Abstract
Purpose
Some prior studies found excess stroke rates among blacks persisted after adjustment for socioeconomic status (SES), fueling speculation regarding racially patterned genetic predispositions to stroke. Prior research was hampered by incomplete SES assessments, without measures of childhood conditions or adult wealth. We assess the role of lifecourse SES in explaining stroke risk and stroke disparities.
Methods
Health and Retirement Study participants age 50+ (n=20,661) were followed on average 9.9 years for self or proxy reported first stroke (2,175 events). Childhood social conditions (southern birthstate, parental SES, self-reported fair/poor childhood health, and attained height), adult SES (education, income, wealth, and occupational status) and traditional cardiovascular risk factors were used to predict first stroke onset using Cox proportional hazards models.
Results
Blacks had 48% higher risk of first stroke incidence than whites (95% CI: 1.33, 1.65). Childhood conditions predicted stroke risk in both blacks and whites, independently of adult SES. Adjustment for both childhood social conditions and adult SES measures attenuated racial differences to marginal significance (HR=1.13; 1.00, 1.28).
Conclusions
Childhood social conditions predict adult stroke risk in American blacks and whites. Additional adjustment for adult SES, in particular wealth, nearly eliminated the disparity in stroke risk between blacks and whites.
Keywords: stroke, lifecourse, social class, aged, United States, racial disparities
Introduction
Stroke is the 3rd leading cause of mortality in the US and a leading cause of major disability in the elderly.(1) Although racial disparities in stroke incidence and mortality are well-documented(2, 3), it is uncertain whether these disparities are attributable to differential socioeconomic conditions of black and white Americans.(4–6) Much of the research on racial disparities in stroke focuses on stroke mortality, but there is evidence that mortality differences mirror disparities in incidence rates(1) and that case fatality rates do not differ substantially by race.(7) Some studies report blacks experienced excess stroke mortality even after statistical adjustment for adult socioeconomic status (SES) and standard cardiovascular risk factors.(6) This has fuelled speculation about possible genetic explanations for racial disparities in stroke and major stroke risk factors, such as hypertension.(8, 9). However, previous research has been hampered by inadequate SES measures, with no adjustment for childhood SES and incomplete measures of adult financial resources. Childhood SES effects are potentially relevant to racial disparities because elderly blacks may have experienced substantial disadvantage throughout the lifecourse.(10) Disadvantage in early childhood may confer increased risk in adulthood, perhaps mediated by infectious diseases, nutritional conditions, or poverty-related stresses.(11, 12) Cardiovascular risk factors are established early in life and begin to diverge in blacks and whites during childhood.(13, 14) Evidence that geographic disparities in stroke risk are associated with place of birth or childhood residence further suggests that early life exposures may shape adult stroke risk.(12, 15–17) Understanding how such differences in social conditions and nativity continue to resonate in the health of today’s elderly is crucial to eliminating racial disparities in stroke.(2, 18) Moreover, if childhood conditions substantially influence adult stroke risk over and above adult characteristics, such factors should be considered in characterizing both black and white patients and populations with respect to stroke risk. Early life exposures may be important to identify, regardless of race.
In this paper, we use data from the Health and Retirement Study (HRS) to examine whether: 1) early life exposures influence stroke risk in both black and white Americans; 2) such exposures contribute to racial/ethnic disparities in adult stroke risk; and 3) the racial disparity in stroke risk persists after full adjustment for childhood social conditions and adult SES.
Methods
Study population
HRS is a longitudinal survey of a national sample of US adults aged 50+ and their spouses. Details of the study are provided elsewhere.(19–21) Enrollment was staggered by birth cohort with enrollments in 1992, 1993 and 1998. Biennial interviews (or proxy interviews for decedent participants) were conducted through 2004. Original survey response rates varied across enrollment cohorts from 70–81%, and at the last interview retention rates ranged from 86–90%. We included all HRS participants born 1900–1947 who were aged 50+ and reported stroke-free at baseline. The HRS is approved by the University of Michigan Health Sciences Human Subjects Committee.
From 23,750 age-eligible respondents who self-reported black or white race, we excluded 281(1.2%) due to unknown stroke status at enrollment; 1,267 (5.3%) respondents who reported prior stroke at enrollment; 539 (2.3%) respondents with unknown date of stroke or death; 325 (1.4%) cases with missing values on adult risk factors and 677 (2.9%) missing values on all childhood social conditions indicators. The final analysis sample was 20,661. Among whites, 12.2% of the age-eligible sample was excluded, compared to 17.6% of age-eligible blacks. The discrepancy was due to a higher prevalence of strokes among blacks at baseline (7% vs 5%) and a higher percentage of blacks missing all childhood SES measures. Average follow-up time was 9.5 years, and 5,031 deaths were reported among the sample as a whole (although only a small fraction of these deaths were related to stroke).
Stroke outcomes
Incident events were defined as first non-fatal or fatal strokes, based on self- or proxy-report of doctors’ diagnoses. Reports of transient ischemic attacks were not coded as strokes. No information on stroke subtypes was obtained. For deceased participants or those unavailable for direct interviews, proxy informants, predominantly spouses, were interviewed. At each assessment except 1994, respondents reported stroke month and year. In 1994, only year was recorded. For these events, December was assigned for strokes in 1992, April for 1993 strokes, and January for strokes in 1994 (based on the distribution of timing of events reported in other interview waves). Stroke events for which the exact month in the two-year interview interval was unknown (n=367) were assigned the median stroke month for events reported by other participants in the same interview wave.
Exposures and demographic characteristics
Self-reported race was categorized as black or white. Southern birth (defined as the US Census Region) was identified based on self-reported state of birth. Preliminary analyses revealed no detectable difference in stroke rates between individuals born in other regions (West, Midwest, or Northeast) or outside the US, so our models compare southern born to all others.
