10.1097/00007890-199205000-00027. [DOI] [PubMed] [Google Scholar]
- 47.Carter V, Shenton BK, Jaques B, Turner D, Talbot D, Gupta A, Chapman CE, Matthews CJ, Cavanagh G. Vimentin Abs: a non-HLA antibody as a potential risk factor in renal transplantation. Transplant Proc. 2005;37:654–657. doi: 10.1016/j.transproceed.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 48.Rose ML. Anti-vimentin Abs are an independent predictor of transplant-associated coronary artery disease following cardiac transplantation. Ital Heart J. 2001;2 3:23S–25S. [PubMed] [Google Scholar]
- 49.Wilkes DS. The role of autoimmunity in the pathogenesis of lung allograft rejection. Arch Immunol Ther Exp (Warsz) 2003;51:227–230. [PubMed] [Google Scholar]
- 50.Fedoseyeva EV, Zhang F, Orr PL, Levin D, Buncke HJ, Benichou G. De novo autoimmunity to cardiac myosin after heart transplantation and its contribution to the rejection process. J Immunol. 1999;162:6836–6842. [PubMed] [Google Scholar]
- 51.Benichou G, Alessandrini A, Charrad RS, Wilkes DS. Induction of autoimmunity after allotransplantation. Front Biosci. 2007;12:4362–4369. doi: 10.2741/2393. [DOI] [PubMed] [Google Scholar]
- 52.Haque MA, Mizobuchi T, Yasufuku K, Fujisawa T, Brutkiewicz RR, Zheng Y, Woods K, Smith GN, Cummings OW, Heidler KM, et al. Evidence for immune responses to a self-antigen in lung transplantation: role of type V collagen-specific T cells in the pathogenesis of lung allograft rejection. J Immunol. 2002;169:1542–1549. doi: 10.4049/jimmunol.169.3.1542. [DOI] [PubMed] [Google Scholar]
- 53.Yoshida S, Haque A, Mizobuchi T, Iwata T, Chiyo M, Webb TJ, Baldridge LA, Heidler KM, Cummings OW, Fujisawa T, et al. Anti-type V collagen lymphocytes that express IL-17 and IL-23 induce rejection pathology in fresh and well-healed lung transplants. Am J Transplant. 2006;6:724–735. doi: 10.1111/j.1600-6143.2006.01236.x. [DOI] [PubMed] [Google Scholar]
- 54.Qu N, de Haan A, Harmsen MC, Kroese FG, de Leij LF, Prop J. Specific immune responses against airway epithelial cells in a transgenic mouse-trachea transplantation model for oblterative airway disease. Transplantation. 2003;76:1022–1028. doi: 10.1097/01.TP.0000080607.28324.A9. [DOI] [PubMed] [Google Scholar]
- 55.Kuo E, Bharat A, Shih J, Street T, Norris J, Liu W, Parks W, Walter M, Patterson GA, Mohanakumar T. Role of airway epithelial injury in murine orthotopic tracheal allograft rejection. Ann Thorac Surg. 2006;82:1226–1233. doi: 10.1016/j.athoracsur.2006.03.122. [DOI] [PubMed] [Google Scholar]
- 56.Zissel G, Ernst M, Rabe K, Papadopoulos T, Magnussen H, Schlaak M, Muller-Quernheim J. Human alveolar epithelial cells Type II are capable of regulating T-cell activity. J Investig Med. 2000;48:66–75. [PubMed] [Google Scholar]
- 57.Tay GK, Witt CS, Christiansen FT, Charron D, Baker D, Herrmann R, Smith LK, Diepeveen D, Mallal S, McCluskey J, et al. Matching for MHC haplotypes results in improved survival following unrelated bone marrow transplantation. Bone Marrow Transplant. 1995;15:381–385. [PubMed] [Google Scholar]
- 58.Harmer AW, Haskard D, Koffman CG, Welsh KI. Novel Abs associated with unexplained loss of renal allografts. Transpl Int. 1990;3:66–69. doi: 10.1007/BF00336205. [DOI] [PubMed] [Google Scholar]
