Abstract
Adiponectin is secreted by white adipose tissue and exists as the most abundant adipokine in the human plasma. Recent research has indicated that plasma adiponectin levels are inversely correlated with body mass index (BMI) and insulin resistance. Reduction of plasma adiponectin levels is commonly observed in the patients with type 2 diabetes (T2D) and/or in those who are obese in comparison with healthy control individuals. The adiponectin (AdipoQ) gene has a moderate linkage disequilibrium (LD), but two small LD blocks are observed, respectively, in the promoter region and the boundary of exon 2-intron 2. Genetic association studies have demonstrated that single nucleotide polymorphisms (SNPs) +45G15G(T/G) in exon 2 and +276G/T in intron 2 of the AdipoQ gene confer the risk susceptibility to the development of T2D, obesity and diabetic nephropathy (DN). The SNPs in the promoter region, including −11426A/G, −11377C/G and −11391G/A, are found to be associated with T2D and DN. Recent research has indicated that the promoter polymorphisms interfere with the AdipoQ promoter activity. The haplotypes constructed by the promoter polymorphisms and SNP +276G/T in intron 2 are associated with circulating adiponectin levels. This review summarises genetic and pathophysiological relevancies of adiponectin and discusses about the biomarkers of adiponectin plasma protein variation and genomic DNA polymorphisms.
Keywords: adiponectin, biomarker, genetic polymorphism, protein variation
Introduction
Adipose tissue or fat is a loose connective tissue composed of adipocytes. Its main role is to store triglyceride (TG) and to release free fatty acid/glycerol in response to changing energy demands. In humans, adipose tissue is located beneath the skin (subcutaneous fat), around internal organs (visceral fat), and in the bone marrow (yellow bone marrow). There exit two types of adipose tissue: white adipose tissue (WAT) and brown adipose tissue (BAT). Adipose tissue also serves as an important endocrine organ,1 because several hormones (adipokines) such as leptin (OMIM 164160),2 adipsin (OMIM 134350),3 tumor necrosis factor-alpha (OMIM 191160),4 and adiponectin (OMIM 605441),5 are found to be secreted by the tissue into the bloodstream. The leptin gene was identified in 1994.6 In one year later, adiponectin was found as the most abundant adipokine and count for 0.01% or 3–30 μg/ml of total plasma protein.7–10 In comparison with leptin research, however, until the recent 8 years, adiponectin has been attracted much attention particularly in the fields of pathophysiology and genetics of type 2 diabetes (T2D), obesity and diabetic nephropathy (DN). In this review, I will summarise genetic and pathophysiological relevancies of adiponectin and discuss about the biomarkers of its plasma protein variation and genomic DNA polymorphisms.
Adipose tissue is an endocrine organ, and adiponectin is the most abundant adipokine secreted by white adipose tissue (WAT) into the bloodstream.
Genomic DNA Structure, mRNA and Protein of Adiponectin
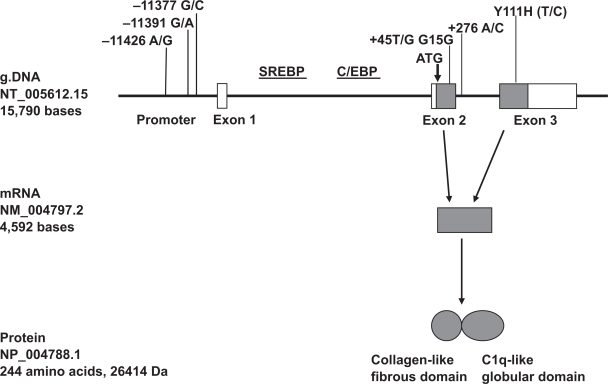
The adiponectin gene (GeneID 9370) has been recently encoded by AdipoQ (adiponectin, C1q and collagen domain containing). The alternative names for adiponectin gene are ACDC, APM1, ACRP30 and GBP28. Figure 1 demonstrates the molecular structures of genomic DNA (NT_005612.15), mRNA (NM_004797.2) and protein (NP_004788.1) of adiponectin. The AdipoQ gene spans 1.579 kb and contains 3 exons. The translation start point is located in exon 2. The promoter region required for the AdipoQ gene includes up-strand sequence, 5’-untraslated region (5’-UTR) and intron 1. The promoter region from −676 to +41 has found to be sufficient for basal transcriptional activity, in which two elements i.e. sterol regulatory binding protein (SREBP, from −431 to −423) and CCAAT/enhancer binding protein (C/EBP, from −230 to −224) reside.11–19 Adiponectin protein is constructed by 244 amino acids at a molecular weight of 26,414 Da, and consists of collagen-like fibrous and C1q-like globular domains. Sequence analysis predicted that the protein has a signal peptide but no transmembrane hydrophobic stretch, and a short N-terminal non-collagenous sequence followed by a short collagen-like motif of G-X-Y repeats. Adiponectin exists mainly as full-length in plasma. The small fragment of globular form is also found in plasma most likely due to the cleavage of adiponectin by leukocyte elastase.20–22 Northern blot analysis detected a 4.5-kb adiponectin transcript in adipose tissue but not in muscle, intestine, placenta, uterus, ovary, kidney, liver, lung, brain or heart.23,24 The AdipoQ mRNA in human (Homo sapiens) has the relatively high identical homology respectively with chimpanzee (Pan troglodytes, 99%), cattle (Bos Taurus, 87%), pig (Sus scrofa, 87%), dog (Canis familiari, 87%), mouse (Mus musculus, 84%) and rat (Rattus norvegicus, 83%). Adiponectin expression is regulated by Peroxisome proliferator-activated receptor-gamma (PPAR γ)-dependent pathways. PPAR γ ligands increase expression and plasma concentration of adiponectin.25–27
Figure 1.
Genomic DNA, mRNA and protein of adiponectin.