Respondents reported mother’s and father’s education (each dichotomized at 8+ years, plus indicators for unknown), father’s occupation (categorized as non-manual (using 1980 census categories 003–285); army; farming, or unknown), retrospective assessment of health “while you were growing up, from birth to age 16” (on a five point scale from excellent to poor), and self-reported height in inches. Childhood health was dichotomized as excellent/good/very good versus fair/poor, based on prior evidence that the measure is highly reliable and not contaminated by current health status with this dichotomization.(22) Adult height has been previously linked to childhood SES and illness.(23) Alternative specifications of parental SES for those with available data, including continuous years of education (0–17) and finer occupational categories, suggested that primary results were not very sensitive to dichotomization of parental SES variables. A substantial number of participants did not report on parental SES (Table 1). Based on a subset of respondents asked directly about childhood living arrangements, a large fraction of those with “unknown” values for a parent’s SES did not live with that parent during childhood. We thus report coefficients for indicators of unknown parental SES, because they may be substantively meaningful, although the interpretation is ambiguous. When questions were not asked at baseline, we used the earliest response available. Childhood health was not routinely asked until the 1998 interview cycle and many people had strokes and died prior to being asked about childhood health, rendering the “unknown” childhood health category uninterpretable.
Table 1.
Characteristics of white and black HRS participants.
| Black Participants | White Participants | |||
|---|---|---|---|---|
| n / mean | % / (std) | n / mean | % / (std) | |
| n | 3,019 | 100 | 17,642 | 100 |
| Mean years of follow-up | 9.3 | (4.2) | 9.5 | (3.9) |
| Total person-years of follow-up | 28,069.2 | 167,250 | ||
| Incident strokes | 399 | 13 | 1,776 | 10 |
| Crude stroke rate/1,000 person-years | 14.2 | 10.6 | ||
| Core Demographic Variables | ||||
| Mean age at enrollment (std) | 63.0 | (10.5) | 64.6 | (10.6) |
| Male | 1,238 | 41 | 8,154 | 46 |
| Hispanic | 36 | 1 | 1,263 | 7 |
| Southern birthstate | 1,952 | 65 | 5,445 | 31 |
| Child Individual Social Condns | ||||
| Mother’s Education | ||||
| < 8 Years | 1,468 | 49 | 7,670 | 43 |
| 8+ Years | 1,174 | 39 | 8,641 | 49 |
| Unknown | 377 | 12 | 1,331 | 8 |
| Father’s Education | ||||
| < 8 Years | 1,513 | 50 | 8,129 | 46 |
| 8+ Years | 876 | 29 | 7,720 | 44 |
| Unkown | 630 | 21 | 1,793 | 10 |
| Father’s Occupation^ | ||||
| Manual/Unskilled | 931 | 31 | 6,605 | 37 |
| Farming | 865 | 29 | 3,473 | 20 |
| Missing | 1,044 | 35 | 3,774 | 21 |
| Childhood Health | ||||
| Excellent, very good, or good | 2,374 | 79 | 14,530 | 82 |
| Fair or poor | 194 | 6 | 911 | 5 |
| Unknown | 451 | 15 | 2,201 | 12 |
| Height | ||||
| Male mean in inches | 69.6 | (2.8) | 69.8 | (2.6) |
| Male unknown | 33 | 1 | 114 | 1 |
| Female mean in inches | 64.3 | (2.8) | 63.9 | (2.4) |
| Female unknown | 52 | 2 | 115 | 1 |
| Low Parental SES Index | 0.55 | (0.40) | 0.50 | (0.39) |
| Adult SES | ||||
| Mean years of education (std) | 10.6 | (3.7) | 12.1 | (3.2) |
| Median wealth (interquartile range)* | 19,419 | (51,926) | 81,388 | (79,339) |
| Median income (interquartile range)* | 11,656 | (17,027) | 20,443 | (20,050) |
| Main Lifetime Occupation^ | ||||
| Manual/Unskilled | 1,332 | 44 | 4,389 | 25 |
| Farming | 59 | 2 | 386 | 2 |
| Missing | 880 | 29 | 5,841 | 33 |
| Cardiovascular risk factors | ||||
| Hypertension | 1,714 | 57 | 7,018 | 40 |
| Diabetes | 543 | 18 | 1,777 | 10 |
| Heart disease | 497 | 16 | 3,346 | 19 |
| Current smoker | 745 | 25 | 3,375 | 19 |
| Vigorous activity | 565 | 19 | 4,475 | 25 |
| Overweight | 1,237 | 41 | 6,990 | 40 |
| Obese | 927 | 31 | 3,210 | 18 |
Sample members were all stroke-free at baseline, born 1900–1947 and aged 50+ at baseline interview.
Income and wealth values are standardized by dividing by the square root of household size.
^ Occupation in the armed services omitted due to small sample sizes.
The three parental SES items were first assessed independently as stroke predictors. An index summarizing parental SES was then constructed based on average responses to the three parental SES questions, including one point each for known mother <8 years of education, father <8 years of education, and father’s occupation manual. The three items were averaged, so the low parental SES index ranges from 0 (best) to 1 (worst). If an item was unknown, this was not considered to indicate low parental SES. The use of summary indexes is an approach adopted successfully in previous research on childhood social disadvantage and adult health.(24, 25) The index provided a single test of whether low parental SES predicted stroke risk after adjustment for adult risk factors. The measures of fair/poor childhood health and height were kept separate from the parental SES index because we considered them to be primarily indicators of physical health. Physical health is conceptually distinct from parental SES and may be either a unique risk factor or an important mediator through which parental resources influence adult stroke risk.
Core demographic variables include age, age-squared, Hispanic ethnicity, and sex. Additional models adjust for the individual’s own attained adult SES (years of education, baseline household income and baseline household wealth (income and wealth in 1992 dollars, adjusted for household size and logged), and occupation held for the longest period, dichotomized as manual/non-manual. For consistency with previous studies, we used standard RAND coding of income (total household) and wealth (total household excluding secondary residence). A small fraction of respondents had missing values on one or more components of wealth or income. Imputations were based on unfolding bracket estimates or other demographic characteristics, and are detailed in RAND technical documentation.(26) Finally, we considered cardiovascular risk factors (first available report of: current smoking status, overweight (body mass index 25–30) or obese (body mass index >30), vigorous physical activity (dichotomised at 3+ times a week), and self-reported baseline diagnoses of hypertension, diabetes and heart disease) as potential mediators between race and stroke risk.
