Abstract
Adult rats and mice born to dams exposed to alcohol (FAE) exhibit enhanced activity of their hypothalamic-pituitary-adrenal (HPA) axis when exposed to stressors. However, the mechanisms responsible for this phenomenon remain uncompletely understood. Here we review two possibilities: one that pertains to nitric oxide (NO), an unstable gas that stimulates the HPA axis; and one that focuses on catecholamines, which also stimulate this axis. We did not observe significant alterations in levels of NO synthase, the enzyme responsible for NO formation, in the PVN of FAE rats. However, the stimulatory influence of this gas on the HPA axis was enhanced in these animals, thereby providing a mechanism likely to participate in the neuroendocrine hyperactivity that is the hallmark of this model. We also recently showed that while the ability of catecholamines to release ACTH was comparable in control rats and rats exposed to alcohol during embryonic development, there was a significant upregulation of the C1 brain stem region when these latter animals were exposed to mild footshocks. As this region sends prominent projections to the PVN, its increased activity may participate in the HPA axis hyperactivity observed in FAE offspring. Finally, we used microarray technology to search for potential differences in genes present in the brains of control and FAE mice. When these brains were collected on day 17.5 of embryonic development, several genes were upregulated while others were downregulated, which may provide potential new candidates that mediate the influence of prenatal alcohol on the HPA axis of adult offspring.
Keywords: alcohol, CRF, ACTH, nitric oxide, catecholamines
The ability of homeostatic challenges present during embryonic development to cause long-term changes in adult offspring is now well recognized. For example, reprogramming of behavioral, metabolic and endocrine functions has been described in rodents born to dams exposed to various stressors, drugs, immune stimuli or food restriction. As elegantly discussed by Simerly,1 “the brain retains the effect of early experience well into adulthood through permanently altered wiring”. This article will address some of the findings obtained in our laboratory in rodents exposed to alcohol during embryonic development, focusing specifically on the ability of this drug to induce a permanent hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis. (Please note that the quoted literature is only meant as illustrative, and not as fully representative of the topics discussed.)
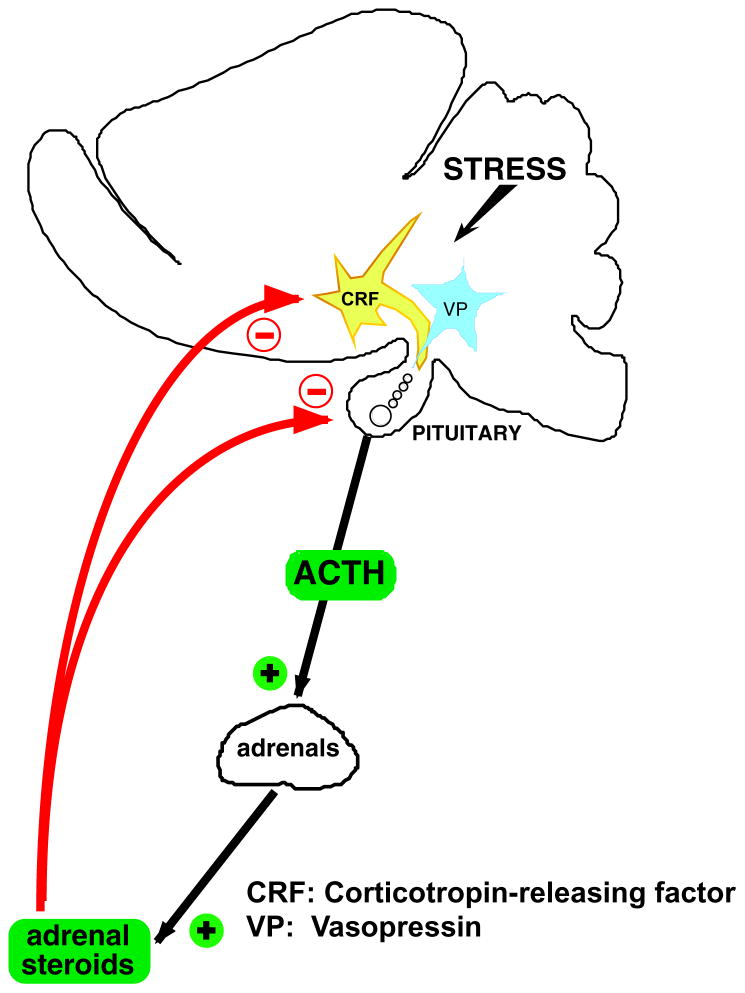
The HPA axis, sometimes called “stress axis”, consists of neurons present in the paraventricular nucleus (PVN) of the hypothalamus, the anterior pituitary and the adrenal glands. As abundantly described elsewhere,2–7 the PVN harbors cell bodies that synthesize the peptides corticotropin-releasing factor (CRF) and vasopressin (VP) that, upon release from terminals in the median eminence, act on cells in the pituitary corticotrophs that release ACTH (Fig. 1). This is followed by secretion of glucocorticosteroids (GC; corticosterone in rats and mice) from the adrenal cortex. Many stimuli, whether they are consciously aversive or represent internal homeostatic threats, activate the HPA axis and if this response is sustained, upregulate the synthesis of CRF and/or VP. The magnitude and duration of HPA axis activation is very tightly controlled by a series of feedback and feedforward mechanisms, and any prolonged dysregulation of these mechanisms results in pathologies.8–10 In the case of CRF, the importance of this peptide in appropriate behavioral responses to stressors,11 in the regulation of sympathetic activity,12, 13 reproductive functions14, 15 and gastrointestinal (GI) functions,16, 17 among others, means that its persistent elevations can lead to mood disorders, disturbances of sympathetic activity, disrupted fertility and diseases of the GI tract. In addition to the consequences of elevated hypothalamic CRF levels, abnormal ACTH/GC secretion due to increased CRF production18 can in themselves disrupt immune cells activity, metabolism, protein synthesis and reproductive functions.
Figure 1.
Cartoon illustrating the HPA axis.