Plasma Protein Levels and Disease Relationships for Adiponectin
There is no significant gender difference of plasma adiponectin levels in gender for newborn infants.28–31 However, plasma adiponectin levels were inversely correlated with obesity and insulin resistance in boys and girls.32 Adiponectin values is found to be decreased in the children whose body mass index (BMI) increased.33 Sex differences in adiponectin are dependent on both puberty stage and adiposity in adolescents.34 In adult, adiponectin expression from adipose tissue is higher in lean subjects and women, and is associated with higher degrees of insulin sensitivity.35
Reduction in plasma adiponectin levels are commonly observed in several metabolic disorders related with insulin resistance, including T2D and/or obesity, in both children and adults.36–41 Pima Indians in Arizona suffer from a high prevalence of T2D, which is associated with obesity. Their levels of plasma adiponectin are significantly depressed.42 Women with prior gestational diabetes mellitus (pGDM) are at increased risk of development of T2D, and they also had lower plasma adiponectin concentrations compared with unaffected women.43,44 Evidence has also indicated that plasma adiponectin levels independently correlate β-cell function in late pregnacy of diabetic patients. Thus, adiponectin may play a role on not only mediating insulin resistance but also β-cell dysfunction in the pathogenesis of diabetes.45 Furthermore, lower plasma adiponectin levels and insulin resistance coexist in a genetically prone subset of first degree African-American relatives before development of impaired glucose tolerance (IGT) and T2D with risk of diabetes.46 The patients with T2D not only have a decreased adiponectin levels in the basal state but also have impaired utilization of adiponectin in the coronary artery and/or the heart, which may promote the development of atherosclerosis.47,48
Plasma adiponectin levels are inversely correlated with body mass index and insulin resistance. Type 2 diabetes patients and obese subjects often have lower plasma adiponectin levels.
Interestingly, plasma adiponectin concentrations in the patients with type 1 diabetes (T1D) are found to be significantly elevated in relation to healthy controls.49–5252 In the patients with macroalbuminuria, progression to end-stage renal disease (ESRD) is found to be associated with higher serum adiponectin levels.53,54 Interrelations between adiponectin and inflammation, dyslipidemia, C-peptide levels and sex appear to be important for complex adiponectin modulation and action. The association between C-peptide and adiponectin is probably one of the reasons for their different respective levels between T1D and T2D. Development of hyperglycemia is often accompanied by dyslipidemia, hypertension heighten inflammation, endothelial dysfunction and coagulant activity, whereas adiponectin is involved in the pathophysiological mechanisms.55–58
Plasma adiponectin levels in type 1 diabetes patients and in the patients with nephropathy are increased compared to non-diabetic individuals or the patients without nephropathy. The possible mechanism concerning different adiponectin levels in type 1 and type 2 diabetes is due to adiponectin modulation and action in related to C-peptide.
Accumulating evidence shows that adiponectin plays an important role on insulin sensitivity, insulin ressistance and β-cell dysfunction. Reduction of plasma/serum adiponectin levels is significantly related to the development of diabetes, obesity and insulin resistance. Adiponectin in human serum forms a wide range of multimers from trimers and hexamers (low and/or medium molecular weight, LMW/MMW) to high molecular weight (HMW) dodecamers and 18 mers. Almost all adiponectin in plasma appears to exist as full-length adiponectin molecule.59–61 Recent research has indicated that HMW adiponectin has bioactivity. Serum HMW adiponectin is related to early postprandial glycemic increases and gastric emptying in T2D patients.61,62 However, a comparative evaluation study with the limited number of subjects (n = 60) does not support the superiority of HMW over total adiponectin in assessing metabolic variables at baseline or in response to physical training.63 Therefore, evaluation of serum adiponectin levels by using the ratio of HMW/(LMW + MMW) in the patients with diabetes, obesity, diabetic nephropathy and healthy individuals is of importance to better understand the role of adiponectin on the development of these complex diseases.
Genetic Polymorphisms of the AdipoQ Gene and Clinically Associated with Diseases
The AdipoQ gene is located on chromosome 3q27. Genome wide scan studies indicate that a susceptibility locus linked to T2D, obesity and coronary heart disease may reside in this chromosomal region.64–71 Based on biological evidences and positional information from linkage studies, the AdipoQ gene is considered as an important candidate for T2D. Mutation screening for the AdipoQ gene in T2D has been done and a total of 42 single nucleotide polymorphisms (SNPs) with minor allele frequency (MAF) > 1.5% are represented in Table 1. The linkage disequilibrium (LD) of the AdipoQ gene is moderate, but there are two small LD blocks, one including SNPs in the promoter region and another one spanning the boundary of exon 2-intron 2.72 In the recent 8 years, a number of genetic association studies for the AdipoQ gene polymorphisms in T2D, obesity and other metabolic disorders have been reported, and the references are selected in this review. Genetic association of SNPs in the AdipoQ gene with T2D, obesity and DN in different ethnic populations is summarized in Table 2. There are two independent association signals, which are represented in these two LD blocks.73 The SNPs i.e. +45T/G and +276G/T in the AdipoQ gene are the common polymorphisms associated with T2D, obesity and insulin resistance.74–81 A haplotype defined by these two SNPs is found to be strongly associated with many components of insulin resistance syndrome.73,82 However, association of +45T/G and +276G/T polymorphisms with T2D is not seen in French and Swedish Caucasians. Instead, SNP −11377C/G, −11426A/G and −11377C/G in the promoter region of the AdipoQ gene are found to be strongly associated with T2D in French and Swedish Caucasians. 83–85 These three promoter polymorphisms are constructed as a LD block and their haplotypes are associated with type 2 diabetes and affect the promoter activity. 86 Non-synonymous SNPs in the C1q-like globular domain have relatively low MAFs (< 1.5%). H111Y is an unique non-synonymous SNP with MAF > 1.5% and found to be significantly associated with low plasma adiponectin levels, BMI and T2D.87 Genetic association studies have clearly demonstrated that the AdipoQ gene DNA polymorphisms have genetic effects on diabetes, obesity and insulin resistance, which are influenced by different genetic backgrounds and environmental factors in different ethnic populations.
Table 1.
Single nucleotide polymorphisms in the adiponectin gene.
