Abstract
Florida red tides, an annual event off the west coast of Florida, are caused by the toxic dinoflagellate, Karenia brevis. K. brevis produces a suite of potent neurotoxins, brevetoxins, which kill fish, sea birds, and marine mammals, as well as sickening humans who consume contaminated shellfish. These toxins become part of the marine aerosol, and can also be inhaled by humans and other animals. Recent studies have demonstrated a significant increase in symptoms and decrease lung function in asthmatics after only one hour of beach exposure during an onshore Florida red tide bloom.
This study constructed a transect line placing high volume air samplers to measure brevetoxins at sites beginning at the beach, moving approximately 6.4 km inland. One non-exposure and 2 exposure studies, each of 5 days duration, were conducted. No toxins were measured in the air during the non-exposure period. During the 2 exposure periods, the amount of brevetoxins varied considerably by site and by date. Nevertheless, brevetoxins were measured at least 4.2 kilometers from the beach and/or 1.6 km from the coastal shoreline. Therefore, populations sensitive to brevetoxins (such as asthmatics) need to know that leaving the beach may not discontinue their environmental exposure to brevetoxin aerosols.
Keywords: brevetoxins, harmful algal blooms (HABs), red tides, Karenia brevis, asthma, air monitoring
1.0 Introduction
Florida red tides are an annual event off the West Coast of Florida and throughout the Gulf of Mexico, caused by blooms of the toxic dinoflagellate, Karenia brevis (K. brevis). K. brevis produces a suite of potent neurotoxins, brevetoxins, leading to significant fish, sea bird, and marine mammal mortality. In addition, these toxins are filtered and accumulated by bivalves such as clams and oysters; people who consume contaminated shellfish become ill with neurotoxic shellfish poisoning (NSP) (Baden et al., 1995; Keynes, 1979; Kirkpatrick et al., 2004; Watkins et al., 2008). K. brevis is an unarmored dinoflagellate that can be easily lysed, releasing brevetoxins into the water. Therefore, K. brevis brevetoxins can be incorporated into the marine aerosol (Pierce et al., 1990). When these toxins are inhaled, brevetoxins can cause upper and lower airway symptoms, particularly in people with chronic respiratory illnesses such as asthma (Fleming, et al., 2005; Fleming et al., 2007; Fleming et al., 2009). For many years, the public health message has been that symptoms of exposure to aerosolized Florida red tide would diminish when people left the beach (Steidinger and Baden, 1984; Baden 1983). However, anecdotal reporting from the community during onshore blooms have suggested that this may not be true for all people, particularly those with underlying respiratory diseases (Kirkpatrick et al., 2006, Kirkpatrick et al., 2009). Therefore, both exposure and health effects from brevetoxins in marine aerosols need to be measured not only at the beach, but also inland from the beach.
Red tide aerosols are marine aerosols containing aerosolized brevetoxins produced in the air/water interface and they are transported by onshore wind to coastal areas (Cheng et al., 2005; Pierce et al., 1990). Pierce and Cheng have reported on the methods to collect and quantify the amount of airborne toxins at the beach during an onshore Florida red tide (Cheng et al., 2005; Pierce et al., 2005). In our previous beach studies, brevetoxin levels measured in the air have ranged from 1.32 ng/m3 (Cheng et al., 2005) to as high as 93 ng/m3 (Backer et al., 2003). This study explored whether brevetoxin exposure continues beyond the beach, and if so, how far inland the brevetoxins travel.
2.0 Methods
This study is part of a larger research program investigating the exposures and health effects in humans and animals from aerosolized brevetoxins (Cheng et al., 2005; Fleming, et al 2005a; Fleming, 2005b; Fleming et al., 2007; Fleming et al., 2009; Pierce et al., 2005). In these “beach studies,” environmental sampling was performed during a 3 day period prior to the beginning of this “transect study” at the beach (Figure 1, site A), and then continued throughout the transect study described below. The environmental sampling was performed to establish the presence or absence of a Karenia bloom (via K brevis cell count in the water, and brevetoxin levels in the water and air samples) as described in prior study publications (Cheng et al., 2005, Pierce et al., 2005).