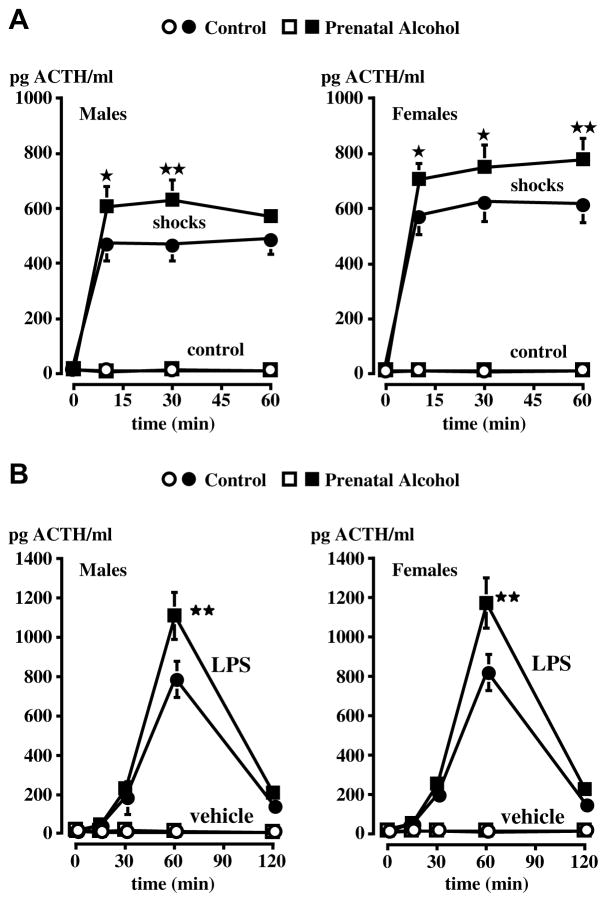
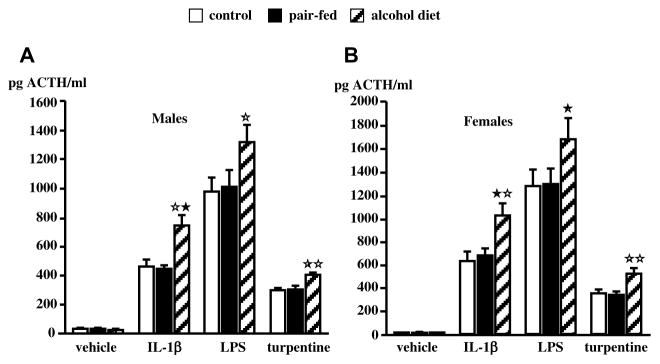
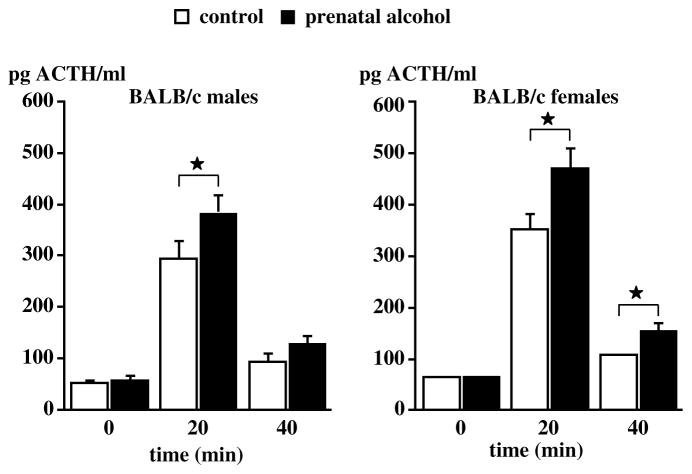
Work carried out in a number of laboratories, including ours, has shown that rats19–24 or mice25 born to dams exposed to alcohol during gestation exhibit abnormally elevated ACTH and corticosterone responses to various stimuli while displaying no or little changes under basal conditions. In the rat, we reported that the drug was most effective when delivered during the second week of embryonic development,26 which corresponds to peak neurogenesis of hypothalamic CRF neurons,27, 28 and that this treatment resulted in increased ACTH release when the adult male or female offspring was exposed to mild footshocks, a pro-inflammatory cytokine or an endotoxin challenge (Fig. 2). This phenomenon is not restricted to offspring born to dams exposed to alcohol vapors, but is also found when the dams were fed alcohol29 (Fig. 3). Finally, in order to show that the ability of prenatal alcohol to alter the offspring’ HPA axis activity was a general phenomenon, i.e., not one restricted to a particular experimental model, we demonstrated that it was also found in mice25 (Fig. 4).
Figure 2.
Upregulated ACTH release in adult male FAE rats exposed to (A) shocks or (B) endotoxin (LPS). In these experiments, the dams were exposed to alcohol vapors during the second week of gestation. A. Effect of the exposure to a 60-min session of mild electrofootshocks (0.5 mA, 1-s duration, 2 shocks/min) on plasma ACTH levels in control (C) and alcohol (E) male or female rats. B. Effect of the iv injection of the vehicle or LPS (0.4 μg/kg) on plasma ACTH levels in C and E male or female rats. Each point represents mean ± SEM of 5–7 animals. *, P<0.05; **, P<0.01 vs C/shocks or C/LPS rats. {Modified by permission from Lee et al.36}
Figure 3.
Upregulated ACTH response to immune stimuli in adult FAE male rats. In these experiments, the dams were fed a liquid alcohol diet during gestation. ACTH released by IL-1β, LPS or turpentine in intact control, pair-fed and alcohol male (A) and female (B) rats tested at 9 weeks of age. Each bar represents the mean ± SEM of 6 animals. Blood samples were obtained 30 min after IL-1β administration, 60 min after LPS injection and 8 h after induction of tissue damage by turpentine. *, P<0.05; **, P<0.01 vs corresponding controls. {Modified by permission from Lee et al.22}
Figure 4.
Upregulated ACTH response to footshocks in adult male mice born to dams exposed to alcohol vapors during the second week of gestation. Male and female mice (8–9 week old) of the BALB/c strain that were born to control dams or dams that were exposed to alcohol during gestation were exposed to a 20 min session of footshocks (0.3 mA, 1-sec duration, 2 shocks/min). The mice were decapitated at the end of the shock session. Each bar represents the mean ± SEM of six (controls) or eight (shocked) animals. *, P<0.05 vs corresponding controls. {Adapted by permission from Kang et al.25}
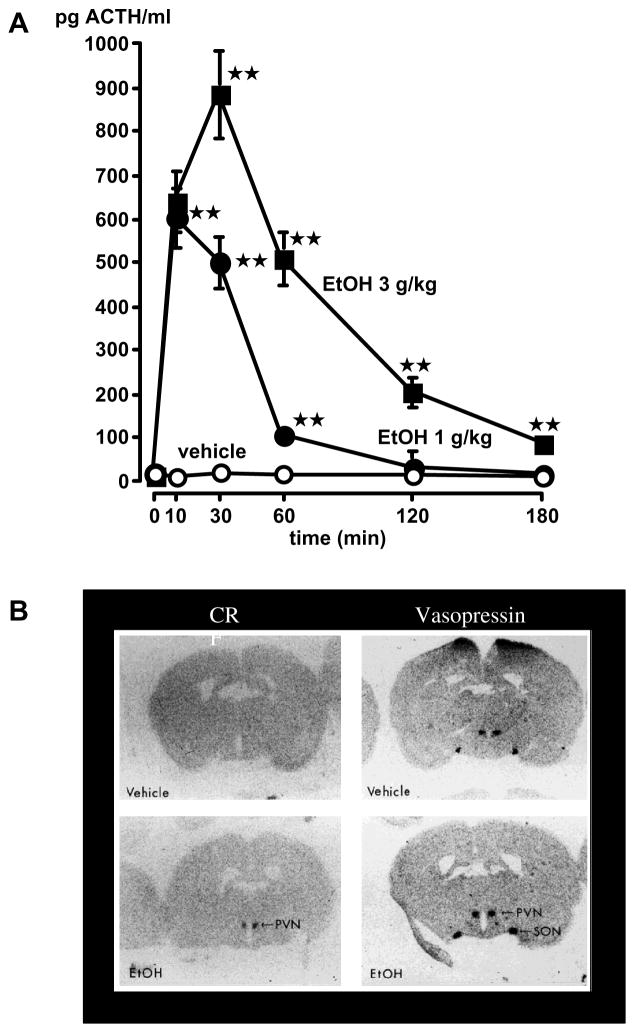
What are the mechanisms that mediate the influence of this drug? Alcohol is a unique drug because it appears to have multiple primary targets that include ligand-gated ion channels such as those associated with GABA, NMDA and serotonin, transporters, neurotransmitters, peptides and steroids. As alcohol readily crosses the placenta, a better understanding of how it activates the HPA axis in naïve animals (i.e., not exposed to the drug during fetal development) may be useful because it may provide a basis for understanding how the drug modifies the HPA axis of the developing fetuses. We have found that acute injection of alcohol elevated plasma ACTH levels and upregulated PVN CRF and VP heteronuclear (hn) RNA gene expression30, 31 (Fig. 5). As removal of endogenous CRF or blockade of its receptors virtually abrogated alcohol-induced ACTH release32 (Fig. 6) as well as alcohol-induced POMC synthesis,33 we concluded that this peptide was required for these responses. While several neurotransmitters likely mediate the sitmulatory influence of alcohol on the HPA axis {see for example 34}, it is also possible that the drug acts directly on the CRF gene. Indeed, we recently reported that a moderate dose of alcohol increased CRF release by cultured hypothalamic cells (Fig. 7A) as well as CRF mRNA levels in these cells, as demonstrated by RT-PCR35 (Fig. 7B).