| SNP ID | SNP type* | Region | Contig position** | Heterozygosity |
|---|---|---|---|---|
| rs16861194 | R = A/G | 5′-end −11426 | 93054575 | 0.283 |
| rs17300539 | R = A/G | 5′-end −11391 | 93054610 | 0.045 |
| rs266729 | S = C/G | 5′-end −11377 | 93054624 | 0.368 |
| rs182052 | R = A/G | Intron 1 | 93055932 | 0.486 |
| rs710445 | R = A/G | Intron 1 | 93056668 | 0.498 |
| rs16861205 | R = A/G | Intron 1 | 93056784 | 0.251 |
| rs16861209 | M = A/C | Intron 1 | 93058264 | 0.052 |
| rs822391 | Y = C/T | Intron 1 | 93058953 | 0.160 |
| rs822393 | Y = C/T | Intron 1 | 93061476 | 0.490 |
| rs16861210 | R = A/G | Intron 1 | 93061648 | 0.131 |
| rs822394 | M = A/C | Intron 1 | 93061878 | 0.121 |
| rs822395 | M = A/C | Intron 1 | 93061957 | 0.417 |
| rs822396 | R = A/G | Intron 1 | 93062027 | 0.238 |
| rs17366499 | R = A/G | Intron 1 | 93062224 | 0.014 |
| rs12495941 | K = G/T | Intron 1 | 93063330 | 0.453 |
| rs7649121 | W = A/T | Intron 1 | 93063935 | 0.422 |
| rs7627128 | M = A/C | Intron 1 | 93063949 | 0.369 |
| rs9877202 | R = A/G | Intron 1 | 93064757 | 0.114 |
| rs2036373 | K = G/T | Intron 1 | 93065341 | 0.061 |
| rs17366568 | R = A/G | Intron 1 | 93065603 | 0.068 |
| rs16861220 | R = A/G | Intron 1 | 93065662 | 0.014 |
| rs16861222 | Y = C/T | Intron 1 | 93065691 | 0.014 |
| rs17366653 | Y = C/T | Intron 1 | 93065966 | 0.014 |
| rs182052 | R = A/G | Intron 1 | 93055932 | 0.486 |
| rs710445 | R = A/G | Intron 1 | 93056668 | 0.498 |
| rs2241766 | K = G/T G45G | exon 2 | 93066942 | 0.397 |
| rs1501299 | M = A/C | Intron 2 +276 | 93066273 | 0.426 |
| rs2241767 | R = A/G | Intron 2 | 93066346 | 0.242 |
| rs3821799 | Y = C/T | Intron 2 | 93066636 | 0.496 |
| rs3774261 | R = A/G | Intron 2 | 93066709 | 0.499 |
| rs3774262 | R = A/G | Intron 2 | 93066964 | 0.239 |
| rs17366743 | Y = C/T H111Y | exon 3 | 93067239 | 0.046 |
| rs4068 | Y = C/T | 3′-UTR | 93067708 | 0.014 |
| rs1501298 | Y = C/T | 3′-UTR | 93067792 | 0.393 |
| rs6444172 | M = A/C | 3′-UTR | 93068221 | 0.014 |
| rs6444174 | Y = C/T | 3′-UTR | 93068339 | 0.117 |
| rs6773957 | R = A/G | 3′-UTR | 93068855 | 0.499 |
| rs1063537 | Y = C/T | 3′-UTR | 93069225 | 0.242 |
| rs2082940 | Y = C/T | 3′-UTR | 93069314 | 0.312 |
| rs1063538 | Y = C/T | 3′-UTR | 93069333 | 0.499 |
| rs1063539 | S = C/G | 3′-UTR | 93070542 | 0.251 |
| rs9842733 | W = A/T | 3′-UTR | 93070632 | 0.074 |
Notes:
The codes of SNP types are designed by American Association of Biochemistry.
The contig reference ID is NT_005612.15. Data are represented from dbSNP database. Those six SNP with bold ID numbers are found to be importantly associated with metabolic disorders.
Table 2.
Association between adiponectin genetic polymorphisms and metabolic disorders.
| SNP | Clinically associated | Ethnic group |
|---|---|---|
| rs16861194 | Adiponectin levels | French Caucasians, Swedish, |
| −11426 A/G | Type 2 diabetes | European Caucasians |
| rs17300539 | Adiponectin levels | French Caucasians |
| −11391 A/G | Type 2 diabetes | UK Caucasian women |
| Obesity | German, Italian | |
| Insulin resistance | Black South Africans | |
| Insulin sensitivity | Spanish, Polish | |
| Diabetic nephropathy | ||
| rs266729 | Adiponectin levels | French Caucasians |
| −11377 C/G | Type 2 diabetes | Swedish, Danish |
| Obesity | German, Italian | |
| Insulin resistance | Black South African | |
| Insulin sensitivity | Chinese, Spanish | |
| Diabetic nephropathy | Polish | |
| rs2241766 | Adiponectin levels | Japanese, Chinese |
| G/T G45G | Fasting glucose levels | Korean, Italian |
| Type 2 diabetes | Quebec family study | |
| Obesity | Uygurs, Swedish, Finnish | |
| Insulin resistance | African Americans | |
| Insulin sensitivity | Spanish, German | |
| Diabetic rentiopathy | European Caucasians | |
| Diabetic nephropathy | Polish | |
| Atherosclerosis | ||
| Cardiovascular diseases | ||
| rs1501299 | Adiponectin levels | European Caucasians |
| +276 A/C | Type 2 diabetes | Japanese, Italian, German |
| Obesity | Chinese, Korean | |
| Insulin resistance | Quebec family study | |
| Insulin sensitivity | Swedish, Finnish | |
| Diabetic nephropathy | African Americans | |
| Polycystic ovary syndrome | Uygurs, Chinese, Spanish Polish | |
| Cardiovascular diseases | ||
| rs17366743 | Adiponectin levels | German, Polish |
| C/T H111Y | Body mass index | Finnish |
| Type 2 diabetes | Framingham Americans |
Both T1D and T2D are heterogeneous disorders and the heterogeneity affects the insulin secretion and insulin action in glucose metabolism. Epidemiological studies indicate that there is the association between T1D and T2D.88 Adiponectin has a role on β-cell dysfunction.89,90 However, whether the AdipoQ gene also confers genetic susceptibility to development of T1D is unknown. A recent genetic association study of the AdipoQ gene in Swedish T1D patients has been performed. Results indicate that SNPs +45G15G(T/G) and +276(G/T) are strongly associated with T1D. Analyses based on haplotypic genotypes (diplotypes) constructed with these two SNPs including +45T/G and +276G/T and another two promoter SNPs i.e. −11426(A/G), −11377(G/C) indicated that common haplotypes are associated with T1D.91 Thus, the AdipoQ gene may confer the common susceptibility in the development of both T1D and T2D.
Interestingly, +276(G/T), as a putative functional SNP (pf-SNP), is found to be associated with plasma adiponectin levels in T2D,74 T1D91 and also in nonalcoholic steatohepatitis (NASH).92 The promoter polymorphisms, including −11426A/G, −11377C/G and −11391G/A, are also found to be associated with are associated with circulating adiponectin levels in diabetes and obesity. The haplotype with the minor allele in all three promoter polymorphisms show a complete loss of promoter activity.83,85,86 Thus, SNPs in the promoter and intron 2 in the AdipoQ gene play a functional role on the regulation of adiponectin.
Evidence indicates that the putative functional SNP (pf-SNP) +276G/T and promoter polymorphisms in the AdipoQ gene are associated with circulating adiponectin levels. These polymorphisms functionally regulate adiponectin promoter activity and its protein levels in blood. Knowledge of cellular mechanism how these polymorphisms regulate plasma adiponectin levels is still limited.