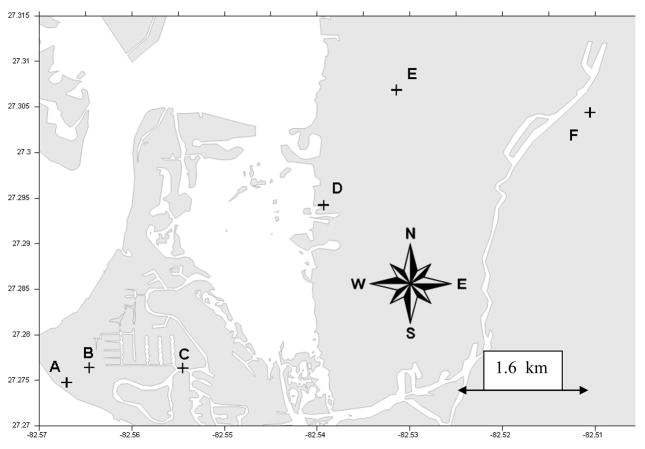
Figure 1.
Inland Transect Aerosol Sampling Locations
2.1 Sampler Locations and Sample Collection
An inland transect line for the placement of the environmental air samplers was established beginning at Siesta Key Beach (Florida) (Site A), moving eastward/inland to a point approximately 6.4 km from the beach study site (Site F). Six high volume samplers (TE-5000, Tisch Environmental Inc., Village of Cleaves, OH, USA) were placed in locations of convenience to provide electrical power for the samplers and ease of changing filters daily (Figure 1). The samplers were fitted with 20 cm × 28 cm glass fiber filters (Whatman EPM 2000, Maidstone, England). Air samples were collected continuously from approximately 8 am to 5 pm daily for a period of 5 days.
2.2 Toxin Analysis
As described in Pierce, et al., (2005), brevetoxins from the marine aerosol were recovered from the glass fiber filters by extraction for 12 h in acetone using a Soxhlet apparatus. The extract was evaporated and transferred to vials for liquid chromatography mass spectroscopy (LC-MS) analysis. Brevetoxin recovery was verified by the addition of standard amounts of brevetoxin PbTx-2 and PbTx-3 to each of the three filters, the filters were run for 4 hours, and subsequently processed for LC-MS analysis.
Brevetoxin analyses were performed by LC-MS using a ThermoFinnigan AqA HPLC/MS obtained from Thermo Electron Corp., Manchester, United Kingdon. The LC consisted of a SpectraSystems, LC Pump P4000, Autosampler AS3000, and a Degasser SCM1000. Mass spectral detection was obtained using an AqA single quad system scanned from 204–1216 AMU with AqA Max 40, and a scan rate of 1.1 scans/second. All analysis was conducted using electrospray with the probe at 3 kV and 250°C. The column was a Phenomenex Security Guard C-18 guard column with a Phenomenex Luna C-18 5Fm 250 mm × 2 mm Analytical Column. The solvent gradient was composed of acidified (0.3% acetic acid) ACN/H2O over 40 minutes. The instrument was calibrated with a standard brevetoxin mix containing PbTx-2 and PbTx-3, obtained from the Center for Marine Science, UNC Wilmington, NC, USA.
3.0 Results
Table 1 lists the environmental conditions and water and air brevetoxin measurements at Site A during the beach studies prior to the 5-day transect studies. Table 2 lists the environmental conditions and air concentration of brevetoxins during the transect studies.
Table 1.
Summary of Beach Studies: Environmental Data
| Date | Temp. (° C) | Humidity (%) | Wind Speed (KPH) | Wind Direction | K. brevis in water (Cells/L)1 | Water Toxin (ug/L) | Air Toxin (ng/m3) |
|---|---|---|---|---|---|---|---|
| Non exposure beach study | |||||||
| October 2004 | 16.0–27.7 | 35–91 | 0–18 | Offshore to Onshore | < 1,000 to 2,000 | < 0.03 | < 0.05 |
| Exposure beach Studies | |||||||
| Feb 2005 | 8.9 – 23.9 | 40 – 97 | 0 – 24 | Offshore | 1,051,000 to 4,639,000 | 13 – 128 | 0.06 to 0.09 |
| March 2005 | 16.7 – 21.0 | 14 – 98 | 0 –19 | Onshore | 59,000 to 200,000 | 2 – 12 | 20 to 38 |
Table 2.