Figure 5.
Acute alcohol releases ACTH (A) and upregulates PVN CRF and VP hnRNA gene expression (B). Results were obtained in adult male rats as the results of their first exposure to the drug. A. The vehicle or alcohol was injected ip and blood samples were removed serially for ACTH measurement. Each point represents the mean ± SEM of 5 rats. **, P<0.01. B. Effect of alcohol on PVN CRF and VP heteronuclear transcript (hnRNA). Left panel: rostrocaudal coronal sections of the PVN of rats injected with the vehicle or alcohol (3 g/kg) that display signals on X-ray film for CRF hnRNA measured 20 min post-injection. Right panel: rostrocaudal sections showing VP hnRNA measured in the PVN 5 min after vehicle or alcohol treatment. Sections were exposed directly to X-ray film for 3 days. {Modified by permission from Rivier et al.30}
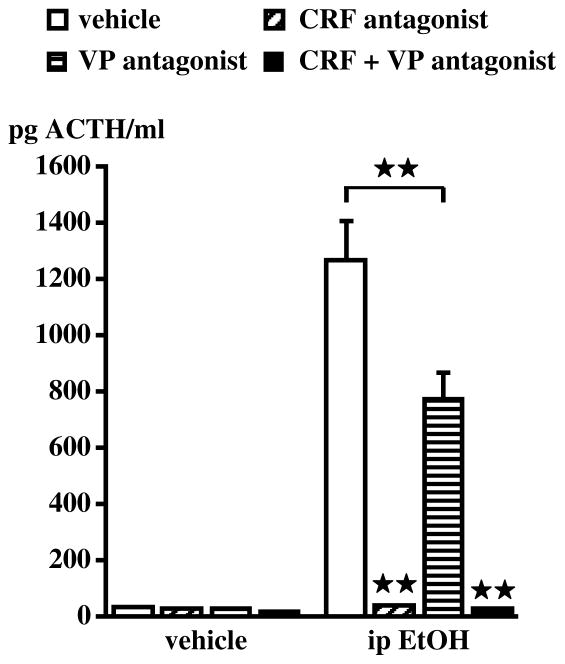
Figure 6.
Blockade of CRF or VP significantly decreases the ACTH response to acute alcohol. Results were obtained in adult male rats as the results of their first exposure to the drug. The CRF antagonist astressin (3 mg/kg) or the VP antagonist dPTyr(Me)VP (0.25 mg/kg) were injected iv and blood samples were obtained 30 min after alcohol administration (3 g/kg, ip). Each bar represents the mean ± SEM of 5–6 animals. **, P<0.01. {Modified by permission from Rivier et al.30 and Ogilvie et al.89}
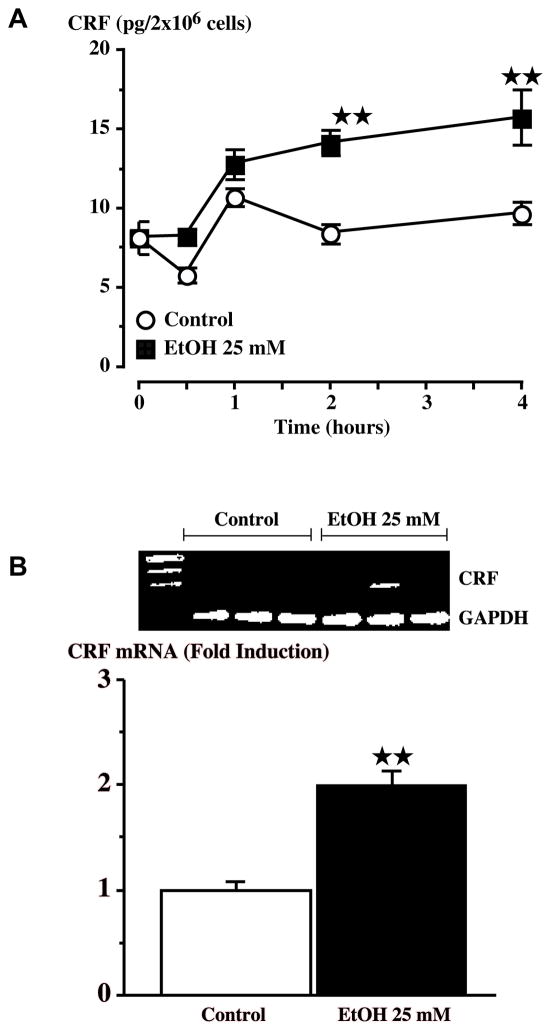
Figure 7.
Alcohol (25 mM) increases CRF release (A) or mRNA levels (B) in primary hypothalamic cell cultures. (A) CRF peptide secretion was detected by RIA after treatment of cells with alcohol. Each point represents the mean ± SEM. (B) RT-PCR analysis of CRF mRNA expression after treatment with 25 mM alcohol. **, P<0.01 vs control. {Modified by permission from Li et al.35}
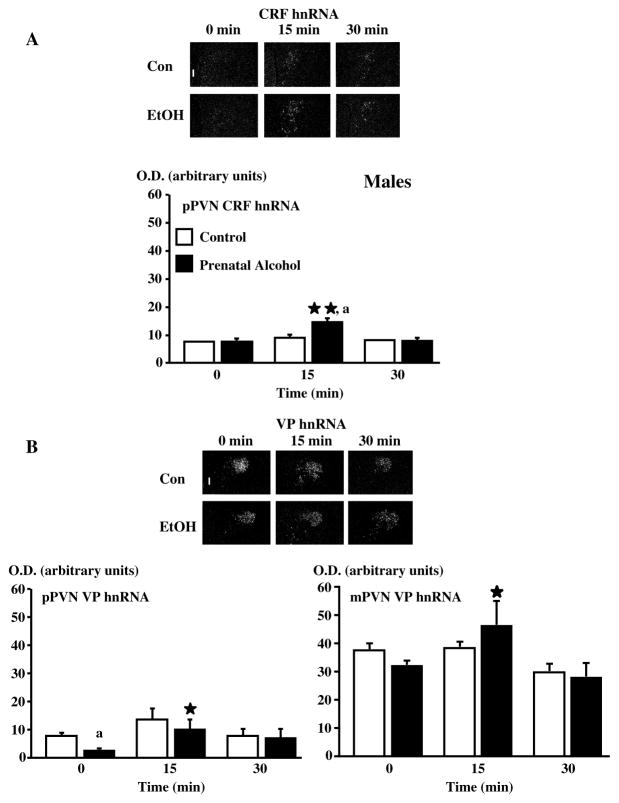
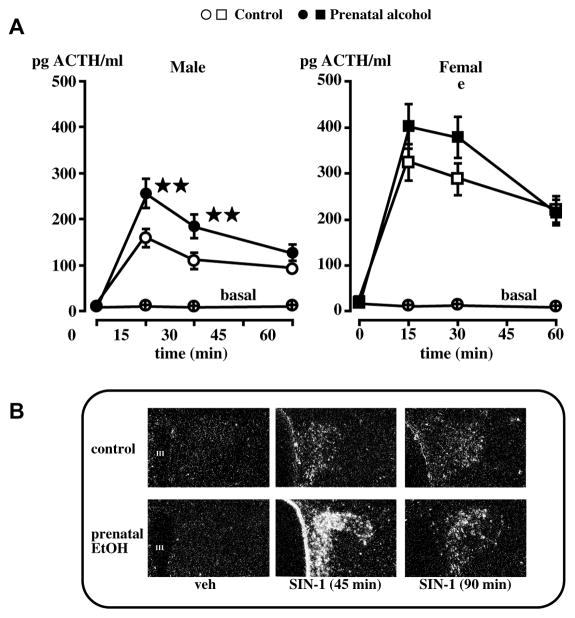
At present, we do not know whether alcohol induces permanent changes in the fetuses’ HPA axis through a direct or indirect effect on PVN CRF, and our studies have therefore focused on the mechanisms through which the adult offspring’ HPA axis was activated. We had found that in adrenal-intact fetal alcohol exposed (FAE) adult offspring, pituitary responsiveness to CRF or VP was unaltered.36 On the other hand, PVN CRF hnRNA gene expression was significantly increased in response to stressors, compared to controls (Fig. 8). Studies of the influence of maternal alcohol on the rat fetus’s developping brain have uncovered a vast array of possible mechanisms, including changes in overall CNS development,37, 38 cell proliferation39, 40 or survival,41 synaptic plasticity,42 levels of retinoids43, 44 and neurotransmitters,45–49 electrophysiological,50 behavioral51 or memory-linked events,52 and poor nutrition,53, 54 to quote only a few. We based our own studies on what we knew about some of the critical signals that influence PVN CRF neuronal activity, and will illustrate two such mechanisms here. The first pertains to nitric oxide (NO), an unstable gas that acts as a transmitter in many parts of the brain, including the hypothalamus.55, 56 We had shown that the injection of NO donors into the brain lateral ventricles rapidly increased plasma ACTH levels, and that this response depended on endogenous CRF.57–59 As there was some evidence that prenatal alcohol might alter brain NO levels in the offspring,46, 60–62 we tested the hypothesis that our FAE model was accompanied by upregulated hypothalamic levels of this gas or by an altered the HPA axis response to NO. We did not measure significant changes in gene expression levels of NO synthase, the enzyme responsible for NO formation, in the PVN of FAE rats.63 On the other hand, we observed that these animals displayed an altered HPA axis response to NO.63 Indeed, as illustrated in Fig. 9, not only did the NO donor SIN-1 cause larger increases in plasma ACTH levels in FAE rats, compared to controls, it also caused a more robust PVN response in terms of CRF neurons.
Figure 8.