Methods of analysis
We used Spearman correlations or tetrachoric correlations(27) to examine associations among measures of childhood social conditions and adult SES.
We present sex and age (linear) adjusted survival curves comparing blacks and whites with the lowest or highest (0 vs 1) values of parental SES index. Cox proportional hazard models were used to estimate excess stroke hazard associated with race, childhood social conditions, adult SES, and cardiovascular risk factors, adjusted for core demographics. Follow-up time was defined as time from baseline interview to date of first stroke, proxy reported death, or last interview. Only strokes were considered events. We first present stratified models (adjusted for demographics and southern birthstate) comparing the relation of childhood social disadvantage and stroke risk in blacks and whites, considering each indicator singly and in combination.
We next examined whether adjustment for southern birthstate, low parental SES, childhood health, adult SES, or adult cardiovascular risk factors attenuated estimated effects of race on stroke risk. Analyses excluding respondents with unknown values for parental SES indicators indicated primary findings were not very sensitive to handling of unknown values.
Analyses were conducted using Stata 9.2. We present 95% confidence intervals (CIs) in lieu of p-values. HRS employed a multi-stage, clustered sample design, which may artificially narrow CIs. However, when we compared conventional CIs to CIs obtained by bootstrapping (1,000 cluster-level resamples), results were nearly identical for all analyses we examined (detailed results available from the authors). We therefore report conventional CIs.
Results
The unadjusted stroke rate for blacks was 14.2 per 1,000 person years (399 events), compared to 10.6 for whites (1,776 events) (Table 1). Black participants had higher scores than whites on most risk factors. Low parental SES was inversely correlated with height, education, wealth, and income and positively correlated with southern birthstate and manual occupation (results from authors) Although most social indicators were associated, the correlations were generally low to moderate, with the strongest relationships between low father’s and mother’s education (tetrachoric rho=0.89) and between adult income and wealth (Spearman’s rho=0.55).
Childhood social conditions and adult stroke risk in black and white HRS participants
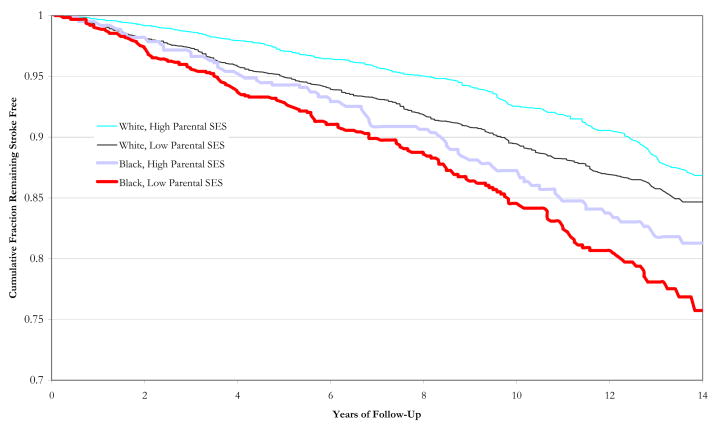
Figure 1 contrasts age and sex-adjusted survival curves for black and white HRS participants with low or high parental SES. Both blacks and whites exposed to low parental SES accumulated strokes more quickly than their unexposed counterparts. However, blacks with high parental SES had higher incidence rates compared to whites with low parental SES.
Figure 1.
Unadjusted survival curves for blacks and whites with lowest or highest parental SES scores.
In Cox models adjusted only for demographics and southern birthstate, stroke risk in blacks was predicted by mother’s education, father’s education, and height (Table 2). In a model including all measures of childhood social conditions, only height remained statistically significant. Patterns were remarkably similar for whites, among whom stroke risk was predicted by mother’s education, father’s education, unknown values for any indicator of parental SES, childhood health, and height. In models simultaneously adjusted for all indicators, father’s education and childhood health retained statistical significance. After adjustment for southern birthstate, childhood health and height, the coefficient for the combined low parental SES index was very similar among blacks (HR=1.31; 95% CI: 0.99, 1.75) and whites (HR=1.27; 1.09, 1.48), but only met conventional statistical significance criteria among whites. In analyses combining blacks and whites, southern birthstate, low parental SES, fair/poor childhood health and height are each independent predictors of adult stroke risk (Table 4, model 4).
Table 2.
Childhood social conditions and hazard ratios for first incident stroke among HRS participants, stratified by race.