It has been demonstrated that SP1 belongs to the SP family of transcriptional factors, and is ubiquitously expressed in mammalian cells.93,94 Barth et al have previously reported that SP1 binding activity is enhanced during adipocyte differentiation, and has stimulatory effects on the adiponectin promoter activity.95 Figure 2 represents the promoter sequence and its polymorphisms in the AdipoQ gene. Recently, evidence has indicated that the allele G of SNP −11377C/G alters the sequence for one of four SP1 binding sites.96 The risk G allele results in abolishing of SP1 binding and subsequently causes reduction of adiponectin activity by ~25%.82 Beside SNP −11377C/G, −11391A/G is another promoter polymorphism and has moderate association with T1D diabetic nephropathy among Danish cohort but not in Finnish and French populations.97 Further investigation for understanding correlation between these two promoter polymorphisms and novel binding site has been taken into our consideration.
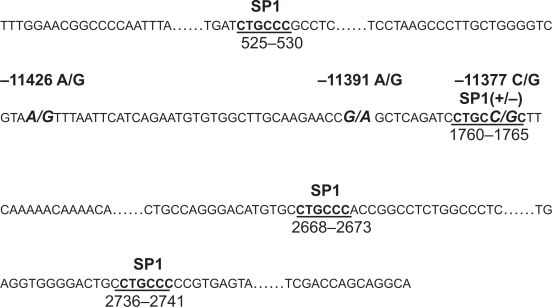
Figure 2.
The adiponectin promoter polymorphisms and the binding sites for transcriptional factor SP1.
Promoter polymorphism −11377C/G resides one of transcriptional stimulatory protein (SP1) binding sites. The G allele interfere the binding function and consequently reduces the adiponectin promoter activity.
Although association of the AdipoQ genetic variation with plasma adiponectin levels in T2D and T1D has been well studied, further investigation is still under consideration in order to fully understand the relationship between genetic role and biological regulation of adiponectin. Indeed, regulation of plasma adiponectin levels is complex and it is under a multigenic control. Recent research has indicated that PPAR γ (P12A) and IRS-1 (G972R) genetic polymorphisms interacts the influence plasma adiponectin concentrations in young Finnish men.98 A linkage analysis reveals that there are two additional loci on chromosomes 14q13 and 5p15 except of the AdipoQ and PPAR γ genes are linked with plasma adiponectin levels.99 Identification of the genes in chromosomal fragments of 14q13 and 5p15 that are linked to plasma adiponectin levels may provide further information for better understanding regulation of adiponectin.
This figure is modified from Zhang et al 2009. The partial sequence from Homo sapiens adiponectin promoter (AJ011119.1, GI: 5823971) is represented. Three adiponectin promoter polymorphisms including −11426A/G, −11391G/A and −11377C/G are shown with block and italic letters. The binding sites for transcriptional factor SP1 are indicated with block letters and underline. Four binding sites for SP1 are located in g.525–530, 1760–1765, 2668–2673 and 2736–2741. SNP-11377C/G is involved in the sequence of SP1 transcriptional binding site at g.1760–1765. SP1 binding activity is enhanced during adipocyte differentiation, and has stimulatory effects on the adiponectin promoter activity (Barth et al 2002). The allele G of SNP −11377C/G alters the sequence for one of SP1 binding sites, which results in reduction of the adiponectin promoter activity by ~25% (Bouatia-Naji et al 2006).
Summary
Taking together, biological and genetic studies have demonstrated that adiponectin plays an important role in pathogenesis of diabetes, obesity and insulin resistance. Plasma adiponectin levels are significantly decreased in T2D patients and obese subjects compared to the healthy individuals. Genetic polymorphisms in LD blocks of the AdipoQ gene, including the promoter region and the boundary of exon 2-intron 2, are associated with T2D, obesity, DN and insulin resistance. Therefore, plasma/serum adiponectin levels and genomic DNA polymorphisms in the AdipoQ gene can be used as the biomarkers for early diagnosis and clinic prediction of diabetes, obesity, diabetic complications and other metabolic disorders. Evaluation of adiponectin levels with the ratio of HMW/(LMW and MMW) and consideration of different ethnic genetic backgrounds are of importance in the translation research of adiponectin.
Databases
OMIM: Online Mendelian Inheritance in Man http://www.ncbi.nlm.nih.gov/sites/entrez?db=OMIMdbSNP: database of Single Nucleotide Polymorphism http://www.ncbi.nlm.nih.gov/SNP/
Abbreviations
- AdipoQ
adiponectin;
- BMI
body mass index;
- BAT
brown adipose tissue;
- DN
diabetic nephropathy;
- ESRD
end stage renal disease;
- IGT
impaired glucose tolerance;
- LD
linkage disequilibrium;
- NASH
nonalcoholic steatohepatitis;
- PPARγ
peroxisome proliferator-activated receptor-gamma;
- pGDM
prior gestational diabetes mellitus;
- PCOS
polycystic ovary syndrome;
- pf-SNP
putative functional SNP;
- SNP
single nucleotide polymorphism;
- T1D
type 1 diabetes;
- T2D
type 2 diabetes;
- SP1
transcriptional stimulatory protein;
- TG
triglyceride;
- UTR
untraslated region,
- WAT
white adipose tissue.
Footnotes
Disclosure
The author reports no conflicts of interest.