The PVN CRF neuronal response in response to footshocks is larger in adult adult male FAE offspring, compared to controls. Dark-field photographs of a representative coronal section through the midportion of the PVN of C or E male rats exposed to a 30-min session of mild electrofootshocks and showing increases in CRF hnRNA (A) levels in the pPVN at 15 min and no overall changes in VP hnRNA (B) levels of the pPVN and the mPVN at 15 and 30 min after shocks. Statistical analysis of the data expressed as arbitrary units for optical density (OD) for mRNA levels. Each point represents the mean ± SEM of 4–5 rats. *, P <0.05 and **, P<0.01 vs t=0; a, P<0.01 vs C at the corresponding time. pPVN, parvocellular division of the paraventricular nucleus, mPVN, magnocellular division of the paraventricular nucleus. Magnification, 220×, III, third ventricle. {Adapted by permission from Lee et al.36}
Figure 9.
Compared to controls, male FAE rats release more ACTH when injected with SIN-1 icv (A) and display enhanced PVN CRF neuronal activity (B). A. Effect of the icv injection of SIN-1 (20 μg) or vehicle on the ACTH response of male and female C and E rats. **, P<0.01. B. Representative dark-field photomicrographs illustrate the immediate early gene NGFI-B transcripts measured before SIN-1 icv injection as well as 45 and 90 min later. III, 3rd ventricle. Magnification: 340×. {Adapted by permission from Lee et al.63}
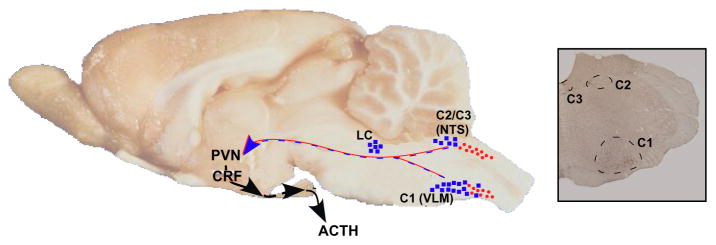
The second hypothesis we tested was that the HPA axis of FAE rats might be more responsive to catecholamines, and/or that adrenergic inputs to the PVN might be upregulated. In agreement with other investigators, we had previously shown that both alpha and beta-adrenergic agonists stimulated the HPA axis in naïve rats {for ref. see 64}. However, when we investigated the potential role of these amines in our prenatal alcohol model, we found that the ACTH response of FAE rats to phenylephrine or propranolol was comparable to that of controls.65 Similarly, the ability of footshocks to stimulate adrenergic neurons in the locus coeruleus, a region with critical adrenergic innervation of the PVN,66 or to elevate the number of PVN cells positive for tyroxine-hydroxylase (TH), a rate-limiting enzyme in catecholamine synthesis, was also comparable in FAE and control rats. In contrast, we made the unexpected observation that the C1 adrenergic region of the brain stem was significantly more activated by shocks in rats exposed to alcohol during embryonic development.65 The C1 brain stem region, which is illustrated in Fig. 10, contributes to the adrenergic innervation of the PVN, in particular those of CRF cells.67 Its influence on PVN neuronal activity is further supported by the fact that lesions transecting the adrenergic projections between the brain stem medulla oblongata and the hypothalamus prevent increased catecholaminergic activity in the brain stem.68 These observations suggest a functional role of C1 neurons on the areas of the PVN that drive the HPA axis. Thus while at present the precise mechanisms through which increased activity of the C1 region participates in the HPA axis hyperactivity observed in our prenatal alcohol model, it is possible that it augments adrenaline release onto CRF-expressing neurons in the parvocellular region of the PVN, thus potentiating footshock-induced increases in the ACTH response observed in FAE rats. This being said, it should be noted that C1 adrenergic neurons have also been shown to influence ACTH via changes in arterial pressure.69, 70 Therefore, increased activity of these neurons could potentially contribute to the enhanced ACTH response to footshocks via changes in afferents to the PVN as well as via altered cardiovascular activity. Regardless of the mechanisms that will be shown important in the FAE model, our results indicate that brainstem adrenergic neurons may exert more influence on the HPA axis than previously understood.
Figure 10.
Cartoon illustrating portions of the ascending catecholaminergic innervation of the PVN. Left panel, sagittal view of the rat brain illustrating the adrenergic (closed squares) and noradrenergic (closed circles) cell groups of the locus coeruleus (LC), ventrolateral medulla (VLM) and nucleus of the solitary tract (NTS). Right panel, coronal section through the rat brain stem illustrating the C1-C3 adrenergic cell populations. (Based on templates published in 3, 90–92.)