| Separate Models | Combined Models | Summary Models | ||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | |
| Blacks (n=3,019) | ||||||
| Mother’s Education | ||||||
| >= 8 Years | reference | reference | ||||
| < 8 Years | 1.46 | (1.13, 1.89) | 1.29 | (0.94, 1.77) | ||
| Unknown | 1.36 | (0.97, 1.91) | 1.14 | (0.77, 1.69) | ||
| Father’s Education | ||||||
| >= 8 Years | reference | reference | ||||
| < 8 Years | 1.46 | (1.10, 1.94) | 1.25 | (0.89, 1.77) | ||
| Unkown | 1.44 | (1.05, 1.98) | 1.40 | (0.97, 2.03) | ||
| Father’s Occupation | ||||||
| Nonmanual/Professional | reference | reference | ||||
| Manual/Unskilled | 0.91 | (0.58, 1.43) | 0.89 | (0.57, 1.40) | ||
| Farming | 0.93 | (0.60, 1.45) | 0.87 | (0.55, 1.36) | ||
| Missing | 1.02 | (0.65, 1.60) | 0.76 | (0.48, 1.21) | ||
| Childhood Health | ||||||
| Excellent, very good, or good | reference | reference | reference | |||
| Fair or poor | 1.29 | (0.90, 1.84) | 1.26 | (0.88, 1.80) | 1.28 | (0.90, 1.84) |
| Unknown | 2.58 | (1.88, 3.54) | 2.89 | (2.02, 4.14) | 2.50 | (1.82, 3.43) |
| Height | ||||||
| Inches | 0.96 | (0.92, 0.99) | 0.96 | (0.93, 1.00) | 0.96 | (0.92, 1.00) |
| Missing | 1.08 | (0.62, 1.87) | 1.04 | (0.59, 1.80) | 1.02 | (0.59, 1.78) |
| Low Parental SES Index | 1.38 | (1.04, 1.83) | 1.31 | (0.99, 1.75) | ||
| Whites (n=17,642) | ||||||
| Mother’s Education | ||||||
| >= 8 Years | reference | reference | ||||
| < 8 Years | 1.22 | (1.06, 1.40) | 1.02 | (0.85, 1.22) | ||
| Unknown | 1.39 | (1.15, 1.67) | 1.19 | (0.95, 1.48) | ||
| Father’s Education | ||||||
| >= 8 Years | reference | reference | ||||
| < 8 Years | 1.31 | (1.14, 1.51) | 1.24 | (1.03, 1.48) | ||
| Unkown | 1.46 | (1.23, 1.75) | 1.33 | (1.08, 1.64) | ||
| Father’s Occupation | ||||||
| Nonmanual/Professional | reference | reference | ||||
| Manual/Unskilled | 1.13 | (0.99, 1.29) | 1.07 | (0.94, 1.23) | ||
| Farming | 1.09 | (0.94, 1.26) | 1.04 | (0.90, 1.21) | ||
| Missing | 1.86 | (1.60, 2.16) | 1.29 | (1.08, 1.53) | ||
| Childhood Health | ||||||
| Excellent, very good, or good | reference | reference | reference | |||
| Fair or poor | 1.34 | (1.11, 1.61) | 1.31 | (1.09, 1.58) | 1.33 | (1.10, 1.60) |
| Unknown | 3.01 | (2.57, 3.51) | 2.56 | (2.12, 3.10) | 2.90 | (2.48, 3.39) |
| Height | ||||||
| Inches | 0.97 | (0.95, 0.99) | 0.98 | (0.96, 0.99) | 0.97 | (0.96, 0.99) |
| Missing | 1.22 | (0.84, 1.77) | 1.20 | (0.83, 1.75) | 1.21 | (0.83, 1.76) |
| Low Parental SES Index | 1.44 | (1.24, 1.68) | 1.27 | (1.09, 1.48) | ||
Separate Models included only one childhood risk factor, without adjustment for other childhood indicators. “Combined Models” simultaneously adjusted for all childhood indicators, and “Summary Models” included the summary index, plus height and fair/poor childhood health. All parameters estimated with Cox proportional hazards models adjusted for age, age-squared, sex, Southern birthstate, and Hispanic ethnicity. Bold text denotes estimates for which the 95% CI did not include the null.
Risk factors from childhood through adulthood and racial disparities in stroke risk
Blacks suffered substantial excess stroke (HR=1.48; 1.33, 1.65) compared to whites in models adjusted only for demographics (Table 4). This excess risk was attenuated after adjusting for southern birth (adjusted HR=1.38), but additional adjustment for parental SES, childhood health, and height resulted in little further attenuation (HR=1.33). After adjusting for adult SES, however, the coefficient for race was substantially attenuated (HR=1.13; 1.00, 1.28). After additional adjustment for cardiovascular risk factors, race did not predict stroke (HR=1.05), but southern birth, low parental SES index, fair/poor childhood health, and height remained significant, independent predictors of stroke risk.
The full complement of social measures was not necessary to effectively explain the racial disparity in these results. The estimated effect of black race was substantially attenuated (HR=1.16; 1.03, 1.31) by adjustment for only three social measures: southern birthstate, log wealth, and years of education. In contrast, even after adjusting for all 6 cardiovascular risk factors but not Southern birthstate, childhood or adult SES, race remained a significant predictor of stroke risk (HR=1.26; 1.12, 1.41).
Discussion
In a unique national longitudinal study, we find that indicators of childhood social conditions predict adult stroke risk in both black and white Americans. Childhood social disadvantage was significantly associated with adult stroke risk independently of adult SES or measured cardiovascular risk factors. Contrary to our original expectations, childhood risk factors did not explain much of the excess stroke risk experienced by blacks, but adjusting for both childhood and adult social conditions nearly entirely attenuated the association between race and stroke risk.
Comparisons with previous studies
Much prior work has documented the extra vulnerability of blacks to stroke. Our findings contribute to understanding the sources of this disparity by demonstrating the important role of two risk factors frequently omitted from stroke studies: birthplace and adult wealth. The US Stroke Belt, a region long associated with excess stroke, substantially overlaps with southern states. Although the Stroke Belt has been well-documented(28, 29), only recently is evidence emerging that risk associated with the Stroke Belt is incurred in childhood.(16, 17, 30) Prior analyses, based on place of adult residence, likely underestimated the role of the Southern Stroke Belt in contributing to national racial disparities. The many older African Americans born in the Stroke Belt who migrated north or west by adulthood would have been effectively misclassified in such studies. (31) To our knowledge, this is the first paper explicitly testing the role of southern birth in explaining racial disparities in stroke incidence. The stroke risk associated with southern birth is similar for blacks and whites, but southern birth is especially important for blacks because the majority of elderly African-Americans were born in the South (authors’ calculations from the 2000 Census Microsample data).(32) Southern blacks in the early 20th century typically faced segregation, poverty, restricted access to education, medical care, and many other health damaging conditions. Dietary differences such as sodium intake may also be important in explaining excess stroke risk among southerners.(33)
Prior findings are inconsistent about whether racial disparities in stroke risk factors and mortality are explained by SES.(4–6) Our results strongly implicate social disadvantage conveyed from childhood through adulthood in creating and maintaining racial disparities. The unusually thorough SES assessments conducted in HRS may ameliorate residual bias affecting studies with briefer SES measures. In our analyses, wealth was key to explaining racial disparities in stroke. Compared to income, wealth more comprehensively reflects economic resources, including lifelong earnings, past financial losses, and intergenerational transfers.(34–36) US blacks average much lower wealth than whites.(37)
Our results contribute to accumulating evidence that early life social conditions affect adult vascular risk.(12, 38, 39) Longitudinal studies demonstrate that cardiovascular risks begin to accumulate in childhood, inducing physiological changes by early adulthood.(14, 40, 41) Prenatal or childhood deprivation may contribute to these enduring physiological or behavioral changes, increasing stroke risk decades later.(12, 42, 43) Some studies suggest childhood effects are almost entirely mediated by adult SES or cardiovascular measures.(38, 39, 44) We found childhood SES predicts adult stroke risk independently of adult SES.