References
- 1.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89(6):2548–56. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 2.http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=164160
- 3.http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=134350
- 4.http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=191160
- 5.http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=605441
- 6.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM.Positional cloning of the mouse obese gene and its human homologue Nature 1994. 1;3726505425–32. [DOI] [PubMed] [Google Scholar]
- 7.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–9. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 8.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose collagen-like factor, apM1. Biochem Biophys Res Commun. 1996;221:286–9. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 9.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 10.Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem. 1996;120:803–12. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- 11.Saito K, Tobe T, Minoshima S, et al. Organization of the gene for gelatinbinding protein (GBP28) Gene. 1999;229:67–73. doi: 10.1016/s0378-1119(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 12.Schaffler A, Orso E, Palitzsch KD, et al. The human apM-1, an adipocyte-specific gene linked to the family of TNF’s and to genes expressed in activated T cells, is mapped to chromosome 1q21.3–q23, a susceptibility locus identified for familial combined hyperlipidaemia (FCH) Biochem Biophys Res Commun. 1999;260:416–25. doi: 10.1006/bbrc.1999.0865. [DOI] [PubMed] [Google Scholar]
- 13.Das K, Lin Y, Widen E, Zhang Y, Scherer PE. Chromosomal localization, expression pattern, and promoter analysis of the mouse gene encoding adipocyte-specific secretory protein Acrp30. Biochem Biophys Res Commun. 2001;280:1120–9. doi: 10.1006/bbrc.2001.4217. [DOI] [PubMed] [Google Scholar]
- 14.Tsao TS, Murrey HE, Hug C, Lee DH, Lodish HF. Oligomerization state-dependent activation of NF-κB signaling pathway by adipocyte complement-related protein of 30 kDa(Acrp30) J Biol Chem. 2002;277:29359–62. doi: 10.1074/jbc.C200312200. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Xu A, Knight C, Xu LY, Cooper GJ. Hydroxylation and glycosylation of the four conserved lysine residues in the collagenous domain of adiponectin. Potential role in the modulation of its insulin-sensitizing activity. J Biol Chem. 2002;277:19521–9. doi: 10.1074/jbc.M200601200. [DOI] [PubMed] [Google Scholar]
- 16.Tsao TS, Tomas E, Murrey HE, et al. Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity. Different oligomers activate different signal transduction pathways. J Biol Chem. 2003;278:50810–7. doi: 10.1074/jbc.M309469200. [DOI] [PubMed] [Google Scholar]
- 17.Pajvani UB, Du X, Combs TP, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–85. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 18.Wong GW, Wang J, Hug C, Tsao TS, Lodish HF. A family of Acrp30/adiponectin structural and functional paralogs. Proc Natl Acad Sci U S A. 2004;101:10302–7. doi: 10.1073/pnas.0403760101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waki H, Yamauchi T, Kamon J, et al. Generation of globular fragment of adiponectin by leukocyte elastase secreted by monocytic cell line THP-1. Endocrinology. 2005;146:790–6. doi: 10.1210/en.2004-1096. [DOI] [PubMed] [Google Scholar]
- 20.Sato C, Yasukawa Z, Honda N, Matsuda T, Kitajima K.Identification and adipocyte differentiation-dependent expression of the unique disialic acid residue in an adipose tissue-specific glycoprotein, adipo Q J Biol Chem 2001. 3;2763128849–56. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Lam KS, Chan L, et al. Post-translational modifications of the four conserved lysine residues within the collagenous domain of adiponectin are required for the formation of its high molecular weight oligomeric complex J Biol Chem 2006. 16;2812416391–400. [DOI] [PubMed] [Google Scholar]
- 22.Waki H, Yamauchi T, Kamon J, et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278(41):40352–63. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 23.Ouchi N, Kihara S, Arita Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100(25):2473–6. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 24.Das K, Lin Y, Widen E, Zhang Y, Scherer PE.Chromosomal localization, expression pattern, and promoter analysis of the mouse gene encoding adipocyte-specific secretory protein Acrp30 Biochem Biophys Res Commun 2001;2;28041120–9. [DOI] [PubMed] [Google Scholar]
- 25.Yu S, Matsusue K, Kashireddy P, et al. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma1) overexpression. J Biol Chem. 2003;278(1):498–505. doi: 10.1074/jbc.M210062200. [DOI] [PubMed] [Google Scholar]
- 26.Kubota N, Terauchi Y, Miki H, et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4(4):597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 27.Yamauchi T, Kamon J, Waki H, et al. The mechanisms by which both heterozygous peroxisome proliferator-activated receptor gamma (PPARgamma) deficiency and PPARgamma agonist improve insulin resistance. J Biol Chem. 2001;276(44):41245–54. doi: 10.1074/jbc.M103241200. [DOI] [PubMed] [Google Scholar]
- 28.Sivan E, Mazaki-Tovi S, Pariente C, et al. Adiponectin in human cord blood: relation to fetal birth weight and gender. J Clin Endocrinol Metab. 2003;88(12):5656–60. doi: 10.1210/jc.2003-031174. [DOI] [PubMed] [Google Scholar]
- 29.Kamoda T, Saitoh H, Saito M, Sugiura M, Matsui A. Serum adiponectin concentrations in newborn infants in early postnatal life. Pediatr Res. 2004;56(5):690–3. doi: 10.1203/01.PDR.0000142711.24999.8A. [DOI] [PubMed] [Google Scholar]
- 30.Tsai PJ, Yu CH, Hsu SP, et al. Cord plasma concentrations of adiponectin and leptin in healthy term neonates: positive correlation with birthweight and neonatal adiposity. Clin Endocrinol (Oxf) 2004;61(1):88–93. doi: 10.1111/j.1365-2265.2004.02057.x. [DOI] [PubMed] [Google Scholar]