Summary of 5 day Inland transect Studies: Environmental Data
| Date | Temp. (° C) | Humidity (%) | Wind Speed (KPH) | Wind Direction | Air Toxin (ng/m3) |
|---|---|---|---|---|---|
| Non exposure 5 day transect | |||||
| October 2004 | 19.4 – 28.3 | 50 – 91 | 0 – 26 | Offshore to Onshore | < 0.05 |
| Exposure 5 day transect | |||||
| Feb 2005 | 8.3 – 22.8 | 46 – 95 | 0 – 42 | Offshore to Onshore | 0.05 – 7.4 |
| March 2005 | 10.0 – 22.2 | 43 – 95 | 0 – 32 | Offshore to Onshore | 0.05 – 16 |
3.1 Non exposure (control) beach studies
Non-exposure beach studies were defined when no K. brevis cells were measured above detectable levels (<1,000cells/L) in the beach waters at Site A (see Figure 1) and were conducted from October 16–18, 2004. Additionally, no brevetoxins were detected in the water or aerosol samples above the limits of detection by LC-MS (<0.05 ng/m3 air, 0.03 μg/L water) as shown in Table 1.
3.2 Exposure beach studies
Exposure beach studies were defined as K. brevis red tide events (cell counts >1,000 cells/L), and were conducted from February 4–6, 2005 and March 11–14, 2005. A summary of the cell counts from water samples and the total brevetoxins detected in water and aerosol samples for the beach studies along with K. brevis cell counts is presented in Table 1. In the beach study of February 4–6, 2005, the cell counts were above 1×106/L, and the brevetoxin concentrations in the water were also high in the range of 13–128 μg/L. However, the air concentrations of brevetoxin were barely detectable (0.06–0.09 ng/m3) because the wind direction during the 3-day beach study periods was offshore. During the exposure beach study of March 11–14, 2005, both the cell counts (59,000 to 200,000 cells/L) and the water concentrations of brevetoxins (2–12 μg/L) were at moderate levels, but the air concentrations of brevetoxin at Siesta Beach were moderate high at 20 to 38 ng/m3.
3.2. Inland transect studies
The analysis of total brevetoxin concentrations in the aerosol samples collected during the October 19 – 23, 2004 inland transect study indicated no detectable levels (<0.05 ng/m3) at any of the sample locations as shown in Table 2. The 5 day non-exposure data are not shown graphically since the levels of brevetoxin were below the limit of detection.
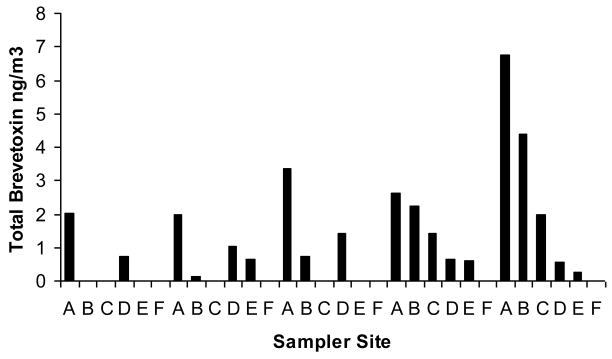
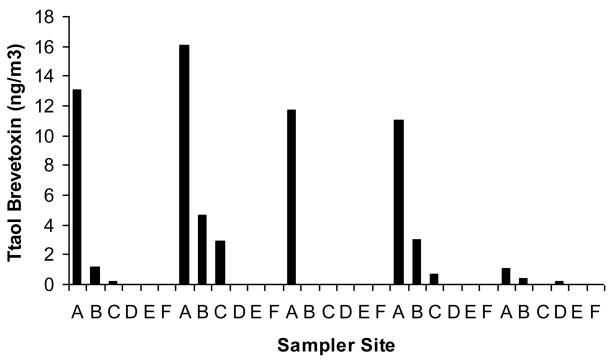
The total brevetoxin concentrations in the aerosol samples collected during the February 7 – 11, and March 15 – 19, 2005 inland transect studies were detectable, and are shown in Figures 2 and 3, respectively. Although the highest levels of total brevetoxins were always measured at the beach site (site A in Figure 1), brevetoxins were detected as far inland as 4.2 km from the beach (site E in Figure 1). The brevetoxin aerosol concentrations in general decreased with increasing distance from the Siesta Beach study site. As the aerosol was transported inland, it was dispersed in the air and some particles were deposited on the ground, resulting in decreased concentration from the beach (Y Chung, unpublished data).
Figure 2.
Inland transect: February 2005
Figure 3.