In addition to the effects of parental SES on adult stroke, the excess stroke risks associated with fair/poor childhood health and height are intriguing. Beyond genetic background, adult height is also affected by childhood illness, SES, and psychosocial trauma.(23, 45) The relative effects of childhood health were similar for blacks and whites, consistent with previous evidence linking childhood health status to numerous adult morbidity measures.(46, 47)
Study limitations and strengths
HRS is uniquely suited for the current investigation, but also has limitations. Self-reported stroke has moderate correspondence with hospital recorded stroke (48–50), and correspondence between proxy and self-reported diagnosed health conditions is good but imperfect.(51) Although there are no national data sources on incidence of first medically verified stroke for comparison, risk factors identified in HRS and overall incidence rates appear consistent with the best currently available evidence from studies with clinical verification (more detailed comparisons available from the authors).(1)(52) It is also possible that some HRS participants who reported themselves to be stroke-free had in fact experienced prior stroke. However, stroke rates are sufficiently low in the population, and likely sensitivity is sufficiently high, that this would probably not introduce a large bias.
We have no data on stroke subtypes. The association between childhood conditions and stroke may vary by subtype(53, 54), but we cannot explore this. Self-reported cardiovascular risk factors, SES, and especially retrospectively reported parental SES, are vulnerable to misreporting or measurement error. Information bias is especially a concern with the childhood SES measures, which were unknown for a much greater fraction of black than white respondents. We consider this likely to reflect differences in family structure, with black fathers less commonly residing with their children in these birth cohorts.(55) For example, among a subsample of respondents asked “before age 16, was there a time of several months or more when your father had no job?”, 7% of white respondents reported that their fathers were deceased or did not live with them at the time, compared to 21% of black respondents. For both whites and blacks, reporting “unknown” for father’s education predicted higher stroke risk; we think this suggests fathers may play important roles in reducing risk of subsequent stroke, perhaps by improving the material standard of living in the household. However, we cannot rule out bias in reporting parental SES as contributing to this finding.
Our adult SES indicators are both more comprehensive and better measured than the childhood measures. This may contribute to the finding that adult SES played a much larger role than parental SES in explaining racial disparities.
Our indicators of childhood social conditions are ambiguous with respect to timing, thus disentangling whether the associations are attributable to prenatal conditions, early childhood or adolescence is not possible with these data.(56) Prior research implicates not only level of socioeconomic position at any single point in time in disease risk, but also the trajectory of change over the lifecourse and socioeconomic instability.(57, 58) We have not explicitly examined these questions in the current analyses.
Some caveats in our study may be inherent to analyses of these research questions; nonetheless they merit explicit mention. The long temporal separation between childhood exposures and HRS enrollment creates the potential for selection bias.(56, 59) Selective survival (or disease-free survival, in this study) is a general problem in lifecourse epidemiology, and it may be especially troubling in disparities research. At baseline, for example, 7% of blacks and 5% of whites reported prevalent stroke and were excluded from the analysis. This selection process would probably tend to underestimate the true excess risk caused by race. Efforts to quantify the direct and indirect effects of race on stroke risk are vulnerable to related biases. Interpretation of coefficients after adjustment for hypothesized mediators relies on very strong assumptions, specifically that the mediators are themselves unconfounded and do not modify the strength of the direct effects of the exposure on the outcome.(60–62)
Despite the limitations, the large size, long follow-up accrued, oversample of blacks and careful adult SES measures in HRS facilitate stratified analyses and make it a unique source of national information on racial disparities.
Implications of findings
Given the great interest in genetic underpinnings of variations in stroke risk(8), it is especially important to evaluate the contributions of social factors to stroke burden and stroke disparities. Understanding population patterns of stroke incidence requires consideration of early life social conditions. Older black and white Americans on average experienced tremendously different social conditions from childhood onwards. Childhood risk factors influence adult stroke risk independently of adult risk factors. Nativity is especially important in understanding black-white disparities in stroke. However, in our analyses racial disparities in stroke are not completely explained by early life risk factors, but were substantially mediated by adult SES.
Table 3.
Hazard ratios for first incident stroke associated with black race, after adjustment for region of birth, childhood disadvantage, adult SES, and adult cardiovascular risk factors.