As it is likely that prenatal alcohol exposure alters many neurotransmitter systems (see above), we recently decided to use microarray analysis to search for candidate genes that might provide us with new hypotheses. A previous study conducted in mice had identified 75 genes that were down-regulated by exposure to alcohol on days 7 and 9 of fetal development, but none that were up-regulated.71 The genes whose levels were altered are linked to cell proliferation, differentiation and apoptosis, and are in general considered to contribute to tissue growth and survival. In our own studies, we used the brain area containing the hypothalamus and the thalamus of 17.5 day-old mice embryos, and compared tissues obtained from controls as well as from animals exposed to alcohol vapors from day 8 of gestation25 (Table 1). Possibly relevant for our model was the finding of slightly upregulated POMC gene expression, which may be linked to stimulation of the fetal HPA axis. Messenger RNA levels for the CRF-related peptide Urocortin 2 (Ucn 2) were significantly decreased by prenatal alcohol while those for the corresponding receptor, CRFR2, were upregulated. However, these results are difficult to interpret because our preliminary findings do not support a role for these receptors in our prenatal alcohol model (S. Lee and C. Rivier, in preparation). Prenatal alcohol also altered levels of the three known vasopressin receptors.72 Among these receptors, the R1b type73 is most relevant for ACTH because it mediates the pituitary effect of its cognate peptide.74 On the other hand, in the periphery, VPR1a75 mediates the vascular influence of vasopressin76 while VPR277 mediates its antidiuretic effects in the kidney.77 While all three receptors have been found in the rat brain, including the hypothalamus,78–86 their role in the central nervous system remains ill defined. Thus, the significance of prenatal alcohol-induced changes in gene expression of these receptors will need to be investigated, and may well extend beyond their possible involvement within the HPA axis. Finally, it was of interest to note that mRNA levels for phenylethanolamine N-methyltransferase (PNMT), an enzyme that converts norepinephrine to epinephrine,67 and mRNA levels for dopamine-β-hydroxylase (DBH), which is required for norepinephrine formation after dopamine side chain hydroxylation and is a marker of epinephrine/norepinephrine neurons, were significantly lowered in the brain of mouse embryos exposed to alcohol. Whether this phenomenon is linked to altered adrenergic influence on the HPA axis, is presently unknown.
Table 1.
Microarray of fetal hypothalami and thalami area (E 17.5) in control or alcohol-exposed mice.
| Gene | Ratio (EtOH/CTL) |
|---|---|
| CRF | ND |
| CRFR1 | 1.007 |
| CRFR2 | 3.725 |
| UCN | ND |
| UCN2 | 0.329 |
| VP | 0.788 |
| VPR1a | 0.171 |
| VPR1b | 0.57 |
| VPR2 | 7.753 |
| VPi1 | 1.08 |
| POMC1 | 1.633 |
| TH | 1.032 |
| DBH | 0.581 |
| PNMT | 0.369 |
| NPY | 1.051 |
| NPYR1 | 0.534 |
| NPYR2 | 0.911 |
| NPYR5 | 0.507 |
| NPYR6 | 2.185 |
In conclusion, we have uncovered two possible mechanisms that may mediate the hyperactivity of the HPA axis of adult FAE rats. The first one pertains to the NO system, a gas whose stimulatory influence is stronger on the HPA axis of FAE, compared to control rats. The second mechanism is represented by a more robust C1 neuronal response to stressors, which may, in turn, induce a stronger activation of PVN cell bodies. As briefly described in the Introduction, brain CRF on one hand, and circulating GC on the other, exert important influences on key responses of the body to homeostatic challenges. Consequently, deregulation of their release is likely to induce significant pathogeneses. Children born to mothers who abused alcohol during pregnancy can display, for example, increased occurrence of infections, disorders in which abnormally elevated GC levels often play a role. These children also often show increased activity levels, attention deficits, higher incidence of drug abuse and/or depression.51, 87 While none of these disorders have been unambiguously demonstrated to result from elevated CRF levels in humans, the influence of this peptide on behavior, drug consumption and mood disorders is well documented in pre-clinical studies (see above). In view of the effects of GC on the mobilization of sugars and fat reserves, as well as the peptides that regulate food intake,8, 88 it is also likely that individuals who consistently release too much cortisol in response to various stimuli may develop metabolic disorders. Experiments carried out in mice lacking CRF or its receptors, or the use of CRF antagonists in human medicine, may allow us to determine the role played by CRF in disorders caused by prenatal alcohol exposure, and to develop therapies that are palliative or restorative.
Acknowledgments
Research supported by NIH grant AA-08924.
References
- 1.Simerly RB. Hypothalamic substrates of metabolic imprinting. Physiol Behav. 2008;94:79–89. doi: 10.1016/j.physbeh.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sawchenko P. Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. In: Mayer E, Saper C, editors. Progress in Brain Research. Vol. 122. Elsevier Sciences; 2000. pp. 61–78. [DOI] [PubMed] [Google Scholar]
- 3.Sawchenko PE, Brown ER, Chan RKW, Ericsson A, Li H-Y, Roland BL, Kovacs KJ. The paraventricular nucleus of the hypothalamus and the functional neuroanatomy of visceromotor responses to stress. In: Holstege G, Bandler R, Saper CB, editors. Emotional Motor System. Vol. 107. Elsevier Science B. V; 1996. pp. 201–222. [DOI] [PubMed] [Google Scholar]
- 4.Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 5.Herman JP, Cullinan WE, Ziegler DR, Tasker JG. Role of the paraventricular nucleus microenvironment in stress integration. Eur J Neurosci. 2002;16:381–385. doi: 10.1046/j.1460-9568.2002.02133.x. [DOI] [PubMed] [Google Scholar]
- 6.Watts AG. The impact of physiological stimuli on the expression of corticotropin-releasing hormone (CRH) and other neuropeptide genes. Front Neuroendocrinol. 1996;17:281–326. doi: 10.1006/frne.1996.0008. [DOI] [PubMed] [Google Scholar]
- 7.Tilbrook AJ, Clarke IJ. Neuroendocrine mechanisms of innate states of attenuated responsiveness of the hypothalamo-pituitary adrenal axis to stress. Front Neuroendocrinol. 2006;27:285–307. doi: 10.1016/j.yfrne.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Dallman MF. Fast glucocorticoid actions on brain: back to the future. Front Neuroendocrinol. 2005;26:103–108. doi: 10.1016/j.yfrne.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Dallman MF. Modulation of stress responses: How we cope with excess glucocorticoids. Exp Neurol. 2007;206:179–182. doi: 10.1016/j.expneurol.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Day TA. Defining stress as a prelude to mapping its neurocircuitry: no help from allostasis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1195–1200. doi: 10.1016/j.pnpbp.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Koob GF, Heinrichs SC, Pich EM, Menzaghi F, Baldwin H, Miczek K, Britton KT. The role of corticotropin-releasing factor in behavioural responses to stress. Ciba Foundation Symposium No. 172 on Corticotropin-Releasing Factor; 1993. pp. 277–295. [DOI] [PubMed] [Google Scholar]