- 31.Kotani Y, Yokota I, Kitamura S, Matsuda J, Naito E, Kuroda Y. Plasma adiponectin levels in newborns are higher than those in adults and positively correlated with birth weight. Clin Endocrinol (Oxf) 2004;61(4):418–23. doi: 10.1111/j.1365-2265.2004.02041.x. [DOI] [PubMed] [Google Scholar]
- 32.Tsou PL, Jiang YD, Chang CC, et al. Sex-related differences between adiponectin and insulin resistance in schoolchildren. Diabetes Care. 2004;27(2):308–13. doi: 10.2337/diacare.27.2.308. [DOI] [PubMed] [Google Scholar]
- 33.Nishimura R, Sano H, Matsudaira T, et al. Changes in body mass index, leptin and adiponectin in Japanese children during a three-year follow-up period: a population-based cohort study. Cardiovasc Diabetol. 2009;8:30. doi: 10.1186/1475-2840-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woo JG, Dolan LM, Daniels SR, Goodman E, Martin LJ. Adolescent sex differences in adiponectin are conditional on pubertal development and adiposity. Obes Res. 2005;13(12):2095–101. doi: 10.1038/oby.2005.260. [DOI] [PubMed] [Google Scholar]
- 35.Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52(7):1779–85. doi: 10.2337/diabetes.52.7.1779. [DOI] [PubMed] [Google Scholar]
- 36.Mannucci E, Ognibene A, Cremasco F, et al. Plasma adiponectin and hyperglycaemia in diabetic patients. Clin Chem Lab Med. 2003;41(9):1131–5. doi: 10.1515/CCLM.2003.175. [DOI] [PubMed] [Google Scholar]
- 37.Silha JV, Krsek M, Skrha J, Sucharda P, Nyomba BL, Murphy LJ. Plasma resistin, leptin and adiponectin levels in non-diabetic and diabetic obese subjects. Diabet Med. 2004;21(5):497–9. doi: 10.1111/j.1464-5491.2004.01178.x. [DOI] [PubMed] [Google Scholar]
- 38.Schulze MB, Rimm EB, Shai I, Rifai N, Hu FB. Relationship between adiponectin and glycemic control, blood lipids, and inflammatory markers in men with type 2 diabetes. Diabetes Care. 2004;27(7):1680–7. doi: 10.2337/diacare.27.7.1680. [DOI] [PubMed] [Google Scholar]
- 39.Shetty GK, Economides PA, Horton ES, Mantzoros CS, Veves A. Circulating adiponectin and resistin levels in relation to metabolic factors, inflammatory markers, and vascular reactivity in diabetic patients and subjects at risk for diabetes. Diabetes Care. 2004;27(10):2450–7. doi: 10.2337/diacare.27.10.2450. [DOI] [PubMed] [Google Scholar]
- 40.Mojiminiyi OA, Abdella NA, Al Arouj M, Ben Nakhi A. Adiponectin, insulin resistance and clinical expression of the metabolic syndrome in patients with Type 2 diabetes. Int J Obes (Lond) 2007;31(2):213–20. doi: 10.1038/sj.ijo.0803355. [DOI] [PubMed] [Google Scholar]
- 41.Aguilar-Salinas CA, García EG, Robles L, et al. High adiponectin concentrations are associated with the metabolically healthy obese phenotype. J Clin Endocrinol Metab. 2008;93(10):4075–9. doi: 10.1210/jc.2007-2724. [DOI] [PubMed] [Google Scholar]
- 42.Lindsay RS, Funahashi T, Krakoff J, et al. Genome-wide linkage analysis of serum adiponectin in the Pima Indian population. Diabetes. 2003;52(9):2419–25. doi: 10.2337/diabetes.52.9.2419. [DOI] [PubMed] [Google Scholar]
- 43.Costacou T, Bosnyak Z, Harger GF, Markovic N, Silvers N, Orchard TJ. Postpartum adiponectin concentration, insulin resistance and metabolic abnormalities among women with pregnancy-induced disturbances. Prev Cardiol. 2008;11(2):106–15. doi: 10.1111/j.1751-7141.2008.07512.x. [DOI] [PubMed] [Google Scholar]
- 44.Heitritter SM, Solomon CG, Mitchell GF, Skali-Ounis N, Seely EW. Subclinical inflammation and vascular dysfunction in women with previous gestational diabetes mellitus. J Clin Endocrinol Metab. 2005;90(7):3983–8. doi: 10.1210/jc.2004-2494. [DOI] [PubMed] [Google Scholar]
- 45.Retnakaran R, Hanley AJ, Raif N, et al. Adiponectin and beta cell dysfunction in gestational diabetes: pathophysiological implications. Diabetologia. 2005;48(5):993–1001. doi: 10.1007/s00125-005-1710-x. [DOI] [PubMed] [Google Scholar]
- 46.Osei K, Gaillard T, Schuster D. Plasma adiponectin levels in high risk African-Americans with normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes. Obes Res. 2005;13(1):179–85. doi: 10.1038/oby.2005.23. [DOI] [PubMed] [Google Scholar]
- 47.Furuhashi M, Ura N, Moniwa N, et al. Possible impairment of transcardiac utilization of adiponectin in patients with type 2 diabetes. Diabetes Care. 2004;27(9):2217–21. doi: 10.2337/diacare.27.9.2217. [DOI] [PubMed] [Google Scholar]
- 48.Takano H, Kodama Y, Kitta Y, et al. Transcardiac adiponectin gradient is independently related to endothelial vasomotor function in large and resistance coronary arteries in humans. Am J Physiol Heart Circ Physiol. 2006;291(6):H2641–6. doi: 10.1152/ajpheart.00702.2006. [DOI] [PubMed] [Google Scholar]
- 49.Perseghin G, Lattuada G, Danna M, et al. Insulin resistance, intramyocellular lipid content, and plasma adiponectin in patients with type 1 diabetes. Am J Physiol Endocrinol Metab. 2003;285(6):E1174–81. doi: 10.1152/ajpendo.00279.2003. [DOI] [PubMed] [Google Scholar]
- 50.Morales A, Wasserfall C, Brusko T, et al. Adiponectin and leptin concentrations may aid in discriminating disease forms in children and adolescents with type 1 and type 2 diabetes. Diabetes Care. 2004;27(8):2010–4. doi: 10.2337/diacare.27.8.2010. [DOI] [PubMed] [Google Scholar]
- 51.Schäffler A, Herfarth H, Paul G, et al. Identification of influencing variables on adiponectin serum levels in diabetes mellitus type 1 and type 2. Exp Clin Endocrinol Diabetes. 2004;112(7):383–9. doi: 10.1055/s-2004-821029. [DOI] [PubMed] [Google Scholar]
- 52.Imagawa A, Funahashi T, Nakamura T, et al. Elevated serum concentration of adipose-derived factor, adiponectin, in patients with type 1 diabetes. Diabetes Care. 2002;25(9):1665–6. doi: 10.2337/diacare.25.9.1665. [DOI] [PubMed] [Google Scholar]
- 53.Lindstrom T, Frystyk J, Hedman CA, Flyvbjerg A, Arnqvist HJ. Elevated circulating adiponectin in type 1 diabetes is associated with long diabetes duration. Clin Endocrinol (Oxf) 2006;65(6):776–82. doi: 10.1111/j.1365-2265.2006.02666.x. [DOI] [PubMed] [Google Scholar]