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | |
| Black race | 1.48 | (1.33, 1.65) | 1.38 | (1.24, 1.55) | 1.36 | (1.21, 1.52) | 1.33 | (1.19, 1.49) | 1.13 | (1.00, 1.28) | 1.05 | (0.93, 1.18) |
| Southern birthstate | 1.27 | (1.16, 1.39) | 1.26 | (1.16, 1.38) | 1.27 | (1.17, 1.39) | 1.24 | (1.14, 1.36) | 1.22 | (1.12, 1.33) | ||
| Low Parental SES Index | 1.46 | (1.28, 1.67) | 1.31 | (1.14, 1.49) | 1.23 | (1.07, 1.41) | 1.19 | (1.04, 1.37) | ||||
| Fair/poor child health | 1.32 | (1.11, 1.55) | 1.27 | (1.08, 1.50) | 1.23 | (1.04, 1.46) | ||||||
| Height in Inches | 0.97 | (0.96, 0.99) | 0.98 | (0.96, 0.99) | 0.98 | (0.97, 1.00) | ||||||
| Adult SES | ||||||||||||
| Years of education | 1.00 | (0.98, 1.01) | 1.00 | (0.99 1.02) | ||||||||
| Income* | 0.96 | (0.93, 0.98) | 0.96 | (0.93, 0.99) | ||||||||
| Wealth* | 0.97 | (0.95, 0.98) | 0.98 | (0.96, 0.99) | ||||||||
| Main Occupation Manual/Unskilled | 1.22 | (1.07, 1.39) | 1.20 | (1.05, 1.37) | ||||||||
| Farming | 1.13 | (0.83, 1.55) | 1.18 | (0.87, 1.62) | ||||||||
| Army | 0.85 | (0.44, 1.65) | 0.80 | (0.41, 1.56) | ||||||||
| Unknown | 1.21 | (1.06, 1.38) | 1.19 | (1.04, 1.35) | ||||||||
| Cardiovascular risk factors | ||||||||||||
| Hypertension | 1.52 | (1.40, 1.67) | ||||||||||
| Current smoker | 1.55 | (1.39 1.73) | ||||||||||
| Vigorous activity | 0.82 | (0.74, 0.91) | ||||||||||
| Overweight | 0.95 | (0.86, 1.05) | ||||||||||
| Obese | 1.01 | (0.89, 1.14) | ||||||||||
| Diabetes | 1.82 | (1.63, 2.04) | ||||||||||
| Heart disease | 1.39 | (1.26, 1.53) | ||||||||||
All models are also adjusted for age, age-squared, sex, Hispanic ethnicity. N=20,661. Bold text denotes estimates for which the 95% CI did not include the null.
Income and wealth values are standardized by dividing by the square root of household size and transformed by taking the natural log.
Acknowledgments
The authors gratefully acknowledge financial support from the US National Institute of Aging (AG023399), and the Robert Wood Johnson Foundation Health and Society Scholars Program. M. Glymour is a Robert Wood Johnson Health and Society Scholar at Columbia University. Dr. Mauricio Avendano is supported by a grant from the Netherlands Organisation for Scientific Research (NWO, grant no. 451-07-001) and a Fellowship from the Erasmus University.
Abbreviations
- HRS
Health and Retirement Study
- SES
Socioeconomic status
- CI
Confidence interval
- HR
Hazard ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart Disease and Stroke Statistics--2008 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Mensah GA. Eliminating disparities in cardiovascular health: six strategic imperatives and a framework for action. Circulation. 2005;111:1332–6. doi: 10.1161/01.CIR.0000158134.24860.91. [DOI] [PubMed] [Google Scholar]
- 3.Broderick J, Brott T, Kothari R, Miller R, Khoury J, Pancioli A, et al. The Greater Cincinnati Northern Kentucky Stroke Study - Preliminary first-ever and total incidence rates of stroke among blacks. Stroke. 1998;29:415–421. doi: 10.1161/01.str.29.2.415. [DOI] [PubMed] [Google Scholar]
- 4.Bravata DM, Wells CK, Gulanski B, Kernan WN, Brass LM, Long J, et al. Racial disparities in stroke risk factors - The impact of socioeconomic status. Stroke. 2005;36:1507–1511. doi: 10.1161/01.STR.0000170991.63594.b6. [DOI] [PubMed] [Google Scholar]
- 5.Casper ML, Barnett EB, Armstrong DL, Giles WH, Banton CJ. Social class and race disparities in premature stroke mortality among men in North Carolina. Ann Epidemiol. 1997;7:146–153. doi: 10.1016/s1047-2797(96)00113-5. [DOI] [PubMed] [Google Scholar]
- 6.Howard G, Russell GB, Anderson R, Evans GW, Morgan T, Howard VJ, et al. Role of social class in excess black stroke mortality. Stroke. 1995;26:1759–63. doi: 10.1161/01.str.26.10.1759. [DOI] [PubMed] [Google Scholar]
- 7.Kissela B, Schneider A, Kleindorfer D, Khoury J, Miller R, Alwell K, et al. Stroke in a Biracial Population The Excess Burden of Stroke Among Blacks. Stroke. 2004;35:426–431. doi: 10.1161/01.STR.0000110982.74967.39. [DOI] [PubMed] [Google Scholar]
- 8.Wolf PA, Kannel WB. Preventing Stroke: Does Race/Ethnicity Matter? Circulation. 2007;116:2099–2100. doi: 10.1161/CIRCULATIONAHA.107.736942. [DOI] [PubMed] [Google Scholar]
- 9.Young J, Chang Y-PC, Kim JD-O, Chretien J-P, Klag M, Levine M, et al. Differential Susceptibility to Hypertension Is Due to Selection during the Out-of-Africa Expansion. PLoS Genet. 2005;1:e82. doi: 10.1371/journal.pgen.0010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams DR, Collins C. US Socioeconomic and Racial-Differences in Health - Patterns and Explanations. Annu Rev Sociol. 1995;21:349–386. [Google Scholar]
- 11.Galobardes B, Lynch JW, Smith GD. Childhood socioeconomic circumstances and cause-specific mortality in adulthood: Systematic review and interpretation. Epidemiol Rev. 2004;26:7–21. doi: 10.1093/epirev/mxh008. [DOI] [PubMed] [Google Scholar]
- 12.Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Ann Epidemiol. 2006;16:91–104. doi: 10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 13.Wang XL, Poole JC, Treiber FA, Harshfield GA, Hanevold CD, Snieder H. Ethnic and gender differences in ambulatory blood pressure trajectories - Results from a 15-year longitudinal study in youth and young adults. Circulation. 2006;114:2780–2787. doi: 10.1161/CIRCULATIONAHA.106.643940. [DOI] [PubMed] [Google Scholar]
- 14.Davis PH, Dawson JD, Riley WA, Lauer RM. Carotid intimal-medial thickness is related to cardiovascular risk factors measured from childhood through middle age - The muscatine study. Circulation. 2001;104:2815–2819. doi: 10.1161/hc4601.099486. [DOI] [PubMed] [Google Scholar]
- 15.Glymour MM, Avendano MP, Berkman LF. Is the stroke belt worn from childhood? Risk of first stroke and state of residence in childhood and adulthood. Stroke. 2007;38:2415–2421. doi: 10.1161/STROKEAHA.107.482059. [DOI] [PubMed] [Google Scholar]
- 16.Lackland DT, Egan BM, Jones PJ. Impact of nativity and race on “stroke belt” mortality. Hypertension. 1999;34:57–62. doi: 10.1161/01.hyp.34.1.57. [DOI] [PubMed] [Google Scholar]
- 17.Fang J, Madhavan S, Alderman MH. The association between birthplace and mortality from cardiovascular causes among black and white residents of New York City. N Engl J Med. 1996;335:1545–1551. doi: 10.1056/NEJM199611213352101. [DOI] [PubMed] [Google Scholar]
- 18.Ovbiagele B, Levine SR. Addressing racial and ethnic disparities in stroke - The time is now. Neurology. 2006;67:1328–1329. doi: 10.1212/01.wnl.0000243251.36208.12. [DOI] [PubMed] [Google Scholar]
- 19.Juster F, Suzman R. An overview of the health and retirement study. J Hum Resur. 1995;30(suppl):S7–S56. [Google Scholar]
- 20.Heeringa SG, Connor J. Technical description of the Health and Retirement Study sample design. 1995 HRS/AHEAD Documentation Report DR-002, Available from: http://hrsonline.isr.umich.edu/docs/userg/HRSSAMP.pdf.