Inland transect: March 2005
4.0 Discussion
The results of this study demonstrate that brevetoxins were transported up to 6.4 km inland from the coastal water source. These data also illustrate the variability from day to day based on cell count (density and/or patchiness of the bloom), wind speed, and wind direction.
The significance of this transect study is that people may still be exposed environmentally to the aerosolized brevetoxins even after they leave the beach. Indeed, if people remain on a barrier island where no point is greater than 1.6 km from a coast, they will most likely be continuing their exposure in any outdoor setting and from all directions if the inland waters also contain K brevis blooms. This is significant as Fleming et al (2005, 2007, 2009) have demonstrated that asthmatics have significantly increased symptoms and decreased lung function after only a 1 hour exposure to aerosolized brevetoxins. Furthermore, aerosolized Florida red tide toxins seem to have more chronic effects, including symptoms and pulmonary function changes in asthmatics for several days following the 1 hour beach exposure (and/or environmental exposure inland), and increased admissions to emergency rooms for respiratory diseases (including pneumonia, bronchitis, and asthma) during active Florida red tides (Kirkpatrick et al., 2006, Kirkpatrick et al., 2009, Kirkpatrick et al., submitted). Currently, the public health message in communities with onshore Karenia blooms has only recommended leaving the beach area to avoid aerosol exposure; this message needs to be re-evaluated based on these new findings to take into account the possibility of inland environmental exposure to brevetoxins, particularly for persons with underlying lung diseases such as asthma.
4.1 Limitations
These findings are based on 1 non-exposure study and 2 exposure studies, therefore these data do not reflect the entire span of environmental conditions that may alter the distance the toxins travel. Environmental monitoring over a much longer time period is needed to fully understand the conditions that optimize inland transport, as well as monitoring even further inland. In addition, studies are needed to explore possible exposure to brevetoxins inside of buildings. Cell count data and water sampling and analysis for brevetoxins at the 2 sites located near the water would enhance the assessment of the bloom intensity and location.
5.0 Conclusions
This study has documented the transport of brevetoxins during Florida red tide blooms from the beach inland by placing a series of air samplers on an inland transect. This study is the first objective documentation of the transport of K. brevis brevetoxins inland. These findings should be considered by healthcare providers, public health officials and policy makers in communities affected by Florida red tide, particularly when making recommendations concerning the avoidance of brevetoxin exposure and health effects for persons with underlying lung disease.
Acknowledgments
This research was supported by the Centers for Disease Control and Prevention and the Florida Department of Health (Cooperative Agreement: U50/CCU423360–02) as well as by the P01 ES 10594 from the National Institute of Environmental Health Sciences. The authors thank the Tropical Breeze Motel (Siesta Key, FL) and the Sarasota residents for allowing air samplers to be placed on their property, and their support of our studies.
Abbreviations
- PbTx
Brevetoxin
- cm
Centimeter
- K. brevis
Karenia brevis
- km
Kilometer
- LCMS
Liquid chromatography mass spectroscopy
- ug/L
microgram per liter
- ng/m3
nanogram per meter cubed
- NSP
neurotoxic shellfish poisoning
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baden DG. Marine food-borne dinoflagellate toxins. International Review Cytology. 1983;82:99–150. doi: 10.1016/s0074-7696(08)60824-4. [DOI] [PubMed] [Google Scholar]
- Baden D, Fleming LE, Bean JA. Marine Toxins. Handbook of Clinical Neurology: Intoxications of the Nervous System Part H. In: DeWolf FA, editor. Natural Toxins and *Drugs. Elsevier Press; Amsterdam: 1995. pp. 141–175. [Google Scholar]