- 54.Saraheimo M, Forsblom C, Fagerudd J, et al. FinnDiane study group. Serum adiponectin is increased in type 1 diabetic patients with nephropathy. Diabetes Care. 2005 Jun;28(6):1410–4. doi: 10.2337/diacare.28.6.1410. [DOI] [PubMed] [Google Scholar]
- 55.Frystyk J, Tarnow L, Hansen TK, Parving HH, Flyvbjerg A. Increased serum adiponectin levels in type 1 diabetic patients with microvascular complications. Diabetologia. 2005;48(9):1911–8. doi: 10.1007/s00125-005-1850-z. [DOI] [PubMed] [Google Scholar]
- 56.Ljubic S, Boras J, Jazbec A, et al. Adiponectin has different mechanisms in type 1 and type 2 diabetes with C-peptide link Clin Invest Med 2009;1;324E271–9. [DOI] [PubMed] [Google Scholar]
- 57.Goldberg RB.Cytokine and Cytokine-like Inflammation Markers, Endothelial Dysfunction and Imbalanced Coagulation in Development of Diabetes and Its Complications J Clin Endocrinol Metab 2009June9[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 58.Nayak S, Soon SQ, Kunjal R, et al. Relationship between adiponectin, inflammatory markers and obesity in type 2 diabetic and non-diabetic Trinidadians. Arch Physiol Biochem. 2009;115(1):28–33. doi: 10.1080/13813450902758785. [DOI] [PubMed] [Google Scholar]
- 59.Waki H, Yamauchi T, Kamon J, et al. Impaired multimerization of human adiponectin mutants associated with diabetes: Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–63. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 60.Pajvani UB, Du X, Combs TP, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–85. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 61.Iwase M, Iino K, Oku M, et al. Serum high-molecular weight adiponectin is related to early postprandial glycemic increases and gastric emptying in patients with type 2 diabetes mellitus. Diabetes Metab Res Rev. 2009;25(4):344–50. doi: 10.1002/dmrr.954. [DOI] [PubMed] [Google Scholar]
- 62.Blüher M, Brennan AM, Kelesidis T, et al. Total and high-molecular weight adiponectin in relation to metabolic variables at baseline and in response to an exercise treatment program: comparative evaluation of three assays. Diabetes Care. 2007;30(2):280–5. doi: 10.2337/dc06-1362. [DOI] [PubMed] [Google Scholar]
- 63.Vionnet N, Hani ElH, Dupont S, et al. Genomewide search for T2D-susceptibility genes in French whites: evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q27-qter and independent replication of a type 2-diabetes locus on chromosome 1q21–q24. Am J Hum Genet. 2000;67:1470–80. doi: 10.1086/316887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Francke S, Manraj M, Lacquemant C, et al. A genome-wide scan for coronary heart disease suggests in Indo-Mauritians a susceptibility locus on chromosome 16p13 and replicates linkage with the metabolic syndrome on 3q27. Hum Mol Genet. 2001;10:2751–65. doi: 10.1093/hmg/10.24.2751. [DOI] [PubMed] [Google Scholar]
- 65.Francke S, Manraj M, Lacquemant C, et al. A genome-wide scan for coronary heart disease suggests in Indo-Mauritians a susceptibility locus on chromosome 16p13 and replicates linkage with the metabolic syndrome on 3q27. Hum Mol Genet. 2001;10:2751–65. doi: 10.1093/hmg/10.24.2751. [DOI] [PubMed] [Google Scholar]
- 66.Chiodini BD, Lewis CM. Meta-analysis of 4 coronary heart disease genome-wide linkage studies confirms a susceptibility locus on chromosome 3q. Arterioscler Thromb Vasc Biol. 2003;23:1863–8. doi: 10.1161/01.ATV.0000093281.10213.DB. [DOI] [PubMed] [Google Scholar]
- 67.DeWan AT, Arnett DK, Atwood LD, et al. A genome scan for renal function among hypertensives: the HyperGEN study. Am J Hum Genet. 2001;68:136–44. doi: 10.1086/316927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moczulski DK, Rogus JJ, Antonellis A, Warram JH, Krolewski AS. Major susceptibility locus for nephropathy in type 1 diabetes on chromosome 3q: results of novel discordant sib-pair analysis. Diabetes. 1998;47(7):1164–9. doi: 10.2337/diabetes.47.7.1164. [DOI] [PubMed] [Google Scholar]
- 69.Bowden DW, Colicigno CJ, Langefeld CD, et al. A genome scan for diabetic nephropathy in African Americans. Kidney Int. 2004;66(4):1517–26. doi: 10.1111/j.1523-1755.2004.00915.x. [DOI] [PubMed] [Google Scholar]
- 70.Chung KW, Ferrell RE, Ellis D, et al. African American hypertensive nephropathy maps to a new locus on chromosome 9q31–q32. Am J Hum Genet. 2003;73(2):420–9. doi: 10.1086/377184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Imperatore G, Hanson RL, Pettitt DJ, Kobes S, Bennett PH, Knowler WC. The Pima Diabetes Genes Group: Sib-pair linkage analysis for susceptibility genes for microvascular complications among Pima Indians with type 2 diabetes. Diabetes. 1998;47:821–30. doi: 10.2337/diabetes.47.5.821. [DOI] [PubMed] [Google Scholar]
- 72.http://www.hapmap.org/cgi-perl/gbrowse/hapmap27_B36/
- 73.Heid IM, Wagner SA, Gohlke H, et al. Genetic architecture of the APM1 gene and its influence on adiponectin plasma levels and parameters of the metabolic syndrome in 1,727 healthy Caucasians. Diabetes. 2006;55(2):375–84. doi: 10.2337/diabetes.55.02.06.db05-0747. [DOI] [PubMed] [Google Scholar]
- 74.Hara K, Boutin P, Mori Y, et al. Genetic variation in the gene encoding adiponectin is associated with an increased risk of type 2 diabetes in the Japanese population. Diabetes. 2002;51:536–40. doi: 10.2337/diabetes.51.2.536. [DOI] [PubMed] [Google Scholar]
- 75.Vasseur F, Helbecque N, Dina C, et al. Single-nucleotide polymorphism haplotypes in the both proximal promoter and exon 3 of the AdipoQ gene modulate adipocyte-secreted adiponectin hormone levels and contribute to the genetic risk for type 2 diabetes in French Caucasians. Hum Mol Genet. 2002;11:2607–14. doi: 10.1093/hmg/11.21.2607. [DOI] [PubMed] [Google Scholar]
- 76.Ohashi K, Ouchi N, Kihara S, et al. Adiponectin I164T mutation is associated with the metabolic syndrome and coronary artery disease J Am Coll Cardiol 2004;7;4371195–200. [DOI] [PubMed] [Google Scholar]
- 77.Zacharova J, Chiasson JL, Laakso M. STOP-NIDDM Study Group. The common polymorphisms (single nucleotide polymorphism [SNP] +45 and SNP +276) of the adiponectin gene predict the conversion from impaired glucose tolerance to type 2 diabetes: the STOP-NIDDM trial. Diabetes. 2005;54(3):893–9. doi: 10.2337/diabetes.54.3.893. [DOI] [PubMed] [Google Scholar]