- 21.Ofstedal MB, Fisher GF, Herzog AR. Documentation of cognitive functioning measures in the health and retirement study. 2005 HRS Documentation Report DR-006, Available from: http://hrsonline.isr.umich.edu/docs/userg/dr-006.pdf.
- 22.Haas S. The long-term effects of poor childhood health: an assessment and application of retrospective reports. Demography. 2007;44:113–135. doi: 10.1353/dem.2007.0003. [DOI] [PubMed] [Google Scholar]
- 23.Kuh D, Wadsworth M. Parental Height: Childhood Environment and Subsequent Adult Height in a National Birth Cohort. Int J Epidemiol. 1989;18:663–668. doi: 10.1093/ije/18.3.663. [DOI] [PubMed] [Google Scholar]
- 24.Kauhanen L, Lakka HM, Lynch JW, Kauhanen J. Social disadvantages in childhood and risk of all-cause death and cardiovascular disease in later life: a comparison of historical and retrospective childhood information. Int J Epidemiol. 2006;35:962–968. doi: 10.1093/ije/dyl046. [DOI] [PubMed] [Google Scholar]
- 25.Yang SM, Lynch JW, Raghunathan TE, Kauhanen J, Salonen JT, Kaplan GA. Socioeconomic and psychosocial exposures across the life course and binge drinking in adulthood: Population-based study. Am J Epidemiol. 2007;165:184–193. doi: 10.1093/aje/kwj357. [DOI] [PubMed] [Google Scholar]
- 26.StClair P, Blake D, Bugliari D, Chien S, Hayden O, Hurd MD, et al. RAND HRS data documentation, Version G 2007. 2007 Available from: http://www.rand.org/labor/aging/dataprod/randhrsg.pdf.
- 27.Drasgow F. Polychoric and polyserial correlations. In: Kotz JN, Editor L, editors. Encyclopedia of statistical sciences. Wiley; New York: 1988. pp. 69–74. [Google Scholar]
- 28.Howard G, Howard VJ, Katholi C, Oli MK, Huston S. Decline in US stroke mortality - An analysis of temporal patterns by sex, race, and geographic region. Stroke. 2001;32:2213–2218. doi: 10.1161/hs1001.096047. [DOI] [PubMed] [Google Scholar]
- 29.Lanska DJ, Peterson PM. Geographic-Variation in Reporting of Stroke Deaths to Underlying or Contributing Causes in the United-States. Stroke. 1995;26:1999–2003. doi: 10.1161/01.str.26.11.1999. [DOI] [PubMed] [Google Scholar]
- 30.Barker DJP, Lackland DT. Prenatal influences on stroke mortality in England and Wales. Stroke. 2003;34:1598–1602. doi: 10.1161/01.STR.0000077257.27430.7E. [DOI] [PubMed] [Google Scholar]
- 31.Tolnay SE. The African American “Great Migration” and beyond. Annu Rev Sociol. 2003;29:209–232. [Google Scholar]
- 32.Ruggles S, Sobek M, Alexander T, Fitch CA, Goeken R, Hall PK, et al. Integrated Public Use Microdata Series: Version 3.0 [Machine-readable database] 2004 [cited; Available from: http://www.ipums.org
- 33.Hajjar I, Kotchen T. Regional variations of blood pressure in the united states are associated with regional variations in dietary intakes: The NHANES-III data. J Nutr. 2003;133:211–214. doi: 10.1093/jn/133.1.211. [DOI] [PubMed] [Google Scholar]
- 34.Krieger N, Williams D, Moss N. Measuring social class in US public health research: concepts methodologies, guidelines. Annu Rev Public Health. 1997;18:341–378. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- 35.Robert S, House JS. SES differentials in health by age and alternative indicators of SES. J Aging Health. 1996;8:359–88. doi: 10.1177/089826439600800304. [DOI] [PubMed] [Google Scholar]
- 36.Oliver ML, Shapiro TM. Black wealth, white wealth: a new perspective on racial inequality. New York: Routledge; 1995. [Google Scholar]
- 37.Conley D. Being black, living in the red: race, wealth, and social policy in America. Berkeley: University of California Press; 1999. [Google Scholar]
- 38.Luo Y, Waite LJ. The impact of childhood and adult SES on physical, mental, and cognitive well-being in later life. J Gerontol B Psychol Sci Soc Sci. 2005;60:S93–S101. doi: 10.1093/geronb/60.2.s93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayward MD, Gorman B. The long arm of childhood: the influence of early-life social conditions on men’s mortality. Demography. 2004;41:87–107. doi: 10.1353/dem.2004.0005. [DOI] [PubMed] [Google Scholar]
- 40.Chen W, Srinivasan SR, Li SX, Xu JH, Berenson GS. Clustering of long-term trends in metabolic syndrome variables from childhood to adulthood in blacks and whites - The Bogalusa Heart Study. Am J Epidemiol. 2007;166:527–533. doi: 10.1093/aje/kwm105. [DOI] [PubMed] [Google Scholar]
- 41.Berenson GS, Wattigney WA, Tracy RE, Newman WP, Srinivasan SR, Webber LS, et al. Atherosclerosis of the Aorta and Coronary-Arteries and Cardiovascular Risk-Factors in Persons Aged 6 to 30 Years and Studied at Necropsy (the Bogalusa Heart-Study) Am J Cardiol. 1992;70:851–858. doi: 10.1016/0002-9149(92)90726-f. [DOI] [PubMed] [Google Scholar]