- 12.Brown M, Fisher L. Regulation of the autonomic nervous system by corticotropin-releasing factor. In: deSouza E, Nemeroff CB, editors. Corticotropin-releasing factor: Basic and clinical studies of a neuropeptide. CRC Press; Boca Raton, FL: 1990. pp. 292–298. [Google Scholar]
- 13.Rivier C, Vale W. Effects of CRF, neurohypophysial peptides and catecholamines on pituitary function. Fed Proc. 1985;44:189–195. [PubMed] [Google Scholar]
- 14.Rivier C, Vale W. Influence of corticotropin-releasing factor (CRF) on reproductive functions in the rat. Endocrinology. 1984;114:914–919. doi: 10.1210/endo-114-3-914. [DOI] [PubMed] [Google Scholar]
- 15.Rivest S, Rivier C. The role of corticotropin-releasing factor and interleukin-1 in the regulation of neurons controlling reproductive functions. Endocr Rev. 1995;16:177–199. doi: 10.1210/edrv-16-2-177. [DOI] [PubMed] [Google Scholar]
- 16.Tache Y, Perdue MH. Role of peripheral CRF signalling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterol Motil. 2004;16(Suppl 1):137–142. doi: 10.1111/j.1743-3150.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 17.Martinez V, Wang L, Rivier J, Grigoriadis D, Tache Y. Central CRF, urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice. J Physiol. 2004;556:221–234. doi: 10.1113/jphysiol.2003.059659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivier CL, Plotsky PM. Mediation by corticotropin-releasing factor (CRF) of adenohypophysial hormone secretion. Ann Rev Physiol. 1986;48:475–494. doi: 10.1146/annurev.ph.48.030186.002355. [DOI] [PubMed] [Google Scholar]
- 19.Lee S, Rivier C. Administration of corticosterone to pregnant adrenalectomized dams does not alter the hypothalamic-pituitary-adrenal axis’ activity of the offspring. Mol Cell Neurosci. 1992;3:118–123. doi: 10.1016/1044-7431(92)90015-t. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Sliwowska JH, Weinberg J. Prenatal alcohol exposure and fetal programming: effects on neuroendocrine and immune function. Exper Biol Med (Maywood) 2005;230:376–388. doi: 10.1177/15353702-0323006-05. [DOI] [PubMed] [Google Scholar]
- 21.Kim CK, Giberson PK, Yui W, Zoeller RT, Weinberg J. Effects of prenatal ethanol exposure on hypothalamic-pituitary-adrenal responses to chronic cold stress in rats. Alcoholism: Clin Exper Res. 1999;23:301–310. [PubMed] [Google Scholar]
- 22.Lee S, Rivier C. Gender differences in the effect of prenatal alcohol exposure on the hypothalamic-pituitary-adrenal axis response to immune signals. Psychoneuroendocrinology. 1996;21:145–156. doi: 10.1016/0306-4530(95)00038-0. [DOI] [PubMed] [Google Scholar]
- 23.Ogilvie K, Rivier C. Prenatal alcohol exposure results in hyperactivity of the hypothalamic-pituitary-adrenal axis of the offspring: Modulation by fostering at birth and postnatal handling. Alcoholism: Clin Exper Res. 1997;21:424–429. doi: 10.1111/j.1530-0277.1997.tb03786.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim C, Turnbull A, Lee S, Rivier C. Effects of prenatal exposure to alcohol on the release of adrenocorticotropic hormone, corticosterone and proinflammatory cytokines. Alcoholism: Clin Exper Res. 1999;23:52–59. [PubMed] [Google Scholar]
- 25.Kang S, Cole M, Lee S, RC Development of individual inhalation chambers for mice: Validation in a model of prenatal alcohol. Alcoholism: Clin Exper Res. 2004;28:1549–1556. doi: 10.1097/01.alc.0000141639.79278.5e. [DOI] [PubMed] [Google Scholar]
- 26.Lee SY, Imaki T, Vale W, Rivier CL. Effect of prenatal exposure to ethanol on the activity of the hypothalamic-pituitary-adrenal axis of the offspring: importance of the time of exposure to ethanol and possible modulating mechanisms. Mol Cell Neurosci. 1990;1:168–177. doi: 10.1016/1044-7431(90)90022-v. [DOI] [PubMed] [Google Scholar]
- 27.Altman J, Bayer S. The development of the rat hypothalamus. Adv Anat Embryol Cell Biol. 1986;100:1–178. [PubMed] [Google Scholar]
- 28.Ifft J. An autoradiographic study of the time of final divisions of neurons in rat hypothalamic nuclei. J Comp Neurol. 1972;144:193–204. doi: 10.1002/cne.901440204. [DOI] [PubMed] [Google Scholar]
- 29.Weinberg J. Hyperresponsiveness to stress: Differential effects of prenatal ethanol on males and females. Alcoholism: Clin Exper Res. 1988;12:647–652. doi: 10.1111/j.1530-0277.1988.tb00258.x. [DOI] [PubMed] [Google Scholar]
- 30.Rivier C, Lee S. Acute alcohol administration stimulates the activity of hypothalamic neurons that express corticotropin-releasing factor and vasopressin. Brain Res. 1996;726:1–10. [PubMed] [Google Scholar]
- 31.Lee S, Lee KF, Bradbury M, Vale W, Rivier C. Alcohol increases steady-state gene expression of corticotropin-releasing factor in the paraventricular nucleus of CRF receptor 1-deficient mice. Alcoholism: Clin Exper Res. 1999;23S:22A. [Google Scholar]
- 32.Rivier C, Bruhn T, Vale W. Effect of ethanol on the hypothalamic-pituitary-adrenal axis in the rat: Role of corticotropin-releasing factor (CRF) J Pharmacol Exper Therap. 1984;229:127–131. [PubMed] [Google Scholar]
- 33.Lee S, Selvage D, Rivier C. Site of action of acute alcohol administration in stimulating the rat hypothalamic-pituitary-adrenal axis: Comparison between the effect of systemic and intracerebroventricular injection of this drug on pituitary and hypothalamic responses. Endocrinology. 2004;145:4470–4479. doi: 10.1210/en.2004-0110. [DOI] [PubMed] [Google Scholar]
- 34.Rivier C. Alcohol stimulates ACTH secretion in the rat: Mechanisms of action and interactions with other stimuli. Alcoholism: Clin Exper Res. 1996;20:240–254. doi: 10.1111/j.1530-0277.1996.tb01636.x. [DOI] [PubMed] [Google Scholar]
- 35.Li Z, Kang SS, Lee S, Rivier C. Effect of ethanol on the regulation of corticotropin-releasing factor (CRF) gene expression. Mol Cell Neurosci. 2005;29:345–354. doi: 10.1016/j.mcn.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Lee S, Schmidt D, Tilders F, Rivier C. Increased activity of the hypothalamic-pituitary-adrenal axis in rats exposed to alcohol in utero: Role of altered pituitary and hypothalamic function. Mol Cell Neurosci. 2000;16:515–528. doi: 10.1006/mcne.2000.0890. [DOI] [PubMed] [Google Scholar]