- 59.al-Hussein KA, Talbot D, Proud G, Taylor RM, Shenton BK. The clinical significance of post-transplantation non-HLA Abs in renal transplantation. Transpl Int. 1995;8:214–220. doi: 10.1007/BF00336540. [DOI] [PubMed] [Google Scholar]
- 60.Ferry BL, Welsh KI, Dunn MJ, Law D, Proctor J, Chapel H, Yacoub MH, Rose ML. Anti-cell surface endothelial Abs in sera from cardiac and kidney transplant recipients: association with chronic rejection. Transpl Immunol. 1997;5:17–24. doi: 10.1016/s0966-3274(97)80021-4. [DOI] [PubMed] [Google Scholar]
- 61.Suberbielle Boissel C, Mirebeau D, Legendre C, Kssentini M, Kreis H, Charron D, Raffoux C. Target antigens of post-rejection non-HLA Abs in renal transplantation. Transplant Proc. 1998;30:2853. doi: 10.1016/s0041-1345(98)00839-2. [DOI] [PubMed] [Google Scholar]
- 62.Martinu T, H D, Davis RD, Steele MP, Palmer SM. Pathologic correlates of bronchiolitis obliterans syndrome in pulmonary retransplant recipients. Chest. 2006;129:1016–1023. doi: 10.1378/chest.129.4.1016. [DOI] [PubMed] [Google Scholar]
- 63.Hirabayashi T, Demertzis S, Schafers J, Hoshino K, Nashan B. Chronic rejection in lung allografts: immunohistological analysis of fibrogenesis. Transpl Int. 1996;9:S293–S295. doi: 10.1007/978-3-662-00818-8_72. [DOI] [PubMed] [Google Scholar]
- 64.Meyer KC, C A, Ziang Z, Cornwell RD, Love RB. Vascular endothelial growth factor in human lung transplantation. Chest. 2001;119:137–143. doi: 10.1378/chest.119.1.137. [DOI] [PubMed] [Google Scholar]
- 65.Krebs R, T J, Nykanen AI, Wood J, Jeltsch M, Yia-Herttuala S, Koskinen PK, Lemstrom KB. Dual role of vascular endothelial growth factor in experimental obliterative bronchiolitis. Am J Respir Crit Care Med. 2005;171:1421–1429. doi: 10.1164/rccm.200408-1001OC. [DOI] [PubMed] [Google Scholar]
- 66.Poli V, Mancini FP, Cortese R. IL-6DBP, a nuclear protein involved in interleukin-6 signal transduction, defines a new family of leucine zipper proteins related to C/EBP. Cell. 1990;63:643–653. doi: 10.1016/0092-8674(90)90459-r. [DOI] [PubMed] [Google Scholar]
- 67.Raymond L, Eck S, Mollmark J, Hays E, Tomek I, Kantor S, Elliott S, Vincenti M. Interleukin-1 beta induction of matrix metalloproteinase-1 transcription in chondrocytes requires ERK-dependent activation of CCAAT enhancer-binding protein-beta. J Cell Physiol. 2006;207:683–688. doi: 10.1002/jcp.20608. [DOI] [PubMed] [Google Scholar]
- 68.Attard FA, Wang L, Potter JJ, Rennie-Tankersley L, Mezey E. CCAAT/enhancer binding protein beta mediates the activation of the murine alpha1(I) collagen promoter by acetaldehyde. Arch Biochem Biophys. 2000;378:57–64. doi: 10.1006/abbi.2000.1803. [DOI] [PubMed] [Google Scholar]
- 69.Rao K, Lund RD, Kunz HW, Gill TJ., 3rd The role of MHC and non-MHC antigens in the rejection of intracerebral allogeneic neural grafts. Transplantation. 1989;48:1018–1021. doi: 10.1097/00007890-198912000-00025. [DOI] [PubMed] [Google Scholar]
- 70.Tanaka Y, Nakamura M, Matsui T, Iizuka N, Kondo H, Tohma S, Masuko K, Yudoh K, Nakamura H, Nishioka K, et al. Proteomic surveillance of autoantigens in relapsing polychondritis. Microbiol Immunol. 2006;50:117–126. doi: 10.1111/j.1348-0421.2006.tb03776.x. [DOI] [PubMed] [Google Scholar]
- 71.Hein S, S T, Schaper J. Ischemia induces early changes to cytoskeletal and contractile proteins in diseased human myocardium. J Thorac Cardiovasc Surg. 1995;110:89. doi: 10.1016/S0022-5223(05)80013-3. [DOI] [PubMed] [Google Scholar]