- 78.Schwarz PE, Govindarajalu S, Towers W, et al. Haplotypes in the promoter region of the AdipoQ gene are associated with increased diabetes risk in a German Caucasian population. Horm Metab Res. 2006;38(7):447–51. doi: 10.1055/s-2006-947842. [DOI] [PubMed] [Google Scholar]
- 79.Bacci S, Menzaghi C, Ercolino T, et al. The +276G/T single nucleotide polymorphism of the adiponectin gene is associated with coronary artery disease in type 2 diabetic patients. Diabetes Care. 2004;27(8):2015–20. doi: 10.2337/diacare.27.8.2015. [DOI] [PubMed] [Google Scholar]
- 80.Lee YY, Lee NS, Cho YM, et al. Genetic association study of adiponectin polymorphisms with risk of Type 2 diabetes mellitus in Korean population. Diabet Med. 2005;22(5):569–75. doi: 10.1111/j.1464-5491.2005.01460.x. [DOI] [PubMed] [Google Scholar]
- 81.González-Sánchez JL, Martínez-Calatrava MJ, Martínez-Larrad MT, et al. Interaction of the −308G/A promoter polymorphism of the tumor necrosis factor-alpha gene with single-nucleotide polymorphism 45 of the adiponectin gene: effect on serum adiponectin concentrations in a Spanish population. Clin Chem. 2006;52(1):97–103. doi: 10.1373/clinchem.2005.049452. [DOI] [PubMed] [Google Scholar]
- 82.Menzaghi C, Ercolino T, Di Paola R, et al. A haplotype at the adiponectin locus is associated with obesity and other features of the insulin resistance syndrome. Diabetes. 2002;51(7):2306–12. doi: 10.2337/diabetes.51.7.2306. [DOI] [PubMed] [Google Scholar]
- 83.Bouatia-Naji N, Meyre D, Lobbens S, et al. ACDC/adiponectin polymorphisms are associated with severe childhood and adult obesity. Diabetes. 2006;55(2):545–50. doi: 10.2337/diabetes.55.02.06.db05-0971. [DOI] [PubMed] [Google Scholar]
- 84.Gu HF, Abulaiti A, Ostenson CG, et al. Single nucleotide SNPs in the proximal promoter region of the adiponectin (APM1) gene are associated with T2D in Swedish caucasians. Diabetes. 2004;53(Suppl 1):31–5. doi: 10.2337/diabetes.53.2007.s31. [DOI] [PubMed] [Google Scholar]
- 85.Vasseur F, Helbecque N, Lobbens S, et al. Hypoadiponectinaemia and high risk of type 2 diabetes are associated with adiponectin-encoding (AdipoQ) gene promoter variants in morbid obesity: evidence for a role of AdipoQ in diabesity. Diabetologia. 2005;48:892–9. doi: 10.1007/s00125-005-1729-z. [DOI] [PubMed] [Google Scholar]
- 86.Laumen H, Saningong AD, Heid IM, et al. Functional characterization of promoter variants of the adiponectin gene complemented by epidemiological data. Diabetes. 2009;58(4):984–91. doi: 10.2337/db07-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Owecki M, Miczke A, Kaczmarek M, et al. The Y111 H (T415C) polymorphism in exon 3 of the gene encoding adiponectin is uncommon in Polish obese patients. Horm Metab Res. 2007;39(11):797–800. doi: 10.1055/s-2007-991155. [DOI] [PubMed] [Google Scholar]
- 88.Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol. 2007;166(5):495–505. doi: 10.1093/aje/kwm106. [DOI] [PubMed] [Google Scholar]
- 89.Kharroubi I, Rasschaert J, Eizirik DL, Cnop M.Expression of adiponectin receptors in pancreatic beta cells Biochem Biophys Res Commun 2003;26;31241118–22. [DOI] [PubMed] [Google Scholar]
- 90.Thamer C, Haap M, Heller E, et al. Beta cell function, insulin resistance and plasma adiponectin concentrations are predictors for the change of postprandial glucose in non-diabetic subjects at risk for type 2 diabetes. Horm Metab Res. 2006;38(3):178–82. doi: 10.1055/s-2006-925204. [DOI] [PubMed] [Google Scholar]
- 91.Ma J, Möllsten A, Falhammar H, et al. Genetic association analysis of the adiponectin polymorphisms in type 1 diabetes with and without diabetic nephropathy. J Diabetes Complications. 2007;21(1):28–33. doi: 10.1016/j.jdiacomp.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 92.Musso G, Gambino R, De Michieli F, Durazzo M, Pagano G, Cassader M. Adiponectin gene polymorphisms modulate acute adiponectin response to dietary fat: Possible pathogenetic role in NASH. Hepatology. 2008;47(4):1167–77. doi: 10.1002/hep.22142. [DOI] [PubMed] [Google Scholar]
- 93.Carninci P, Sandelin A, Lenhard B, et al. Genome-wide analysis of mammalian promoter architecture and evolution. Nat Genet. 2006;38(6):626–35. doi: 10.1038/ng1789. [DOI] [PubMed] [Google Scholar]
- 94.Li L, He S, Sun JM, Davie JR. Gene regulation by Sp1 and Sp3. Biochem Cell Biol. 2004;82(4):460–71. doi: 10.1139/o04-045. [DOI] [PubMed] [Google Scholar]
- 95.Barth N, langmann T, Scholmerich J, Schmitz G, Schaffler A. Identification of regulatory elements in the human adipose most abundant gene transcript-1 (apM-1) promoter: role of SP1/SP3 and TNF-alpha as regulatory pathways. Diabetologia. 2002;45:1425–33. doi: 10.1007/s00125-002-0895-5. [DOI] [PubMed] [Google Scholar]
- 96.Zhang D, Ma J, Brismar K, Efendic S, Gu HF. A single nucleotide polymorphism alters the sequence of SP1 binding site in the adiponectin promoter region and is associated with diabetic nephropathy among type 1 diabetic patients in the Genetics of Kidneys in Diabetes Study. J Diabetes Complications. 2009;23(4):265–72. doi: 10.1016/j.jdiacomp.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 97.Vionnet N, Tregouet D, Kazeem G, et al. Analysis of 14 candidate genes for DN on chromosome 3q in European populations: strongest evidence for association with a variant in the promoter region of the adiponectin gene. Diabetes. 2006;55:3166–74. doi: 10.2337/db06-0271. [DOI] [PubMed] [Google Scholar]
- 98.Mousavinasab F, Tähtinen T, Jokelainen J, et al. Common polymorphisms in the PPARgamma2 and IRS-1 genes and their interaction influence serum adiponectin concentration in young Finnish men. Mol Genet Metab. 2005;84(4):344–8. doi: 10.1016/j.ymgme.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 99.Menzaghi C, Ercolino T, Salvemini L, et al. Multigenic control of serum adiponectin levels: evidence for a role of the APM1 gene and a locus on 14q13 Physiol Genomics 2004;4;192170–4. [DOI] [PubMed] [Google Scholar]