- 37.Maier SE, Chen WJA, Miller JA, West JR. Fetal alcohol exposure and temporal vulnerability: Regional differences in alcohol-induced microencephaly as a function of the timing of binge-like alcohol exposure during rat brain development. Alcoholism: Clin Exper Res. 1997;21:1418–1428. doi: 10.1111/j.1530-0277.1997.tb04471.x. [DOI] [PubMed] [Google Scholar]
- 38.Livy DJ, Miller EK, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol Teratol. 2003;25:447–458. doi: 10.1016/s0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- 39.Miller MW. Effects of prenatal exposure to ethanol on neocortical development: II. Cell proliferation in the ventricular and subventricular zones of the rat. J Comp Neurol. 1989;287:326–338. doi: 10.1002/cne.902870305. [DOI] [PubMed] [Google Scholar]
- 40.Guerri C. Neuroanatomical and neurophysiological mechanisms involved in central nervous system dysfunctions induced by prenatal alcohol exposure. Alcoholism: Clin Exper Res. 1998;22:304–312. doi: 10.1111/j.1530-0277.1998.tb03653.x. [DOI] [PubMed] [Google Scholar]
- 41.Sarkar DK, Kuhn P, Marano J, Chen C, Boyadjieva N. Alcohol Exposure during the Developmental Period Induces {beta}-Endorphin Neuronal Death and Causes Alteration in the Opioid Control of Stress Axis Function. Endocrinology. 2007;148:2828–2834. doi: 10.1210/en.2006-1606. [DOI] [PubMed] [Google Scholar]
- 42.Sutherland RJ, McDonald R, Savage DD. Prenatal exposure to moderate levels of ethanol can have long-lasting effects on hippocampal synaptic plasticity in adult offspring. Hippocampus. 1997;7:232–238. doi: 10.1002/(SICI)1098-1063(1997)7:2<232::AID-HIPO9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 43.Grummer MA, Langhough RE, Zachman Rd. Maternal ethanol ingestion effects on fetal rat brain vitamin A as a model for fetal alcohol syndrome. Alcoholism: Clin Exper Res. 1993;17:592–597. doi: 10.1111/j.1530-0277.1993.tb00805.x. [DOI] [PubMed] [Google Scholar]
- 44.Deltour L, Ang HL, Duester G. Ethanol inhibition of retinoic acid synthesis as a potential mechanism for fetal alcohol syndrome. FASEB J. 1996;10:1050–1057. [PubMed] [Google Scholar]
- 45.Gottesfeld Z, Garcia C, Lingham R, Chronister R. Prenatal ethanol exposure impairs lesion-induced plasticity in a dopaminergic synapse after maturity. Neuroscience. 1989;29:715–723. doi: 10.1016/0306-4522(89)90143-7. [DOI] [PubMed] [Google Scholar]
- 46.Druse MJ. Effects of in utero ethanol exposure on the development of neurotransmitter systems. Dev Central Nervous Syst. 1992:139–167. [Google Scholar]
- 47.Becker HC, Hale RL, Boggan WO, Randall CL. Effects of prenatal ethanol exposure on later sensitivity to the low-dose stimulant actions of ethanol in mouse offspring: possible role of catecholamines. Alcoholism: Clin Exper Res. 1993;17:1325–1335. doi: 10.1111/j.1530-0277.1993.tb05249.x. [DOI] [PubMed] [Google Scholar]
- 48.Spuhler-Phillips K, Lee YH, Hughes P, Randoll L, Leslie SW. Effects of prenatal ethanol exposure on brain region NMDA-mediated increase in intracellular calcium and the NMDAR1 subunit in forebrain. Alcoholism: Clin Exper Res. 1997;21:68–75. [PubMed] [Google Scholar]
- 49.Naassila M, Daoust M. Effect of prenatal and postnatal ethanol exposure on the developmental profile of mRNAs encoding NMDA receptor subunits in rat hippocampus. J Neurochem. 2002;80:850–860. doi: 10.1046/j.0022-3042.2002.00755.x. [DOI] [PubMed] [Google Scholar]
- 50.Kaneko WM, Riley EP, Ehlers CL. Electrophysiological and behavioral findings in rats prenatally exposed to alcohol. Alcohol. 1993;10:169–178. doi: 10.1016/0741-8329(93)90099-a. [DOI] [PubMed] [Google Scholar]
- 51.Riley EP. The long-term behavioral effects of prenatal alcohol exposure in rats. Alcoholism: Clin Exp Res. 1990;14:670–673. doi: 10.1111/j.1530-0277.1990.tb01225.x. [DOI] [PubMed] [Google Scholar]
- 52.Vaglenova J, Petkov VV. Fetal alcohol effects in rats exposed pre- and postnatally to a low dose of ethanol. Alcoholism: Clin Exper Res. 1998;22:697–703. [PubMed] [Google Scholar]
- 53.Weinberg J. Effects of ethanol and maternal nutritional status on fetal development. Alcoholism: Clin Exper Res. 1985;9:49–55. doi: 10.1111/j.1530-0277.1985.tb05049.x. [DOI] [PubMed] [Google Scholar]
- 54.Dreosti IE. Nutritional factors underlying the expression of the fetal alcohol syndrome. Ann NY Acad Sci. 1993;678:193–204. doi: 10.1111/j.1749-6632.1993.tb26122.x. [DOI] [PubMed] [Google Scholar]
- 55.Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 56.Bredt DS, Snyder SH. Nitric oxide: A physiologic messenger molecule. Annu Rev Biochem. 1994;63:175–95. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- 57.Rivier C, Shen G. In the rat, endogenous nitric oxide modulates the response of the hypothalamic-pituitary-adrenal axis to interleukin-1β, vasopressin and oxytocin. J Neurosci. 1994;14:1985–1993. doi: 10.1523/JNEUROSCI.14-04-01985.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee S, Kim C, Rivier C. Nitric oxide stimulates ACTH secretion and the transcription of the genes encoding for NGFI-B, corticotropin-releasing factor, corticotropin-releasing factor receptor type 1 and vasopressin in the hypothalamus of the intact rat. J Neurosci. 1999;19:7640–7647. doi: 10.1523/JNEUROSCI.19-17-07640.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seo DO, Rivier C. Microinfusion of a nitric oxide donor in discrete brain regions activates the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol. 2001;13:925–933. doi: 10.1046/j.1365-2826.2001.00690.x. [DOI] [PubMed] [Google Scholar]
- 60.Kimura KA, Parr AM, Brien JF. Effect of chronic maternal ethanol administration on nitric oxide synthase activity in the hippocampus of the mature fetal guinea pig. Alcohol: Clin Exper Res. 1996;20:948–953. doi: 10.1111/j.1530-0277.1996.tb05276.x. [DOI] [PubMed] [Google Scholar]
- 61.Kimura KA, Brien JF. Hippocampal nitric oxide synthase in the fetal guinea pig: effects of chronic prenatal ethanol exposure. Dev Brain Res. 1998;106:39–46. doi: 10.1016/s0165-3806(97)00184-3. [DOI] [PubMed] [Google Scholar]
- 62.Jang MH, Lee MH, Kim H, Lee SJ, Sim YJ, Kim CJ, Park SK, Kim J, Kim EH. Maternal alcohol administration suppresses expression of nitric oxide synthase in the hippocampus of offspring rats. J Pharmacol Sci. 2005;98:459–462. doi: 10.1254/jphs.sc0050060. [DOI] [PubMed] [Google Scholar]
- 63.Lee S, Blanton C, Rivier C. Prenatal ethanol exposure alters the responsiveness of the rat hypothalamic-pituitary-adrenal axis to nitric oxide. Alcoholism: Clin Exper Res. 2003;27:962–969. doi: 10.1097/01.ALC.0000076120.67014.ED. [DOI] [PubMed] [Google Scholar]
- 64.Seo D, Lee S, Rivier C. Role of specific adrenergic receptors in mediating the ACTH response to increased nitric oxide levels. J Neuroendocrinol. 2003;15:530–537. doi: 10.1046/j.1365-2826.2003.01027.x. [DOI] [PubMed] [Google Scholar]