- Cheng YS, Zhou Y, Irvin CM, Pierce R, Naar J, Backer LC, Fleming LE, Kirkpatrick B, Baden D. Characterization of Marine Aerosol for Assessment of Human Exposure to Brevetoxins. Environmental Health Perspectives. 2005;113(5):638–643. doi: 10.1289/ehp.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer LC, Fleming LE, Rowan A, Cheng Y-S, Benson J, Pierce R, Zaias J, Bean J, Bossart G, Baden DG. Recreational Exposure to Aerosolized Brevetoxins During Florida Red Tide Events. Harmful Algae. 2003;2:19–28. [Google Scholar]
- Fleming LE, Kirkpatrick B, Backer LC, Bean JA, Wanner A, Dalpra D, Tamer R, Zaias J, Cheng YS, Pierce R, Naar J, Abraham W, Clark R, Zhou Y, Henry MS, Johnson D, Van de Bogart G, Bossart GD, Harrington M, Baden DG. Initial Evaluation of the Effects of Aerosolized Florida Red Tide Toxins (Brevetoxins) in Persons with Asthma. Environ Health Persp. 2005;113(5):650–7. doi: 10.1289/ehp.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming LE, Bean JA, Kirkpatrick B, Chung YS, Pierce R, Naar J, Nierenberg K, Backer LC, Wanner A, Reich A, Zhou Y, Watkins S, Henry M, Zaias J, Abraham WM, Benson J, Cassedy A, Hollenbeck J, Kirkpatrick G, Clarke T, Baden DG. Exposure and Effect Assessment of Aerosolized Red Tide Toxins (Brevetoxins) and Asthma Env Health Persp in press. [Google Scholar]
- Fleming LE, Kirkpatrick B, Backer LC, Bean JA, Wanner A, Reich A, Dalpra D, Zaias J, Cheng YS, Pierce R, Naar J, Abraham WM, Baden DG. Aerosolized Red-Tide Toxins (Brevetoxins) and Asthma. Chest. 2007;131(1):187–194. doi: 10.1378/chest.06-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming LE, Backer LC, Baden DG. Overview of Aerosolized Florida Red Tide Toxins: Exposures and Effects. Environmental Health Perspectives. 2005;113(5):618–620. doi: 10.1289/ehp.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes RD. Ion channels in the nerve cell membrane. Sci Am. 1979;240:125–135. doi: 10.1038/scientificamerican0379-126. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Fleming LE, Backer LC, Bean JA, Tamer R, Kirkpatrick G, Kane T, Wanner A, Dalpra D, Reich A, Baden DG. Environmental exposures to Florida Red Tides: Effects on Emergency Room Respiratory Diagnoses Admissions. Harmful Algae. 2006;5:526–533. doi: 10.1016/j.hal.2005.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Fleming LE, Bean JA, Nierenberg K, Backer LC, Chung YS, Pierce R, Reich A, Naar J, Zaias J, Wanner A, Hollenbeck J, Baden DG. Aerosolized Red Tide Toxins (Brevetoxins) and Asthma: Continued health effects after 1 hour beach exposure. doi: 10.1016/j.hal.2010.08.005. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Fleming LE, Squicciarini D, Backer LC, Clark RC, Abraham W, Benson J, Cheng YS, Johnson D, Pierce R, Zaias J, Bossart GD, Baden DG. Literature Review of Florida Red Tide: Implications for Human Health Effects. Harmful Algae. 2003;3(2):99–115. doi: 10.1016/j.hal.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Bean JA, Fleming LE, Backer LC, Akers R, Wanner A, Dalpra D, Nierenberg K, Reich A, Baden DG. In: Moestrup, et al., editors. Aerosolized Red Tide Toxins (Brevetoxins) and Asthma: A 10 day follow up after 1 hour acute beach exposure; Proceedings of the 12th International Conference on Harmful Algae. International Society for Harmful Algae and Intergovernmental Oceanographic Commission of UNESCO; Copenhagen. 2009. pp. 297–299. [Google Scholar]
- Pierce RH, Henry MS, Proffitt LS, Hasbrouck PA. Red tide toxin (brevetoxin) enrichment in marine aerosol. In: Graneli E, Sundstron S, Elder L, Anderson DM, editors. Toxic Marine Phytoplankton. Elsevier Scientific Publishing; New York: 1990. pp. 397–402. [Google Scholar]
- Pierce R, Henry MS, Blum PC, Hamel SL, Kirkpatrick B, Cheng YS, Zhou Y, Irvin CM, Naar J, Weidner A, Fleming LE, Backer LC, Baden DG. Brevetoxin composition in water and marine aerosol along a Florida beach: Assessing potential human exposure to marine biotoxins. Harmful Algae. 2005;4(6):965–972. [Google Scholar]
- Steidinger KA, Baden DG. Toxic marine dinoflagellates. In: Spector DL, editor. Dinoflagellates. Academy Press; New York: 1984. pp. 201–261. [Google Scholar]
- Watkins SM, Reich A, Fleming LE, Hammond R. Neurotoxic Shellfish Poisoning. Marine Drugs. 2008;6(3):431–455. doi: 10.3390/md20080021. [DOI] [PMC free article] [PubMed] [Google Scholar]