- 42.Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301:1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lackland DT, Bendall HE, Osmond C, Egan BM, Barker DJP. Low birth weights contribute to the high rates of early-onset chronic renal failure in the southeastern United States. Arch Intern Med. 2000;160:1472–1476. doi: 10.1001/archinte.160.10.1472. [DOI] [PubMed] [Google Scholar]
- 44.Wannamethee SG, Whincup PH, Shaper G, Walker M. Influence of fathers’ social class on cardiovascular disease in middle-aged men. Lancet. 1996;348:1259–1263. doi: 10.1016/S0140-6736(96)02465-8. [DOI] [PubMed] [Google Scholar]
- 45.Gunnell D. Commentary: Can adult anthropometry be used as a ‘biomarker’ for prenatal and childhood exposures? Int J Epidemiol. 2002;31:390–394. [PubMed] [Google Scholar]
- 46.Blackwell DL, Hayward MD, Crimmins EM. Does childhood health affect chronic morbidity in later life? Soc Sci Med. 2001;52:1269–84. doi: 10.1016/s0277-9536(00)00230-6. [DOI] [PubMed] [Google Scholar]
- 47.Kuh D, Ben-Shlomo Y, editors. A lifecourse approach to chronic disease epidemiology: tracing the origins of ill-health from early to adult life. Oxford University Press; Oxford: 1997. [Google Scholar]
- 48.Engstad T, Bonaa KH, Viitanen M. Validity of self-reported stroke - The Tromso study. Stroke. 2000;31:1602–1607. doi: 10.1161/01.str.31.7.1602. [DOI] [PubMed] [Google Scholar]
- 49.Beckett M, Weinstein M, Goldman N, Lin YH. Do health interview surveys yield reliable data on chronic illness among older respondents? Am J Epidemiol. 2000;151:315–323. doi: 10.1093/oxfordjournals.aje.a010208. [DOI] [PubMed] [Google Scholar]
- 50.Bergmann MM, Byers T, Freedman DS, Mokdad A. Validity of self-reported diagnoses leading to hospitalization: A comparison of self-reports with hospital records in a prospective study of American adults. Am J Epidemiol. 1998;147:969–977. doi: 10.1093/oxfordjournals.aje.a009387. [DOI] [PubMed] [Google Scholar]
- 51.Magaziner J, Bassett SS, Hebel JR, Gruber Baldini A. Use of proxies to measure health and functional status in epidemiologic studies of community-dwelling women aged 65 years and older. Am J Epidemiol. 1996;143:283–292. doi: 10.1093/oxfordjournals.aje.a008740. [DOI] [PubMed] [Google Scholar]
- 52.Glymour M, Avendano M. Can Self-Reported Strokes be Used to Study Stroke Incidence and Risk Factors? Evidence from the Health and Retirement Study. Stroke Forthcoming. doi: 10.1161/STROKEAHA.108.529479. [DOI] [PubMed] [Google Scholar]
- 53.McCarron MO, Smith GD, McCarron P. Secular stroke trends: early life factors and future prospects. Qjm-an International Journal of Medicine. 2006;99:117–122. doi: 10.1093/qjmed/hcl008. [DOI] [PubMed] [Google Scholar]
- 54.Hart CL, Smith GD. Relation between number of siblings and adult mortality and stroke risk: 25 year follow up of men in the Collaborative study. J Epidemiol Community Health. 2003;57:385–391. doi: 10.1136/jech.57.5.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruggles S. The Origins of African-American Family Structure. Am Sociol Rev. 1994;59:136–151. [Google Scholar]
- 56.Glymour MM. Commentary: Selected samples and nebulous measures: some methodological difficulties in life-course epidemiology. Int J Epidemiol. 2007;36:566–568. doi: 10.1093/ije/dym099. [DOI] [PubMed] [Google Scholar]
- 57.McDonough P, Duncan GJ, Williams D, House J. Income dynamics and adult mortality in the United States, 1972 through 1989. Am J Public Health. 1997;87:1476–1483. doi: 10.2105/ajph.87.9.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benzeval M, Judge K. Income and health: the time dimension. Soc Sci Med. 2001;52:1371–1390. doi: 10.1016/s0277-9536(00)00244-6. [DOI] [PubMed] [Google Scholar]
- 59.Hernán MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–25. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 60.Robins JM, Greenland S. Identifiability and exchangeability for direct and indirect effects. Epidemiology. 1992;3:143–55. doi: 10.1097/00001648-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 61.Kaufman J, Maclehose R, Kaufman S. A further critique of the analytic strategy of adjusting for covariates to identify biologic mediation. Epidemiol Perspect Innov. 2004;1:4. doi: 10.1186/1742-5573-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cole SR, Hernán MA. Fallibility in estimating direct effects. Int J Epidemiol. 2002;31:163–5. doi: 10.1093/ije/31.1.163. [DOI] [PubMed] [Google Scholar]