- 65.Choi I, Lee S, Rivier C. Novel role of adrenergic neurons in the brain stem in mediating the hypothalamic-pituitary axis hyperactivity caused by prenatal alcohol exposure. Neuroscience. 2008 doi: 10.1016/j.neuroscience.2008.04.081. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sawchenko PE, Cunningham ET, Bittencourt JC. Aminergic and peptidergic pathways subserving the stress response. In: Kvetnansky R, McCarty R, Axelrod J, editors. Stress: Neuroendocrine and Molecular Approaches. Gordon and Breach; New York: 1992. pp. 15–27. [Google Scholar]
- 67.Cunningham E, Bohn M, Sawchenko P. Organization of adrenergic inputs to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol. 1990;292:651–667. doi: 10.1002/cne.902920413. [DOI] [PubMed] [Google Scholar]
- 68.Li HY, Ericsson A, Sawchenko PE. Distinct mechanisms underlie activation of hypothalamic neurosecretory neurons and their medullary catecholaminergic afferents in categorically different stress paradigms. Proc Natl Acad Sci USA. 1996;93:2359–2364. doi: 10.1073/pnas.93.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baertschi AJ, Ward DG, Gann DS. Role of atrial receptors in the control of ACTH. Am J Physiol. 1976;231:692–699. doi: 10.1152/ajplegacy.1976.231.3.692. [DOI] [PubMed] [Google Scholar]
- 70.Reis DJ. The C1 area of rostra 1 ventrolateral medulla: Role in tonic and reflex regulation of arterial pressure. In: Magro A, Osswalk W, Reis D, Vanhoutte P, editors. Central and peripheral mechanisms of cardiovascular regulation. Kluwer Academic Pub; New York: 1986. pp. 487–502. [Google Scholar]
- 71.Hard M, Abdolell M, Robinson B, Koren G. Gene-expression analysis after alcohol exposure in the developping mouse. J Lab Clin Med. 2005;145:47–54. doi: 10.1016/j.lab.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 72.Jard S. Vasoresspin receptors. Front Horm Res. 1985;13:89–105. [Google Scholar]
- 73.Saito M, Sugimoto T, Tahara A, Kawashima H. Molecular cloning and characterization of rat V1B vasopressin receptor: evidence for its expression in extra-pituitary tissues. Biochem Biophys Res Comm. 1995;212:751–757. doi: 10.1006/bbrc.1995.2033. [DOI] [PubMed] [Google Scholar]
- 74.Antoni FA. Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Front Neuroendocrinol. 1993;14:76–122. doi: 10.1006/frne.1993.1004. [DOI] [PubMed] [Google Scholar]
- 75.Morel A, O’Carroll AM, Brownstein MJ, Lolait SJ. Molecular cloning and expression of a rat V1a arginine vasopressin receptor. Nature. 1992;356:523–526. doi: 10.1038/356523a0. [DOI] [PubMed] [Google Scholar]
- 76.Howl J, Wheatley M. Molecular pharmacology of Vla vasopressin receptors. Gen Pharmacol. 1995;26:1143–1152. doi: 10.1016/0306-3623(95)00016-t. [DOI] [PubMed] [Google Scholar]
- 77.Lolait SJ, O’Carroll AM, McBride OW, Konig M, Morel A, Brownstein MJ. Cloning and characterization of a vasopressin V2 receptor and possible link to nephrogenic diabetes insipidus. Nature. 1992;357:336–339. doi: 10.1038/357336a0. [DOI] [PubMed] [Google Scholar]
- 78.Ostrowski NL, Lolait SJ, Bradley DJ, O’Carroll AM, Brownstein MJ, Young WF., III Distribution of V1a and V2 vasopressin receptor messenger ribonucleic acids in rat liver, kidney, pituitary, and brain. Endocrinology. 1992;131:533–535. doi: 10.1210/endo.131.1.1535312. [DOI] [PubMed] [Google Scholar]
- 79.Lolait SJ, O’Carroll AM, Mahan LC, Felder CC, Button DC, Young WS, III, Mezey E, Brownstein MJ. Extrapituitary expression of the rat V1b vasopressin receptor gene. Proc Natl Acad Sci, USA. 1995;92:6783–6787. doi: 10.1073/pnas.92.15.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hirasawa A, Hashimoto K, Tsujimoto G. Distribution and developmental change of vasopressin V1A and V2 receptor mRNA in rats. Eur J Pharmacol. 1994;267:71–75. doi: 10.1016/0922-4106(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 81.Ostrowski N, Lolait S, Young W. Cellular localization of vasopressin V1a receptor messenger ribonucleic acid in adult male rat brain, pineal, and brain vasculature. Endocrinology. 1994:135. doi: 10.1210/endo.135.4.7925112. [DOI] [PubMed] [Google Scholar]
- 82.Young WSI, Kovács K, Lolait SJ. The diurnal rhythm in vasopressin V1a receptor expression in the suprachiasmatic nucleus is not dependent on vasopressin. Endocrinology. 1993;133:585–590. doi: 10.1210/endo.133.2.8344200. [DOI] [PubMed] [Google Scholar]
- 83.Hurbin A, Boissin-Agasse L, Orcel H, Rabié A, Joux N, Desarménien MG, Richard P, Moos FC. The V1a and V1b, but not V2, vasopressin receptor genes are expressed in the supraoptic nucleus of the rat hypothalamus, and the transcripts are essentially colocalized in the vasopressinergic magnocellular neurons. Endocrinology. 1998;139:4701–4707. doi: 10.1210/endo.139.11.6320. [DOI] [PubMed] [Google Scholar]
- 84.Saito R, Ishiharada N, Ban Y, Honda K, Takano Y, Kamiya H. Vasopressin V1 receptor in rat hippocampus is regulated by adrenocortical functions. Brain Res. 1994;646:170–174. doi: 10.1016/0006-8993(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 85.Fahrenholz F, Jurzak M, Gerstberger R, Haase W. Renal and central vasopressin receptors: immunocytochemical localization. Ann NY Acad Sci. 1993;689:194–206. doi: 10.1111/j.1749-6632.1993.tb55548.x. [DOI] [PubMed] [Google Scholar]
- 86.Patchev VK, Almeida OFX. Corticosteroid regulation of gene expression and binding characteristics of vasopressin receptors in the rat brain. Eur J Neurosci. 1995;7:1579–1583. doi: 10.1111/j.1460-9568.1995.tb01153.x. [DOI] [PubMed] [Google Scholar]
- 87.Riley EP, Mattson SN, Li TK, Jacobson SW, Coles CD, Kodituwakku PW, Adnams Cm, Korkman MI. Neurobehavioral consequences of prenatal alcohol exposure: an international perspective. Alcoholism: Clin Exper Res. 2003;27:362–373. doi: 10.1097/01.ALC.0000052703.38558.B2. [DOI] [PubMed] [Google Scholar]
- 88.Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun. 2005;19:275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 89.Ogilvie K, Lee S, Weiss B, Rivier C. Mechanisms mediating the influence of alcohol on the hypothalamic-pituitary-adrenal axis responses to immune and nonimmune signals. Alcoholism: Clin Exper Res. 1998;22:243S–247S. doi: 10.1097/00000374-199805001-00005. [DOI] [PubMed] [Google Scholar]
- 90.Sawchenko PE, Swanson LW. Organization of CRF immunoreactive cells and fibers in the rat brain: Immunohistochemical studies. In: DeSouza EB, Nemeroff CB, editors. Corticotropin-Releasing Factor: Basic and Clinical Studies of a Neuropeptide. CRC Press, Inc; Boca Raton, FL: 1990. pp. 29–51. [Google Scholar]
- 91.Ericsson A, Kovács KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li H-Y, Sawchenko PE. Hypothalamic effector neurons and extended circuitries activated in “neurogenic” stress: A comparison of footshock effects exerted acutely, chronically, and in animals with controlled glucocorticoid levels. J Comp Neurol. 1998;393:244–266. [PubMed] [Google Scholar]